Abstract
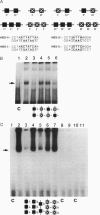
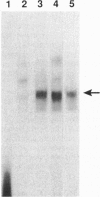
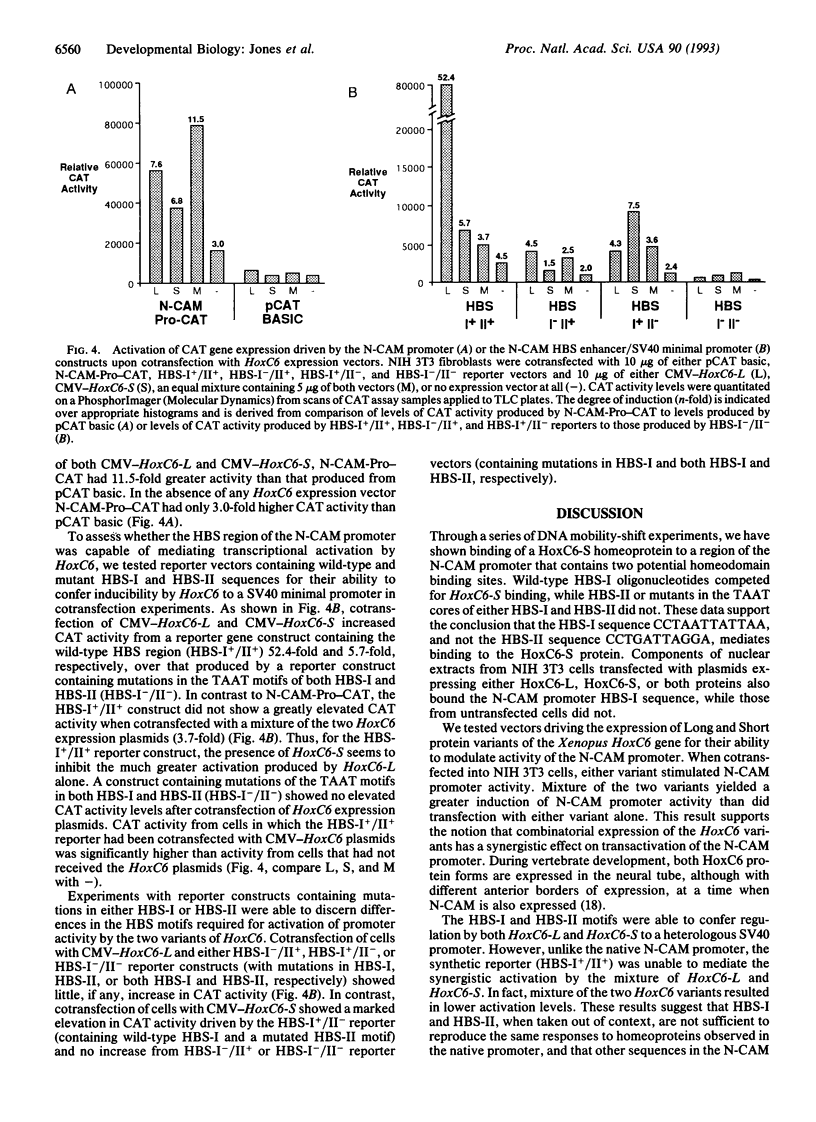
Scores of homeobox gene-encoded transcription factors are expressed in a definite spatiotemporal pattern during embryogenesis and regulate a series of as yet unidentified target genes to help coordinate the morphogenetic process. We have suggested that homeobox gene products modulate the expression of adhesion molecule genes and have shown in cotransfection experiments that the promoters for the neural cell adhesion molecule (N-CAM) and cytotactin/tenascin genes respond to cues from different homeobox-containing genes. In this study, we show that the HoxC6 (Hox-3.3)-encoded homeoprotein binds to a DNA sequence in the N-CAM promoter CCTAATTATTAA, designated homeodomain binding site I (HBS-I). To test whether HoxC6 regulated N-CAM promoter activity, we cotransfected the Long and Short reading frame variants of Xenopus HoxC6 (CMV-HoxC6-L and CMV-HoxC6-S) driven by the human cytomegalovirus (CMV) promoter together with a chloramphenicol acetyltransferase (CAT) reporter gene driven by the mouse N-CAM promoter (N-CAM-Pro-CAT). Cotransfection of NIH 3T3 cells with either of the CMV-HoxC6 expression vectors stimulated N-CAM promoter-driven CAT expression. A 47-bp region from the N-CAM promoter that included HBS-I and an adjacent potential HBS, HBS-II, conferred HoxC6 regulation on a simian virus 40 minimal promoter. HBS-I was sufficient for transactivation of the minimal promoter by CMV-HoxC6-S. However, transcriptional activation by CMV-HoxC6-L required both HBS-I and HBS-II, inasmuch as mutation of either HBS-I, HBS-II, or both motifs abolished the response. These studies suggest that HBS-I is a target site for binding and transcriptional control of the N-CAM promoter by homeoproteins, although accessory DNA sequences (such as HBS-II) may also be required. Together with previous studies, these results support the notion that N-CAM gene expression may be controlled by different combinations of homeoproteins that appear in a place-dependent manner during embryogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell. 1989 May 5;57(3):347–349. doi: 10.1016/0092-8674(89)90909-4. [DOI] [PubMed] [Google Scholar]
- Ananthan J., Baler R., Morrissey D., Zuo J., Lan Y., Weir M., Voellmy R. Synergistic activation of transcription is mediated by the N-terminal domain of Drosophila fushi tarazu homeoprotein and can occur without DNA binding by the protein. Mol Cell Biol. 1993 Mar;13(3):1599–1609. doi: 10.1128/mcb.13.3.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D. J., Scott M. P. Downstream of the homeotic genes. New Biol. 1992 Jan;4(1):5–15. [PubMed] [Google Scholar]
- Bloch-Gallego E., Le Roux I., Joliot A. H., Volovitch M., Henderson C. E., Prochiantz A. Antennapedia homeobox peptide enhances growth and branching of embryonic chicken motoneurons in vitro. J Cell Biol. 1993 Jan;120(2):485–492. doi: 10.1083/jcb.120.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco A. E., McGinnis W., Gehring W. J., De Robertis E. M. Cloning of an X. laevis gene expressed during early embryogenesis coding for a peptide region homologous to Drosophila homeotic genes. Cell. 1984 Jun;37(2):409–414. doi: 10.1016/0092-8674(84)90371-4. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Goetz J., Wright C. V., Fritz A., Hardwicke J., De Robertis E. M. Differential utilization of the same reading frame in a Xenopus homeobox gene encodes two related proteins sharing the same DNA-binding specificity. EMBO J. 1988 Jul;7(7):2139–2149. doi: 10.1002/j.1460-2075.1988.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C. M., Oliver G., Ting S. A., Jegalian B. G., Chen H. M., De Robertis E. M. Gradients of homeoproteins in developing feather buds. Development. 1990 Dec;110(4):1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Jones F. S. Cytotactin: a morphoregulatory molecule and a target for regulation by homeobox gene products. Trends Biochem Sci. 1992 Jun;17(6):228–232. doi: 10.1016/0968-0004(92)90383-k. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulation. Dev Dyn. 1992 Jan;193(1):2–10. doi: 10.1002/aja.1001930103. [DOI] [PubMed] [Google Scholar]
- Galang C. K., Hauser C. A. Cooperative DNA binding of the highly conserved human Hox 2.1 homeodomain gene product. New Biol. 1992 May;4(5):558–568. [PubMed] [Google Scholar]
- Gaunt S. J., Coletta P. L., Pravtcheva D., Sharpe P. T. Mouse Hox-3.4: homeobox sequence and embryonic expression patterns compared with other members of the Hox gene network. Development. 1990 Jun;109(2):329–339. doi: 10.1242/dev.109.2.329. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. P., Brookman J. J., Strutt D. I., White R. A. Targets of homeotic gene control in Drosophila. Nature. 1990 Nov 22;348(6299):308–312. doi: 10.1038/348308a0. [DOI] [PubMed] [Google Scholar]
- Gould A. P., White R. A. Connectin, a target of homeotic gene control in Drosophila. Development. 1992 Dec;116(4):1163–1174. doi: 10.1242/dev.116.4.1163. [DOI] [PubMed] [Google Scholar]
- Graham A., Maden M., Krumlauf R. The murine Hox-2 genes display dynamic dorsoventral patterns of expression during central nervous system development. Development. 1991 May;112(1):255–264. doi: 10.1242/dev.112.1.255. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hirsch M. R., Gaugler L., Deagostini-Bazin H., Bally-Cuif L., Goridis C. Identification of positive and negative regulatory elements governing cell-type-specific expression of the neural cell adhesion molecule gene. Mol Cell Biol. 1990 May;10(5):1959–1968. doi: 10.1128/mcb.10.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M., Goodman C. S. Cell and substrate adhesion molecules in Drosophila. Annu Rev Cell Biol. 1991;7:505–557. doi: 10.1146/annurev.cb.07.110191.002445. [DOI] [PubMed] [Google Scholar]
- Joliot A., Pernelle C., Deagostini-Bazin H., Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Chalepakis G., Gruss P., Edelman G. M. Activation of the cytotactin promoter by the homeobox-containing gene Evx-1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2091–2095. doi: 10.1073/pnas.89.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Prediger E. A., Bittner D. A., De Robertis E. M., Edelman G. M. Cell adhesion molecules as targets for Hox genes: neural cell adhesion molecule promoter activity is modulated by cotransfection with Hox-2.5 and -2.4. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2086–2090. doi: 10.1073/pnas.89.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Nose A., Mahajan V. B., Goodman C. S. Connectin: a homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. Cell. 1992 Aug 21;70(4):553–567. doi: 10.1016/0092-8674(92)90426-d. [DOI] [PubMed] [Google Scholar]
- Oliver G., Wright C. V., Hardwicke J., De Robertis E. M. Differential antero-posterior expression of two proteins encoded by a homeobox gene in Xenopus and mouse embryos. EMBO J. 1988 Oct;7(10):3199–3209. doi: 10.1002/j.1460-2075.1988.tb03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F., Tomaselli K. J. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser S. J., Kenyon C. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature. 1992 Jan 16;355(6357):255–258. doi: 10.1038/355255a0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P. Vertebrate homeobox gene nomenclature. Cell. 1992 Nov 13;71(4):551–553. doi: 10.1016/0092-8674(92)90588-4. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Rathjen F. G. Regulation of axonal growth in the vertebrate nervous system by interactions between glycoproteins belonging to two subgroups of the immunoglobulin superfamily. J Cell Biol. 1992 Dec;119(6):1387–1394. doi: 10.1083/jcb.119.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Treisman J., Harris E., Wilson D., Desplan C. The homeodomain: a new face for the helix-turn-helix? Bioessays. 1992 Mar;14(3):145–150. doi: 10.1002/bies.950140302. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Cho K. W., Hardwicke J., Collins R. H., De Robertis E. M. Interference with function of a homeobox gene in Xenopus embryos produces malformations of the anterior spinal cord. Cell. 1989 Oct 6;59(1):81–93. doi: 10.1016/0092-8674(89)90871-4. [DOI] [PubMed] [Google Scholar]