Abstract
Objectives
To determine the influence of epidemiologic factors and the influence of genetic variants affecting FKBP5, a protein known to modulate hypothalamic-pituitary-adrenocortical (HPA) axis function, on the severity of somatic symptoms commonly termed “post-concussive” six and twelve months after motor-vehicle collision (MVC).
Methods
European Americans 18–65 years of age who presented to one of eight emergency departments (ED) after MVC were enrolled. Exclusion criteria included hospital admission. Blood samples were collected in the ED for genotyping. Participants completed evaluations including an adapted Rivermead Post-Concussive Symptoms Questionnaire in the ED and at six weeks, six months, and one year. Repeated measures analysis of covariance were used to evaluate the association between epidemiologic factors (sociodemographic, pre-MVC health, collision characteristics, head injury, peritraumatic pain and stress), FKBP5 genetic variants, and post-concussive symptom severity.
Results
Among 943 patients recruited in the ED, follow-up was completed on 835 (88%) at six months and 857 (90%) at one year. Self-reported head impact during collision was not associated with chronic post-concussive symptom severity. After correction for multiple testing, three FKBP5 single nucleotide polymorphisms (rs3800373, rs7753746, and rs9380526) predicted chronic post-concussive symptom severity, with the average symptom severity of 1.10 (95% CI 0.96–1.24), 1.36 (1.21–1.51), and 1.55 (1.23–1.88) for one, two or three copies of minor allele at rs3800373 (p=0.001). Similar effect sizes were observed for the minor alleles of rs7753746 and rs9380526.
Conclusions
Post-concussive symptoms after minor MVC are not generally related to the severity of mild brain injury. This study shows that neurobiologic stress systems may play a role in pathogenesis of post-concussive symptoms.
Keywords: post-concussive symptoms, minor traumatic brain injury, FKBP5, stress response
Introduction
Post-concussive (PC) symptoms are common after traumatic and stressful events such as a non-life threatening motor vehicle collision (MVC) (1). PC symptoms may persist for months or years after trauma exposure, and include symptoms such as headache, dizziness, fatigue, insomnia, and problems with concentration or memory (2–5). Costs due to PC symptoms have been estimated at over 17 billion dollars annually in the United States alone (6).
PC symptoms after non-life threatening motor vehicle collision (MVC) have traditionally been attributed to minor traumatic brain injury (MTBI) (2–4), however available evidence does not support an association between minor head injury and PC symptoms (5, 7–11). The absence of empiric support for the hypothesis that MTBI causes PC symptoms, and of studies examining other potential etiologic mechanisms, has resulted in a contemporary void in understanding the pathogenesis of PC symptoms. This lack of understanding is a barrier to the development of more effective secondary preventive interventions and treatments.
In addition to potentially causing tissue injury, stressful events such as MVC activate neurobiological stress systems which have been shown to be powerful modulators of neurosensory processing (12). If stress system activation contributes to PC symptom development after MVC, then genetic variants which influence the function of important stress systems would be expected to be associated with PC symptom vulnerability. The identification of such genetic associations would provide important evidence that stress systems contribute to PC symptom development, and could also suggest the involvement of specific physiologic systems. However, to date very few genetic association studies have been performed, and none have evaluated physiologic systems of central importance to the stress response such as the hypothalamic-pituitary-adrenocortical (HPA) axis.
This study had two goals. First, we sought to replicate findings from other studies that head injury is not associated with PC symptom severity after non-life threatening MVC in a large cohort with excellent follow-up. Second, we performed a novel genetic association analysis evaluating the association between genetic variants increasing the expression of the gene coding for glucocorticoid receptor co-chaperone FK506 binding protein 51 (FKBP5) and PC symptom burden six months and one year after MVC. Increased FKBP5 expression reduces glucocorticoid receptor sensitivity (13), resulting in decreased negative feedback inhibition of the HPA axis and elevated glucocorticoid levels (13, 14). Elevated glucocorticoid levels have been shown to cause neuronal sensitization and stress-induced hyperalgesia (15), and we hypothesized that glucocorticoid-mediated neuronal sensitization also contributes to the development of persistent PC symptoms.
2. Methods
2.1. Study Design and Setting
Details regarding the methodology of this multicenter emergency department (ED)-based prospective observational cohort study have been described elsewhere (16). Recruitment took place between February 2009 and October 2011 at eight EDs in four no-fault insurance states in the U.S (Massachusetts, Florida, Michigan, and New York). Institutional Review Board approval for the study was obtained at each study site, and each participant provided written informed consent.
2.2. Selection of Participants
Adults aged 18–65 years who were evaluated in the ED after MVC and were alert, oriented, and clinically stable were eligible for study enrollment. Exclusion criteria included evidence of intracranial or spinal injury, fractures other than phalanges, use of β-adrenoceptor antagonists, and/or use of >20mg of morphine or equivalent daily. Pregnant women, prisoners, and non-English speaking patients were also excluded. Patients reporting a loss of consciousness >30 minutes and/or amnesia >24 hours were excluded in order to remove subjects with potential traumatic brain injury (2). Enrollment was limited to individuals of European American ethnicity to reduce the risk of population stratification bias in genetic analyses (17). Participant ethnicity was obtained via self-report; previous studies have shown that race obtained via self-report and genetic assessment are highly correlated (18).
2.3. Data Collection and Measurements
2.3.1 ED Interview
Patients were screened and recruited by research assistants at each ED site. Research assistants’ training involved the completion of a study module followed by an interview with a standardized mock ED patient (error rate of 1.3% across research assistants). After written informed consent was obtained, study participants completed a web-based survey in the ED followed by telephone or web-based follow-up questionnaires six weeks, six months, and one year after MVC. Study participants were compensated $80, $50, $60, and $70 for completing each of these assessments, respectively.
2.3.2 Participant Demographics
Demographic information was extracted from the medical record or obtained via ED interview.
2.3.3 Pre-MVC Health Status
Participants’ health status during the month prior to the collision was assessed in the ED via several questionnaires: the Short-Form Health Survey (SF-12) Mental and Physical Component Summary (19), the Center for Epidemiological Studies Depression (CESD) Scale (20) (score ≥16 corresponds to moderate depression (20), score ≥27 corresponds to severe depression (21)), the State Trait Personality Inventory (STPI-Y) Anxiety and Anger subscales (22, 23).
2.3.4 Collision Characteristics
MVC characteristics were collected using a questionnaire developed with biomechanics experts at the University of Michigan Transportation Research Institute. This questionnaire assesses direction of vehicle impact, head impact occurrence, airbag deployment, and extent of vehicle damage. Self-report responses on this questionnaire have been shown to be strongly correlated with police report data (24).
2.3.5 Symptoms Immediately After the Collision
Current overall pain severity in the ED was assessed via a 0–10 numeric rating scale (NRS) and subsequently grouped into categories of no pain (0), mild pain (0.5–3.5), moderate pain (4–6.5), and severe pain (7–10) using well-established cut-offs (25). The Peritraumatic Distress Inventory (PDI) (26) (score ≥23 corresponds to clinically significant distress (27)), and the Michigan Critical Events Perception Scale (MCEPS) (28) (score ≥15 corresponds to clinically significant dissociation (28)) were also assessed in the ED.
2.3.6 DNA Collection and Processing
DNA collection and FKBP5 genotyping methods for this cohort have been described (29). In brief, blood (8.5 cc) was collected in the ED using PAXgene DNA storage tubes (QIAGEN), then refrigerated and shipped in batches every two weeks to Beckman Coulter Genomics, Inc. (Morrisville, NC) for DNA extraction (PAXgene blood DNA kit, Qiagen, Valencia, CA) and genotyping (Sequenom, Inc., San Diego, CA). Two Hapmap samples and two repeat samples were included in each genotyping batch (96 samples) to ensure genotypic accuracy and reliability. Repeated genotyping demonstrated greater than 98% call agreement. Genotype data that did not show call agreement were set to missing and were subsequently excluded from the analyses. Thirty-three FKBP5 single nucleotide polymorphisms (SNPs) were genotyped in this cohort and selected to cover haplotype diversity at the FKBP5 gene locus. Six SNPs were excluded due to minor allele frequency <0.05. Fourteen tagging SNPs were then selected by using the Tagger procedure (Haploview software) and threshold r2=0.8 to avoid redundant analyses of highly associated SNPs. Among these selected SNPs, six FKBP5 SNPs were found by our research group to be associated with post-MVC pain outcomes (29). These six SNPs (rs3800373, rs7753746, rs2817040, rs9380526, rs9394314, and rs2817032) were selected for analysis in the present study.
2.4 Outcomes
The PC syndrome is a constellation of somatic symptoms that occur after a traumatic event which traditionally involves initial head trauma. A lack of consensus exists regarding the definition of PC syndrome and the specific symptoms that constitute the syndrome (e.g., DSM-IV criteria (30) versus ICD-10 criteria (31)). The WHO (ICD-10) defines ‘postconcussional syndrome’ by a history of head trauma with loss of consciousness preceding the onset of symptoms by up to 4 weeks together with the presence of 3 or more of the following: (a) unpleasant sensations such as headache, dizziness, fatigue, noise intolerance, or malaise, (b) emotional changes such as irritability, depression, or anxiety, (c) difficulty concentrating, memory impairment, (d) insomnia, (e) reduced tolerance to alcohol, and (f) preoccupation with the above symptoms (31). In contrast, the DSM-IV research definition of ‘postconcussional disorder’ is (a) a significant cerebral concussion (which may involve unconsciousness, post traumatic amnesia, or seizures), (b) evidence of concentration difficulty or memory deficit, (c) at least 3 of the following symptoms for at least 3 months following the injury: fatigue, sleep disturbance, headache, dizziness, irritability, anxiety or depression, personality change, or apathy, (d) the symptoms must start after the head trauma or represent a substantial worsening of preexisting symptoms, (e) the symptoms cause significant functional or social impairment, and (f) the symptoms are not due to another disorder (30). Due to this lack of consensus regarding the definition of PC syndrome, we assessed PC symptom burden rather than PC syndrome presence or absence.
PC symptoms were assessed using twelve symptoms from the Rivermead Post-Concussion Symptoms Questionnaire (32): headache, dizziness, nausea, noise sensitivity, fatigue, insomnia, poor concentration, taking longer to think, blurred vision, light sensitivity, double vision, and restlessness. Participants were asked to report the severity of each symptom on a 0–10 scale, where 0 represented no problem and 10 represented a major problem. Current PC symptom severity and PC symptoms during the month prior to the MVC were assessed in the ED; PC symptoms during the past month were assessed via telephone or web-based questionnaire six weeks, six months, and one year after MVC.
Average PC symptom severity was calculated as the average of the twelve somatic symptom scales assessed. This outcome was chosen because of the lack of consistent PC syndrome definition or scale in the literature (2–4). Six month and one year timepoints were set pre-hoc as primary outcome timepoints for chronic PC symptoms based on evidence that most acute PC symptoms resolve within three months of injury (3). Posttrauamatic stress disorder symptoms (PTSD, Impact of Events Scale Revised (IESR) (33)) and depressive symptoms (CESD) (20) were also assessed at six months and one year.
2.5 Statistical Analysis
2.5.1 Participant Characteristics
The association between participant/collision-related characteristics and average PC symptom severity was evaluated using repeated measures analysis of covariance (ANCOVA) at the six month and one year timepoints post-MVC. All analytic models (epidemiologic and genetic) were adjusted for pre-MVC symptom severity because of our focus on symptoms due to MVC, and for study site as a major study design factor contributing to patient heterogeneity in our sample. Adjustment for study site in genetic models was also necessary to correct for potential genetic stratification between the study sites. Analyses of participant demographic characteristics were additionally adjusted for sex. Finally, analyses of pre-MVC and post-accident psychological characteristics were additionally adjusted for sex, education, and body mass index, as these are potential confounders of these associations.
2.5.2 Genetic Characteristics
FKBP5 SNPs were assessed for Hardy-Weinberg equilibrium (HWE) (chi-square test). Levontin D’ and squared correlation r2 using HaploView (34) were calculated to assess the linkage disequilibrium between SNPs.
Associations between FKBP5 polymorphisms and average PC symptom severity six months and one year post-MVC were evaluated using repeated measures ANCOVA, adjusted for study site and for pre-MVC PC symptoms. An additive genetic model (genotype coded as the number of minor alleles, i.e. 0, 1, or 2) was used to assess for genotype effects. In order to adjust for multiple testing, the method of spectral decomposition was used to estimate the effective number of tests among correlated genotypes (35, 36). Applying this approach to FKBP5 SNPs identified the effective number of independent tests as three, with a corrected alpha of 0.0167 (=0.05/3).
3. Results
3.1 Characteristics of Study Subjects
A total of 10,629 patients were screened in the ED, 1416 were eligible, and 948 were enrolled. Five patients (0.5%) whose loss of consciousness lasted longer than 30 minutes were excluded from analyses. No patient had amnesia lasting more than 24 hours. Follow up data were obtained from 855 (90%) participants at 6 weeks post-MVC, from 835 (88%) participants at 6 months post-MVC, and from 857 (90%) participants at 1 year post-MVC. (Supplemental Digital Content 1, Figure S1, depicting a flow diagram of participant enrollment and exclusion criteria.) Participant baseline characteristics are presented in Table 1.
Table 1.
Baseline Participant Characteristics in Emergency Department
| Frequency in Emergency Department (n=943) n (%) |
||
|---|---|---|
| Age (years) | Mean 35.6 (SD 13.3) | |
| Range 18–65 | ||
| Sex | Female | 574 (60.9) |
| Male | 369 (39.1) | |
| Body Mass Index | Underweight or normal | 350 (38.3) |
| Overweight | 287 (31.4) | |
| Obese | 277 (30.3) | |
| Education | High school or less | 224 (23.8) |
| Some college or trade school | 366 (38.9) | |
| College graduate or more | 351 (37.3) | |
| Annual Incomea | ≤ $19,999 | 117 (13.9) |
| $20,000 – 39,999 | 176 (21.0) | |
| $40,000 – 79,999 | 274 (21.6) | |
| ≥ $80,000 | 273 (32.5) | |
| Collision Type | Rear end | 339 (35.9) |
| Front end (not rear) | 369 (39.9) | |
| Other or no damage | 217 (23.5) | |
| Body Contacta | Hit head during collision | 368 (42.5) |
| Did not hit head during collision | 497 (57.5) | |
| Emergency Department | No pain | 38 (4.1) |
| Mild pain | 149 (16.0) | |
| Moderate pain | 405 (43.4) | |
| Severe pain | 342 (36.6) |
Greater than 5% of data missing; SD=Standard deviation
3.2 PC Symptoms Over Time After MVC
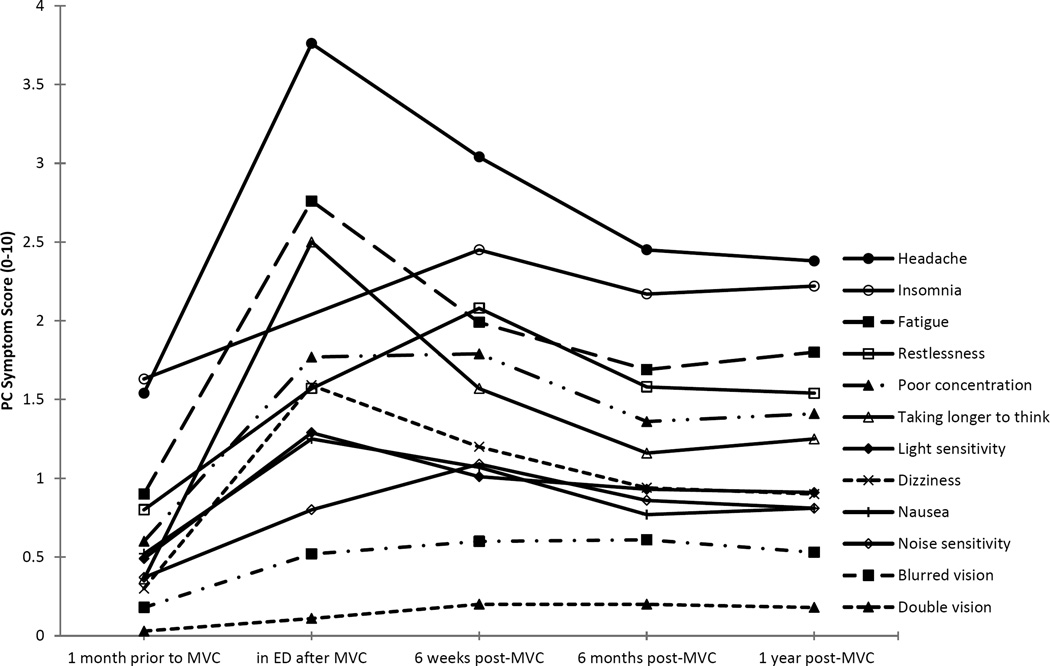
Mean PC symptom scores over time in the cohort are shown in Figure 1. PC symptoms were highest in the hours after MVC, improved at six weeks, and generally stabilized after six months. Compared with reported pre-MVC symptoms, all individual PC symptoms remained significantly elevated at each post-MVC timepoint (non-parametric Wilcoxon signed rank test p<0.001 for each timepoint compared to pre-MVC). Pre-MVC symptoms which showed the greatest increase in severity six months post-MVC were headache, taking longer to think, and fatigue (Figure 1 and Supplemental Digital Content 1, Table S1, describing the progression of average PC symptom severity over time).
Figure 1. Progression of Average PC Symptom Severity over Time.
Progression of twelve PC symptoms depicted over time (pre-MVC, shortly after MVC, at six weeks, six months, and one year post-MVC). Dashed line represents the absence of data at the ED timepoint for insomnia. ED=emergency department, MVC=motor vehicle collision, PC=post-concussive
3.3 Associations Between Sociodemographic Characteristics and Chronic Post-MVC PC Symptoms
Associations between baseline sociodemographic and pre-MVC health characteristics and average PC symptom severity at six months and one year follow-up are shown in Table 2. Female sex, higher body mass index, and lower education predicted chronic PC symptoms. Worse reported pre-MVC physical health and greater reported pre-MVC depressive, anger, and anxiety symptoms also predicted increased chronic PC symptom severity.
Table 2.
Associations Between Participant Sociodemographic, Pre-MVC, Collision-Related, and Post-MVC Characteristics and Average PC Symptom Severity at Six and Twelve Months After MVC
| Frequency at 6 months (n=835) |
Average PC Symptom Severitya | |||
|---|---|---|---|---|
| Mean (95% CI) | P value | |||
| BASELINE DEMOGRAPHIC CHARACTERISTICS | ||||
| Age | 18–27 years | 294 | 1.14 (0.97–1.30) | 0.20 |
| 28–41 years | 249 | 1.27 (1.09–1.45) | ||
| 42–65 years | 292 | 1.34 (1.18–1.50) | ||
| Sex | Female | 520 | 1.37 (1.24–1.49) | 0.002 |
| Male | 315 | 1.05 (0.89–1.21) | ||
| Body Mass Index | Underweight or normal | 304 | 1.27 (1.06–1.48) | <0.001b |
| Overweight | 253 | 1.31 (1.10–1.53) | ||
| Obese | 250 | 1.73 (1.53–1.93) | ||
| Education | High school or less | 184 | 1.68 (1.45–1.91) | 0.003b |
| Some college or trade school | 322 | 1.46 (1.27–1.66) | ||
| College graduate or more | 328 | 1.24 (1.02–1.46) | ||
| Annual Incomed | ≤ $19,999 | 95 | 1.51 (1.21–1.82) | 0.063b |
| $20,000 – 39,999 | 150 | 1.42 (1.16–1.68) | ||
| $40,000 – 79,999 | 252 | 1.52 (1.30–1.74) | ||
| ≥ $80,000 | 251 | 1.20 (0.97–1.42) | ||
| PRE-MVC HEALTH CHARACTERISTICS | ||||
| Mental Health Tertilese | Lower | 270 | 1.40 (1.22–1.57) | 0.031c |
| Middle | 273 | 1.08 (0.91–1.25) | ||
| Higher | 287 | 1.18 (1.01–1.35) | ||
| Physical Health Tertilesf | Lower | 285 | 1.32 (1.15–1.49) | 0.087c |
| Middle | 274 | 1.25 (1.09–1.42) | ||
| Higher | 271 | 1.07 (0.89–1.24) | ||
| Depressiong | None | 667 | 1.12 (1.00–1.24) | 0.002c |
| Moderate | 106 | 1.51 (1.26–1.77) | ||
| Severe | 60 | 1.67 (1.29–2.05) | ||
| Anger Trait Tertilesh | Lower | 335 | 1.12 (0.96–1.28) | 0.001c |
| Middle | 252 | 1.07 (0.89–1.24) | ||
| Higher | 222 | 1.51 (1.32–1.70) | ||
| Anxiety Trait Tertilesi | Lower | 332 | 1.09 (0.93–1.24) | 0.002c |
| Middle | 224 | 1.11 (0.92–1.29) | ||
| Higher | 263 | 1.47 (1.30–1.65) | ||
| COLLISION CHARACTERISTICS | ||||
| Vehicle Damage | Minor | 118 | 1.32 (1.07–1.57) | 0.30 |
| Moderate | 252 | 1.31 (1.14–1.49) | ||
| Severe (not drivable) | 438 | 1.16 (1.03–1.30) | ||
| Collision Type | Rear end | 300 | 1.33 (1.16–1.49) | 0.27 |
| Front end (not rear) | 328 | 1.15 (0.99–1.30) | ||
| Other or no damage | 191 | 1.27 (1.07–1.47) | ||
| Airbag Deployed | Yes | 572 | 1.21 (1.02–1.39) | 0.51 |
| No | 229 | 1.28 (1.16–1.41) | ||
| Head Injuryd | Hit head during collision | 322 | 1.25 (1.09–1.40) | 0.89 |
| Did not hit head during collision | 447 | 1.26 (1.12–1.40) | ||
| POST-ACCIDENT CHARACTERISTICS IN ED | ||||
| Dissociationj | Yes | 290 | 1.46 (1.29–1.62) | <0.001c |
| No | 539 | 1.09 (0.97–1.22) | ||
| Distressk | Yes | 309 | 1.49 (1.31–1.63) | <0.001c |
| No | 515 | 1.06 (0.94–1.19) | ||
| EDl | No pain | 33 | 0.97 (0.52–1.42) | <0.001c |
| Mild pain | 133 | 0.85 (0.61–1.09) | ||
| Moderate pain | 358 | 1.04 (0.90–1.19) | ||
| Severe pain | 302 | 1.61 (1.44–1.77) | ||
Repeated measures analysis of covariance (ANCOVA) model using six months and one year outcomes adjusted for symptoms prior to MVC, ED site, and other covariates when indicated by subsequent footnotes
Adjusted for sex
Adjusted for sex, body mass index, and education
Greater than 5% of data missing
Short-Form Health Survey (SF-12) Mental Component Score, 0–100 normalized scale, divided into tertiles: lower (<48), middle (48–57), higher (>57); lower scores represent poorer mental health,
Short-Form Health Survey (SF-12) Physical Component Score, 0–100 normalized scale, divided into tertiles: lower (<52), middle (52–57), higher (>57); lower scores represent poorer physical health,
Center for Epidemiological Studies Depression (CES-D) Scale, range 0–60, with cutoffs 16–26 for moderate depression and ≥ 27 for severe depression.
State Trait Personality Inventory (STPI-Y) Anger subscale, range 10–40, divided into tertiles: lower (<15), middle (14–17), higher (>18); higher scores indicate more frequent symptoms
State Trait Personality Inventory (STPI-Y) Anxiety subscale, range 10–40, divided into tertiles: lower (<14), middle (14–17), higher (>17); higher scores indicate more frequent symptoms
Michigan Critical Events Perception Scale (MCEPS), range 5–25 with cutoff ≥15 for clinically significant dissociation
Peritraumatic distress inventory (PDI), range 0–52 with cutoff ≥ 23 for clinically significant distress
0–10 numeric rating scale, categorized by no pain (0), mild pain (0.5–3.5), moderate pain (4–6.5), and severe pain (7–10)
CI=confidence interval; ED=emergency department; MVC=motor vehicle accident; PC=post-concussive
3.4 Associations Between Collision Characteristics, Head Injury, and Chronic Post-MVC PC Symptoms
Associations between collision characteristics and average PC symptom severity six months and one year after MVC are shown in Table 2. Extent of vehicle damage, type of collision, and airbag deployment did not predict chronic PC symptom burden. Head impact during collision also did not predict chronic PC symptoms.
3.5 Associations Between Peritraumatic Pain and Psychological Symptoms and Chronic Post-MVC PC Symptoms
In contrast to collision characteristics and head injury, psychological and pain symptoms during the peritraumatic period were strong predictors of chronic PC symptoms severity (Table 2). Individuals with substantial peritraumatic dissociation and distress had significantly higher PC symptoms six months and one year after MVC. Similarly, higher pain symptoms in the peritraumatic period predicted greater chronic PC symptom burden.
3.6 Association Between Genotypic FKBP5 Variants and Chronic Post-MVC PC Symptoms
All genotyped FKBP5 SNPs were in HWE (p>0.05) and had excellent call rates (≥99%). They were all in high linkage disequilibrium with average pairwise D’=0.88 (Supplemental Digital Content 1, Figure S2). Three of the six assessed SNPs (rs3800373, rs7753746, and rs9380526) predicted chronic post-MVC PC symptom severity (Table 3). The presence of one copy of the risk allele increased mean symptom severity by approximately 0.5 points on a 0–10 point scale, after adjusting for ED site, symptoms existing prior to MVC, and multiple testing.
Table 3.
Associations Between FKBP5 Genetic Variants and PC Symptom Severity Six and Twelve Months After MVC
| FKBP5 SNP | Genotype | n | Average PC symptom severity, 0–10 scale (95% CI) |
P valuea |
|---|---|---|---|---|
| rs3800373 | T/T | 447 | 1.10 (0.96–1.24) | 0.001b |
| T/G | 413 | 1.36 (1.21–1.51) | ||
| G/G | 80 | 1.55 (1.23–1.88) | ||
| rs7753746 | A/A | 611 | 1.17 (1.05–1.29) | 0.006b |
| A/G | 297 | 1.37 (1.19–1.54) | ||
| G/G | 29 | 1.84 (1.30–2.39) | ||
| rs9380526 | T/T | 402 | 1.12 (0.97–1.27) | 0.007b |
| T/C | 437 | 1.31 (1.16–1.45) | ||
| C/C | 100 | 1.53 (1.24–1.83) | ||
| rs2817040 | G/G | 512 | 1.20 (1.07–1.33) | 0.32 |
| G/A | 366 | 1.31 (1.15–1.46) | ||
| A/A | 62 | 1.31 (0.92–1.69) | ||
| rs9394314 | A/A | 447 | 1.16 (1.02–1.30) | 0.10 |
| A/G | 414 | 1.32 (1.18–1.47) | ||
| G/G | 80 | 1.36 (1.03–1.69) | ||
| rs2817032 | T/T | 471 | 1.17 (1.03–1.31) | 0.13 |
| T/C | 395 | 1.33 (1.18–1.48) | ||
| C/C | 75 | 1.33 (0.98–1.67) | ||
Repeated measures analysis of covariance (ANCOVA) model (additive genetic model) using six months and one year outcomes adjusted for ED site and symptoms prior to MVC
Significant after correction for multiple testing (the effective number of tests was estimated using the method of spectral decomposition); three effective tests estimated for FKBP5, alpha = 0.05/3 = 0.0167
CI=confidence interval; SNP=single nucleotide polymorphism; MVC=motor vehicle collision; PC=post-concussive
5. Discussion
In this large prospective cohort study of adult European Americans followed for one year after presenting to the ED for care after MVC, somatic symptoms were common. All of the somatic symptoms evaluated showed marked increases in intensity in the immediate aftermath of MVC, with increased symptoms persisting in the study cohort at six weeks, six months, and one year. Together with data from previous studies (3, 5, 9, 10, 37), the results of the present study, which is one of the largest to date and had excellent follow-up rates, provide strong evidence that chronic increased somatic symptoms such as headache, dizziness, fatigue, insomnia, and cognitive difficulties are indeed a common sequelae among individuals who are seen in the ED after MVC and discharged to home after evaluation.
In contrast to this robust evidence that chronic somatic symptoms are a common sequelae of MVC, results from both previous studies (5, 7–11) and the present study do not support the hypothesis that these somatic symptoms result from minor traumatic brain injury. These findings confirm that biomechanical factors related to body movement during the collision in general, and head movement/injury in particular, are not prominent causes of somatic symptoms after MVC in this population.
If chronic somatic symptoms after MVC are a real phenomenon, but are not the result of head concussion/brain trauma, then how might they arise? Epidemiologic evidence from the current study supports the hypothesis that the activation of neurobiologic stress response systems is involved in the development of somatic symptoms in this population. Reported pre-MVC psychological symptoms and peritraumatic psychological and pain symptoms predicted chronic somatic symptom development in the present study. Psychological symptoms prior to trauma (9, 37–39) and in the immediate aftermath of trauma (37, 39) have also been found to predict persistent post-traumatic somatic symptoms in previous studies. These symptoms are known to be modulated by neurobiologic stress systems (40–43). The activation of such systems has been shown to affect memory, e.g. (44), psychological responses, e.g. (41, 45), and pain processing, e.g. (46–48), in a time-dependent and sometimes sex-dependent, e.g. (49, 50) manner. These findings support the hypothesis that the activation of neurobiologic stress systems is involved in the development of chronic somatic symptoms in this population.
In order to further evaluate this hypothesis, we analyzed the association between genetic variants known to affect the function of a physiologic system of central importance to the stress response, the HPA axis, and post-traumatic somatic symptoms. Three genetic variants in FKBP5 (SNP rs3800373, rs7753746, rs9380526), a gene that codes for a co-chaperone of the glucocorticoid receptor which modulates glucocorticoid receptor sensitivity (13) and influences HPA axis negative feedback inhibition (13), were found to predict PC symptom severity six months and one year after MVC. The minor alleles of these three FKBP5 polymorphisms tag risk haplotypes have an overall inhibitory effect on glucocorticoid receptor sensitivity by promoting a receptor complex with a lower affinity for cortisol (14) and by having increased induction by glucocorticoids and other stress hormones (51). Because glucocorticoid receptor sensitivity is essential for HPA axis negative feedback inhibition, carriers of these FKBP5 risk alleles associated with increased PC symptoms in the present study would be expected to experience more persistent elevation of glucocorticoids in response to MVC (29).
To our knowledge this is the first study to identify an association between FKBP5, or any stress system-related gene, and PC symptom outcome. Variants in FKBP5 have been shown to increase vulnerability to persistent pain (29) and mental disorders such as depression (51, 52) and anxiety (53) after trauma. The association between FKBP5 risk variants and PC symptoms adds support to the theory that stress systems play a role in the pathogenesis of PC symptoms after a non-life threatening MVC. Of note, study participants with the rs3800373 risk allele also had increased PTSD and depressive symptoms six months and one year post-MVC (data not shown). In addition, our data results persisted when headache score was removed from our PC symptom summary score (p=0.0167), indicating that pain was not the major driver of the association between FKBP5 and PC symptom outcomes.
FKBP5 polymorphisms rs1360780 (in high linkage disequilibrium with the SNPs in our study) and rs3800373 have previously been shown to interact with early life stressors and predict adult psychological disorders such as PTSD (54, 55). Both PTSD and the post-concussive symptoms occur commonly after MVC (1, 56). Overlapping PTSD and PC symptoms include insomnia, difficulty concentrating, and irritability, however their diagnostic criteria differ significantly. Several studies have demonstrated an association between PTSD and PC symptoms in the months or years after trauma (57–59), indicating potential shared vulnerability. At present the shared and distinct biologic mechanisms mediating the symptom clusters denoting these two conditions remain poorly understood (60).
Several limitations should be considered when interpreting our study results. First and foremost, our study focused on chronic post-traumatic somatic symptom development among individuals presenting to the ED after MVC and discharged to home after evaluation (this population accounts for approximately 90% of all individuals who present to the ED after MVC (61)). The generalizability of our findings to other populations, such as individuals with sport-related head injury or more severe head injury, is unknown. Second, we used head impact as a marker of concussion/mild brain injury. It is possible that other mechanisms, such as rotational movements of the head, could have caused brain injury in the absence of head impact. However, collision characteristics associated with the severity and direction of collision were also not associated with chronic somatic symptom development. In addition, collisions that occur in other non-threatening settings (e.g. in bumper cars) exert the same biomechanical stress as a low speed MVC (62), yet prolonged symptoms after bumper car collisions are rare (63). Third, we did not directly assess the relationship between physiologic measures of stress (e.g., cortisol level) and PC symptoms. Although there is no single validated psychological index of stress, we did evaluate psychological measures of stress including distress and dissociation symptoms in the ED and PTSD and depressive symptoms six and twelve months after MVC, and these psychological measures of stress were associated with worse PC symptoms over time. In addition, we could only assess pre-MVC health and function after the MVC, and the amount of bias introduced by retrospective recall in this setting is unknown. Also, our study excluded MVC patients with significant brain injury (e.g., those hospitalized due to traumatic brain injury), therefore how PC symptoms in this group compare to those of study participants is unknown. In addition, since we did not assess ancestry using genetic markers, there is a possibility for residual population stratification. However, as noted above, self-report has shown to be a reliable method of assessing ancestry (18), and all assessed polymorphisms were in HWE in our study, suggesting at most negligible population stratification. Finally, given that our study was limited to European Americans age 18–65 years old, generalizability to children, elderly patients, and other races and ethnicities is unknown.
In summary, our findings indicate that PC symptoms are common after non-life threatening MVC but are very unlikely to be related to mild brain trauma. Instead, both epidemiologic and genetic association data support the hypothesis that neurobiologic stress systems are involved in chronic somatic symptom development after MVC. The term “post-concussive” should be avoided when referring to post-MVC somatic symptoms in this population, since this delineation perpetuates a hypothesis refuted by the evidence. Further studies investigating neurobiologic mechanisms contributing to chronic somatic symptoms after MVC are warranted. Only by correctly identifying pathophysiologic mechanisms mediating post-MVC somatic symptom outcomes can we develop interventions to prevent these common and morbid outcomes.
Supplementary Material
Acknowledgements
We would like to thank the participants for taking part in this study. Dr. McLean had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. McLean received funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number 1RO1 AR056328. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Auvergne received funding through the Scott Neil Schwirck Research Fellowship at the University of North Carolina School of Medicine.
Abbreviations
- PC
post-concussive
- MVC
motor vehicle collision
- MTBI
minor traumatic brain injury
- HPA
hypothalamic-pituitary-adrenocortical
- FKBP5
FK506 binding protein 51
- ED
emergency department
- SF-12
Short-Form Health Survey
- CESD
Center for Epidemiological Studies Depression
- IESR
Impact of Events Scale Revised
- STPI-Y
State Trait Personality Inventory
- PDI
Peritraumatic Distress Inventory
- MCEPS
Michigan Critical Events Perception Scale
- NRS
numeric rating scale
- SNPs
single nucleotide polymorphisms
- HWE
Hardy-Weinberg equilibrium
Footnotes
The authors of this manuscript have no conflicts of interest to disclose.
Author Contributions: SM and LA conceived the study. SM obtained research funding and supervised the conduct of data collection. DP, JJ, RS, RD, DL, NR, and PH supervised emergency department data collection at their respective study sites. JU managed the data, including quality control. JU, AB, and SM supervised statistical analyses, LA analyzed the data, LA and SM drafted the manuscript, and all authors contributed to its revision. SM takes responsibility for the paper as a whole.
References
- 1.Bazarian JJ, McClung J, Shah MN, Ting Cheng Y, Flesher W, Kraus J. Mild traumatic brain injury in the United States, 1998–2000. Brain Injury. 2005;19:85–91. doi: 10.1080/02699050410001720158. [DOI] [PubMed] [Google Scholar]
- 2.McAllister TW. Mild Brain Injury. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of Traumatic Brain Injury. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2011. [Google Scholar]
- 3.Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pepin M. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 4.Reuben A, Sampson P, Harris AR, Williams H, Yates P. Postconcussion syndrome (PCS) in the emergency department: predicting and pre-empting persistent symptoms following a mild traumatic brain injury. EMJ. 2013 doi: 10.1136/emermed-2012-201667. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy JD, Cancelliere C, Carroll LJ, Cote P, Hincapie CA, Holm LW, Hartvigsen J, Donovan J, Nygren-de Boussard C, Kristman VL, Borg J. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95:S132–S151. doi: 10.1016/j.apmr.2013.08.299. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 7.Iverson GL, Lange RT. Examination of "postconcussion-like" symptoms in a healthy sample. Appl Neuropsychol. 2003;10:137–144. doi: 10.1207/S15324826AN1003_02. [DOI] [PubMed] [Google Scholar]
- 8.Iverson GL, McCracken LM. 'Postconcussive' symptoms in persons with chronic pain. Brain Inj. 1997;11:783–790. doi: 10.1080/026990597122990. [DOI] [PubMed] [Google Scholar]
- 9.McLean SA, Kirsch NL, Tan-Schriner CU, Sen A, Frederiksen S, Harris RE, Maixner W, Maio RF. Health status, not head injury, predicts concussion symptoms after minor injury. Am J Emerg Med. 2009;27:182–190. doi: 10.1016/j.ajem.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, Chapman J, Gurka J, Dawson K, Capon L, Marosszeky JE. Mild traumatic brain injury does not predict acute postconcussion syndrome. J Neurol Neurosurg Psychiatry. 2008;79:300–306. doi: 10.1136/jnnp.2007.126565. [DOI] [PubMed] [Google Scholar]
- 11.Lagarde E, Salmi LR, Holm LW, Contrand B, Masson F, Ribereau-Gayon R, Laborey M, Cassidy JD. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. JAMA Psychiatry. 2014;71:1032–1040. doi: 10.1001/jamapsychiatry.2014.666. [DOI] [PubMed] [Google Scholar]
- 12.McLean SA. The potential contribution of stress systems to the transition to chronic whiplash-associated disorders. Spine (Phila Pa 1976) 2011;36:S226–S232. doi: 10.1097/BRS.0b013e3182387fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Bull DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, Hartmann J, Hausch F, Schmidt MV, Holsboer F, Ising M, Cox MB, Schmidt U, Rein T. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes, Brain, and Behavior. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- 15.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of Neuroscience. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platts-Mills TF, Ballina L, Bortsov AV, Soward A, Swor RA, Jones JS, Lee DC, Peak DA, Domeier RM, Rathlev NK, Hendry PL, McLean SA. Using emergency department-based inception cohorts to determine genetic characteristics associated with long term patient outcomes after motor vehicle collision: methodology of the CRASH study. BMC Emerg Med. 2011;11:14. doi: 10.1186/1471-227X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diatchenko L, Slade GD, Nackley AG, Maixner W. Responses to Drs. Kim and Dionne regarding comments on Diatchenko, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2007;129:366–370. doi: 10.1016/j.pain.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, Pankow JS, Province MA, Hunt SC, Boerwinkle E, Schork NJ, Risch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. Appl Psych Meas. West Publishing Co.; 1977. The CES-D scale: A self report depression scale for research in the general population; pp. 385–401. [Google Scholar]
- 21.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13:163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) 1979 [Google Scholar]
- 23.Spielberger CD, Reheiser EC. Assessment of Emotions: Anxiety, Anger, Depression, and Curiosity. Appl Psychol Health Well Being. 2009;1:271–302. [Google Scholar]
- 24.Lee YM, Platts-Mills TF, Macwilliams JB, Sochor MR, Jones JS, Domeier RM, Schneider LW, McLean SA. Descriptions of motor vehicle collisions by participants in emergency department-based studies: are they accurate? West J Emerg Med. 2012;13:329–334. doi: 10.5811/westjem.2011.9.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22:1453–1458. doi: 10.1007/s11606-007-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, Marmar CR. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 27.Nishi D, Matsuoka Y, Yonemoto N, Noguchi H, Kim Y, Kanba S. Peritraumatic Distress Inventory as a predictor of post-traumatic stress disorder after a severe motor vehicle accident. Psychiatry Clin Neurosci. 2010;64:149–156. doi: 10.1111/j.1440-1819.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- 28.Michaels AJ, Michaels CE, Moon CH, Smith JS, Zimmerman MA, Taheri PA, Peterson C. Posttraumatic stress disorder after injury: impact on general health outcome and early risk assessment. J Trauma. 1999;47:460–467. doi: 10.1097/00005373-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS, Holbrook D, Rathlev NK, Foley KA, Lee DC, Collette R, Domeier RM, Hendry PL, McLean SA. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013;154:1419–1426. doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 31.World Health Organization, editor. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic criteria for research. Geneva: World Health Organization; 1993. [Google Scholar]
- 32.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 33.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale - Revised. Behav Res Ther. 2003;41:1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 35.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 37.Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma. 2009;66:289–296. doi: 10.1097/TA.0b013e3181961da2. discussion 96–7. [DOI] [PubMed] [Google Scholar]
- 38.Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A, Schonberger M. Predictors of postconcussive symptoms 3 months after mild traumatic brain injury. Neuropsychology. 2012;26:304–313. doi: 10.1037/a0027888. [DOI] [PubMed] [Google Scholar]
- 39.Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, Chapman J, Gurka J, Marosszeky JE. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. 2011;25:454–465. doi: 10.1037/a0022580. [DOI] [PubMed] [Google Scholar]
- 40.McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101–107. doi: 10.1016/j.jpain.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pervanidou P, Chrousos GP. Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149–160. doi: 10.1016/S0079-6123(10)82005-9. [DOI] [PubMed] [Google Scholar]
- 43.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 44.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. J Pain. 2011;12:1069–1079. doi: 10.1016/j.jpain.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Ballina LE, Ulirsch JC, Soward AC, Rossi C, Rotolo S, Linnstaedt SD, Heafner T, Foley KA, Batts J, Collette R, Holbrook D, Zelman S, McLean SA. mu-Opioid receptor gene A118G polymorphism predicts pain recovery after sexual assault. J Pain. 2013;14:165–171. doi: 10.1016/j.jpain.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–155. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 49.Bortsov AV, Diatchenko L, McLean SA. Complex multilocus effects of catechol-o-methyltransferase haplotypes predict pain and pain interference 6 weeks after motor vehicle collision. Neuromolecular Med. 2014;16:83–93. doi: 10.1007/s12017-013-8255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology. 2009;34:587–596. doi: 10.1016/j.psyneuen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 51.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 52.Kang JI, Chung HC, Jeung HC, Kim SJ, An SK, Namkoong K. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: a controlled and prospective study. Psychoneuroendocrinology. 2012;37:1569–1576. doi: 10.1016/j.psyneuen.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 54.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryb GE, Dischinger PC, Read KM, Kufera JA. PTSD after severe vehicular crashes. Annals of advances in automotive medicine / Annual Scientific Conference Association for the Advancement of Automotive Medicine Association for the Advancement of Automotive Medicine Scientific Conference. 2009;53:177–193. [PMC free article] [PubMed] [Google Scholar]
- 57.Bryant RA, Harvey AG. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. The Journal of Nervous and Mental Disease. 1999;187:302–305. doi: 10.1097/00005053-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90:1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 59.Bryant RA. Posttraumatic stress disorder and traumatic brain injury: can they co-exist? Clinical Psychology Review. 2001;21:931–948. doi: 10.1016/s0272-7358(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 60.Bryant RA. Posttraumatic stress disorder and mild brain injury: controversies, causes and consequences. Journal of Clinical and Experimental Neuropsychology. 2001;23:718–728. doi: 10.1076/jcen.23.6.718.1024. [DOI] [PubMed] [Google Scholar]
- 61.Platts-Mills TF, Hunold KM, Esserman DA, Sloane PD, McLean SA. Motor vehicle collision-related emergency department visits by older adults in the United States. Acad Emerg Med. 2012;19:821–827. doi: 10.1111/j.1553-2712.2012.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer S, Hugemann RE, Weber M. Zur Belastung der HWS dutch Auffahrkollisionen. Verkehrsunfall und Fahrzeugtechnik. 1994;32:187–199. [Google Scholar]
- 63.Castro WH. Correlation between exposure to biomechanical stress and Whiplash Associated Disorders (WAD) Pain Res Manag. 2003;8:76–78. doi: 10.1155/2003/425714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.