Abstract
BACKGROUND
The feasibility, safety, and efficacy of prolonged use of an artificial beta cell (closed-loop insulin-delivery system) in the home setting have not been established.
METHODS
In two multicenter, crossover, randomized, controlled studies conducted under free-living home conditions, we compared closed-loop insulin delivery with sensor-augmented pump therapy in 58 patients with type 1 diabetes. The closed-loop system was used day and night by 33 adults and overnight by 25 children and adolescents. Participants used the closed-loop system for a 12-week period and sensor-augmented pump therapy (control) for a similar period. The primary end point was the proportion of time that the glucose level was between 70 mg and 180 mg per deciliter for adults and between 70 mg and 145 mg per deciliter for children and adolescents.
RESULTS
Among adults, the proportion of time that the glucose level was in the target range was 11.0 percentage points (95% confidence interval [CI], 8.1 to 13.8) greater with the use of the closed-loop system day and night than with control therapy (P<0.001). The mean glucose level was lower during the closed-loop phase than during the control phase (difference, −11 mg per deciliter; 95% CI, −17 to −6; P<0.001), as were the area under the curve for the period when the glucose level was less than 63 mg per deciliter (39% lower; 95% CI, 24 to 51; P<0.001) and the mean glycated hemoglobin level (difference, −0.3%; 95% CI, −0.5 to −0.1; P=0.002). Among children and adolescents, the proportion of time with the nighttime glucose level in the target range was higher during the closed-loop phase than during the control phase (by 24.7 percentage points; 95% CI, 20.6 to 28.7; P<0.001), and the mean nighttime glucose level was lower (difference, −29 mg per deciliter; 95% CI, −39 to −20; P<0.001). The area under the curve for the period in which the day-and-night glucose levels were less than 63 mg per deciliter was lower by 42% (95% CI, 4 to 65; P=0.03). Three severe hypoglycemic episodes occurred during the closed-loop phase when the closed-loop system was not in use.
CONCLUSIONS
Among patients with type 1 diabetes, 12-week use of a closed-loop system, as compared with sensor-augmented pump therapy, improved glucose control, reduced hypoglycemia, and, in adults, resulted in a lower glycated hemoglobin level. (Funded by the JDRF and others; AP@home04 and APCam08 ClinicalTrials.gov numbers, NCT01961622 and NCT01778348.)
Intensive insulin therapy is the standard of care for type 1 diabetes but is limited by the risk of hypoglycemia,1 which leads to failure in achieving treatment goals for most patients in all age groups.2,3 Among patients with type 1 diabetes, hypoglycemia is common, has a major effect on patients’ quality of life and psychological well-being,4 and may cause seizures, which is of particular concern during the overnight hours in children and adolescents.5 New approaches (e.g., continuous glucose monitoring) can improve glycemic control when the patient wears the sensors on a regular basis.6,7 If insulin delivery is linked to sensor glucose levels during the use of an insulin pump that has a threshold-suspend feature,8 which temporarily interrupts insulin delivery at preset glucose levels, the risk of hypoglycemia may be reduced.
The artificial beta cell, or closed-loop insulin-delivery system, expands on the concept of sensor-responsive insulin delivery. The closed-loop system differs from conventional pump therapy and threshold-suspend approaches in that it uses a control algorithm that autonomously and continually increases and decreases the subcutaneous delivery of insulin on the basis of real-time sensor glucose levels.9
After extensive studies under controlled laboratory settings,10-14 investigations of closed-loop systems in transitional outpatient settings that incorporated remote monitoring and supervision by research staff members in hotels15 or at diabetes camps16,17 have shown improved glucose control and a reduced risk of hypoglycemia.16-19 However, studies involving patients under at-home, free-living conditions have been limited to 1-week, day-and-night use of the closed-loop system in adults20 and to overnight use of a closed-loop system for 3 to 6 weeks in adolescents and adults.21-23 The evaluation of the closed-loop system in children 12 years of age or younger in free-living settings is also desirable.
Here we present the results of two multicenter, 12-week, free-living home trials — one involving the day-and-night use of a closed-loop system in adults and the other the overnight use of a closed-loop system in children and adolescents. We hypothesized that the extended use of closed-loop insulin delivery without remote monitoring or close supervision would be feasible, improve glycemic control, and minimize the risk of hypoglycemia.
METHODS
STUDY PARTICIPANTS
All the participants had type 1 diabetes, as defined by the World Health Organization, and had received insulin-pump therapy for at least 6 months. We recruited adults who were at least 18 years of age and had a glycated hemoglobin level of 7.5 to 10% (58 to 86 mmol per mole of nonglycated hemoglobin) and children and adolescents who were 6 to 18 years of age and had a glycated hemoglobin level of less than 10%. Details regarding the inclusion and exclusion criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
STUDY OVERSIGHT
The two study protocols were approved by independent research ethics committees (one central ethics committee for the study involving children and one ethics committee in each country for the study involving adults) and by the regulatory authority in the United Kingdom (Medicines and Healthcare Products Regulatory Agency). The study involving adults also received approval from regulatory authorities in Germany (Federal Institute for Drugs and Medical Devices) and Austria (Austrian Agency for Health and Food Safety).
All the adult participants provided written informed consent. In the study involving children and adolescents, participants who were 16 years of age or older and the parents or guardians of participants who were younger than 16 years of age provided written informed consent; written assent was obtained from participants younger than 16 years of age. The safety aspects of the two studies were overseen by an independent data and safety monitoring board.
Abbott Diabetes Care supplied discounted continuous glucose-monitoring devices, sensors, and details of the communication protocol to facilitate real-time connectivity. Diasend provided discounted hardware and software platforms for data upload. Abbott Diabetes Care read the manuscript before it was submitted for publication but had no role in its revision. No sponsor had any other role in the design of the study, the collection, analysis, or interpretation of the data, or the writing of the report. The authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocols. Two of the authors hold patents related to closed-loop insulin-delivery systems and systems for insulin delivery that use various measurement-error models.
STUDY DESIGN
Characteristics of the Two Studies
The two studies were open-label, multicenter, crossover, randomized, controlled trials (Fig. S1 in the Supplementary Appendix). The study involving adults was conducted in the United Kingdom, Germany, and Austria,24 and the study involving children and adolescents was conducted at three sites in the United Kingdom. All the participants were randomly assigned to receive either 12 weeks of automated closed-loop insulin delivery (intervention period) first and sensor-augmented pump therapy (control period) second, or vice versa. The analysis of the closed-loop phase included data from the combined closed-loop phases of the two randomization sequences; the analysis of the control phase also included combined data. The study protocols are available at NEJM.org.
Identical insulin pumps and continuous glucose-monitoring devices were used during the two treatment periods in the two trials; continuous glucose-monitoring devices were worn during the two study periods. Participants were not remotely monitored or supervised, and they performed their usual free-living daily activities. Participants were free to consume any meals of their choice. As a precaution, during the first 2 weeks of the study periods, participants were advised against international travel and the use of the closed-loop system during exercise. All the participants were provided with a telephone number for a 24-hour helpline to contact the study team in the event of study-related issues.
Each participant had an identical number of planned contacts with the study team during the two treatment periods. Blood samples were obtained for the measurement of glycated hemoglobin levels at enrollment and before and after each treatment period. C-peptide levels were measured at enrollment during a period when participants did not have hypoglycemia (i.e., had a blood glucose level ≥72 mg per deciliter [4.0 mmol per liter]).
The two treatment interventions were separated by a washout period (lasting 4 to 6 weeks in adults and 3 to 4 weeks in children and adolescents). During the washout period, the participants could continue using the study insulin pump with their standard pump settings.
Study Involving Adults
After receiving training regarding the use of the insulin pump and the continuous glucose-monitoring device, participants underwent a run-in period lasting 4 to 6 weeks. During this period, participants came to the research center at weekly intervals for adjustment of pump therapy by the study team according to a prespecified sequence of written instructions for basal and meal-related insulin-dose adjustments. Participants were required to use the study devices for at least 10 days during the last 2 weeks of the run-in period before randomization.
During the intervention period, participants used the closed-loop system during the day and night while they were at home, at work, and during holidays. Participants performed insulin meal-priming bolus calculations by entering carbohydrate amount and fingerstick capillary glucose measurements into the standard bolus calculator.
Study Involving Children and Adolescents
After receiving training regarding the use of the insulin pump and the continuous glucose-monitoring device, participants underwent a run-in period lasting 2 to 8 weeks. Data obtained during this period were used for adjustment of the therapy. Participants were required to use the study devices for at least 12 days during the run-in period before randomization.
During the intervention period, participants used the closed-loop system overnight while at home during school terms and holidays. Participants were instructed to initiate the system at home after their evening meal or at bedtime at the latest and to discontinue it before breakfast the next morning.
CLOSED-LOOP SYSTEM
An identical, individually adapting, model-predictive-control treat-to-target algorithm (a control approach relying on a dynamic model of glucose regulation to calculate the insulin delivery that is predicted to achieve desirable glucose levels) was used in the two studies. Every 12 minutes, the control algorithm calculated an insulin infusion rate that was automatically sent wirelessly to the study insulin pump (Figs. S2 and S3 in the Supplementary Appendix). A hybrid closed-loop approach was applied in the day-and-night study involving adults, in which participants additionally administered prandial insulin using the standard bolus calculator. The control algorithm was initialized with the use of a preprogrammed basal insulin delivery downloaded from the study pump, and the participant’s weight and total daily insulin dose were entered at setup. Further details are provided in the Supplementary Appendix.
STUDY END POINTS
The primary end point in the study involving adults was the proportion of time that the glucose level, as measured by the continuous glucose-monitoring device, was in the target range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter) during the 12-week study periods. The primary end point in the study involving children and adolescents was the proportion of time that the nocturnal glucose level, as detected by the sensor, was in the target glucose range of 70 to 145 mg per deciliter (3.9 to 8.0 mmol per liter) during the 12-week study periods. Secondary end points were insulin delivery, the glycated hemoglobin level, mean sensor glucose levels, the variability of the glucose level, and the time spent below and above the relevant glucose ranges during day-and-night, daytime, and overnight periods (see the Supplementary Appendix).
STATISTICAL ANALYSIS
Statistical analyses were performed on an intention-to-treat basis. We compared the respective values obtained during the 12-week intervention and control periods using a least-squares repeated-measures regression model, adjusting for the period effect as a covariate and accounting for the correlated data from the same participant with the use of an unstructured covariance matrix. The hypothesis testing was ordered first to consider the primary end points at the 0.05 level and then to move to testing the secondary end points individually at the 0.05 level without any control for multiplicity. A sensitivity analysis was used to assess the effect of withdrawal of participants from the study. All P values are two-sided (see the Supplementary Appendix).
RESULTS
PARTICIPANTS
We screened 75 patients, of whom 58 were eligible and underwent randomization (Table 1). All the participants had a C-peptide level of less than 33 pmol per liter during the initial assessment, measured at a time when they did not have hypoglycemia (i.e., had a blood glucose level ≥72 mg per deciliter), except for four participants in the study involving children and adolescents who had levels of 40, 40, 170, and 530 pmol per liter. Four adults had stable microvascular complications, and no adult had any macrovascular complications; none of the children and adolescents had either microvascular or macrovascular complications. One adult participant and one adolescent participant voluntarily withdrew during the washout phase because of issues unrelated to the closed-loop study (Fig. S4 in the Supplementary Appendix).
Table 1. Characteristics of the Study Participants at Baseline.*.
| Characteristic | Adults (N = 33) |
Children and Adolescents (N = 25) |
|---|---|---|
| Sex — no. (%) | ||
| Female | 15 (45) | 11 (44) |
| Male | 18 (55) | 14 (56) |
| Age — yr | 40.0±9.4 | 12.0±3.4 |
| Weight — kg | 77.5±15.0 | 43.9±16.6 |
| BMI† | 25.5±4.4 | 18.9±3.5 |
| BMI z score | — | 0.3±1.0 |
| Duration of diabetes — yr | 20.9±9.3 | 4.7±2.6 |
| Duration of pump use — yr | 7.8±5.9 | 3.3±1.8 |
| Total daily insulin dose — U/kg/day | 0.62±0.15 | 0.89±0.24 |
| Glycated hemoglobin at screening | ||
| Percent | 8.5±0.7 | 8.1±0.9 |
| Millimoles per mole of non- glycated hemoglobin |
69±7 | 65±10 |
Plus–minus values are means ±SD.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
END POINTS IN THE STUDY INVOLVING ADULTS
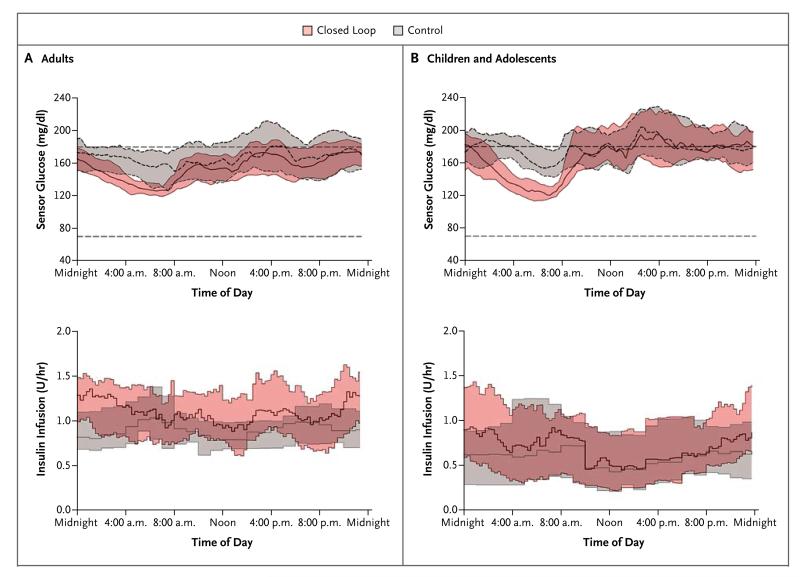
Table 2 details the primary and secondary end points in the study involving adults. The sensor glucose levels and insulin-delivery profiles are shown in Figure 1A. The proportion of time that the glycated hemoglobin level was in the target range (primary end point) was significantly greater during the intervention period than during the control period — by a mean of 11.0 percentage points (95% confidence interval [CI], 8.1 to 13.8; P<0.001). The mean glucose level was significantly lower with day-and-night use of the closed-loop system than with the control system (P<0.001), as was the time spent above the target range (P<0.001). The time that the glucose level was less than 70 mg per deciliter and less than 50 mg per deciliter (2.8 mmol per liter) was significantly less with the closed-loop system than with the control system (P=0.02 and P<0.001, respectively). The relative burden of hypoglycemia, as measured by the area under the curve when the sensor glucose level was less than 63 mg per deciliter (3.5 mmol per liter), was significantly lower by 39% (95% CI, 24 to 51) during the intervention period than during the control period (P<0.001). After adjustment of the sensor-augmented pump therapy during the run-in phase, the glycated hemoglobin level was lower during the day-and-night closed-loop insulin-delivery period than during the control period (P=0.002).
Table 2. Comparison of Day-and-Night and Overnight Glucose Control during Closed-Loop and Control Periods.*.
| End Point |
Adults
|
Children and Adolescents
|
||||||
|---|---|---|---|---|---|---|---|---|
| Closed-Loop Period (N = 32) |
Control Period (N = 33) |
Paired Difference or Paired Ratio (95% CI)† |
P Value | Closed-Loop Period (N = 25) |
Control Period (N = 24) |
Paired Difference or Paired Ratio (95% CI)† |
P Value | |
| Day and night | ||||||||
| Percent of time with glucose level in range | ||||||||
| 70 to 180 mg/dl‡ | 67.7±10.6 | 56.8±14.2 | 11.0 (8.1 to 13.8) | <0.001 | 61.2±11.9 | 51.6±11.8 | 8.9 (5.9 to 11.8) | <0.001 |
| >180 mg/dl | 29.2±11.4 | 38.9±16.6 | −9.6 (−13.0 to −6.3) | <0.001 | 36.0±12.5 | 44.5±12.7 | −7.7 (−11.0 to −4.4) | <0.001 |
| <70 mg/dl — median (interquartile range) | 2.9 (1.4 to 4.5) | 3.0 (1.8 to 6.1) | 0.81 (0.68 to 0.96) | 0.02 | 3.1 (1.7 to 3.5) | 3.8 (1.6 to 5.2) | 0.83 (0.62 to 1.10) | 0.18 |
| <50 mg/dl — median (interquartile range) | 0.3 (0.1 to 0.7) | 0.4 (0.1 to 0.9) | 0.45 (0.31 to 0.65) | <0.001 | 0.2 (0.1 to 0.4) | 0.4 (0.2 to 0.7) | 0.47 (0.22 to 1.00) | 0.05 |
| AUC during day with glucose <63 mg/dl — median (interquartile range)§ |
169 (35 to 344) | 198 (74 to 479) | 0.61 (0.49 to 0.76) | <0.001 | 147 (75 to 209) | 221 (97 to 385) | 0.58 (0.35 to 0.96) | 0.03 |
| Glucose (mg/dl) | 157±19 | 168±28 | −11 (−17 to −6) | <0.001 | 172±28 | 182±28 | −9 (−16 to −2) | 0.01 |
| Within-day SD of glucose (mg/dl) | 61±12 | 65±12 | −5 (−7 to −3) | <0.001 | 77±18 | 78±14 | −1 (−4 to 2) | 0.54 |
| Coefficient of variation of glucose (%) | ||||||||
| Within day | 39±4 | 39±4 | −0 (−2 to 1) | 0.41 | 45±5 | 43±5 | 2 (0 to 3) | 0.07 |
| Between days | 15±3 | 18±4 | −3 (−4 to −2) | <0.001 | 20±5 | 22±4 | −2 (−3 to 0) | 0.08 |
| Nighttime | ||||||||
| Percent of time with glucose level in range | ||||||||
| 70 to 145 mg/dl¶ | 59.1±9.5 | 39.4±13.3 | 19.4 (15.5 to 23.3) | <0.001 | 59.7±11.5 | 34.4±11.0 | 24.7 (20.6 to 28.7) | <0.001 |
| >145 mg/dl | 38.0±9.4 | 55.6±15.8 | −17.1 (−21.4 to −12.8) | <0.001 | 37.2±12.1 | 60.7±13.2 | −22.9 (−28.2 to −17.6) | <0.001 |
| <70 mg/dl — median (interquartile range) | 2.4 (1.1 to 3.7) | 4.0 (1.8 to 5.8) | 0.59 (0.44 to 0.78) | 0.001 | 2.2 (1.8 to 4.3) | 3.5 (1.2 to 5.9) | 0.91 (0.56 to 1.48) | 0.70 |
| <50 mg/dl — median (interquartile range) | 0.3 (0.1 to 0.6) | 0.4 (0.2 to 1.2) | 0.46 (0.28 to 0.76) | 0.004 | 0.3 (0.1 to 0.5) | 0.6 (0.1 to 1.1) | 0.64 (0.26 to 1.55) | 0.31 |
| AUC during day with glucose <63 mg/dl — median (interquartile range)§ |
151(45 to 262) | 214 (117 to 620) | 0.41 (0.27 to 0.60) | <0.001 | 137 (57 to 297) | 295 (81 to 553) | 0.78 (0.38 to 1.61) | 0.48 |
| No. of nights with glucose <63 mg/dl — median (interquartile range) |
7 (3 to 12) | 10 (5 to 17) | 0.62 (0.45 to 0.87) | 0.007 | 7 (4 to 10) | 7 (5 to 16) | 1.06 (0.56 to 2.02) | 0.85 |
| Glucose (mg/dl) | 143±13 | 162±25 | −19 (−25 to −13) | <0.001 | 146±22 | 176±29 | −29 (−39 to −20) | <0.001 |
| Within-night SD of glucose (mg/dl) | 53±11 | 60±12 | −8 (−11 to −5) | <0.001 | 60±17 | 71±13 | −11 (−17 to −6) | <0.001 |
| Coefficient of variation of glucose (%) | ||||||||
| Within night | 37±5 | 37±5 | −1 (−3 to 1) | 0.25 | 40±7 | 41±7 | 0 (−4 to 4) | 0.95 |
| Between nights | 25±5 | 29±6 | −5 (−7 to −3) | <0.001 | 28±8 | 33±7 | −6 (−10 to −2) | 0.007 |
| Glycated hemoglobin (%) | ||||||||
| Before the intervention | 7.6±0.9 | 7.6±0.8 | — | — | 7.8±0.7 | 7.8±0.6 | — | — |
| After the intervention | 7.3±0.8 | 7.6±1.1 | −0.3 (−0.5 to −0.1) | 0.002 | 7.6±1.1 | 7.9±0.6 | −0.3 (−0.6 to 0.1) | 0.17 |
Plus–minus values are means ±SD. Adults used the closed-loop system day and night, and children and adolescents used the closed-loop system overnight only. Data for the 24-hour period were calculated from sensor glucose measurements that were recorded throughout the study (day and night) for the children and adolescents.
Normally distributed data are presented as mean differences of values (closed-loop phase minus control phase), with 95% confidence intervals for the mean. A positive value indicates that the measurement was higher during the closed-loop period than it was during the control period. Nonnormally distributed data are presented as the ratio of values in the closed-loop phase over those in the control phase, with 95% confidence intervals for the ratio. Values greater than 1 indicate that the measurement was higher during the closed-loop period than during the control period.
This assessment was the primary end point for the closed-loop study involving adults.
The area under the curve (AUC) is for a glucose level of less than 63 mg per deciliter (3.5 mmol per liter) per 24-hour period and was calculated as milligrams per deciliter, multiplied by minutes.
This assessment was the primary end point for the closed-loop study involving children and adolescents.
Figure 1. Sensor Glucose Levels and Insulin Delivery.
Shown are the median sensor glucose levels and the median values for insulin delivery during the day-and-night closed-loop study involving adults (Panel A) and the overnight closed-loop study involving children and adolescents (Panel B). The bands indicate interquartile ranges. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Glucose variability, measured both as the standard deviation of the sensor glucose level and as the coefficient of variation of the sensor glucose level between days, was significantly lower with day-and-night use of the closed-loop system than with the control system. During the intervention period, the time during which the glucose level was in target range was greater and the mean overnight glucose level and the glycated hemoglobin level were lower with the closed-loop system than with the control system, without an increase in the total daily insulin use (P=0.57) (Table S1 in the Supplementary Appendix). Higher basal insulin delivery during the intervention period than during the control period (P<0.001) was offset by lower bolus delivery during the intervention period (P=0.002), presumably owing to lower glucose levels that resulted in reduced correction boluses.
Insulin delivery during the daytime (8:01 a.m. to 11:59 p.m.) and overnight (midnight to 8:00 a.m.) were similar during the two study periods. The day-and-night closed-loop system was used for a median of 20.2 hours per day, and participants wore the continuous glucose monitor for a median of 22.7 hours per day. During the control period, participants wore the continuous glucose monitor for a median of 22.9 hours per day.
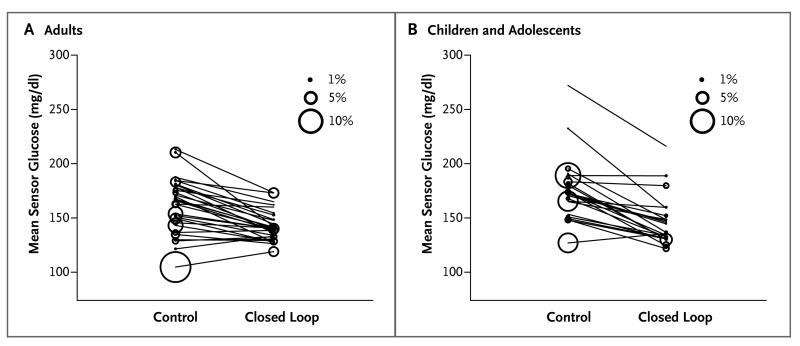
Overnight end points were similar to those during the 24-hour period. The overnight mean glucose level was significantly lower with the closed-loop system than with the control system (P<0.001), and the proportion of time that the glucose level was within the overnight target range was greater with the closed-loop system (P<0.001) (Fig. 2). The proportion of time that patients had hypoglycemia, the number of nights that the sensor glucose level was less than 63 mg per deciliter for at least 20 minutes, and the area under the curve when the sensor glucose level was less than 63 mg per deciliter were all significantly lower with the closed-loop system than with the control system.
Figure 2. Overnight Glucose Levels.
Shown are the individual overnight mean sensor glucose levels in adults (Panel A) and in children and adolescents (Panel B). Adults used the closed-loop systems day and night and children and adolescents used the closed-loop systems overnight. The size of the bubble indicates the proportion of time overnight during which the glucose level was below 50 mg per deciliter (2.8 mmol per liter).
The daytime end points are shown in Table S2 in the Supplementary Appendix. We observed in a lower mean glucose level, an increased proportion of time spent within the target range, and a reduced proportion of time spent above the target range. The time that the glucose level was less than 50 mg per deciliter was significantly lower with the closed-loop system than with the control therapy (P=0.02).
END POINTS IN THE STUDY INVOLVING CHILDREN AND ADOLESCENTS
The primary and secondary end points in the study involving children and adolescents are shown in Table 2. The sensor glucose levels and insulin-delivery profiles are shown in Figure 1B. The proportion of nocturnal time that the glucose level was in the target range (primary end point) was significantly greater during the intervention period than during the control period by a mean of 24.7 percentage points (95% CI, 20.6 to 28.7; P<0.001). The mean overnight glucose level was significantly lower with the closed-loop system than with the control system (P<0.001) (Fig. 2), as was the time spent above the target range (P<0.001). The proportion of time that the sensor glucose level indicated a blood glucose level below 70 mg per deciliter was less than 4%, and the proportion of the time that the sensor glucose level indicated a blood glucose level below 50 mg per deciliter was less than 1%; these values were similar during the two study periods.
Glucose variability, as measured by the standard deviation of the overnight sensor glucose level and the coefficient of variation between nights, was significantly less with the closed-loop system than with the control system. Nocturnal glucose levels were lower during the intervention period than during the control period without an increase in the total overnight insulin dose (P=0.11) (Table S1 in the Supplementary Appendix). Daytime insulin delivery and the total daily insulin dose were similar during the two study periods. The overnight closed-loop system was operating for a median of 9.3 hours per day. Participants wore the continuous glucose monitor for a median of 22.1 hours per day during the intervention period and for a median of 20.3 hours per day during the control period (Table S3 in the Supplementary Appendix).
End points calculated over the 24-hour period are shown in Table 2. The 24-hour mean glucose level was significantly lower with overnight use of the closed-loop system than with the sensor-augmented pump therapy (P=0.01), and the proportion of time spent within the wider (70 to 180 mg per deciliter) target range was significantly greater with the closed-loop system (P<0.001). The time that the glucose level was below 50 mg per deciliter over the 24-hour period tended to be lower with the closed-loop system than with the control system (P=0.05). The burden of hypoglycemia during the 24-hour period, as measured by the area under the curve when the sensor glucose level was less than 63 mg per deciliter, was significantly lower by 42% (95% CI, 4 to 65) during the intervention period than during the control period (P=0.03).
The comparison of end points during daytime is shown in Table S2 in the Supplementary Appendix. The mean glucose level and the proportions of time spent within, above, and below the wider target range were similar during the two study periods. The area under the curve when the sensor glucose level was less than 63 mg per deciliter was significantly lower during the intervention period than during the control period (P=0.04). The time that the glucose level was below 50 mg per deciliter tended to be lower during the intervention period than during the control period (P=0.07), a finding that was attributed to the fact that the amount of time that the level was below 50 mg per deciliter was 79% (95% CI, 34 to 93) lower in the intervention period than in the control period during the post-breakfast time period (8:01 a.m. to 11:59 a.m.) (P=0.01).
ADVERSE EVENTS
Details of all the adverse events are provided in Table 3. One episode of severe hypoglycemia occurred in an adult participant during the intervention period when the closed-loop system was not in use because of loss of connectivity (low battery) and the participant was receiving insulin at the rate supplied by the study insulin pump (Fig. S5 in the Supplementary Appendix). In the study involving children and adolescents, one adolescent participant had two severe hypoglycemic episodes (seizures) during the intervention period; these episodes required third-party assistance but did not result in hospital admission (Fig. S6 and S7 in the Supplementary Appendix). During the two episodes, the closed-loop system was not in use (closed-loop system not turned on and lack of pump connectivity) and the participant was using sensor-augmented pump therapy. The adult and adolescent participants both recovered fully, without clinical sequelae.
Table 3. Adverse Events.*.
| Event |
Adults (N = 33)
|
Children and Adolescents (N = 25)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Run-in Period |
Closed-Loop Period |
Control Period |
Washout Period |
Run-in Period |
Closed-Loop Period |
Control Period |
Washout Period |
|
| number (percent) | ||||||||
| Respiratory tract infection | 0 | 6 (18) | 6 (18) | 0 | 0 | 0 | 0 | 1 (4) |
| Gastroenteritis | 0 | 2 (6) | 3 (9) | 0 | 0 | 0 | 0 | 0 |
| Shingles | 0 | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0 |
| Knee-joint arthralgia | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Fractured finger | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 | 0 |
| Inflammation at site of sensor insertion |
0 | 2 (6) | 4 (12) | 0 | 0 | 1 (4) | 0 | 0 |
| Ketonemia related to inter- current illness |
0 | 1 (3) | 1 (3) | 0 | 0 | 2 (8) | 0 | 0 |
| Hyperglycemia related to infusion-set occlusion |
0 | 6 (18) | 4 (12) | 0 | 0 | 2 (8) | 0 | 0 |
| Severe hypoglycemia | 0 | 1 (3) | 0 | 0 | 0 | 2 (8) | 0 | 0 |
| Hospitalization | ||||||||
| Due to inguinal hernia | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Due to renal calculi | 0 | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0 |
| Due to peritonsillar abscess | 0 | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0 |
| Due to gastroenteritis | 0 | 0 | 0 | 0 | 1 (4) | 0 | 0 | 0 |
Adults used the closed-loop system day and night, and children and adolescents used the closed-loop system overnight only. In the two studies, the closed-loop (intervention) and the control periods each lasted 12 weeks. In the study involving adults, the run-in period lasted 4 to 6 weeks and the washout period lasted 4 to 6 weeks. In the study involving children and adolescents, the run-in period lasted 2 to 8 weeks and the washout period lasted 3 to 4 weeks.
DISCUSSION
Our findings show the feasibility, safety, and efficacy of 12-week day-and-night application of closed-loop insulin delivery in adults and overnight application in children and adolescents under free-living conditions. Among adults, glucose control was better with day-and-night use of a hybrid closed-loop system than with a control system consisting of a sensor-augmented pump: the proportion of time that the glucose level was in the target range was greater with the closed-loop system, and the mean glucose level and the risk of hypoglycemia were lower; the glycated hemoglobin level was also lower. Among children and adolescents, the amount of time that the nocturnal sensor glucose level was within the target range was greater with overnight use of the closed-loop system than with the control system and the mean glucose level was lower. Extended benefits from overnight use of the closed-loop system in children and adolescents were seen over the full 24-hour period, and the reduced burden of hypoglycemia was attributed mainly to the post-breakfast period.
Hypoglycemia is a key factor that limits the use of intensive insulin therapy in patients with type 1 diabetes.1 Systems with threshold-suspend control25 and predictive low-glucose suspend control26 may reduce the risk of hypoglycemia, but the systems are not designed to step up insulin delivery and do not address the issue of hyperglycemia. The advantage of a closed-loop system is the responsive, graduated modulation of insulin delivery, both below and above the preset pump regimen, which allows for improvements in the proportion of time spent in target glucose range and the lowering of the mean glucose level without increasing the risk of hypoglycemia. Even though the pump therapy was appropriately adjusted in adults during the run-in period, the glycated hemoglobin level was further reduced from the end of the run-in period with the closed-loop system. More consistent glucose values were observed with the closed-loop system than with the control therapy, despite high day-to-day variability in insulin requirements. No apparent trend was seen in the proportion of time during which the glucose level was in the target range over the 12-week intervention period, which implies rapid day-to-day adaptation by the control algorithm (Fig. S8 in the Supplementary Appendix).
The current study extends and confirms findings from our previous, shorter trials during free daily living in adults and adolescents.20,22,23 Other studies of closed-loop systems in the outpatient or home setting have been performed over a shorter duration and under conditions of remote monitoring or close supervision.21,27,28 Adults and adolescents in an outpatient setting had a lower mean glucose level with the use of a dual-hormone (insulin and glucagon) closed-loop system for 5 days than with conventional therapy, with a lower risk of hypoglycemia in adults but not in adolescents.18 Among children and adolescents at a diabetes camp, the risk of hypoglycemia was lower with a dual-hormone system used for 3 nights than with an insulin-alone closed-loop system, with a similar sensor glucose level.19 Dual-hormone systems may provide additional protection against hypoglycemia29 but are currently limited by the need to reconstitute glucagon daily and by the use of a second pump to deliver glucagon through a separate infusion set, which increases the burden and complexity.
The strengths of our studies are the multicenter design and, in the study involving adults, the multinational design, which support generalizability. In an effort to assess the real-world use and applicability of a new technology, we did not apply remote monitoring or close supervision. We did not restrict participants’ dietary intake or, after the initial 2 weeks, physical activity or geographical movements. Participants were allowed to travel and to use the system when driving. The comparator was sensor-augmented insulin-pump therapy. An efficient crossover design was adopted; confounding study-period or carryover effects were not detected for the primary end points (Tables S4 and S5 in the Supplementary Appendix). We applied clinically relevant and commonly adopted target glucose ranges to differentiate between fasting or overnight conditions and nonfasting conditions. Adherence to wearing the glucose sensor in the two participant groups was high and similar (>20 hours per day) in the two study periods. The study was limited by the number of devices each participant had to use. A more adaptive control algorithm might further enhance daytime benefits.
In conclusion, we found that extended use of a closed-loop system at home over a period of 12 weeks during free daily living without close supervision is feasible in adults, children, and adolescents with type 1 diabetes. Improvements in glucose control and reductions in the burden of hypoglycemia were observed. Among adults, the glycated hemoglobin level was lower with the use of a closed-loop system day and night than with a sensor-augmented insulin pump, even when the insulin pump was adjusted appropriately.
Supplementary Material
Acknowledgments
Supported by grants from the JDRF (22-2011-668) and Seventh Framework Program of the European Union (ICT FP7- 247138), with additional support for the artificial pancreas work from a National Institute for Health Research Cambridge Biomedical Research Centre and Wellcome Strategic Award (100574/Z/12/Z).
We thank the study volunteers for their participation; the staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility; Jasdip Mangat and John Lum, Jaeb Center, for development and validation of the closed-loop system; Josephine Hayes, University of Cambridge, for administrative support; Yue Ruan, University of Cambridge, for data management; Karen Whitehead, University of Cambridge, for laboratory support; the staff at Profil, including Krisztina Schmitz-Grozs for serving as a research physician, Martina Haase for serving as an insulin-pump expert during the adjustment phase, and Maren Luebkert, Kirstin Kuschma, and Elke Przetak for administrative, coordinating, and documentation support; and Keith Burling, Core Biochemical Assay Laboratory at University of Cambridge, and Gareth Dunseath, Institute of Life Sciences at Swansea University, for biochemical analyses.
APPENDIX
The authors’ full names and academic degrees are as follows: Hood Thabit, M.D., Martin Tauschmann, M.D., Janet M. Allen, R.N., Lalantha Leelarathna, Ph.D., Sara Hartnell, B.Sc., Malgorzata E. Wilinska, Ph.D., Carlo L. Acerini, M.D., Sibylle Dellweg, M.D., Carsten Benesch, Ph.D., Lutz Heinemann, Ph.D., Julia K. Mader, M.D., Manuel Holzer, M.Sc., Harald Kojzar, B.Sc., Jane Exall, R.N., James Yong, M.D., Jennifer Pichierri, M.Sc., Katharine D. Barnard, Ph.D., Craig Kollman, Ph.D., Peiyao Cheng, M.P.H., Peter C. Hindmarsh, M.D., Fiona M. Campbell, M.D., Sabine Arnolds, M.D., Thomas R. Pieber, M.D., Mark L. Evans, M.D., David B. Dunger, M.D., and Roman Hovorka, Ph.D., for the APCam Consortium and AP@home Consortium
The authors’ affiliations are as follows: the Wellcome Trust–Medical Research Council Institute of Metabolic Science, University of Cambridge (H.T., M.T., J.M.A., L.L., M.E.W., M.L.E., D.B.D., R.H.), the Department of Diabetes and Endocrinology, Cambridge University Hospitals NHS Foundation Trust (H.T., L.L., S.H., M.L.E.), and the Department of Paediatrics, University of Cambridge (M.T., J.M.A., M.E.W., C.L.A., D.B.D., R.H.), Cambridge, Leeds Children’s Hospital, Leeds (J.E., J.Y., F.M.C.), the Institute of Child Health, University College London Hospital, London (J.P., P.C.H.), and the Faculty of Health and Social Sciences, Bournemouth University, Bournemouth (K.D.B.) – all in the United Kingdom; Profil, Neuss, Germany (S.D., C.B., L.H., S.A.); the Department of Internal Medicine, Medical University of Graz, Graz, Austria (J.K.M., M.H., H.K., T.R.P.); and the Jaeb Center for Health Research, Tampa, FL (C.K., P.C.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–76. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036–50. doi: 10.1111/dme.12676. [DOI] [PubMed] [Google Scholar]
- 4.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20:22–5. doi: 10.2337/diacare.20.1.22. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabet Med. 2013;30:1126–31. doi: 10.1111/dme.12247. [DOI] [PubMed] [Google Scholar]
- 6.Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 7.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224–32. doi: 10.1056/NEJMoa1303576. [DOI] [PubMed] [Google Scholar]
- 9.Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7:385–95. doi: 10.1038/nrendo.2011.32. [DOI] [PubMed] [Google Scholar]
- 10.Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61:2230–7. doi: 10.2337/db11-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-Logic Artificial Pancreas System: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072–6. doi: 10.2337/dc09-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–7. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148–55. doi: 10.2337/dc12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovorka R. Artificial Pancreas Project at Cambridge 2013. Diabet Med. 2015;32:987–92. doi: 10.1111/dme.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care. 2013;36:1851–8. doi: 10.2337/dc12-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824–33. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 17.Ly TT, Breton MD, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37:2310–6. doi: 10.2337/dc14-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313–25. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haidar A, Legault L, Matteau-Pelletier L, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:595–604. doi: 10.1016/S2213-8587(15)00141-2. [DOI] [PubMed] [Google Scholar]
- 20.Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931–7. doi: 10.2337/dc13-2911. [DOI] [PubMed] [Google Scholar]
- 21.Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37:3025–32. doi: 10.2337/dc14-0835. [DOI] [PubMed] [Google Scholar]
- 22.Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014;2:701–9. doi: 10.1016/S2213-8587(14)70114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–11. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leelarathna L, Dellweg S, Mader JK, et al. Assessing the effectiveness of 3 months day and night home closed-loop insulin delivery in adults with suboptimally controlled type 1 diabetes: a randomised crossover study protocol. BMJ Open. 2014;49:e006075. doi: 10.1136/bmjopen-2014-006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310:1240–7. doi: 10.1001/jama.2013.277818. [DOI] [PubMed] [Google Scholar]
- 26.Buckingham BA, Raghinaru D, Cameron F, et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38:1197–204. doi: 10.2337/dc14-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789–96. doi: 10.2337/dc13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205–11. doi: 10.2337/dc14-3073. [DOI] [PubMed] [Google Scholar]
- 29.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3:17–26. doi: 10.1016/S2213-8587(14)70226-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.