It has been shown that chronic graft-versus-host disease (cGVHD) incidence is significantly increased in male recipients receiving allogeneic hematopoietic cell transplant from female donors (F→M HCT).1,2 B cell immunity plays a critical role in cGVHD development,3,4 and we have so far investigated clinically relevant immune responses against minor antigens encoded by the Y-chromosome (HY antigens) following F→M HCT.5–7 Recently, we have demonstrated that in F→M HCT, antibody responses against HY antigens (HY-Ab) are correlated with cGVHD and that the cumulative number of HY-Abs three months post-HCT could significantly predict cGVHD development and non-relapse mortality.8
In this study, we investigated whether HY-Ab detected in female donors prior to donation can predict cGVHD or other clinical outcomes following F→M HCT. We hypothesized that HY seropositive female grafts would lead to antigen-specific HY-Ab production in male recipients along with a higher incidence of cGVHD. We first measured HY-Abs in 289 female donors and tested their correlation with clinical outcomes of male recipients in collaboration with the National Marrow Donor Program (NMDP)/Center for International Blood and Marrow Transplant Registry (CIBMTR). Thereafter, we studied adoptive HY-Ab development in our Stanford F→M HCT cohort (n=90).8
Briefly, we measured IgG against five HY antigens using our protein microarray technology (Online Supplementary Document)9 in pre-donation sera collected from 289 female donors of 8/8 HLA-matched HCT with myeloablative conditioning, facilitated by the NMDP between 1990 and 2002. Almost all patients in the NMDP cohort (93%) received a bone marrow graft from an HLA-matched unrelated donor (Online Supplementary Table S1). Furthermore, we separately studied pre-donation plasma from 90 adult female donors and their corresponding male recipients who underwent HCT at Stanford University Hospital between 2005 and 2012. Almost all of the Stanford patients (97%) received a peripheral blood graft, for which an HLA-matched related donor was selected in 76%, and reduced-intensity conditioning was used for 57% (Online Supplementary Table S1). The Stanford data confirmed female donor HY-Ab prevalence and assessed the association between the presence of HY-Ab in female donors and post-HCT HY-Ab development in male recipients. This study was approved by The Institutional Review Board of Stanford University and the NMDP. All patients gave written informed consent for the cryopreservation and analyses of blood samples in accordance with the Declaration of Helsinki.
In the 289 NMDP female unrelated donor cohort, half of the female donors (49%) were found to have at least one of the five HY-Abs (Figure 1A), and antibody was detected most frequently against UTY and DBY which are the immune-dominant HY antigens following F→M HCT.5,8 Of the 289 patients, 75 patients (26%) had one HY-Ab, 39 patients (13%) had two HY-Abs, and 29 patients (10%) had three or more HY-Abs. A similar distribution of HY-Abs was observed in the 90 Stanford female donor cohort (mainly from related sisters, Figure 1A) and the prevalence of individual HY-Abs did not significantly differ from the NMDP cohort.
Figure 1.
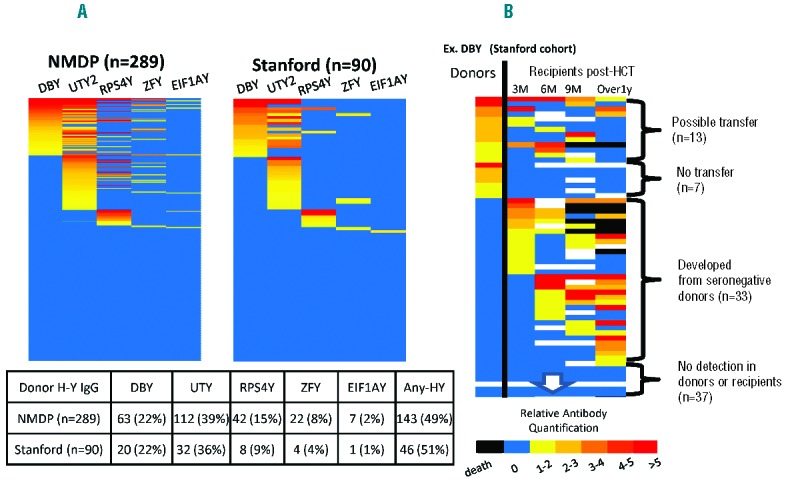
(A) Prevalence of female donor HY antibodies in the NMDP (n=289, left) and the Stanford cohorts (n=90, right). The various heat colors indicate relative antibody quantification as a factor of each HY seropositive threshold. UTY2 are shown as representative of the three UTY1-3 fragments. No statistical difference was observed between the cohorts. (B) Specific HY antibody in female donors and its temporal changes in corresponding recipients post-transplant. DBY-IgG is shown as representative. From left to right, the columns represent donors and recipients at 3, 6, and 9 months, and beyond 1 year post-transplant. The various heat colors indicate relative antibody quantification as a factor of each HY seropositive threshold. Black denotes death. For each donor-recipient pair, one of the following four patterns was observed: 1) HY humoral immunity was possibly transferred, 2) never transferred, 3) developed from seronegative donors, and 4) HY-Ab was detected in neither donors nor recipients.
Next, we assessed the association of donor HY-Abs with donor parity and donor age (range: 18–60) in the NMDP cohort. Given that women who give birth to multiple boys are reported to have anti-HY immunity,10,11 we expected multiparous female donors would have higher levels of HY seropositivity. However, detection of any HY-Abs in female donors was not associated with donor parity (Online Supplementary Table S2). One possible explanation for the absence of association is that the information on the gender of births had not been collected. Further information on birth gender might reveal a positive association, although privacy concerns should be considered. Alternatively, HY immunity may result from other male interactions besides childbirth.12,13 While there was also no difference in age between female donors testing seropositive and seronegative for any HY-Abs, the donors who had three or more HY-Abs detected (high HY score) were significantly older (P=0.02, Online Supplementary Table S2). We believe the increased HY score in older female donors may have resulted from cumulative male exposures and not isolated birth events. Analysis of the data from the Stanford cohort similarly showed no association between donor age and individual HY-Ab seropositivity in female donors. Parity information was not available in the Stanford cohort.
As our primary research endpoint, cGVHD incidence was assessed in F→M HCT recipients in the NMDP cohort. Overall cGVHD incidence was 46% (95%CI: 40–52%). Analysis was first carried out for individual HY-Ab detection in female donors prior to transplant. There was no statistical difference in cGVHD incidence in male recipients according to the detection of individual HY-Ab in female donors (Figure 2A). Analysis was then expanded to include detection of multiple HY-Abs (as reported by the HY score) in female donors, and no significant association with cGVHD incidence in male recipients was observed in either univariate or multivariate analyses (Figure 2B and Online Supplementary Table S3). Furthermore, we did not find any association between donor HY score and other recipient clinical outcomes (Online Supplementary Figure S1 and Online Supplementary Table S3). This absence of association between HY score in female donors and cGVHD development in corresponding male recipients was similarly observed both in the analysis of the Stanford cohort and when the two cohorts were combined for analysis.
Figure 2.
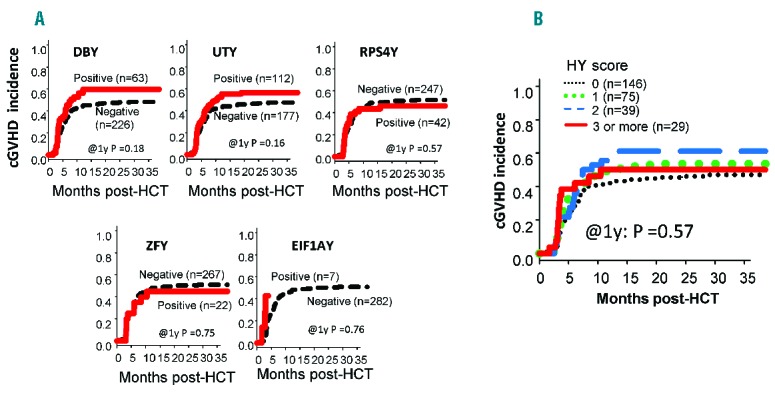
Chronic GVHD incidence (A) according to the detection of individual HY antibodies in female donors prior to transplant and (B) according to the detection of multiple HY antibodies in female donors (donor HY score) prior to transplant. Neither the detection of individual donor HY-Abs prior to transplant nor increased donor HY score prior to transplant was associated with chronic GVHD development in male recipients. (Figures are for the NMDP cohort).
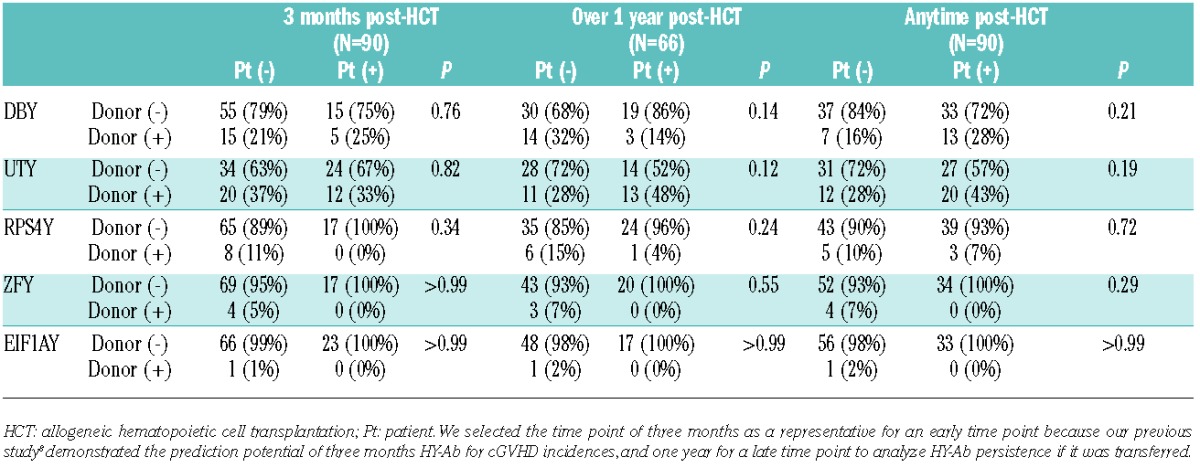
This absence of female donor HY-Ab association with male recipient clinical outcomes raised the question of whether specific humoral immunity against each HY antigen could be truly transferred from female donors to male recipients. To address this directly, HY-Ab was tested in the 90 Stanford female donors and their male recipients. Figure 1B shows DBY-IgG development as representation of all five HY antigens. For each donor-recipient pair, one of the four following patterns was observed: 1) HY humoral immunity was possibly transferred, 2) never transferred, 3) developed from seronegative donors, and 4) HY-Ab was detected in neither the donor nor recipient in the pair (Figure 1B). Of note, antibody detected in female donors against each specific HY antigen was not associated with post-HCT development in their corresponding male recipients (Table 1). Rather, individual HY-Abs post-HCT seem to develop frequently from donors who were seronegative for the specific HY antigen, and the positive predictive value for HY-Ab development three months post-HCT was 25% in DBY-IgG, 38% in UTY-IgG, and 0% in the other three antigens (Table 1). Thus, these results provide little evidence that adoptive humoral HY immunity transfers from female donors to male recipients.
Table 1.
Association between individual donor HY seropositivities and their development post-HCT in the Stanford cohort.
Consistent with our HY-Ab findings, a prior CIBMTR analysis showed that the greatest increase in cGVHD risk in F→M HCT was due to sex mismatch disparity itself rather than donor parity (41% for multiparous female donors vs. 39% for nulliparous female donors vs. 30% for male donors).2 This lack of difference in cGVHD incidence between those with nulliparous and parous female donors can be supported by the absence of HY immunity transfer from female donors to male recipients in our current study. Together, this clinical study and our donor HY-Ab analysis suggests alloimmunity results primarily from de novo HY antigen stimulation following F→M HCT, and that female donor HY-Ab testing does not improve donor selection. The de novo development of humoral HY immunity post-F→M HCT predicts cGVHD development, while female donor HY-Ab status does not. Furthermore, the identification and monitoring of allogeneic HY-Ab development post-HCT may help assess the biological effects of novel therapeutic agents in order to optimize when and how to use these B cell targeted drugs.
The absence of adoptive humoral HY immune transfer might raise a concern for the efficacy of donor vaccination strategies to augment humoral graft-versus-leukemia benefits. Similar to HY immunity, presensitized donor anti-tumor humoral immunity may not be effectively transferred to recipients. Human clinical trials in multiple myeloma have included donor immunization followed by a recipient booster vaccination. In a recent trial, an increase of anti-idiotype IgG was limited to only 3 out of 10 recipients following a post-HCT booster vaccination, even though it could be induced and detected in most similarly vaccinated donors.14 Post-HCT vaccination/sensitization may be critical for the development of effective allo- and anti-tumor humoral immunity.
Our study may have some limitations. First, the current study was retrospective, but the two different cohorts independently produced similar results, providing strong evidence for the absence of an association of donor HY seropositivity with clinical outcomes. Second, the sample size of this study might have been too small to detect a significant impact of donor HY seropositivity. If a sample size larger than the almost 300 patients in this study is needed to detect an association, the impact for female donor HY seropositivity is unlikely to be clinically significant or influential for donor selection criteria. Third, measuring HY-Abs is an indirect tool to assess the transfer of HY reactive B cells. Perhaps the direct clone identification of HY reactive B cells via high throughput sequence analysis would reveal adoptive B cell transfer from donors to recipients post-HCT.15 The absence of HY-Ab transfer from female donors to male recipients was assessed only in the Stanford cohort, where donors predominately provided G-CSF mobilized grafts (97%). It would be warranted to confirm the HY-Ab transfer in the case of bone marrow transplantation.
In summary, half of the female donors were shown to be HY seropositive using our novel microarray system. However, there was little evidence to suggest that female donor HY serostatus prior to transplant was associated with post-HCT HY-Ab development or clinical outcomes following F→M HCT. Most humoral HY immunity may develop post F→M HCT from naïve B cells being stimulated by HY antigen following sex mismatched transplantation.
Acknowledgments
We thank the nursing staff, social workers, case managers, physical therapists, dieticians, and data managers who made the care of the patients described herein possible. The care of these patients, and the management of data were supported by NIH P01CA049605, R21 HL084318 and K08HL69132.
Footnotes
Funding: the CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and National Institute of Allergy and Infectious Diseases (NIAID); a grant/cooperative agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Abbott Laboratories; Aetna; American International Gropu, Inc.; American Red Cross; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; AnorMED, Inc.; Astellas Pharma US, Inc.; Baxter International, Inc.; Berlex Laboratories, Inc.; Biogen IDEC, Inc.; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bristol- Myers Squibb Company; BRT Laboratories, Inc.; Cangene Corporation; Celgene Corporation; CellGenix, Inc.; Cell Therapeutics, Inc.; CelMed Biosciences; Cylex Inc.; Cytonome, Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline, Inc.; Histogenetics, Inc.; HKS Medical Information Systems; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; MultiPlan, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Pharmaceuticals, Inc.; Osiris Therapeutics, Inc.; Pall Medical; Pfizer, Inc.; Pharmion Corporation; PDL BioPharma, Inc; Roche Laboratories; Sanofi-aventis; Schering Plough Corporation; StemCyte, Inc.; StemSoft Software, Inc.; SuperGen, Inc.; Sysmex; The Marrow Foundation; THERAKOS, Inc.; University of Colorado Cord Blood Bank; ViaCell, Inc.; ViraCor Laboratories; Wellpoint, Inc.; and Zelos Therapeutics, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
The online version of this paper contains a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. [DOI] [PubMed] [Google Scholar]
- 2.Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(7):758–769. [DOI] [PubMed] [Google Scholar]
- 3.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakasone H, Sahaf B, Miklos DB. Therapeutic benefits targeting B-cells in chronic graft-versus-host disease. Int J Hematol. 2015;101(5):438–451. [DOI] [PubMed] [Google Scholar]
- 5.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahaf B, Yang Y, Arai S, Herzenberg LA, Miklos DB. H-Y antigen-binding B cells develop in male recipients of female hematopoietic cells and associate with chronic graft vs. host disease. Proc Natl Acad Sci U S A. 2013;110(8):3005–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popli R, Sahaf B, Nakasone H, Lee JY, Miklos DB. Clinical impact of H-Y alloimmunity. Immunol Res. 2014;58(2–3):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakasone H, Tian L, Sahaf B, et al. Allogeneic HY antibodies detected 3 months after female-to-male HCT predict chronic GVHD and nonrelapse mortality in humans. Blood. 2015;125(20):3193–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadia PP, Sahaf B, Miklos DB. Recombinant antigen microarrays for serum/plasma antibody detection. Methods Mol Biol. 2011;723:81–104. [DOI] [PubMed] [Google Scholar]
- 10.Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103(5):1961–1964. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen HS, Wu F, Aghai Z, et al. H-Y antibody titers are increased in unexplained secondary recurrent miscarriage patients and associated with low male : female ratio in subsequent live births. Hum Reprod. 2010;25(11):2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YX, Zhu WJ, Jiang H. Sperm exposure during menses is a risk factor for developing antisperm antibody (ASA) in female. Arch Gynecol Obstet. 2013;288(5):1145–1148. [DOI] [PubMed] [Google Scholar]
- 13.Peters B, Whittall T, Babaahmady K, Gray K, Vaughan R, Lehner T. Effect of heterosexual intercourse on mucosal alloimmunisation and resistance to HIV-1 infection. Lancet. 2004;363(9408):518–524. [DOI] [PubMed] [Google Scholar]
- 14.Foglietta M, Neelapu SS, Kwak LW, et al. Neoantigen and tumor antigen-specific immunity transferred from immunized donors is detectable early after allogeneic transplantation in myeloma patients. Bone Marrow Transplant. 2013;48(2):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan A, Sahaf B, Zhang B, et al. Impaired B Cell Clonotype Diversification After Allogeneic Hematopoietic Cell Transplantation Predicts Graft-Versus-Host Disease. Biol Blood Marrow Transplant. 2013;19(2):S148–149 [Google Scholar]


