Abstract
Introduction
The Failure to Rescue (FTR) rate is the probability of death after a major complication and was defined in elective surgical cohorts. In elective surgery, the precedence rate (proportion of deaths preceded by major complications) approaches 100%, but recent studies in trauma report rates of only 20–25%. We hypothesized that use of high quality data would result precedence rates in higher than those derived from national datasets, and we further sought to characterize the nature of those deaths not preceded by major complications.
Methods
Prospectively collected data from 2006–2010 from a single level I trauma centre were used. Patients age >16 years with AIS ≥2 who survived beyond the trauma bay were included. Complications, mortality, FTR, and precedence rates were calculated. Chart abstraction was performed for registry deaths without recorded complications to verify the absence of complications and determine the cause of death, after which outcomes were re-calculated.
Results
A total of 8004 patients were included (median age 41(IQR 25–75), 71% male, 82% blunt, median ISS 10(IQR5–18)). Using registry data the precedence rate was 55%, with 132/293 (45%) deaths occurring without antecedent major complications. On chart abstraction, 11/132 (8%) patients recorded in the registry as having no complication prior to death were found to have major complications. Complication and FTR rates after chart abstraction were statistically significantly different than those derived from registry data alone (complications 16.5% vs. 16.3, FTR 12.3 vs.13p=0.001), but this difference was unlikely to be clinically meaningful. Patients dying without complications predominantly (87%) had neurologic causes of demise.
Conclusions
Use of data with near-complete ascertainment of complications results in precedence rates much higher than those from national datasets. Patients dying without precedent complications at our centre largely succumbed to progression of neurologic injury. Attempts to use FTR to compare quality between centres should be limited to high quality data.
Level of Evidence
Level III
Keywords: failure to rescue, outcomes, methodology
Historically reported outcome metrics after trauma include mortality and adverse occurrence rates, but recent work has examined the role of the Failure to Rescue (FTR) in trauma. This metric was originally described by Silber et al. in a cohort of patients undergoing elective surgery[1] and refers to the conditional probability of death after an adverse occurrence. Mathematically, this concept can be considered as the probability of death occurring after an adverse occurrence (p(d|a)) multiplied by the probability of an adverse occurrence (p(a)) plus the chance of dying after no adverse occurrence (p(d|no a)) multiplied by the probability of not having an adverse occurrence (1-p(a)), or:
In this equation, the first term describes the fraction of death attributable to dying after an adverse occurrence, while the second term describes the fraction of death attributable to dying without an adverse occurrence. In the elective surgical population, it is highly unlikely that a death would occur without a preceding serious adverse occurrence, and so the second term of the equation should reduce to zero. For this reason, any deaths that occur without a coded antecedent adverse occurrence are highly suspect for incomplete ascertainment of adverse occurrences and the “precedence rate”, or percentage of deaths that are preceded by a serious adverse occurrence should approximate 100%. Under these conditions, variations in mortality between centres are due to variations in adverse occurrence rates, variations in FTR rates, or both.
While FTR rates have great potential to inform trauma care, application of the original FTR metric to the trauma population is clouded by the second term in the equation above [p(d|no a)*(1-p(a))], which, unlike in elective surgical populations, is not expected to approach zero. Many trauma patients who die will do so secondary to progression of disease or through changes in level of the aggressiveness of care and not as result of an adverse occurrence. This is problematic because when the precedence rate is not 100%, those deaths in the second term of the equation (deaths not preceded by an adverse occurrence, or “non-precedence” deaths) could represent progression of disease, a decision to withdraw care, or a misclassified case of FTR secondary to an unrecorded adverse occurrence. Deaths secondary to progression of disease or withdrawal of care will not impact the FTR rate, but misclassification of FTR cases clearly influences FTR rates and hence estimates of the quality of care between centres.
Previous work has used data from the United States National Trauma Data Bank (NTDB) to examine FTR rates in the trauma population [2–5]. While more granular than administrative data, these registry data do not ensure complete ascertainment of adverse occurrences. In our analysis, we used institutional registry data along with chart review to ensure a detailed understanding of the hospital course. We hypothesized that the precedence rate using this granular data would not approach 100% but would be much higher the 20–25% rates that have been previously reported using NTDB data [2, 3]. Using chart abstraction, we also sought to characterize the nature, cause of, and circumstances surrounding non-precedence deaths.
Patients and Methods
This retrospective review was conducted in accordance with the ethical standards of the Perelman School of Medicine at the University of Pennsylvania and was approved by the Institutional Review Board.
The Hospital of the University of Pennsylvania is an academic level 1 trauma centre located in urban Philadelphia which participates in the Pennsylvania Trauma Outcomes Study (PTOS), a state-wide data registry. This database is maintained by the Pennsylvania Trauma Systems Foundation (PTSF), which is responsible for accreditation and quality of trauma centres in Pennsylvania. To ensure the quality of data collection at the centre-level, specially trained registrars at each trauma centre prospectively abstract detailed data from the medical chart of each patient meeting inclusion criteria into the PTOS registry. These data are collected according to standardized definitions put forth by the PTSF and a subset of charts is re-reviewed to ensure inter-rater reliability by registrars. Centrally, the PTSF assures the quality of the data by submitting it to range, logic, and missingness checks. Additionally, subsets of submitted data are re-abstracted by the PTSF during site accreditation visits to verify accuracy. As data quality is linked to accreditation, centres are strongly incentivized to accurately report data and rates of missing data are low. We performed a 5-year query of our institutional PTOS data from 1 January 2006 to 31 December 2010. Demographic data, presenting vital signs, mechanism of injury, Abbreviated Injury Scale (AIS) scores, Injury Severity Scores (ISS), serious adverse occurrences, and morality were abstracted. Patients were included if they were >16 years of age and were admitted to an inpatient setting. Patients who died in the trauma bay, died in the operating room after resuscitation in the trauma bay, had a maximum AIS of <2, were less than 16 years of age, were pregnant, or were prisoners were excluded. Patients were considered to have unstable vital signs if they presented with a systolic blood pressure of <90mmHg or a pulse rate of >100 beats per minute.
The mortality rate and the serious adverse occurrence rate were calculated for the included population. Adverse occurrences were defined in accordance with the PTOS data definitions (available online at http://www.ptsf.org/upload/2014_PTOS_Manual_Tab_1_through_4_Final.doc). Consistent with the original FTR work, we used the definition of serious adverse occurrences that captured the greatest fraction of overall deaths [1, 6]. Adverse occurrences were considered serious adverse occurrences if they were included by definitions initially put forth by Silber et al. or if they were found in univariate logistic regression analysis to be associated with mortality (p < 0.1). The failure to rescue rate, defined as the probability of death after serious adverse occurrence, was calculated. Non-precedence deaths, defined as those patients who died without recorded adverse occurrences were then isolated. The medical records of this subset of patients were abstracted to determine the proportion who did in fact have an adverse occurrence prior to death (false negative FTR), the proportion who presented with pre-existing Do-Not-Resuscitate (DNR) orders, the proportion undergoing withdrawal of care, and the proportion expiring due to progression of disease. Death secondary to progression of disease was defined as the occurrence of a brain death examination consistent with brain death or death that occurred in the setting of unsuccessful ongoing resuscitative efforts. Mortality rates, adverse occurrences, and FTR were recalculated after excluding patients who died in less than 48 hours in order to examine the impact of early deaths.
Categorical variables were compared between the FTR and non-FTR groups using Fisher’s exact test. Continuous variables were assessed for normality using Shapiro-Wilk test. Those that were found to be non-normally distributed were compared using Mann-Whitney (2 groups) or Kruskal-Wallis test (more than 2 groups). Continuous variables that were normally distributed were compared between groups using t-test. Mortality, serious adverse occurrence, and FTR rates were compared before and after chart abstraction using one or two sample test of proportions as appropriate, with 95% exact confidence intervals. Two-tailed statistical significance was set at p=0.05. All statistical analyses were conducted using Stata v13.1 (College Station, TX).
Results
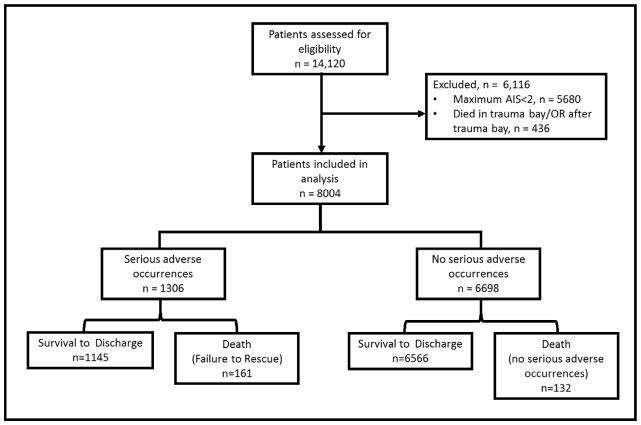
A total of 14,120 patients were seen at our centre over the study period, of which 5680 were excluded for a maximum AIS of <2. An additional 436 patients were excluded for death in trauma bay or death in the OR immediately after the trauma bay leaving 8004 patients for analysis (Figure 1). Patients had a mean age of 41 (IQR 25–75), were 71% male, were 47% African American, and suffered chiefly from blunt mechanisms of injury (82%). The most common comorbidities were hypertension (24%), alcohol abuse (14.3%), drug abuse (12.1%), and Type II diabetes (12%). Baseline demographics of the cohort can be seen in Table 1.
Figure 1.
Flow chart of numbers of patients eligible and included in the study.
Table 1.
Demographics and mechanisms of injury for the overall cohort.
| Overal Cohort n= 8004 | |
|---|---|
|
| |
| Age in years | 41 (IQR 25–75) |
| Male gender | 5,709 (71%) |
| Race | |
| African American | 3,737 (47%) |
| Caucasian | 3674 (46%) |
| Other | 593 (7.4%) |
| Blunt Mechanism | 6589 (82%) |
| Unstable vitals | 2,209 (28%) |
| ISS | 10 (IQR 5–18) |
| Max AIS | 3 (IQR 2–4) |
| Surgical Intervention | 926 (12%) |
| Comorbidities | |
| Hypertension | 1973 (24.7%) |
| ETOH abuse | 1142 (14.3%) |
| Drug abuse | 972 (12.1%) |
| DM II | 525 (6.6%) |
| CAD | 383 (4.8%) |
| CVA | 250 (3.1%) |
| Warfarin use | 239 (3.0%) |
| Malignancy | 216 (2.7%) |
Non-parametric continuous values expressed as median (Interquartile Range); Categorical values expressed as n (%). ISS= Injury Severity Score; AIS= Abbreviated Injury Scale, ETOH = Ethanol; DM II = Diabetes Mellitus Type II; CAD = Coronary Artery Disease; CVA = Cerebrovascular Accident.
There were 3,163 adverse occurrences that occurred in 1,395 patients for an overall adverse occurrence rate of 17.4%. After univariate analysis with mortality and inclusion of definitions from previous work, the final list of serious adverse occurrences included Acute Respiratory Distress Syndrome (ARDS), acute respiratory failure, aspiration pneumonia, atelectasis, pneumonia, pneumothorax, pulmonary embolism, deep venous thrombosis, arrhythmia, coagulopathy, pleural effusion, hypothermia, post-operative haemorrhage, cardiopulmonary arrest, acute kidney injury, hepatic failure, stroke, empyema, sepsis, septicaemia, gastrointestinal bleed, organ/vessel/nerve damage, decubitus ulcer, urinary tract infection, and wound infection. Associations between adverse occurrences and death can be seen in Table 2. When restricted to serious adverse occurrences, as defined above, there were 2,901 serious adverse occurrences in 1,306 patients for a serious adverse occurrence rate of 16.3%. The overall number of deaths and serious adverse occurrences in the study cohort can be seen in Figure 1. Restricting the cohort to those patients who survived for >48 hours did not significantly change these estimates, indicating that the majority of patients who died before this time point did so without recorded adverse occurrences.
Table 2.
Adverse occurrences and their univariate associations with mortality.
| Adverse Occurrence | n | Association with mortality | ||
|---|---|---|---|---|
| RR | 95%CI | p | ||
| ARDS | 59 | 10.40 | (7.23– 14.94) | 0.00 |
| Acute Respiratory Failure | 240 | 4.39 | (3.16– 6.1) | 0.00 |
| Apiration Pneumonia | 205 | 3.86 | (2.67– 5.59) | 0.00 |
| Atelectasis | 154 | 2.94 | (1.83– 4.75) | 0.00 |
| Pneumonia | 422 | 3.01 | (2.2– 4.11) | 0.00 |
| Pneumothorax | 30 | 3.68 | (1.47– 9.23) | 0.02 |
| Pulmonary Embolism | 57 | 2.91 | (1.36– 6.27) | 0.02 |
| Extremity Compartment Syndrome | 46 | 1.79 | (.6– 5.38) | 0.24 |
| Deep Venous Thrombosis | 289 | 1.54 | (.94– 2.52) | 0.11 |
| Arrhythmia | 149 | 7.62 | (5.62– 10.33) | 0.00 |
| Transfusion Reaction | 3 | 9.13 | (1.84– 45.43) | 0.11 |
| Coagulopathy | 95 | 13.55 | (10.44– 17.58) | 0.00 |
| Pleural Effusion | 64 | 2.59 | (1.2– 5.6) | 0.03 |
| Hypothermia | 5 | 16.55 | (8.02– 34.15) | 0.00 |
| Post-operative Haemorrhage | 23 | 4.80 | (1.96– 11.79) | 0.01 |
| Cardiopulmonary Arrest | 79 | 16.79 | (13.2– 21.34) | 0.00 |
| Acute Kidney Injury | 74 | 13.60 | (10.27– 18.01) | 0.00 |
| Hepatic Failure | 5 | 16.55 | (8.02– 34.15) | 0.00 |
| Dehiscence | 35 | 1.56 | (.41– 6.04) | 0.37 |
| Stroke | 37 | 2.98 | (1.17– 7.57) | 0.05 |
| Empyema | 14 | 3.92 | (1.08– 14.22) | 0.09 |
| Sepsis | 63 | 11.76 | (8.48– 16.3) | 0.00 |
| Septicemia | 116 | 3.67 | (2.26– 5.97) | 0.00 |
| Gastrointestinal Bleed | 52 | 5.40 | (3.06– 9.54) | 0.00 |
| Organ, Vessel, Nerve Damage | 51 | 2.71 | (1.17– 6.27) | 0.04 |
| Decubitus | 138 | 1.81 | (.95– 3.43) | 0.10 |
| Urinary Tract Infection | 323 | 1.46 | (.91– 2.36) | 0.13 |
| Wound Infection | 149 | 1.86 | (1.01– 3.43) | 0.07 |
RR= Relative Risk, CI = Confidence Interval, ARDS=Acute Respiratory Distress Syndrome. P values for Fisher’s exact test.
Of the 1306 patients who developed serious adverse occurrences, 161 died for a FTR rate of 12.3% (95%CI 10.6% – 14.3%). Demographic and clinical characteristics of the patients who died with and without a coded serious adverse occurrences were similar in age, race, mechanism, and injury severity. Patients who died FTR deaths were more likely to be male (79% vs. 65%, p<0.001) and had higher rates of pre-existing conditions (Table 3).
Table 3.
Demographics, mechanism of injury, physiology and injury severity between patients dying with (FTR) and without (non-precedented) serious adverse occurrences.
| Died | p | ||
|---|---|---|---|
| With Serious Adverse | Without Serious Adverse | ||
| Occurrences n=161 | Occurrences n=132 | ||
| Age in years | 59 (IQR 31–78) | 55 (IQR 31–78) | 0.87 |
| Male gender | 137 (79%) | 115 (65%) | <0.01 |
| Race | 0.13 | ||
| African American | 69 (40%) | 61 (35%) | |
| Caucasian | 87 (50%) | 105 (60%) | |
| Other | 17 (10%) | 10 (6%) | |
| Blunt Mechanism | 134(79%) | 141 (80%) | 0.60 |
| Unstable vitals | 73 (42%) | 75 (43%) | 1.00 |
| ISS | 29 (IQR 25–43) | 27 (IQR 25 –34) | 0.18 |
| Max AIS | 5 (IQR 4–5) | 5 (IQR 5–5) | 0.04 |
| Co-morbidities | |||
| COPD | 5 (2.9%) | 0 (0%) | 0.03 |
| Cirrhosis | 8 (4.6%) | 1 (0.6%) | 0.02 |
| Prior MI | 13 (7.5%) | 1 (0.6%) | <0.01 |
| Chemotherapy | 17 (9.8%) | 8 (4.6%) | 0.06 |
Non-parametric continuous values expressed as median (Interquartile Range); Categorical values expressed as n (%). P values are for Mann-Whitney or Fisher’s exact test, as appropriate. ISS= Injury Severity Score; AIS= Abbreviated Injury Scale; COPD = Chronic Obstructive Pulmonary Disease; MI = Myocardial Infarction.
Results of chart review for patients with non-precedence deaths can be seen in table 4. Of these, 67/132 (51%) patients were made withdrawal of care and 48 (35%) patients had a positive formal brain death evaluation, 9 (7%) were DNR on admission, and 8(6%) died of progression of injury. In total, 115/132 were deemed to have a neurologic cause of death. Only 11/132 (8%) of these deaths were discovered to have sustained a serious adverse occurrence prior to death (misclassified FTR), indicating that the vast majority of patients coded as ‘no adverse occurrence’ in this cohort did in fact expire without adverse occurrences. Repeating these analyses after restricting the cohort to those surviving >48 hours reduced the overall number of patients dying without a coded adverse occurrence and the number of patients who succumbed to brain death.
Table 4.
Factors surrounding deaths for patients who died without a recorded serious adverse event (non-precedented deaths) in the database.
| Registry deaths with no recorded serious adverse occurrences | ||
|---|---|---|
| Cause of death on chart review | Overall Cohort n= 132 | Deaths after 48 hours |
| Withdrawal of care | 67 (51%) | 41 (70%) |
| Brain Death | 48 (36%) | 14 (22%) |
| DNR on admission | 9 (7%) | 3 (5%) |
| Progression of injury | 8 (6%) | 2 (3%) |
| Serious adverse occurrence on chart review | 11 (8%) | 8 (13%) |
When examining all deaths that occurred after the trauma bay, inclusion of the misclassified FTR cases increased the serious adverse occurrence rate and the FTR rate (Table 5). Given the relatively large sample size and paired nature of the data, McNemar’s test was statistically significant, but the magnitude of the difference was unlikely to be of clinical significance (serious adverse occurrence rate 16.3% (95%CI 15.5% – 17.1%) vs. 16.5% (95%CI 15.6% – 17.2%), p=0.001), FTR rate 12.3% (95%CI 10.6% – 14.2%) vs. 13.1% (95%CI 11.2% –15.0%) p=0.001). After restricting to deaths that occurred after 48 hours and inclusion of misclassified FTR cases, similar findings were noted. After including those patients who died after serious adverse occurrences not recorded in the registry but discovered on chart review as FTR cases, there was a modest increase in the precedence rate from 55% (95%CI 49%–61%) to 58% (95% CI 53%–64%), p=0.001.
Table 5.
Mortality, FTR, and serious adverse occurrence rates before and after chart abstraction of cases who died without a serious adverse occurrence, in the overall cohort and restricting to those patients who died after 48 hours.
| Overall Cohort | |||
|---|---|---|---|
| n=8004 | |||
| Overall Cohort | After chart abstraction | p | |
| Serious adverse occurrences | 1306 (16.3, 95%CI 15.5–17.1) | 1317 (16.5, 95%CI 15.6–17.2) | 0.001 |
| Deaths | 293 (3.7, 95%CI 3.3–4.1) | 293 (3.7, 95%CI 3.3–4.1) | 1.00 |
| FTR | 161 (12.3, 95%CI 10.6–14.2) | 172 (13.1, 95%CI 11.2–15.0) | 0.001 |
| Deaths occuring after 48 hours | |||
|---|---|---|---|
| n=7891 | |||
| Overall Cohort | After chart abstraction | p | |
| Serious adverse occurrences | 1265 (16.0, 95%CI 15.2–16.9) | 1273 (16.1, 95%CI 15.3–16.9) | 0.002 |
| Deaths | 180 (2.2, 95%CI 2.0–2.6) | 180 (2.2, 95%CI 2.0–2.6) | 1 |
| FTR | 120 (9.5, 95%CI 7.9–11.2) | 128 (10.1, 95%CI 8.5–11.8) | 0.002 |
Categorical variables expressed as n (%) with 95% exact confidence intervals. P value for McNemar’s test.
Discussion
Failure to rescue as a quality metric has been in existence for over 20 years, but has only recently been explored in the trauma population. Widespread adoption of FTR in outcomes literature [7–10] may reflect the fact that the FTR metric enjoys several favourable qualities compared to examining adverse occurrences alone. First, FTR is more strongly associated with variables that are thought to represent hospital quality, such as nurse to bed ratios, board certification status of physicians, and technology indices than are adverse occurrence rates, which are more closely associated with patient-level variables [1, 11, 12]. Second, FTR rates are more closely associated with mortality rates than are adverse occurrence rates, which have been repeatedly demonstrated to show no clear association with mortality [11, 13, 14]. Third, the FTR rate can be considered as measure of the way a medical centre rises to meet the needs of a patient who has taken a turn for the worse. This concept may resonate deeply with trauma practitioners, as it could be fairly argued that the ideas of rescue and failure to rescue are the principles which underpin the development of the trauma system as a whole.
Despite these attractive properties, the application of the original iteration of the FTR metric to trauma populations must be used with caution. This metric was derived in the elective surgery cohort and it is assumed that these patients are not actively dying when they present for their elective operation. By the classical FTR methodology, patients who die in the hospital after surgery with no recorded adverse occurrences are assumed to have suffered missed adverse occurrences and are thus included as FTR cases[15]. By contrast, patients who die in the hospital after injury may be actively dying upon presentation or may die after arrival of progression of unsurvivable injury. Inclusion of these deaths as FTR cases may unfairly penalize centres with high proportions of unsurvivable injuries and may not truly reflect ability of those centres to rescue salvageable patients. Additionally, the FTR rate is critically sensitive to the ascertainment of adverse occurrences because patients who die without recorded serious adverse occurrences will not be included as FTR cases. Instead, these non-precedence cases will be included in the second term of the equation . In an elective surgical cohort these deaths would be highly suspect for an unrecorded adverse occurrence, but in trauma the second term of this equation is not expected to be zero as at least some fraction of deaths will occur secondary to progression of disease. Thus, for trauma patients, the second term of the FTR equation will contain both patients who died from disease progression plus any patients who died from unrecorded adverse occurrences. The validity of using FTR as a quality metric to compare quality of care between trauma centres hinges upon the assumption that the second term is comprised only of patients who died secondary to progression of disease.
In previous work using the FTR metric in trauma patients, Glance et al. examined the differences in adverse occurrence and FTR rates in high and low performing hospitals, with performance level being defined by risk-adjusted mortality rates. Unadjusted rates of adverse occurrences were similar between low, average, and high mortality centres (5.92% vs. 3.99% vs. 5.49%) but FTR rates increased monotonically across this spectrum (overall rates 13.2% vs. 16.9% vs. 27.5%) [2]. In a similar study, Haas et al. found that the risk-adjusted adverse occurrence rate increased monotonically across quintiles of increasing mortality risk, as did risk-adjusted mortality rates after adverse occurrences[3]. Despite different inclusion criteria and modelling, the association between mortality and FTR persisted. Both of these studies used the 2007 NTDB National Trauma Data Bank (NTDB) as the data source. Careful examination of the reported mortality, adverse occurrence, and FTR rates in these studies reveals that the overall precedence rate was only 20–25%, indicating that 70–75% of patients died without recorded antecedent serious adverse occurrences, thus excluding them from FTR analysis.
Using our own trauma registry with chart review of all deaths without a coded adverse occurrence we found a precedence rate of 55–58%. There are two possibilities for this discrepancy between rates and those reported using the NTDB. First, it may be that a national rates of death secondary to progression of disease are much higher than those observed at our centre. While some degree of variation in death secondary to progression of disease is likely to occur between centres, it is unlikely that any single centre should have rates that are twice the national average. A more likely explanation is that serious adverse occurrences are under-coded in the NTDB from which these national precedent rates were derived. Not all trauma centres that contribute data to the NTDB report adverse occurrences, and rates between centres that do are highly variable. This variation is felt by some authors to be more likely to be more reflective of quality of reporting rather than of care [16, 17] but comparisons between centres using this data will not reflect this distinction. Maintenance of trauma centre accreditation in the state of Pennsylvania requires mandatory reporting of data to the Pennsylvania State Outcomes Study (PTOS) registry. As described by in the methods section, this data is collected prospectively by trained registrars at each centre and undergoes rigorous quality assurance including range, logic, and missingness checks.
While our precedence rate was significantly higher than that reported using NTDB data, it is still much lower than the theoretical optimum of 100% which would be expected in elective surgery. This then begs the methodologic question of how to treat the large fraction of deaths in the trauma population that appear to occur without preceding serious adverse occurrences. One approach to is to include deaths that are not preceded by serious adverse occurrences as FTR cases under the assumption that these deaths resulted from missed serious adverse occurrences. This approach has face validity in the elective surgery cohort, but our data indicates that the vast majority of non-precedented deaths did in fact die without sustaining a serious adverse event. Inclusion of these deaths in the FTR rate therefore punishes centres who care for patients with non-survivable injuries and thus reflects neither the quality of care rendered nor the intention of the original FTR metric. A second approach to these non-precedented deaths is to simply ignore them and calculate FTR rates based only on those patients who had serious adverse events. Ignoring a subset of deaths may lead to reductions in the validity and reliability of the FTR metric[7]. Beyond this, poor quality data in may not capture all serious adverse events. These false negative FTR cases will not be included in FTR calculations, and thus may lead to biased estimates FTR rates secondary to misclassification of outcomes. A third and perhaps preferable approach would be to define a trauma-specific FTR metric that excludes deaths occurring secondary to factors such as non-survivable injury and pre-existing DNR orders from analysis prior to calculating FTR rates. The vast majority of patients who died without recorded serious adverse occurrences at our centre did so secondary to progression of neurologic injury. These deaths most often occurred after a positive brain death examination or secondary to withdrawal of care after a discussion of dismal neurologic prognosis. The centre-level contribution to the outcomes of such patients is expected to be negligible, so excluding these deaths a priori from FTR analysis should not compromise conclusions about quality of care between centres. Under this approach the precedence rate could rise to approximate elective surgical models and thus reduce the threat of miss-classified FTR cases. While conceptually appealing, the feasibility of this approach is yet unproven. In our analysis, comparison of simple demographic, injury, and physiologic variables between those patients who expired after FTR and those who died secondary to progression of disease did not reveal differences clear enough to reliably discriminate the two groups. While the approaches outlined above may hold promise in increasing the reliability and validity of the FTR metric in the trauma population, the best methods of doing so have yet to be defined.
As a single institution study, our work has limitations which must be discussed. First, our progression of disease estimate may not be applicable to other institutions with different case mixes. Specifically, 27% of patients who expired with no coded adverse occurrences suffered a gunshot wound to the head which may result in lower progression of disease estimates in centres with lower volumes of penetrating trauma. Excluding patients who died in trauma bay may limit the inclusion of iatrogenic adverse occurrences in the analysis, but as the majority of these patients cannot reasonably be expected to have had time to develop the most of the adverse occurrences captured by PTOS and other major databases, exclusion of these patients does not appreciably change the FTR rate. Although the registry portion of the data for this study was collected in a rigorous prospective fashion, ultimately this remains a retrospective cohort study and standard caveats to this approach apply. Notably, we cannot say with 100% certainty that all adverse occurrences were correctly ascertained, and misclassification of adverse vs. serious adverse occurrences could influence the apparent rates of serious adverse occurrences and FTR. However, of the occurrences defined by PTOS, the only ones which did not meet criteria for ‘serious’ as described in the methods section of our paper were extremity compartment syndrome, transfusion reaction, dehiscence, and urinary tract infection. Given that these are relatively distinct clinical entities, it seems unlikely that misclassification is a significant issue. Finally, one of the major limitations of retrospective cohort studies is that although associations can be described, no assumptions about causality can be made. As such, for patients who sustained more than one adverse occurrence the first serious adverse occurrence was used to define entry into FTR group, and we cannot infer that it was this adverse occurrence that was ultimately responsible for their demise.
Conclusions
In conclusion, while the precedence rate in FTR studies in trauma patients of deaths is not expected to approach 100%, using high quality data we found a precedence rate over twice that which has been reported using U.S. national data. Development of a trauma-specific metric may improve the validity of comparisons of trauma centres using FTR but his metric should account for deaths secondary to progression of disease and pre-existing DNR orders and be grounded in high-quality data.
Acknowledgments
This project was supported by Award Number K12 HL 109009 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Medical Care. 1992;30:615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Glance LG, Dick AW, Meredith JW, Mukamel DB. Variation in hospital complication rates and failure-to-rescue for trauma patients. Annals of Surgery. 2011;253:811–6. doi: 10.1097/SLA.0b013e318211d872. [DOI] [PubMed] [Google Scholar]
- 3.Haas B, Gomez D, Hemmila MR, Nathens AB. Prevention of complications and successful rescue of patients with serious complications: Characteristics of high-performing trauma centers. Journal of Trauma - Injury, Infection and Critical Care. 2011;70:575–82. doi: 10.1097/TA.0b013e31820e75a9. [DOI] [PubMed] [Google Scholar]
- 4.Almoudaris AM, Mamidanna R, Faiz O. Failure to rescue in trauma patients: Operative interventions must be considered. Annals of Surgery. 2014:259. doi: 10.1097/SLA.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 5.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–35. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 6.Silber JH. Failure to rescue[2] JAMA Surgery. 2014;149:747–8. doi: 10.1001/jamasurg.2014.589. [DOI] [PubMed] [Google Scholar]
- 7.Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue comparing definitions to measure quality of care. Medical Care. 2007;45:918–25. doi: 10.1097/MLR.0b013e31812e01cc. [DOI] [PubMed] [Google Scholar]
- 8.Ghaferi AA, Dimick JB. Variation in Mortality After High-Risk Cancer Surgery. Failure to Rescue. Surgical Oncology Clinics of North America. 2012;21:389–95. doi: 10.1016/j.soc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: The role of failure to rescue. JAMA Surgery. 2014;149:119–23. doi: 10.1001/jamasurg.2013.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. New England Journal of Medicine. 2002;346:1715–22. doi: 10.1056/NEJMsa012247. [DOI] [PubMed] [Google Scholar]
- 11.Silber JH, Rosenbaum PR, Schwartz JS, Ross RN, Williams SV. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. Journal of the American Medical Association. 1995;274:317–23. [PubMed] [Google Scholar]
- 12.Silber JH, Kennedy SK, Even-Shoshan O, Chen W, Mosher RE, Showan AM, et al. Anesthesiologist board certification and patient outcomes. Anesthesiology. 2002;96:1044–52. doi: 10.1097/00000542-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Silber JH, Rosenbaum PR. A Spurious Correlation between Hospital Mortality and Complication Rates: The Importance of Severity Adjustment. Medical Care. 1997;35:OS77–OS92. doi: 10.1097/00005650-199710001-00011. [DOI] [PubMed] [Google Scholar]
- 14.Waits SA, Sheetz KH, Campbell DA, Ghaferi AA, Englesbe MJ, Eliason JL, et al. Failure to rescue and mortality following repair of abdominal aortic aneurysm. J Vasc Surg. 2014;59:909–14. e1. doi: 10.1016/j.jvs.2013.10.078. [DOI] [PubMed] [Google Scholar]
- 15.Silber JH, Kennedy SK, Even-Shoshan O, Chen W, Koziol LF, Showan AM, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93:152–63. doi: 10.1097/00000542-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Kardooni S, Haut ER, Chang DC, Pierce CA, Efron DT, Haider AH, et al. Hazards of benchmarking complications with the national trauma data bank: Numerators in search of denominators. Journal of Trauma - Injury, Infection and Critical Care. 2008;64:273–7. doi: 10.1097/TA.0b013e31816335ae. [DOI] [PubMed] [Google Scholar]
- 17.Hemmila MR, Jakubus JL, Wahl WL, Arbabi S, Henderson WG, Khuri SF, et al. Detecting the blind spot: Complications in the trauma registry and trauma quality improvement. Surgery. 2007;142:439–49. doi: 10.1016/j.surg.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]