Abstract
Alzheimer’s disease (AD) is an age-related progressive neurodegenerative disorder. Several types of treatments have been tested to block or delay the onset of the disease, but none have been completely successful. Diet, lifestyle and natural products are currently the main scientific focuses. Here, we evaluate the effects of oral administration of the monoterpene linalool (25 mg / kg), every 48 hours for 3 months, on aged (21–24 months old) mice with a triple transgenic model of AD (3xTg-AD) mice. Linalool-treated 3xTg-AD mice showed improved learning and spatial memory and greater risk assessment behavior during the elevated plus maze. Hippocampi and amygdalae from linalool-treated 3xTg-AD mice exhibited a significant reduction in extracellular β-amyloidosis, tauopathy, astrogliosis and microgliosis as well as a significant reduction in the levels of the pro-inflammatory markers p38 MAPK, NOS2, COX2 and IL-1β. Together, our findings suggest that linalool reverses the histopathological hallmarks of AD and restores cognitive and emotional functions via an anti-inflammatory effect. Thus, linalool may be an AD prevention candidate for preclinical studies.
Keywords: Alzheimer’s disease, Linalool, Behavior function, Inflammation
1. Introduction
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is the most common form of dementia. AD is responsible for a considerable human, social and economic burden around the world (Association, 2014). In Latin America, the main causes of dementia are a sedentary lifestyle, metabolic disorder, and cardiovascular and cerebrovascular diseases (Kalaria et al., 2008). In general, AD patients present with a gradual deterioration of episodic memory, a global decline in cognitive function, and behavioral changes. AD symptoms are the clinical manifestations of a progressive accumulation of intra- and extracellular β-amyloid, the formation of neurofibrillary tangles (NFTs) and the extensive oxidative stress associated with neuron and synapse loss (Ittner and Gotz, 2011; Reitz and Mayeux, 2014).
The current standard pharmacotherapy for cognitive improvement in AD patients includes acetylcholinesterase inhibitors, such as galantamine, and the N-methyl-D-aspartate (NMDA) antagonist memantine, which promote cognitive function in patients with moderate to severe AD (Kang et al., 2014). However, the approval of these drugs has not been based on their ability to slow disease progression but on their ability to improve clinical symptomatology. Hence, only symptomatic drugs with transient benefits have been approved for clinical use in AD patients by the US Food and Drug Administration (FDA) (Bassil and Grossberg, 2009). The use of alternative therapies for neuroprotection is increasing (Silva et al, 2014); these alternatives include natural products, such as monoterpenes (Dinda et al., 2009; Tabassum et al., 2015).
(-)Linalool, one enantiomer of the naturally occurring monoterpene, is a major volatile component of essential oils in several aromatic plant species, such as Lavandula angustifolia Mill., Melissa officinalis L., Rosmarinus officinalis L. and Cymbopogon citratus DC. Interestingly, many linalool-producing species are traditionally used in folk medicine and in aromatherapy to relieve symptoms and to cure a variety of acute and chronic ailments (Batista et al., 2010; Elisabetsky et al., 1995). Linalool is widely used in the manufacture of fragrances for shampoos, soaps and detergents and in pharmaceutical drug formulations (Letizia et al., 2003; Mitic-Culafic et al., 2009).
Linalool exhibits a variety of pharmacological effects, including antimicrobial, antileishmanial, anti-inflammatory, anti-oxidant and cardiovascular effects, in normotensive and hypertensive rats (Anjos et al., 2013; Beier et al., 2014; Celik and Ozkaya, 2002; Huo et al., 2013; Wu et al., 2014). The strong antioxidant activity of linalool inhibits LDL oxidation; this inhibition enhances cholesterol uptake via macrophage scavenger receptors. Linalool significantly reduced plasma TG, total cholesterol and HMG-CoA levels, demonstrating in vivo anti-atherogenic activity (Cho et al., 2011; Chung et al., 2008). Linalool has remarkable effects on the central nervous system (CNS), acting as a sedative, antinociceptive, anticonvulsant and anxyolitic (Batista et al., 2010; de Almeida et al., 2009; Elisabetsky et al., 1999; Linck et al., 2009). Linalool also modulates glutamatergic neurotransmission both in vitro and in vivo, possibly through NMDA receptor interactions (Elisabetsky et al., 1995; Silva Brum et al., 2001). However, nothing is known about linalool’s effect on AD neuropathology and behavioral impairments, which is the goal of the present study.
2. Materials and methods
2.1. Animals
Homozygous triple transgenic AD model (3xTg-AD) and non-transgenic (Non-Tg) mice (Oddo et al., 2003) from the in-house colony at the University of Antioquia maintained in the SIU (Sede de Investigación Universitaria) specific pathogen-free vivarium in Medellín, Colombia were used at ages from 18–21 months to obtain a homogenous penetrance of tauopathy. The mice were maintained on a 12:12 hour dark:light cycle and received food and water ad libitum. The animals were handled according to Colombian standards (law 84/1989 and resolution 8430/1993) and guidelines. Special care was taken to minimize animal suffering and to reduce the number of animals used.
2.2. Administration of drugs
Linalool (Sigma Aldrich, Cat: L2602) was dissolved in phosphate-buffered saline (PBS). The 3xTg-AD mice received 25 mg/kg linalool or saline solution (vehicle) orally every 48 hours for 3 consecutive months beginning at 18–21 months of age and were sacrificed at ages of 21–24 months (Figure 1). The linalool dose (25 mg/kg) is not toxic (Brickers et al., 2003; Api et al., 2015), and the interval between the final drug treatment and the assays were selected based on previous in vivo studies (Huo et al., 2013; Mehri et al., 2014; Nascimento et al., 2014). We carefully monitored the general health of the mice throughout the linalool treatment and did not observe any adverse effects.
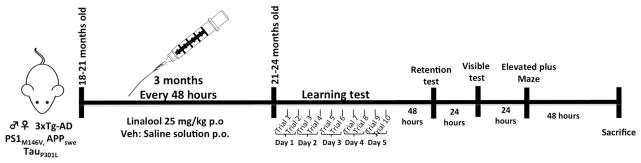
Figure 1. Experimental design.
Linalool (25 mg/kg) or saline were administered orally by gavage to 18- to 21-month-old Non-Tg and 3xTg-AD mice for 3 months, every 48 hours. Learning and memory were evaluated in the Morris water maze test (five days, ten trials) at 21–24 months of age. Afterward, the elevated plus maze was performed over two days. Then, the mice were sacrificed for histological and biochemical analyses.
2.3. Histology
Twenty-four hours after the final behavioral test, the animals were anesthetized intraperitoneally using a mixture of ketamine (50 mg/kg) plus xylazine (20 mg/kg) and were perfused with normal saline and 4% paraformaldehyde (0.1 M PBS, pH 7.4). Brains were removed and post-fixed with 4% paraformaldehyde at 4°C for 48 hours and then cryopreserved with 30% sucrose and stored at −20°C. The brains were sectioned (50 μm) with a Leica VT1000S vibrating blade microtome (Leica Microsystems, Germany).
2.4. Immunohistochemistry
The coronal sections (50 μm) were permeabilized and blocked with 0.3% Triton X-100 and 1% BSA in PBS and were then probed with primary antibodies: anti-beta amyloid antibody (beta amyloid 1–16 (6E10) Monoclonal #SIG-39320, Covance, 1:500), anti-phospho-PHF-tau (pSer202/Thr205 Antibody (AT8) #MN1020, Thermo Scientific, 1:500), anti-GFAP (Monoclonal Anti-Glial Fibrillary Acidic Protein, #G 3893, Sigma, 1:500), anti-Iba1 (Rabbit Anti-Iba1 (Ionized calcium binding adaptor molecule) #019–19741, Wako, 1:500) and the appropriate secondary antibodies (1:250 concentration, goat anti-rabbit IgG (H+L) biotin-conjugated, Pierce #31822 or goat Anti-Mouse IgG (H+L) Biotin Conjugated Pierce #31800). Later, tissues were incubated with avidin biotin complex (ABC Standard Peroxidase Staining Kit, Pierce #32020, 1:250 reagent A:B) for 1 hour. Once the complex was removed, diaminobenzidine (DAB) was used as developer. The sections were dehydrated with alcohol, cleared with xylene and sealed with Consul-mount. The immunoreactivity in the tested areas was quantified at 10x or 40x magnification and analyzed using ImageJ 1.45 software (NIH, USA). The absence of primary antibody did not result in immunoreactivity. The CA1 and subiculum (hippocampus), entorhinal cortex (EC) and amygdala were evaluated at bregma 1.76 mm posterior to bregma (Paxinos and Franklin, 2004).
2.5. Immunofluorescence
Sections at the level of the bregma were rinsed in 0.1 M PBS and incubated for 10 min with 50 mM ammonium chloride to prevent autofluorescence. The sections were preincubated for 60 min at room temperature with Triton X-100 in PBS (TXPBS) and 3% BSA and were then incubated overnight at 4°C with the following primary antibodies: anti-beta amyloid antibody (beta amyloid 1–16 (6E10) Monoclonal #SIG-39320, Covance, 1:500) and anti-Iba1 (Rabbit Anti-Iba1 (Ionized calcium binding adaptor molecule) #019–19741, Wako, 1:500). The primary antibodies were diluted in TXPBS and 1% BSA. Then, the sections were washed four times in 0.1 M PBS and incubated for 90 min at room temperature with mouse Alexa Fluor 488 or rabbit Alexa Fluor 594 secondary antibodies (1:1000; Molecular Probes, Eugene, OR). The sections were stained for 15 min at room temperature with Hoechst (1:1,000; Sigma), washed four times in buffer, mounted on slides, and coverslipped with Gel Mount (Biomeda, Foster City, CA). The sections were observed and photographed with a motorized spinning disk confocal microscope (DSU; Olympus IX 81). Omission of the primary antibodies resulted in no staining.
2.6. Morris water maze test
Forty-eight hours after the final treatment, the animals were evaluated in the Morris water maze (MWM). A white plastic tank 1 m in diameter and 30 cm in height was filled with water (22 ± 2°C) to a depth of 20 cm. The platform (7 cm diameter) was 1.5 cm below the surface of the water during spatial learning and 1.5 cm above the surface of the water during the visible session. Extra-maze visual cues around the room remained in a fixed position throughout the experiment. Ten sessions or trials were performed, two complete sessions per day over five days. “Each session consisted of four successive subtrials (30 s inter-trial interval), and each subtrial began with the mouse placed pseudo-randomly in one of four starting locations. The animals had been trained to stay on the platform for 30 s prior to the initial trial. The latency to reach the platform was evaluated using a visible platform to control for any difference in visual-motor abilities or motivation between the experimental groups. If a mouse did not locate the platform after a maximum of 60 s, it was gently guided to the platform. Then, the animals were then provided with 48 h of retention time, followed by a probe trial of spatial reference memory, in which the animals were placed in the tank without the platform for 60 s (Figure 1). The latency to reach the exact former platform location and the number of crossings of the platform target quadrant were recorded during the probe trial. An automated system (Viewpoint, Lyon, France) recorded the behavior of the animals” (Sabogal-Guaqueta et al., 2015).
2.7. Elevated plus maze
Twenty-four hours after the visible test in the Morris water maze, the animals were exposed to the elevated plus maze (EPM). The apparatus was made of white Plexiglas and illuminated using approximately 30–40 lux. The EPM consisted of two open arms (30 × 5 × 0.25 cm) and two closed arms (30 × 5 × 15 cm) extending from a common central platform (5 × 5 cm), and the entire apparatus was elevated by a single central support to a height of 60 cm above floor level. Each mouse was placed in the middle section facing an open arm and left to explore the maze for a single 5 min session with the experimenter out of view. After each trial, the floor was wiped clean with 10% alcohol.
The frequency of open entries (arm entry defined as all four paws into an arm) and the amount of time spent by the animals in open sections of the maze were recorded. We also calculated the % open entries (open entries/total entries × 100) and the % time spent in open arms (open time/300 × 100). We also recorded the rearing frequency and duration (all rearing occurred against the walls of the enclosed arms), the frequency of discrete behaviors, such as head dipping (exploratory movement of head/shoulders over sides of the maze), and the duration of grooming (species-typical sequence beginning with the snout, progressing to the ears, and ending with whole-body grooming). Each experiment was videotaped using a high-resolution video camera. These data were collected using X-Plo-Rat 2005 software (Taverna-Chaim, 2008).
2.8. Western blotting
After behavior testing, the animals were sacrificed by decapitation, and the hippocampus and amygdala were dissected and immediately frozen in liquid nitrogen and stored at −80°C until analysis. The ti ssues were dissected and homogenized in lysis buffer according to a described previously protocol (Cardona-Gomez et al., 2004). Membranes were incubated overnight with the following primary antibodies: PHF-1 monoclonal antibody, which recognizes Tau pSer-396/404 and was donated by P. Davies (Feinstein Institute for Medical Research, Manhasset, NY); anti-phospho-PHF-tau (pSer202/Thr205 Antibody (AT8) #MN1020, Thermo Scientific, 1:500); anti-NOS2 (C-11) Ms mAb (# Sc 7271, Santa Cruz Biotechnology, 1:100); rabbit polyclonal anti-COX2 (#AB15191, Abcam, 1:1000); rabbit phospho-p38 MAPK Thr180/Tyr182 (# 9215, Cell Signaling, 1:1000); and tubulin (Mouse monoclonal anti-βIII tubulin antibody, #G712A, Promega, 1:10000) as a loading control. IRDye 800CW goat anti-mouse or rabbit (LI-COR; diluted 1:10000) was used as a secondary probe. The blots were visualized using an Odyssey Infrared Imaging System (LI-COR Biosciences, United States).
2.9. β-Amyloid and IL-1β ELISA
Brain levels of soluble Aβ 1–40 and 1–42 were measured using ELISA. Soluble Aβ levels were measured by sandwich capture ELISA using Colorimetric BetaMark - β Amyloid x-40 (SIG-38954- Covance Laboratories) and x-42 ELISA (SIG-38956-Covance Laboratories) kits. IL-1β was measured with a Quantikine ELISA Mouse IL-1β (Cat. #MLB00C, R&D Systems, Minneapolis, USA) kit following the manufacturer’s protocol at a protein concentration of 50 μg/mL.
2.10. Statistics
At least 3 mice were used for each histological study; 4–6 mice were used for each biochemical study; and 4–5 mice and 10–12 mice were used for EPM and MWM, respectively. Parametric data were evaluated with analysis of variance (ANOVA) to compare the 4 groups and then with Tukey’s test for post hoc multiple comparison between-group analyses. Nonparametric data were evaluated using the Kruskal-Wallis test. The escape latency during the training and the transfer test was tested using two-way ANOVA followed by a Dunnett’s post hoc test for multiple comparisons. The statistical analysis was performed using GraphPad Prism software (version 6.0), and the results were considered significant when p≤0.05. The values are expressed as the means ± SEM.
3. Results
3.1. Linalool treatment reversed spatial memory impairment in old 3xTg-AD mice
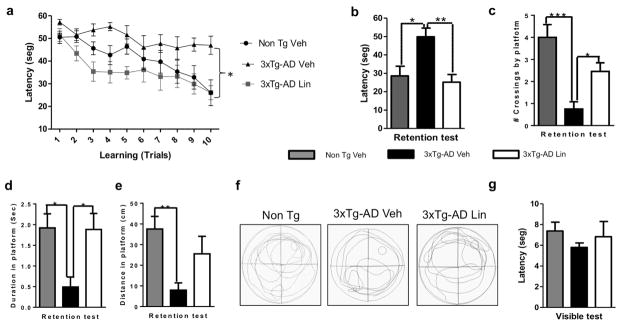
The MWM test is one of the most widely accepted behavioral tests for monitoring spatial learning and memory skills, which primarily depend on the hippocampus. Non-Tg mice treated with vehicle and 3xTg-AD mice treated with vehicle or linalool were assessed. At the beginning of the test, all groups showed a similar performance during trials 1 and 2 on the first day. The average latency to the target platform for all groups during the 5 days of training is shown in Figure 2 a. As previously demonstrated, 3xTg-AD mice exhibit a higher latency to locate the platform in the MWM test because of the synaptic dysfunction and long-term potentiation (LTP) deficits in these animals (Cantanelli et al., 2014). Interestingly, spatial learning abilities were restored in 3xTg-AD mice treated with linalool for 3 months. Starting at the third trial, treated 3xTg-AD mice were similar to Non-Tg mice treated with vehicle (Figure 2 a). During the retention test, the hidden platform was removed for measurements of the hippocampus-dependent memory 48 hours after the final learning trial. Non-Tg mice and the 3xTg-AD mice treated with linalool exhibited a higher retention performance than that of the 3xTg-AD vehicle-treated mice (Figure 2 b). The linalool-treated 3xTg-AD mice crossed the platform a significantly higher number of times, spent more time reaching the platform, and swam a longer distance in the the platform location than did the vehicle-treated 3xTg-AD mice; these values were similar to those of the Non-Tg mice (Figure 2 c–e). Representative images of the travel routes during the retention test are provided in Figure 2 f, which shows that 3xTg-AD animals treated with linalool remained closer to the hidden platform than did the 3xTg-AD animals treated with vehicle. Thus, the linalool treatment successfully reversed spatial memory impairment. The visible test did not reveal visual, motor or motivational deficits (Figure 2 g).
Figure 2. Linalool treatment prevented spatial memory impairment in 3xTg- AD mice.
a) Mean latency to reach the hidden platform during the spatial learning task. b) Latency, c) number of crossings, d) time spent reaching the platform and e) distance to reach the platform in the retention test. f) Representative images of the route of travel during the retention test. g) No differences were detected between the experimental groups in the visual, motor or motivational skills of the animals during the visible test. The data are expressed as the means ± SEM. n=8–16. *Difference between the 3xTg-AD vehicle-treated group and the other groups *p<0.05.
3.2. Anxiolytic activity after oral administration of linalool in aged 3xTg-AD mice
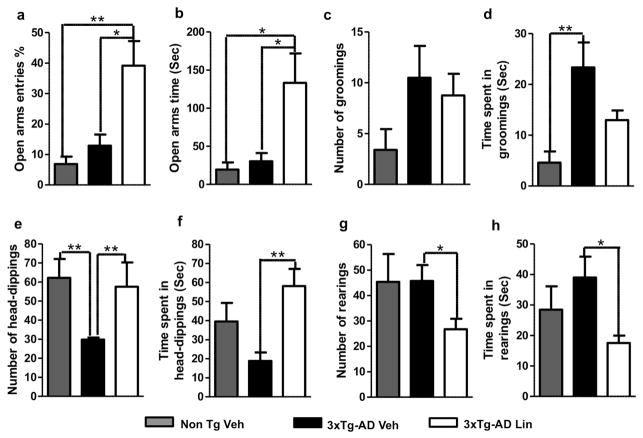
We analyzed the effect of linalool on anxiety using the EPM test (Campos et al., 2013). Our data show a significant increase in the percentage of open-arm entries and the time spent in the open arms when comparing the 3xTg-AD mice treated with linalool to the Non-Tg and 3xTg-AD vehicle-treated mice, which rarely visited the open arms (Figure 3 a–b). Additionally, 3xTg-AD vehicle mice showed a trend towards an increased number of grooming events and a significantly long amount of time spent grooming compared to those of the Non-Tg mice and the 3xTg-AD linalool-treated mice (Figure 3 c–d). The number of occurrences of and time spent head-dipping were reduced in 3xTg-AD mice; in 3xTg-AD linalool-treated mice, these values were restored to levels similar to those of the Non-Tg control mice (Figure 3 e–f). This head-dipping behavior was inversely proportional to the number of rearing and the time spent rearing, which predominantly occurred in the closed arms. Therefore, rearing was significantly lower in the linalool-treated 3xTg-AD mice than in the other experimental groups (Figure 3 g–h).
Figure 3. Anxiolytic effect of linalool oral administration on 3xTg-AD mice.
ab) Relative frequency of open arm entries and the time spent in the open arms. c–d) Number of grooming behaviors and the time spent grooming. e–f) Number of head-dipping behaviors and the time spent head-dipping. g–h) Number of rearing actions and the time spent rearing at the end of the EPM test performed two weeks after the final dose of vehicle or linalool. The data are expressed as the means ± SEM. n=4–5. *p<0.05.
3.3. Aged 3xTg-AD linalool-treated mice show reduced β-amyloidosis
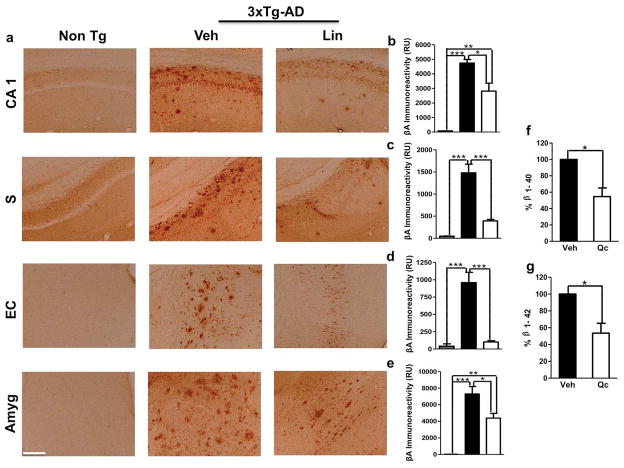
3xTg-AD vehicle-treated mice showed the typical amyloid deposition found in the Non-Tg mice (Oddo et al., 2003), and this deposition was significantly reduced in the hippocampus, entorhinal cortex and amygdala of the 3xTg-AD linalool-treated mice (Figure 4 a–e). The linalool treatment did not completely eliminate β-amyloidosis in the 3xTg-AD mice. The decrease in β-amyloid levels was accompanied by a significant reduction in β-amyloid 1–40 and 1–42 protein levels in the hippocampal lysates of the linalool-treated 3xTg-AD mice compared to the vehicle-treated 3xTg-AD mice (Figure 4 f–g). Together, these data show that cerebral amyloidosis, including amyloid deposits and β-amyloid peptide abundance, was delayed by the oral administration of linalool in old 3xTg-AD mice.
Figure 4. 3xTg-AD linalool-treated mice show reduced β-amyloidosis.
a) Representative images of βA (anti-βA 6E10) immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of vehicle- and linalool-treated 3xTg-AD and vehicle-treated Non-Tg mice at 21–24 months of age. Magnification: 10x; scale bar: 50 μm. b) The values in the bar graph are expressed in densitometric relative units (RU) of βA immunoreactivity in the CA1 area, c) he subiculum, d) the EC and e) the amygdala. f) The relative βA 1–40 and g) βA 1–42 fragment levels were analyzed in hippocampal lysates by ELISA. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4–5. *p<0.05; **p<0.01; ***p<0.001.
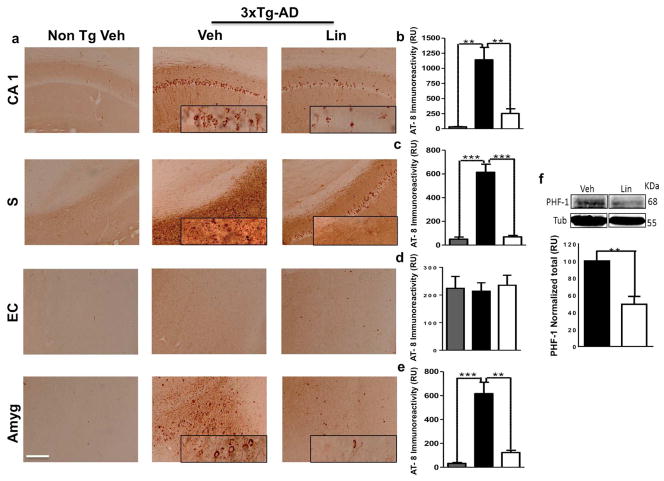
3.4. Linalool treatment ameliorated tau hyperphosphorylation in aged 3xTg-AD mice
Several abnormal tau hyperphosphorylation sites in AD have been identified using the increased tau aggregation at 18 months in the 3xTg-AD model (Oddo et al., 2003) We explored the effect of linalool on tau pathology in the brains of old 3xTg-AD mice using AT-8 immunoreactivity. 3xTg-AD mice treated with linalool displayed a significant decrease in pair helical filaments (PHFs) in the CA1 area, the subiculum and the amygdala but not in the EC. These findings were similar to the AT-8 immunoreactivity observed in the Non-Tg vehicle-treated mice (Figure 5 e). We also found a significant reduction in PHF-1 protein levels in the hippocampal lysates from linalool-treated 3xTg-AD mice compared with vehicle-treated mice, suggesting a reduction in NFTs (Figure 5 f).
Figure 5. Linalool treatment attenuated tau hyperphosphorylation in old 3xTg-AD mice.
a) Representative images of AT8 (anti-tau pSer202/Thr205) immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non-Tg and 3xTg-AD mice treated with vehicle or linalool. Magnification: 10x; scale bar: 50 μm. b) The values in the bar graph are expressed in densitometric relative units (RU) of AT8 immunoreactivity in the CA1 area, c) the subiculum, d) the EC and e) the amygdala. f) Representative bands and densitometric intensities of PHF-1 in hippocampal lysates. Tubulin was used as a loading control. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4. *p<0.05; **p<0.01; ***p<0.001.
3.5. Linalool decreased the inflammatory response in old 3xTg-AD mice
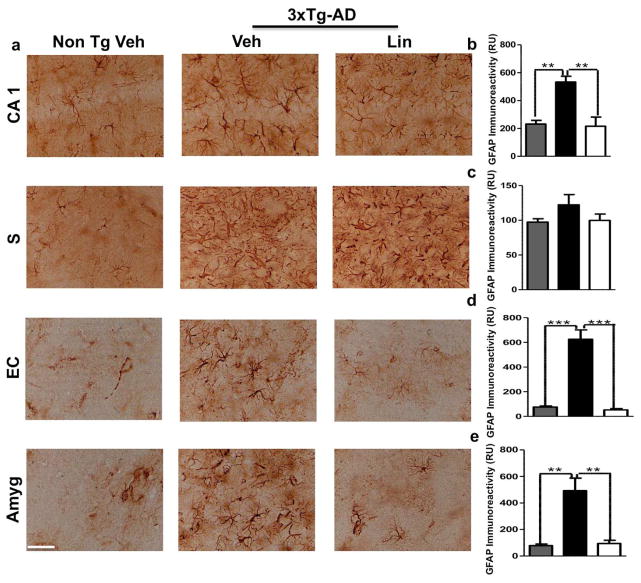
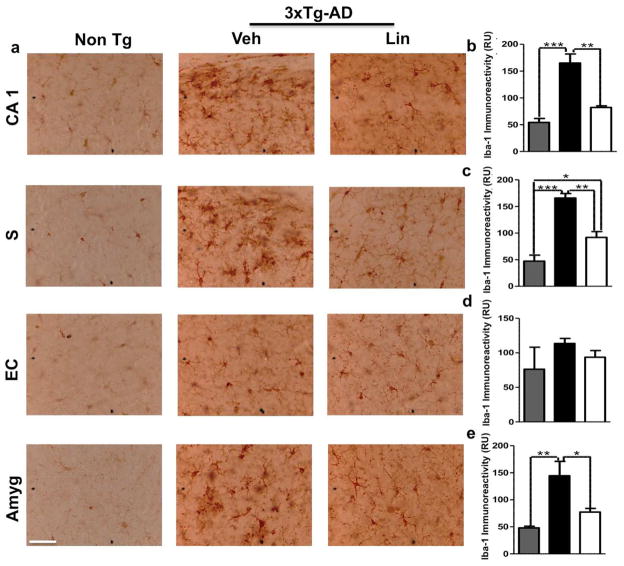
Chronic activation of glial cells around amyloid plaques is associated with AD pathophysiology via the production of numerous neurotoxic reactants, proinflammatory cytokines, and immunostimulatory molecules. Reactive gliosis and increased expression of proinflammatory cytokines have been demonstrated in old transgenic mice with a model of cerebral amyloid deposition (Birch et al., 2014). To test whether linalool has an immunomodulatory effect on 3xTg-AD mice, we examined astrogliosis and microgliosis by evaluating GFAP and Iba1 immunoreactivity, respectively. Our data showed a significant increase in GFAP immunoreactivity in 3xTg-AD mice compared to the Non-Tg mice; however, linalool treatment significantly reduced the GFAP immunoreactivity in the CA1 hippocampal area, in the EC and in the amygdala compared to that in 3xTg-AD vehicle-treated mice (Figure 6 a–e). We did not observe changes in the subiculum (Figure 6 a, c). Microglial activation is an AD hallmark associated with amyloidosis and tauopathy (Lee et al., 2013). We found that the linalool-treated 3xTg-AD mice displayed microglial immunoreactivity that was significantly decreased in the CA1 area, in the subiculum and in the amygdala compared to that in the vehicle-treated 3xTg-AD mice (Figure 7 a–e). However, we did not find changes in the EC (Figure 7 d).
Figure 6. Linalool decreased astrogliosis in old 3xTg-AD mice.
a) Representative images of GFAP immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non-Tg and 3xTg-AD mice treated with vehicle or Linalool. Magnification: 40x. b) The values in the bar graphs are expressed in densitometric relative units (RU) of GFAP immunoreactivity in the CA1 area, c) the subiculum, d) the EC and e) the amygdala. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=3–4. **p<0.01; ***p<0.001.
Figure 7. Linalool ameliorated microgliosis in 3xTg-AD mice.
a) Representative images of GFAP immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non-Tg and 3xTg-AD mice treated with vehicle or linalool. Magnification: 40x. b) The values in the bar graphs are expressed in densitometric relative units (RU) of Iba1 immunoreactivity in the CA1 area, c) the subiculum, d) the EC and e) the amygdala. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=3–4. *p<0.05 **p 0.01; ***p<0.001.
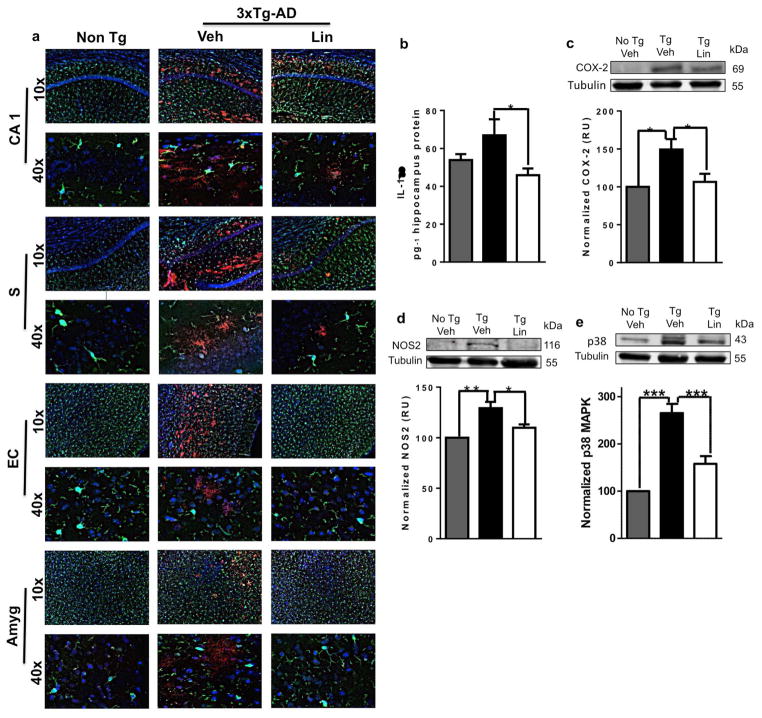
Microglial activation was related to βA immunolabeling (Figure 8 a) and to the hippocampal upregulation of proinflammatory cytokines and the levels of associated marker proteins, such as IL-1β, iNOS, COX-2, and p38 MAPK, which were significantly upregulated in untreated 3xTg-AD mice compared to the Non-Tg group. This inflammatory response was reversed by linalool treatment (Figure 8 be) (p<0.05 - p<0.001) (Figure 8 b–e), confirming previous reports of the anti-inflammatory effects of linalool (Huo et al., 2013; Li et al., 2015)
Figure 8. Linalool reduced the proinflammatory response in 3xTg-AD mice.
Morphological characterization showing nuclei stained with Hoechst (blue), β-amyloid stained with Alexa Fluor 594 phalloidin dye (red) and microglia visualized with Iba1 (green). Magnification, 10x and 60x. a) Representative images of CA1, subiculum, EC, and the amygdala. b) Quantification of IL-1β in hippocampal lysates. Representative bands and densitometric intensities of c) COX-2, d) NOS2 and e) p38 MAPK in hippocampal lysates. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4–5. *p<0.05; **p<0.01‚ ***p<0.001.
4. Discussion
Oral administration of the monoterpene linalool ameliorated the histopathological hallmarks of AD and reversed the associated cognitive and emotional deficits in aged triple transgenic AD model mice. Our data suggests that linalool could be a pharmacological therapy for attenuating the neurotoxicity in neurodegenerative diseases. Few studies have reported linalool-mediated neuroprotection. Linalool protects against glucose/serum deprivation (GSD) in PC12 cells (Alinejad et al., 2013) and against acrylamide (ACR)-induced neurotoxicity in Wistar rats, increasing the glutathione (GSH) content while decreasing the ACR-induced lipid peroxidation in rat brain tissue (Mehri et al., 2014). However, other studies show that monoterpenes such as thymol and carvacrol and its derivates, inhibit acetylcholinesterase activity in vitro more strongly than linalool does (Jukic et al., 2007; Perry et al., 2000), suggesting that linalool does not interfere with impaired cholinergic function. Also, thymol and carvacrol enhanced the performance on the MWM test after the injection of βA peptide in rat brains (Azizi et al., 2012). While linalool impaired the acquisition of long-term memory without interfering with short-term memory in rats in an object recognition task. This effect was associated with an antagonist capacity, similar to a glutamate antagonist (Coelho et al., 2011). Nevertheless, higher doses (i.p. 50–100 mg/kg) of linalool were used than those in our study (v.o. 25 mg/kg), which could explain our divergent results. Therefore, although linalool has been suggested to be a neuroprotective agent, its potential therapeutic role in AD has not been demonstrated previously.
EPM is one of the most widely used tests to measure anxiety and is based on the natural tendency of rodents to explore novel environments and their innate avoidance of unprotected, bright, and elevated places (Espejo et al, 1997). These behaviors can be regulated by the amygdala, a region of the limbic system involved in a variety of emotional and cognitive functions (Campos et al., 2013). Interestingly, our data showed that linalool-treated 3xTg-AD mice exhibited an increased frequency of entry into the open arms, increased head-dipping and reduced grooming and rearing frequencies compared with those of vehicle-treated 3xTg-AD mice. These results suggest reduced anxiety or a “risk assessment” behavior when the mice visit the open arms of the maze (Walf and Frye, 2007), which is unexpected considering that these results were obtained nine days after the final dose of linalool. Our data confirm the results of previous studies reporting that inhaled linalool has anxiolytic properties, increases social interaction, and decreases aggressive behavior (de Almeida et al., 2009; Linck et al., 2010). Although, other study did not report any difference in the number of entries to the open arms using 125 mg/kg linalool (Cline et al., 2008).
Linalool also reduced β-amyloidosis and tauopathy in the hippocampus (CA1, subiculum) and amygdala of aged 3xTg-AD linalool-treated mice compared to untreated mice. These data represent the first evidence that regular administration of linalool can prevent age-related cognitive impairments and β-amyloid accumulation in 3xTg-AD mice. The decreased β-amyloid level was accompanied by a reduction in β-amyloid 1–40 and 1–42 protein levels. These data are supported by studies, where a mixture of linalool and the monoterpene 2,3,4,4-tetramethyl-5-methylenecyclopent-2-enone led to a significant improvement in the reduction in brain-soluble Aβ 40 (Videira et al., 2014). Linalool is able to cross the blood-brain barrier (Cheng et al., 2015); however, the exact molecular mechanism of the linalool-mediated reduction in β-amyloid needs further study. Tauopathy is detected in pyramidal neurons in CA1 at 15 months of age (Oddo et al., 2003), and in our homozygous mouse colony, it is strongly detected at 18 months of age (Castro-Alvarez et al., 2014). Tauopathy is associated with degenerative symptoms, including significant deficits in hippocampus-dependent cognitive task performance. However, we did not detect immunoreactivity in the EC, which is consistent with some (Mastrangelo and Bowers, 2008; Sabogal-Guaqueta et al., 2015) but not all (Khan et al., 2014) previous studies.
Hyperactived astrocytes and microglial proliferation accompanies βA deposition in the brains of AD model mice (Birch et al., 2014). In the present study, GFAP and Iba1 immunoreactivity was reversed in aged 3xTg-AD mice by oral treatment with linalool. In addition, linalool treatment reduced inflammatory markers, down-regulating IL-1β and the downstream molecules p38 MAPK, COX-2 and iNOS. In previous studies, linalool reduced the serum and hepatic TNF-α, IL-6, iNOS and COX-2 expression by inhibiting NF-κB activation (Li et al., 2014). Moreover, linalool dramatically inhibited the LPS-induced phosphorylation of ERK, JNK, and p38 in RAW 264.7 cells (Huo et al., 2013). Linalool also has antioxidant properties and shows protective effects against hydrogen peroxide-induced oxidative stress in brain tissue (Celik and Ozkaya, 2002), which together could lead to fewer β-amyloid plaques and reduced tauopathy. Together, these findings suggest that monoterpenes, such as linalool, or the essential oils of several aromatic plants rich in this compound could be beneficial for AD patients or could be used as a chemical basis for the further development novel drugs to treat tauopathies.
5. Conclusion
In summary, our findings suggest that oral administration of linalool at an advanced stage of AD in 3xTg-AD model mice reversed the histopathological hallmarks of AD and restored cognitive and emotional functions. Thus, linalool may be a good candidate for further preclinical studies and future translational studies on AD.
Highlights.
Oral linalool reverses spatial memory impairment in old 3xTg-AD mice
Anxiolytic activity of linalool in aged 3xTg-AD mice
Aged 3xTg-AD linalool-treated mice reduces β-amyloidosis
Linalool ameliorates tau hyperphosphorylation in aged 3xTg-AD mice
Linalool decreases inflammatory response in old 3xTg-AD mice
Acknowledgments
The authors thank the Cellular and Molecular Neurobiology Area of the Neuroscience Group of Antioquia, the Group of Bioactive Substances, Professor Jose Ramirez from the Group of Immunomodulation of University of Antioquia and Professor Marisol Lamprea from the Neuroscience Laboratory at National University of Colombia for their scientific and technical support during the experiments. This research was funded by grants from COLCIENCIAS # 11565740581 (GPC-G), CODI University of Antioquia, Young Investigator Programme 2011-2012 Colciencias (AMS-G) and Project 1 R01 AG029802-01 NIA/NIH, Subcontract 2011-2012 (GPC-G). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alinejad B, Ghorbani A, Sadeghnia HR. Effects of combinations of curcumin, linalool, rutin, safranal, and thymoquinone on glucose/serum deprivation-induced cell death. Avicenna J Phytomed. 2013;3:321–328. [PMC free article] [PubMed] [Google Scholar]
- Anjos PJ, Lima AO, Cunha PS, De Sousa DP, Onofre AS, Ribeiro TP, Medeiros IA, Antoniolli AR, Quintans-Junior LJ, Santosa MR. Cardiovascular effects induced by linalool in normotensive and hypertensive rats. Z Naturforsch C. 2013;68:181–190. [PubMed] [Google Scholar]
- Association, A. s. Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia. 2014;10 doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 2012;23:241–249. doi: 10.1097/FBP.0b013e3283534301. [DOI] [PubMed] [Google Scholar]
- Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatment of Alzheimer’s disease. CNS Drugs. 2009;23:293–307. doi: 10.2165/00023210-200923040-00003. [DOI] [PubMed] [Google Scholar]
- Batista PA, Werner MF, Oliveira EC, Burgos L, Pereira P, Brum LF, Story GM, Santos AR. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J Pain. 2010;11:1222–1229. doi: 10.1016/j.jpain.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Beier RC, Byrd JA, 2nd, Kubena LF, Hume ME, McReynolds JL, Anderson RC, Nisbet DJ. Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: effects on poultry. Poult Sci. 2014;93:267–272. doi: 10.3382/ps.2013-03254. [DOI] [PubMed] [Google Scholar]
- Birch AM, Katsouri L, Sastre M. Modulation of inflammation in transgenic models of Alzheimer’s disease. J Neuroinflammation. 2014;11:25. doi: 10.1186/1742-2094-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr. 2013;35(Suppl 2):S101–111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- Cantanelli P, Sperduti S, Ciavardelli D, Stuppia L, Gatta V, Sensi SL. Age-Dependent Modifications of AMPA Receptor Subunit Expression Levels and Related Cognitive Effects in 3xTg-AD Mice. Front Aging Neurosci. 2014;6:200. doi: 10.3389/fnagi.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004;25:363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Castro-Alvarez JF, Uribe-Arias SA, Kosik KS, Cardona-Gomez GP. Long- and short-term CDK5 knockdown prevents spatial memory dysfunction and tau pathology of triple transgenic Alzheimer’s mice. Front Aging Neurosci. 2014;6:243. doi: 10.3389/fnagi.2014.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik S, Ozkaya A. Effects of intraperitoneally administered lipoic acid, vitamin E, and linalool on the level of total lipid and fatty acids in guinea pig brain with oxidative stress induced by H2O2. J Biochem Mol Biol. 2002;35:547–552. doi: 10.5483/bmbrep.2002.35.6.547. [DOI] [PubMed] [Google Scholar]
- Cline M, Taylor JE, Flores J, Bracken S, McCall S, Ceremuga TE. Investigation of the anxiolytic effects of linalool, a lavender extract, in the male Sprague-Dawley rat. AANA J. 2008;76:47–52. [PubMed] [Google Scholar]
- Coelho VR, Gianesini J, Von Borowski R, Mazzardo-Martins L, Martins DF, Picada JN, Santos AR, Brum LF, Pereira P. (-)-Linalool, a naturally occurring monoterpene compound, impairs memory acquisition in the object recognition task, inhibitory avoidance test and habituation to a novel environment in rats. Phytomedicine. 2011;18:896–901. doi: 10.1016/j.phymed.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Cheng BH, Lee-Yan Sheen, Shang-Tzen C. Evaluation of anxiolytic potency of essential oil and S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves in mice. Journal of Traditional and Complementary Medicine. 2015;5:27–34. doi: 10.1016/j.jtcme.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Jun HJ, Lee JH, Jia Y, Kim KH, Lee SJ. Linalool reduces the expression of 3-hydroxy-3-methylglutaryl CoA reductase via sterol regulatory element binding protein-2- and ubiquitin-dependent mechanisms. FEBS Lett. 2011;585:3289–3296. doi: 10.1016/j.febslet.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Chung MJ, Park KW, Kim KH, Kim CT, Baek JP, Bang KH, Choi YM, Lee SJ. Asian plantain (Plantago asiatica) essential oils suppress 3-hydroxy-3-methyl-glutaryl-co-enzyme A reductase expression in vitro and in vivo and show hypocholesterolaemic properties in mice. Br J Nutr. 2008;99:67–75. doi: 10.1017/S0007114507798926. [DOI] [PubMed] [Google Scholar]
- de Almeida ER, Rafael KR, Couto GB, Ishigami AB. Anxiolytic and anticonvulsant effects on mice of flavonoids, linalool, and alpha-tocopherol presents in the extract of leaves of Cissus sicyoides L. (Vitaceae) J Biomed Biotechnol. 2009;2009:274740. doi: 10.1155/2009/274740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinda B, Chowdhury DR, Mohanta BC. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, part 3. Chem Pharm Bull (Tokyo) 2009;57:765–796. doi: 10.1248/cpb.57.765. [DOI] [PubMed] [Google Scholar]
- Elisabetsky E, Brum LF, Souza DO. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine. 1999;6:107–113. doi: 10.1016/s0944-7113(99)80044-0. [DOI] [PubMed] [Google Scholar]
- Elisabetsky E, Marschner J, Souza DO. Effects of Linalool on glutamatergic system in the rat cerebral cortex. Neurochem Res. 1995;20:461–465. doi: 10.1007/BF00973103. [DOI] [PubMed] [Google Scholar]
- Huo M, Cui X, Xue J, Chi G, Gao R, Deng X, Guan S, Wei J, Soromou LW, Feng H, Wang D. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. Journal of Surgical Research. 2013;180:e47–54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Jukic M, Politeo O, Maksimovic M, Milos M, Milos M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother Res. 2007;21:259–261. doi: 10.1002/ptr.2063. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P World Federation of Neurology Dementia Research G. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Ryoo NY, Shin DW, Trojanowski JQ, Shaw LM. Role of cerebrospinal fluid biomarkers in clinical trials for Alzheimer’s disease modifying therapies. Korean J Physiol Pharmacol. 2014;18:447–456. doi: 10.4196/kjpp.2014.18.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Rizer J, Hunt JB, Selenica ML, Gordon MN, Morgan D. Review: experimental manipulations of microglia in mouse models of Alzheimer’s pathology: activation reduces amyloid but hastens tau pathology. Neuropathol Appl Neurobiol. 2013;39:69–85. doi: 10.1111/nan.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letizia CS, Cocchiara J, Lalko J, Api AM. Fragrance material review on linalool. Food Chem Toxicol. 2003;41:943–964. doi: 10.1016/s0278-6915(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang X, Huang H. Protective effect of linalool against lipopolysaccharide/D-galactosamine-induced liver injury in mice. Int Immunopharmacol. 2014;23:523–529. doi: 10.1016/j.intimp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Lv O, Zhou F, Li Q, Wu Z, Zheng Y. Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem Res. 2015 doi: 10.1007/s11064-015-1629-7. [DOI] [PubMed] [Google Scholar]
- Linck VM, da Silva AL, Figueiro M, Caramao EB, Moreno PR, Elisabetsky E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 2010;17:679–683. doi: 10.1016/j.phymed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Linck VM, da Silva AL, Figueiro M, Piato AL, Herrmann AP, Dupont Birck F, Caramao EB, Nunes DS, Moreno PR, Elisabetsky E. Inhaled linalool-induced sedation in mice. Phytomedicine. 2009;16:303–307. doi: 10.1016/j.phymed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MA, Bowers WJ. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008;9:81. doi: 10.1186/1471-2202-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehri S, Meshki MA, Hosseinzadeh H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem Toxicol. 2014:1–5. doi: 10.3109/01480545.2014.919585. [DOI] [PubMed] [Google Scholar]
- Mitic-Culafic D, Zegura B, Nikolic B, Vukovic-Gacic B, Knezevic-Vukcevic J, Filipic M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem Toxicol. 2009;47:260–266. doi: 10.1016/j.fct.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Nascimento SS, Camargo EA, DeSantana JM, Araujo AAS, Menezes PP, Lucca W, Albuquerque RLC, Bonjardim LR, Quintans LJ. Linalool and linalool complexed in beta-cyclodextrin produce anti-hyperalgesic activity and increase Fos protein expression in animal model for fibromyalgia. Naunyn-Schmiedebergs Archives of Pharmacology. 2014;387:935–942. doi: 10.1007/s00210-014-1007-z. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2004. [Google Scholar]
- Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol. 2000;52:895–902. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabogal-Guaqueta AM, Munoz-Manco JI, Ramirez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gomez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93C:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Brum LF, Emanuelli T, Souza DO, Elisabetsky E. Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochem Res. 2001;26:191–194. doi: 10.1023/a:1010904214482. [DOI] [PubMed] [Google Scholar]
- Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ahmed ME, Ashafaq M, Khan MB, Islam F, Safhi MM, Islam F. Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-kappaB in middle cerebral artery occlusion rats. Eur J Pharmacol. 2015;747:190–199. doi: 10.1016/j.ejphar.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Taverna-Chaim K, Morato S. X-Plo-Rat 2005 1.1.0. Ribeirao Preto: Universidade de Sao Paulo; 2008. [Google Scholar]
- Videira R, Castanheira P, Graos M, Resende R, Salgueiro L, Faro C, Cavaleiro C. Dose-dependent inhibition of BACE-1 by the monoterpenoid 2,3,4,4-tetramethyl-5-methylenecyclopent-2-enone in cellular and mouse models of Alzheimer’s disease. J Nat Prod. 2014;77:1275–1279. doi: 10.1021/np400903w. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Yu L, Qiu J, Shen B, Wang D, Soromou LW, Feng H. Linalool attenuates lung inflammation induced by Pasteurella multocida via activating Nrf-2 signaling pathway. Int Immunopharmacol. 2014;21:456–463. doi: 10.1016/j.intimp.2014.05.030. [DOI] [PubMed] [Google Scholar]