Abstract
Metabolic disease, specifically obesity, has now become the greatest challenge to improving cardiovascular health. The renin-angiotensin system (RAS) exists as both a circulating hormone system and as a local paracrine signaling mechanism within various tissues including the brain, kidney, and adipose, and this system is strongly implicated in cardiovascular health and disease. Growing evidence also implicates the RAS in the control of energy balance, supporting the concept that the RAS may be mechanistically involved in the pathogenesis of obesity and obesity hypertension. Here, we review the involvement of the RAS in the entire spectrum of whole organism energy balance mechanisms, including behaviors (food ingestion and spontaneous physical activity) and biological processes (digestive efficiency and both aerobic and nonaerobic resting metabolic rates). We hypothesize that opposing, tissue-specific effects of the RAS to modulate these various components of energy balance can explain the apparently paradoxical results reported by energy-balance studies that involve stimulating, versus disrupting, the RAS. We propose a model in which such opposing and tissue-specific effects of the RAS can explain the failure of simple, global RAS blockade to result in weight loss in humans, and hypothesize that obesity-mediated uncoupling of endogenous metabolic rate control mechanisms can explain the phenomenon of obesity-related hypertension.
Keywords: angiotensin, energy, hypertension, metabolism, obesity
the renin-angiotensin system (RAS) is well-recognized for its contributions to cardiovascular control physiology. The RAS exists as a classic, circulating hormone system, but also locally within individual tissue types as a paracrine, autocrine, and even intracrine signaling modality (29, 36, 48). Circulating RAS activity positively correlates with body mass in both human and animal models (7, 20, 31, 34, 38, 82), and weight loss in humans is associated with reduced RAS activity (31, 134). As detailed below, genetic and pharmacological manipulation of components of the RAS in rodents results in altered energy balance and/or sensitivity to weight gain. This is mediated through various combinations of effects on ingestive behavior, digestive efficiency, physical activity, and resting metabolic rate (Table 1), though the actions of the RAS on these contributors to energy balance appear to be site and ligand/receptor specific. Collectively, these findings lead to the hypothesis that the RAS is a critical contributor to the development of obesity and obesity-associated cardiovascular dysfunctions such as hypertension.
Table 1.
Examples of the effects of genetic and pharmacological targeting of RAS components on metabolic functions
| Energy Intake |
Energy Expenditure |
||||||
|---|---|---|---|---|---|---|---|
| Targeted RAS Component | Manipulation (Ref) | Model | Weight Gain or Adiposity | Food intake | Digestive efficiency | Physical activity | Resting metabolism |
| Renin | Global knockout (127) | C57BL/6 mouse | ↓ | ↔ | ↓ | ↔ | ↑ |
| Aliskiren infusion (124) | C57BL/6 mouse | ↓ | ↔ | ??? | ??? | ??? | |
| Global transgenic (44) | SD rat | ↑ | ↑ | (↓)? | ??? | ↑ | |
| Angiotensinogen | Global knockout (83) | ICR-CD1 mouse | ↓ | ↔ | ↔ | ↑ | ↔ |
| Adipose knockout (144) | C57BL/6 mouse | ↔ | ??? | ??? | ??? | ??? | |
| Adipose transgenic on global knockout (82) | ICR-CD1 mouse | ↑ | ↔ | ??? | ↓ | ??? | |
| Adipose transgenic (147) | C57BL/6 mouse | ↑ | ??? | ??? | ??? | ??? | |
| Angiotensin-converting enzyme | Global knockout (65) | C57BL/6 mouse | ↓ | ↔ | ↔ | ↑ | ↑ |
| Captopril in drink (137) | C57BL/6 mouse | ↓ | (↓)? | ??? | ??? | ??? | |
| Perindopril in drink (136) | SD rat | ↓ | (↔)? | ??? | ??? | ??? | |
| Enalapril in drink (113) | Wistar rat | ↓ | ↓ | ↑ | ??? | ??? | |
| AT1/AT1A receptor | Global knockout (70) | C57BL/6 mouse | ↓ | ↑ | ??? | ??? | ↑ |
| Candesartan in drink (150) | Wistar rat | ↓ | ↓ | ??? | ??? | ??? | |
| SIM1-specific knockout (27) | C57BL/6 mouse | ↑ | ↑ | ??? | ↔ | ↓ | |
| AT2 receptor | Global knockout (147) | C57BL/6 mouse | ↓ | ↓ | ??? | (↑)? | (↑)? |
| Global knockout ± Adipose AGT transgenic (148) | C57BL/6 mouse | ↓ | ??? | ??? | ??? | ??? | |
| Mas receptor | Global knockout (114) | FVB/N mouse | (↑)? | ↔ | ??? | ??? | ??? |
| Peptides | Subcutaneous ANG II infusion (16) | SD rat | ↓ | ↓ | ??? | ??? | ↓ |
| Global transgenic (47) | C57BL/6 mouse | ↔ | ↔ | ??? | ??? | ??? | |
| ICV ANG II infusion (104) | SD rat (young) | ↓ | ↓ | ??? | (↑)? | (↑)? | |
| ICV ANG II infusion (105) | SD rat (aged) | ↓ | ↓ | ??? | (↑)? | (↑)? | |
| ICV ANG II infusion (26) | Long-Evans rat | ↓ | ↓ | ??? | ??? | ↑ | |
| Brain-specific transgenic (46) | C57BL/6 mouse | ↓ | ↔ | ??? | ↔ | ↑ | |
| DOCA-salt (45) | C57BL/6 mouse | ↓ | ↔ | ??? | ↓ | ↑ | |
???, Not determined; ↑, increased; ↓, decreased; ↔, no change; (↑)?, possible increase; (↓)?, possible decrease; (↔)?, possible no change. ICV, intracerebroventricular.
Obesity and Obesity-Related Hypertension
According to the American Heart Association's annual statistical update, 30% of children and 70% of adults in the United States are now overweight or obese (90). Simultaneously, roughly 33% of Americans experience hypertension, and there is gross overlap between these two groups (90). Evidence tends to support a causal role for obesity in the pathogenesis of hypertension, as weight loss interventions such as chronic orlistat use can reduce blood pressure (118). Despite substantial investments by various governmental, industrial, and nonprofit organizations, the cardiovascular research community is poised to fall far short of the highly publicized American Heart Association 2020 Impact Goal (to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular diseases and stroke by 20%) (78), almost exclusively due to obesity and related metabolic disease (61). Thus it is clear that improving cardiovascular health depends on improving metabolic health.
Obesity as a Chronic Energy Imbalance
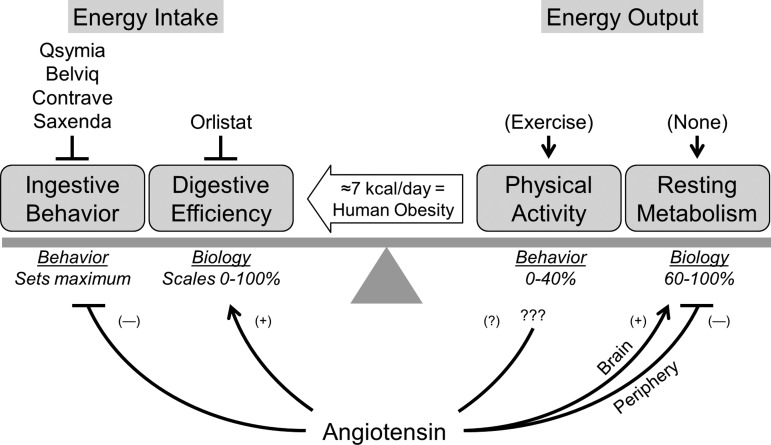
Obesity can be conceptualized as the consequence of a chronic imbalance between energy input and output. At the population level, it has been estimated that as little as a 7.2 kcal/day imbalance between energy input and output is sufficient to account for the current human obesity epidemic in the United States (51). When compared with a standard 2,000 kcal/day diet, 7.2 kcal/day represents <0.5% of total daily energy turnover and is the equivalent to ingesting one teaspoon of ketchup or roughly four Tic Tac candies, or failure to perform seven sit-up exercises per day. While this estimate represents a gross oversimplification that assumes no compensatory changes in various systems during sustained weight gain, an excess 7.2 kcal/day accumulation in the body over 40 years would equate to 26 to 58 lbs. (11 to 26 kg) if converted directly to lean (1 g/4 kcal) or fat (1 g/9 kcal) tissue. Such gains are in line with observed excess weight gain in adults in the United States (71). Regardless of the exact precision of the 7.2 kcal/day estimate, however, the very small magnitude of the gap highlights the sensitivity and reliability of the physiological systems in place to control energy balance. Thus it follows that relatively small adjustments in any aspect of energy balance may represent favorable means of correcting obesity. We hypothesize that the RAS is integrally involved in the regulation of all aspects of energy balance, but also that this control is mediated through complex, opposing, and tissue-specific actions.
RAS in Energy Balance: Behaviors Versus Biological Processes
At the most basic level, obesity is due to an imbalance between energy “input” and “output,” which includes behavioral mechanisms such as food intake and physical activity and biological mechanisms such as gastrointestinal absorption efficiency and resting metabolism. Energy homeostasis at the whole animal level is achieved through modulation of both “input” and “output,” though this is often inappropriately oversimplified and paraphrased as merely adjusting “diet” and “exercise.” In subsections below, we detail the site-, ligand-, and receptor-specific effects of the RAS upon individual components of energy balance.
Food Intake
Caloric intake (food ingestion) is unquestionably a major contributor to energy balance, as this behavior precedes the expenditure of the calories in question and maximally limits the energy to be balanced. Not surprisingly, the modulation of intake behavior (“dieting” behavior and pharmacological appetite suppressants) are frequently the first approaches used to combat obesity. As of late 2015, there are four FDA-approved, appetite-suppressant drugs available in the United States. Qsymia (phentermine/topiramate) is a combination of a phenethylamine (similar to methamphetamine) and an anticonvulsant that was approved in 2012, though postapproval cardiovascular safety trials are underway. Belviq (lorcaserin) is a serotonin 5-HT2C agonist also approved in 2012, though it is considered a Schedule IV drug by the United States Drug Enforcement Administration (DEA) because of hallucinogenic properties. Contrave (bupropion/naltrexone) is a combination norepinephrine-dopamine reuptake inhibitor-nicotinic receptor antagonist and opioid receptor antagonist that was approved in 2014, and its postapproval safety evaluation is ongoing. Saxenda/Victoza (liraglutide) is a glucagon-like peptide-1 receptor agonist that was approved in late 2014 that likely works through suppressing appetite, but its complete mechanism is not yet clearly documented. Collectively, over the last few years this array of new appetite-suppressant compounds has hit the American market, and the medical community is bracing to see if these new compounds are sufficiently efficacious to risk various potential side effects.
The RAS is known to modulate food intake behavior. Infusion of exogenous angiotensin II either peripherally or centrally results in reduced food intake, presumably through actions within the hypothalamus. Cassis et al. (17) found that in rats, chronic peripheral infusion of angiotensin reduced body mass primarily through suppression of food intake. Similarly de Kloet et al. (26) found that in rats, intracerebroventricular (ICV) infusion of angiotensin reduced food intake, and this was associated with increased expression of anorexic hormones in the hypothalamus. Using FVB mice, Yoshida et al. (146) found that peripheral and central delivery of angiotensin both caused reduced food intake and suppressed orexigenic gene expression in the hypothalamus. Jointly, these findings indicate an important role of angiotensin actions to suppress food consumption both peripherally and centrally.
The involvement of various angiotensin II receptor subtypes in the control of ingestive behavior is complicated. Ohinata et al. (95) examined the involvement of AT1 and AT2 receptors in the anorexic behavior induced by ICV delivery of angiotensin II. This group determined that genetic or pharmacological inhibition of the AT2 receptor abolished the anorexic effect, whereas manipulation of the AT1 receptor had much smaller effects (95). New colocalization studies from de Kloet et al. (28) have documented expression of AT2 receptors with GABA- and acetylcholine-synthetic enzymes in brain regions well recognized as contributing to ingestive behavior, including the nucleus of the solitary tract, the area postrema, and the median preoptic nucleus. Thus it appears that AT2 receptors may play a dominant role in the control of food intake by the RAS within the brain. Conversely, de Kloet et al. (27) identified that deletion of the AT1 receptors within the paraventricular nucleus increased food intake. However, various studies have implicated AT1 receptor subtypes and its second-messenger systems in the control of sodium intake behavior (23, 24), and therefore future studies must be carefully designed to delineate sodium seeking behavior versus energy seeking behavior.
Digestive Efficiency
Total caloric intake is bounded by caloric ingestion (a behavior), but this value is scaled from 0 to 100% by the modulation of digestive efficiency (a biological function). Various pathological states (e.g., diarrhea, gastric motility disorders, or malabsorption disorders), medical interventions (e.g., various bariatric surgeries), and dietary components (e.g., fiber, sodium, carbohydrates, fats) modulate digestive efficiency. Furthermore, the gut microbiota also majorly contributes to the regulation of digestive efficiency (30, 66, 128). It follows that investigations into the control of energy balance in vivo require assessments of digestive efficiency to fully account for caloric input, and that targeting digestive efficiency may prove effective as an antiobesity therapy. Indeed, the longest-running FDA-approved antiobesity drug available in the United States is orlistat (tetrahydrolipstatin), approved for prescription use in 1999 and now sold over the counter as Alli. The molecular mechanism of orlistat's efficacy is to reduce digestive efficiency by inhibiting pancreatic lipase activity in the gastrointestinal tract. It has been suggested, however, that the major mechanism of weight loss in human users is actually the learned avoidance of fatty foods. This behavioral modification (changes in food choices away from fatty foods toward predominantly carbohydrate-laden foods to avoid unpleasant side effects) may also explain the limited long-term efficacy of orlistat, as roughly half of initial weight lost with the use of this compound is regained within a few years (130).
As Garg et al. (37) recently reviewed, virtually all components of the RAS are expressed throughout the gastrointestinal tract in rodents as well as humans. Angiotensinogen is expressed in the small intestine, colon, and stomach (13, 52, 139); renin is expressed in intestines and colon (57, 117); angiotensin-converting enzymes (ACE) 1 and 2 are expressed in the intestine and colon (10, 53, 54, 57, 129); and angiotensin receptors AT1 and AT2 are expressed in the intestine (33, 121, 122). It is interesting to note that the highest human tissue concentrations of ACE and ACE2 expression are in the gastrointestional tract (54, 129).
Functionally, manipulation of the RAS can have large effects on digestive efficiency. Takahashi et al. (127) demonstrated that mice deficient for renin exhibit reduced digestive efficiency that correlated with reduced lipase/colipase expression in the pancreas and decreased weight gain during high-fat feeding. In addition, our group recently examined the effect of dietary sodium on weight gain during high-fat feeding in wild-type mice (135). Weight gain in C57BL/6J mice with a 45% high-fat diet was suppressed in a dose-dependent manner by dietary sodium. This was not mediated through any changes in food intake, physical activity, or resting metabolism but instead appeared to be completely dependent on the suppression of digestive efficiency. Chronic infusion (replacement) of angiotensin II reversed the digestive efficiency-suppressing effect of high dietary sodium, and the effect was reconstituted specifically by antagonism of AT2 (not AT1) receptors. Finally, digestive efficiency was suppressed in mice lacking AT2 receptors on either the FVB/NCrl or C57BL/6J backgrounds. Thus an increased appreciation for the role of the RAS in the control of digestive efficiency may lead to the development of novel therapeutics. Furthermore, involvement of the RAS in its control should bring increased attention and scrutiny of digestive efficiency (a major and often-ignored regulator of energy input) to studies of energy balance.
Physical Activity
A common strategy to induce weight loss is to increase energy expenditure via exercising. Physical activity has numerous beneficial effects including reducing adiposity and the risk for cardiovascular disease. After initial weight loss, physical activity is considered necessary for weight maintenance. Although most overweight or obese individuals are successful in losing weight through diet and exercise behaviors, studies have shown that only approximately 20% of these individuals maintain the weight loss for at least 1 yr (84, 138). A major problem in showing the benefits of exercise is the lack of long-term adherence (63). Animal models have shown that exercise reduces energy intake and increases energy expenditure (79, 123). However, in normal weight humans exercise causes compensatory increases in ingestive behaviors (58). Therefore, it is unclear how exercise influences food intake and preference or energy expenditure. Furthermore, there is still debate on the beneficial effects of different types of exercise such as aerobic versus resistance training (80). Thus approaches to increase adherence to exercise regimens and increased understanding of the costs and benefits of specific types of exercise are needed to improve the maintenance of weight loss at the population level.
Massiera et al. (82, 83) observed that mice harboring a null allele for angiotensinogen have increased locomotor activity, and that mice with adipose-specific manipulation of angiotensinogen also exhibit altered spontaneous locomotor activity. Therefore, the RAS appears to modulate locomotor activity; however, further studies are needed to better understand this effect. For example, other genetic or pharmacological manipulations of the RAS have not been reported to exhibit significant effects on physical activity. Rodents with global ACE deficiency, pharmacological blockade of ACE or AT1R, or angiotensin II treatment have not been linked to changes in locomotor activity (15, 62, 65, 106, 112). In contrast, Takada et al. (126) found that olmesartan treatment improved exercise capacity in mice fed a high-fat diet (126). Thus control of spontaneous locomotor activity by the RAS is possible, but the nuances of this mechanism are unclear.
Interestingly, any interaction between physical activity and the RAS appears to be bidirectional because exercise can alter the RAS. Gomes-Santos et al. (42) found that exercise training in rats reduced plasma angiotensin II and increased angiotensin-(1–7) levels. In addition to changes in the circulating RAS, rodent studies have shown that chronic exercise can lead to a reduction in brain and cardiac RAS activity (3, 5, 100). Additional studies to clarify whether exercise directly modulates the RAS versus whether this modulation is secondary to improved cardiovascular or metabolic control and reduced adiposity are warranted.
Resting Metabolism
Just as food consumption behavior is only one component of energy input, exercise behavior is only one component of energy expenditure. A large fraction of the calories expended by endothermic organisms is spent in the form of resting metabolic rate (RMR). Depending on the equation used to estimate RMR [an estimate of basal metabolic rate (BMR)] and the level of physical activity of the subject (i.e., extreme athlete versus sedentary), RMR accounts for between 60 and >90% of the total energy expenditure (75, 85, 111). Thus this biological controller of energy expenditure represents yet another potential therapeutic target for obesity. Debatably the single most potent antiobesity drug ever used clinically was 2,4-dinitrophenol (DNP). This compound is a mitochondrial proton ionophore, acting as an artificial uncoupling protein to destroy the proton gradient across the inner membrane of mitochondria (125). As cells scramble to utilize available fuel sources to rebuild the proton gradient, energy is lost in the form of heat. The drug indiscriminately affects all cell types and exhibits highly undesirable pharmacokinetic properties that can lead to cataracts and even death. The clinical use of DNP has a fascinating history, and it has the dubious honor of helping to precipitate the passage of the United States Federal Food, Drug and Cosmetic Act of 1938, which gave the FDA its legal powers to ban dangerous drugs such as DNP (49). Recent studies using oral, extended-release formulations of DNP have demonstrated the utility of this compound (presumably through its actions to increase RMR) to protect the liver of rats from nonalcoholic fatty liver disease (101). Collectively, the previous use of DNP in humans and its beneficial effects in various disease models serve as potent proof-of-principle demonstrations that increasing RMR represents a robust method to combat obesity and other metabolic diseases. We simply need safer drugs, which will come from a deeper understanding of the biological controllers of RMR.
Contributions of the RAS to the control of RMR have been demonstrated using genetic and pharmacological methods. Genetic deletion of renin (127), ACE (65), the angiotensin AT1A receptor (70), and the angiotensin AT2 receptor (147) are all associated with increased RMR. Pharmacological inhibition of ACE by captopril (137) or antagonism of AT1A receptors by candesartan (150) both appear to increase RMR. In contrast, genetic disruption of the angiotensin-(1–7) receptor Mas may reduce RMR (114). These studies collectively implicate the RAS in the control of RMR, but as we detail below, there appear to be site-specific and antagonistic effects of the circulating versus tissue versions of the RAS in RMR control.
Notably, most studies investigating a role for RMR energy balance utilize easily accessible, turn-key instrumentation such as oxygen/carbon dioxide respirometry to assess the impact of manipulations upon metabolic rate. Unfortunately these methods rely upon assumptions that are simply untenable, such as a lack of physiological significance of anaerobic (fermentation and nitrogenous) metabolic rates (68, 75, 85). In contrast, other methods such as direct calorimetry, which account for all forms of metabolism, have also been used to investigate the effects of RAS manipulation upon RMR. Using a custom-built combined direct calorimeter (to measure total RMR) and respirometer (to measure aerobic RMR), we recently documented that genetic disruption of the angiotensin AT2 receptor in FVB/NCrl mice has a robust effect on “nonaerobic” RMR (the difference in results between direct calorimetry and respirometry and logically a measure of the combination of anaerobic processes) (12). Interestingly, we also found that placing wild-type C57BL/6J mice on a 45% high-fat diet (a manipulation previously shown to have stimulatory effects on the circulating and adipose RAS) also has a major suppressive effect on “nonaerobic” RMR (11). Such findings are strikingly similar to those reported from humans by Pittet et al. (102, 103) who used a combined direct calorimeter and respirometer to examine resting metabolism (at rest after a 12-h fast) in lean and obese humans. Studies published by this group in the mid-1970s demonstrated that this “nonaerobic” RMR contributed roughly 6–12% of the total RMR in lean men and women. Obese women, in comparison, exhibited a complete loss of the “nonaerobic” fraction of total RMR (102). Similarly, Seale and Rumpler (116) demonstrated that total daily energy turnover by respirometric methods underestimated the values calculated using isotope dilution methods by 16% (≈425 kcal/day). Together these studies support the concept that respirometry/aerobic RMR is ignoring some pathophysiologically relevant component of energy expenditure in humans (51). Collectively then, it is reasonable to hypothesize that the RAS may contribute to the control of both the aerobic (i.e., processes that can be measured by respirometry) and “nonaerobic” (i.e., processes to which respirometry-based methods are blind) RMR. Improved methodologies to assess both aerobic and “nonaerobic” components of RMR stand to greatly challenge our understanding of the control of resting energy expenditure by the RAS and other hormone systems and may identify “nonaerobic” processes, which are modulated by the RAS, as novel and robust targets for obesity therapeutics (11, 67).
The applicability of studies of RMR in rodents toward our understanding of human RMR control is often questioned. Many studies have illustrated allometric scaling principles across species; for example, it has been demonstrated that the relationship between metabolic rate and organism mass follows a simple positive scaling function across species differing in body mass by a factor of ∼1018 (39). Nonetheless, before the late 2000s, because brown fat had not yet been identified in adult humans, and the function of this tissue in adaptive thermogenic mechanisms in rodents had long been known, it was assumed that resting metabolic processes would function quite differently. Then in 2009 several studies identified functional brown adipose tissue in adult humans following acute cold exposure (22, 133). Subsequently, molecular analyses have aimed to clarify the identity and to understand the function of such “brown/beige/brite” adipose in humans (25, 92, 141). Thus the similarities across species appear to be strong, supporting the continued use of rodent species to dissect the physiology of RMR. Careful consideration must always be given, however, to species-specific differences in thermoregulatory setpoints such as differences in upper- and lower-critical temperatures when translating findings between species (1).
Opposing, Tissue-Specific Metabolic Regulatory Actions of the RAS
Descriptions of the metabolic effects of global RAS disruption in rodents have led many to hypothesize that inhibiting the RAS may have beneficial antiobesity effects in humans. Unfortunately, almost no published studies support this concept, as pharmacological RAS interference largely fails to modulate body mass in humans (2, 31, 55). One study examining the effects of ACE inhibitors perindopril, enalapril, losartan, and telmisartan in obese hypertensive human subjects documented significant beneficial effects on body mass index, waist-hip-ratio, and body fat percentage (93). Unfortunately, many more studies have failed to document beneficial effects of candesartan (43, 76), valsartan (86), ramipril (8), irbesartan (9), telmisartan (60, 89, 120), and olmesartan (81) in humans.
One hypothesis for the lack of consistent beneficial metabolic effects of indiscriminant RAS inhibition on obesity in human patients is rooted in the established independent (and occasionally opposing) effects of local paracrine tissue versions of the RAS, versus the circulating hormone version of this system. For example, within the brain, all components of the RAS are expressed and regulated independently of the circulating and peripheral tissue versions of this system (48, 73). Delivery of angiotensin II into the brain via ICV cannula results in increased RMR and reduced body mass (26, 104, 105). When animals are treated with chronic delivery of the steroid deoxycorticosterone and maintained on a high dietary sodium regimen (the DOCA-salt model), the brain RAS is stimulated while the circulating RAS is suppressed (115). We previously demonstrated that treating wild-type C57BL/6J mice with DOCA-salt resulted in a robust increase in RMR, and that inhibiting AT1 receptors within the brain attenuated this effect (45). Ongoing work in our lab focuses on the dissociation of controlling blood pressure versus energy balance by the RAS within the brain (19). Similarly, mice with transgenic hyperactivity of the brain RAS (sRA mice) exhibit increased RMR that is correlated with suppressed circulating RAS activity (46). As expected, inhibition of β-adrenergic signaling in sRA mice attenuated RMR, implicating the sympathetic nervous system in the elevated energy expenditure of these animals. Surprisingly, however, we discovered that chronic replacement of circulating angiotensin II levels via subcutaneous infusion also completely normalized RMR of sRA mice. Complementing these findings, Yiannikouris et al. (144, 145) has demonstrated that adipose tissue produces RAS components in sufficient amounts to contribute to blood pressure control. These data together highlight critical, opposing effects of the circulating RAS and local brain RAS in the control of RMR. Perhaps the failure of whole body RAS inhibition to oppose obesity in humans stems from the effects of anti-RAS compounds to simultaneously modulate both stimulatory and inhibitory tissue-specific mechanisms, resulting in no net change in energy balance (Fig. 1). By way of analogy, simultaneously and firmly depressing both the accelerator and brake pedals of an automobile has little net effect on the movement of the vehicle, yet the engine may burn out. In much the same way, indiscriminately activating or suppressing the RAS may have no net effect on energy balance, yet the cardiovascular system may pay a hefty price for such stimulation.
Fig. 1.
Renin-angiotensin system (RAS) and energy balance. Energy balance is maintained by the regulation of behaviors (food ingestion and physical activity) and biological mechanisms (digestive efficiency and resting metabolism), and very small but sustained imbalances mediate human obesity. The RAS has been documented to exhibit modulatory effects on each of these major contributors to energy balance. The complex, simultaneous, and frequently opposing modulation of all of these systems by angiotensin may explain why simple RAS inhibition does not reliably cause weight loss in humans. (−), Inhibition; (+), stimulation; (?), remains unclear.
Above we have documented potent, opposing tissue-specific effects of the RAS on components of energy balance. In particular, the effects of the RAS appear to largely oppose the effects of the circulating and adipose RAS. One resulting hypothesis to explain the failure of RAS blockade to reduce body mass in humans focuses on the compartmental distribution of RAS inhibitors in overweight/obese human patients that are examined in such clinical studies. Very few of the RAS inhibitors can cross an intact blood-brain barrier (BBB). Captopril exhibits low permeability through the BBB in normotensive Wistar rats (64). Outside the circumventricular organs, lisinopril and benazepril also appear to exhibit low permeability through the BBB, though high doses of perindopril do exhibit a low level of permeability in selected regions (18). Candesartan appears to readily cross the BBB, whereas losartan, irbesartan, and telmisartan all exhibit much lower permeability (21, 40, 41, 132). While losartan exhibits low permeability through the BBB, the active metabolite EXP 3174 may more efficiently cross (110). Critically, these assessments of BBB permeability have been performed almost exclusively in lean, otherwise healthy animals. The tacit assumption that the BBB is intact and functioning normally in obese or obese hypertensive patients is probably inappropriate.
RAS components themselves, and the various pharmacological modulators of the RAS, are all known to affect BBB permeability. Astrocyte-derived angiotensin II acts via AT1 receptors on BBB endothelial cells to modulate permeability (140). Interestingly, angiotensin-(1–7) (142) and the activation of AT2 receptors (88) also modulate permeability. Peripheral angiotensin II infusion in mice also frequently results in increased BBB permeability, which is reduced in AT1a knockout mice (91, 98) or by tempol (149). The effect of angiotensin II to increase BBB permeability is also amplified with coadministration of nitro-l-arginine methyl ester (69). Angiotensin II acts directly on microvessel endothelial cells and this effect can be ameliorated by telmisartan but not the AT2 antagonist PD-123,319 (35). Dahl salt-sensitive rats exhibit increased BBB permeability, and a nondepressor dose of olmesartan reduces permeability (98, 99). Spontaneously hypertensive rats exhibit disrupted BBB, resulting in increased permeability and increased angiotensin II extravasation into brain tissue; this can be corrected with losartan but not hydralazine treatment (6). Similar to losartan, candesartan also reduces BBB permeability (74, 97). Enalapril appears to reduce permeability following middle cerebral artery occlusion-induced ischemia (96), whereas captopril increases permeability in normotensive rats (119). Interestingly, DOCA-salt hypertension does not appear to grossly modulate BBB permeability (108, 109), which may reflect the tissue-specific effects of DOCA-salt treatment on RAS activity.
Alterations of the BBB not only occur with hypertension but also with metabolic disorders including obesity and diabetes. Mice fed high-fat diets exhibit deterioration of the BBB (59, 131). Body mass is also positively correlated with markers of reduced BBB function in elderly women (50). Although leptin transport across the BBB is reduced in obesity (14), leptin is a large peptide (146 amino acids) and likely not reflective of changes in permeability to other smaller peptides (such as the 8 amino acid angiotensin II) and small nonpeptide modulators of the RAS. Streptozotocin-induced Type 1 diabetes results in increased BBB permeability in rats, which can be reversed by candesartan treatment (4). KKA(y) Type 2 diabetic mice exhibit increased BBB permeability that is reversed by telmisartan; blocking the activation of PPAR-γ via GW9662 blocked this beneficial effect (87). Losartan was less effective than telmisartan to reduce BBB permeability, and GW9662 had no modulatory effects on the losartan effect.
Collectively these data are consistent with the hypothesis that a dysfunctional BBB during obesity and obesity-related hypertension may eliminate beneficial compartmentalization of RAS hyper- versus hypoactivity, resulting in loss of tissue-specific metabolic control by the RAS. Future studies investigating the utility of RAS modulators that cannot cross “weakened” BBB layers during obese states may thus directly test this hypothesis and result in the development of RAS-focused metabolic therapeutic compounds.
Hypothesis: Uncoupled Feedback and Obesity-Related Hypertension
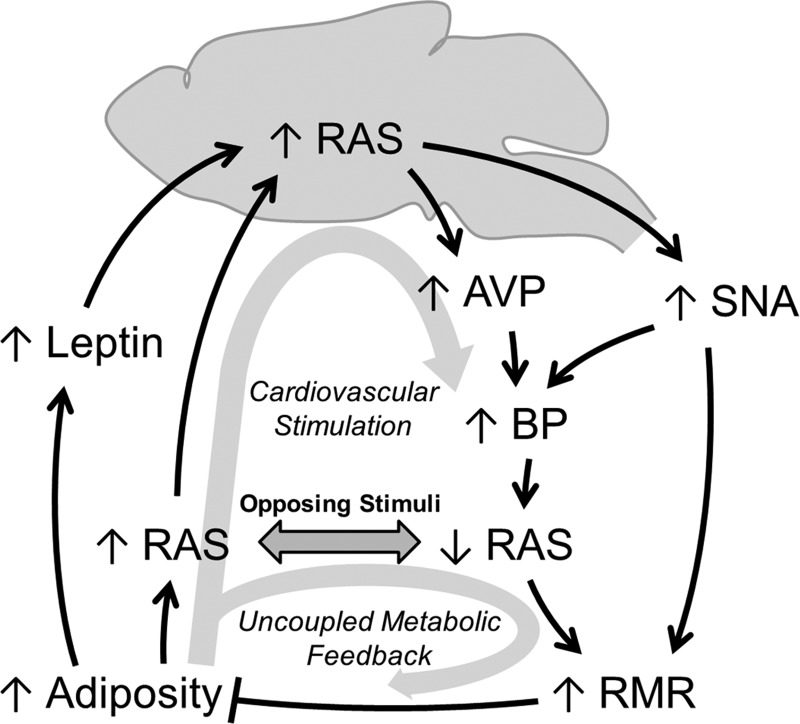
Obesity is correlated with increased circulating RAS activity, increased levels of various other hormones such as leptin, and increased sympathetic nerve activity (SNA) (32, 143–145). Angiotensinogen derived from adipose tissue is increased in obese humans (143), and RAS components from adipose tissue contribute to blood pressure control (144, 145). Circulating RAS activity is a well-established stimulant of the brain RAS (94). Similarly, work from the Mark group (56) demonstrated that SNA responses to leptin were completely dependent on AT1 receptors in the brain, as ICV infusion of losartan attenuated SNA activity responses to leptin injection, to both interscapular brown adipose and kidney. These data highlight the dependence of thermogenic and renal SNA responses to various obesity-related stimuli upon brain AT1 signaling. As we previously documented, the brain RAS (through AT1 signaling) is critically involved in the stimulation of RMR (45, 46), and this stimulation requires suppression of the circulating RAS via increased blood pressure (46, 47, 77). Thus it is conceivable that obesity uncouples the hypertension-mediated suppression of the circulating RAS, thereby disrupting the stimulation of RMR by obesity (Fig. 2). Ultimately, these effects would culminate in unchecked weight gain and increased blood pressure.
Fig. 2.
Hypothesized mechanism for the uncoupling of resting metabolic rate control by the circulating RAS, leading to obesity-related hypertension. Increased adiposity causes increases in leptin and the circulating RAS, both of which stimulate brain RAS activity. The brain RAS, through arginine vasopressin (AVP) and sympathetic nervous activity (SNA), stimulates blood pressure (BP). Elevated BP causes a reflexive suppression of the circulating RAS. Reduced circulating RAS activity and SNA synergistically act to increase resting metabolic rate, which ultimately antagonizes obesity. We hypothesize that the obesity-associated increase in circulating RAS activity uncouples the control of resting metabolism by the brain RAS, resulting in maintained stimulation of blood pressure, but loss of feedback inhibition of adiposity and weight gain, which presents clinically as obesity-related hypertension.
Conclusions and Ongoing Questions
The lack of a unique “obesity-specific” mechanism of hypertension is highlighted by the finding that normal-weight hypertensive, overweight hypertensive, and obese hypertensive patients have the same blood pressure responses to antihypertensive mediations (107). In the early 1970s, Laragh and colleagues (72) documented the relative abundance of essential hypertensive patients with high-, normal-, and low-circulating renin. These studies and several subsequent replications have demonstrated that roughly 16% of hypertensive patients exhibit high-renin hypertension (where the RAS is presumably the primary cause of the hypertension), whereas roughly 57% exhibit normal and 27% exhibit low circulating levels of renin. We posit that comorbid obesity and hypertension may represent an enrichment of the high-RAS hypertensive population, in which elevated RAS activity (in the context of an obesigenic environment) may spill over and contribute to the pathogenesis of obesity.
Herein we hypothesize a relatively simple model of the pathogenesis of obesity and obesity-related hypertension that may result from opposing, tissue-specific effects of the RAS on energy balance. The RAS is involved in the control or regulation of all of the cardinal metabolic processes (food intake behavior, digestive efficiency, physical activity, and resting metabolism; Fig. 1), and the production of RAS components by (and action upon) adipose tissue may ultimately explain the pathogenesis of obesity and the paradox of why stimulation of the RAS and inhibition of the RAS can both result in a negative energy balance. Much more work is needed to understand the mechanisms by which the RAS contributes to energy homeostasis, and how it functions in concert with cardiovascular control, to develop potent dual antihypertensive/antiobesity therapeutics.
GRANTS
N. K. Littlejohn was supported by a predoctoral fellowship from the American Heart Association (14PRE18330015). This work was supported by grants from the National Heart, Lung, and Blood Institute (HL098276, HL084207), American Heart Association (14IRG18710013, 15SFRN23730000), American Diabetes Association (1-14-BS-079), the University of Iowa Office of the Vice President for Research and Economic Development, the Fraternal Order of Eagles' Diabetes Research Center, and the Center for Hypertension Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.K.L. and J.L.G. conception and design of research; N.K.L. and J.L.G. prepared figures; N.K.L. and J.L.G. drafted manuscript; N.K.L. and J.L.G. edited and revised manuscript; N.K.L. and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the American Physiological Society's Water and Electrolyte Homeostasis Section for selecting J. L. Grobe to receive the 2015 Young Investigator Award, for which this review was invited, and Drs. Curt D. Sigmund and Allyn L. Mark for nominating J. L. Grobe for this award.
REFERENCES
- 1.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab 4: 461–470, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuissa H, Jones PG, Marso SP, O'Keefe JH Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol 46: 821–826, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol 106: 1069–1085, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad AS. Role of AT1 receptors in permeability of the blood-brain barrier in diabetic hypertensive rats. Vasc Pharmacol 45: 141–147, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Barretti DL, Magalhaes Fde C, Fernandes T, do Carmo EC, Rosa KT, Irigoyen MC, Negrao CE, Oliveira EM. Effects of aerobic exercise training on cardiac renin-angiotensin system in an obese Zucker rat strain. PLos One 7: e46114, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63: 572–579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloem LJ, Manatunga AK, Tewksbury DA, Pratt JH. The serum angiotensinogen concentration and variants of the angiotensinogen gene in white and black children. J Clin Invest 95: 948–953, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR. Effect of ramipril on the incidence of diabetes. N Engl J Med 355: 1551–1562, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Boschmann M, Engeli S, Adams F, Franke G, Luft FC, Sharma AM, Jordan J. Influences of AT1 receptor blockade on tissue metabolism in obese men. Am J Physiol Regul Integr Comp Physiol 290: R219–R223, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Bruneval P, Hinglais N, Alhenc-Gelas F, Tricottet V, Corvol P, Menard J, Camilleri JP, Bariety J. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry 85: 73–80, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Burnett CM, Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Mol Metab 3: 460–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am J Physiol Endocrinol Metab 305: E916–E924, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell DJ, Habener JF. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest 78: 31–39, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci 59: 416–423, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Cassis L, Helton M, English V, Burke G. Angiotensin II regulates oxygen consumption. Am J Physiol Regul Integr Comp Physiol 282: R445–R453, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA. Mechanisms contributing to angiotensin II regulation of body weight. Am J Physiol Endocrinol Metab 274: E867–E876, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Chai SY, Perich R, Jackson B, Mendelsohn FA, Johnston CI. Acute and chronic effects of angiotensin-converting enzyme inhibitors on tissue angiotensin-converting enzyme. Clin Exp Pharmacol Physiol Suppl 19: 7–12, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Claflin KE, Grobe JL. Control of energy balance by the brain renin-angiotensin system. Curr Hypertens Rep 17: 38, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Cooper R, McFarlane-Anderson N, Bennett FI, Wilks R, Puras A, Tewksbury D, Ward R, Forrester T. ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J Hum Hypertens 11: 107–111, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Culman J, von Heyer C, Piepenburg B, Rascher W, Unger T. Effects of systemic treatment with irbesartan and losartan on central responses to angiotensin II in conscious, normotensive rats. Eur J Pharmacol 367: 255–265, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels D. Frontiers in neuroscience. diverse roles of angiotensin receptor intracellular signaling pathways in the control of water and salt intake. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration, edited by De Luca LA, Menani JV, and Johnson AK. Boca Raton, FL: CRC, 2014. [PubMed] [Google Scholar]
- 24.Daniels D, Yee DK, Fluharty SJ. Angiotensin II receptor signalling. Exp Physiol 92: 523–527, 2007. [DOI] [PubMed] [Google Scholar]
- 25.de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab 308: E1085–E1105, 2015. [DOI] [PubMed] [Google Scholar]
- 26.de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, Seeley RJ, Woods SC. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab 301: E1081–E1091, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Structure Function, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Mello WC. Is an intracellular renin-angiotensin system involved in control of cell communication in heart? J Cardiovasc Pharmacol 23: 640–646, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem 24: 1663–1677, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 45: 356–362, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Ewert S, Spak E, Olbers T, Johnsson E, Edebo A, Fandriks L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol (Oxf) 188: 33–40, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Faloia E, Gatti C, Camilloni MA, Mariniello B, Sardu C, Garrapa GG, Mantero F, Giacchetti G. Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. J Endocrinol Invest 25: 309–314, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab 29: 640–647, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Fleming I, Kohlstedt K, Busse R. The tissue renin-angiotensin system and intracellular signalling. Curr Opin Nephrol Hypertens 15: 8–13, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther 35: 414–428, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giacchetti G, Faloia E, Mariniello B, Sardu C, Gatti C, Camilloni MA, Guerrieri M, Mantero F. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens 15: 381–388, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science 293: 2248–2251, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Gohlke P, Von Kugelgen S, Jurgensen T, Kox T, Rascher W, Culman J, Unger T. Effects of orally applied candesartan cilexetil on central responses to angiotensin II in conscious rats. J Hypertens 20: 909–918, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, Culman J, Unger T. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther 298: 62–70, 2001. [PubMed] [Google Scholar]
- 42.Gomes-Santos IL, Fernandes T, Couto GK, Ferreira-Filho JC, Salemi VM, Fernandes FB, Casarini DE, Brum PC, Rossoni LV, de Oliveira EM, Negrao CE. Effects of exercise training on circulating and skeletal muscle renin-angiotensin system in chronic heart failure rats. PLos One 9: e98012, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Dell'Oro R, Trevano FQ, Bombelli M, Scopelliti F, Facchini A, Mancia G, Study C. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens 21: 1761–1769, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Gratze P, Boschmann M, Dechend R, Qadri F, Malchow J, Graeske S, Engeli S, Janke J, Springer J, Contrepas A, Plehm R, Klaus S, Nguyen G, Luft FC, Muller DN. Energy metabolism in human renin-gene transgenic rats: does renin contribute to obesity? Hypertension 53: 516–523, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grobe JL, Rahmouni K, Liu X, Sigmund CD. Metabolic rate regulation by the renin-angiotensin system: brain vs. body. Pflügers Arch 465: 167–175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology 23: 187–193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Tox 7: 205–212, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med 262: 643–650, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 378: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallersund P, Elfvin A, Helander HF, Fandriks L. The expression of renin-angiotensin system components in the human gastric mucosa. J Renin Angiotensin Aldosterone Sys 12: 54–64, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532: 107–110, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Hasvold LP, Bodegard J, Thuresson M, Stalhammar J, Hammar N, Sundstrom J, Russell D, Kjeldsen SE. Diabetes and CVD risk during angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment in hypertension: a study of 15,990 patients. J Hum Hypertens 28: 663–669, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 303: H197–H206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirasawa K, Sato Y, Hosoda Y, Yamamoto T, Hanai H. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem 50: 275–282, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med 48: 1472–1476, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci 6: 88, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsueh W, Davidai G, Henry R, Mudaliar S. Telmisartan effects on insulin resistance in obese or overweight adults without diabetes or hypertension. J Clin Hypertens (Greenwich) 12: 746–752, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation 125: 2595–2602, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irvine RJ, White JM, Head RJ. The renin angiotensin system and nociception in spontaneously hypertensive rats. Life Sci 56: 1073–1078, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med 168: 1550–1559; discussion 1559–1560, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarden JO, Barry DI, Juhler M, Graham DI, Strandgaard S, Paulson OB. Cerebrovascular aspects of converting-enzyme inhibition II: Blood-brain barrier permeability and effect of intracerebroventricular administration of captopril. J Hypertens 2: 599–604, 1984. [DOI] [PubMed] [Google Scholar]
- 65.Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA 105: 6531–6536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94: 58–65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaiyala KJ. What does indirect calorimetry really tell us? Mol Metab 3: 340–341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaiyala KJ, Ramsay DS. Direct animal calorimetry, the underused gold standard for quantifying the fire of life. Comp Biochem Physiol A Mol Integr Physiol 158: 252–264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalayci R, Kaya M, Uzun H, Bilgic B, Ahishali B, Arican N, Elmas I, Kucuk M. Influence of hypercholesterolemia and hypertension on the integrity of the blood-brain barrier in rats. Int J Neurosci 119: 1881–1904, 2009. [DOI] [PubMed] [Google Scholar]
- 70.Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology 146: 3481–3489, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Kuczmarski RJ. Prevalence of overweight and weight gain in the United States. Am J Clin Nutr 55: 495S–502S, 1992. [DOI] [PubMed] [Google Scholar]
- 72.Laragh JH. Biochemical profiling and the natural history of hypertensive diseases: low-renin essential hypertension, a benign condition. Circulation 44: 971–974, 1971. [DOI] [PubMed] [Google Scholar]
- 73.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Li Z, Cao Y, Li L, Liang Y, Tian X, Mo N, Liu Y, Li M, Chui D, Guo X. Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem Biophys Res Commun 449: 74–80, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Lighton JRB. Measuring Metabolic Rates. New York: Oxford University, 2008. [Google Scholar]
- 76.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertens 21: 1563–1574, 2003. [DOI] [PubMed] [Google Scholar]
- 77.Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol 304: R81–R828, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 121: 586–613, 2010. [DOI] [PubMed] [Google Scholar]
- 79.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 297: R793–R802, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum M, Rothman AJ, Ryan D. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 23: 7–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marinik EL, Frisard MI, Hulver MW, Davy BM, Rivero JM, Savla JS, Davy KP. Angiotensin II receptor blockade and insulin sensitivity in overweight and obese adults with elevated blood pressure. Ther Adv Cardiovasc Dis 7: 11–20, 2013. [DOI] [PubMed] [Google Scholar]
- 82.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15: 2727–2729, 2001. [DOI] [PubMed] [Google Scholar]
- 83.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology 142: 5220–5225, 2001. [DOI] [PubMed] [Google Scholar]
- 84.McGuire MT, Wing RR, Hill JO. The prevalence of weight loss maintenance among American adults. Int J Obes Relat Metab Disord 23: 1314–1319, 1999. [DOI] [PubMed] [Google Scholar]
- 85.McLean JA, Tobin G. Animal and Human Calorimetry. New York: Cambridge University, 1988. [Google Scholar]
- 86.McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 362: 1477–1490, 2010. [DOI] [PubMed] [Google Scholar]
- 87.Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K, Iwanami J, Horiuchi M. Peroxisome proliferator-activated receptor-gamma activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension 59: 1079–1088, 2012. [DOI] [PubMed] [Google Scholar]
- 88.Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, Kan-No H, Wang XL, Chisaka T, Bai HY, Iwanami J, Horiuchi M. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens 27: 1036–1044, 2014. [DOI] [PubMed] [Google Scholar]
- 89.Mori Y, Itoh Y, Tajima N. Telmisartan improves lipid metabolism and adiponectin production but does not affect glycemic control in hypertensive patients with type 2 diabetes. Adv Ther 24: 146–153, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation 131: e29–e322, 2015. [DOI] [PubMed] [Google Scholar]
- 91.Nagai M, Terao S, Vital SA, Rodrigues SF, Yilmaz G, Granger DN. Role of blood cell-associated angiotensin II type 1 receptors in the cerebral microvascular response to ischemic stroke during angiotensin-induced hypertension. Exp Transl Stroke Med 3: 15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Nedogoda SV, Ledyaeva AA, Chumachok EV, Tsoma VV, Mazina G, Salasyuk AS, Barykina IN. Randomized trial of perindopril, enalapril, losartan and telmisartan in overweight or obese patients with hypertension. Clin Drug Invest 33: 553–561, 2013. [DOI] [PubMed] [Google Scholar]
- 94.Northcott CA, Watts S, Chen Y, Morris M, Chen A, Haywood JR. Adenoviral inhibition of AT1a receptors in the paraventricular nucleus inhibits acute increases in mean arterial blood pressure in the rat. Am J Physiol Regul Integr Comp Physiol 299: R1202–R1211, 2010. [DOI] [PubMed] [Google Scholar]
- 95.Ohinata K, Fujiwara Y, Fukumoto S, Iwai M, Horiuchi M, Yoshikawa M. Angiotensin II and III suppress food intake via angiotensin AT(2) receptor and prostaglandin EP(4) receptor in mice. FEBS Lett 582: 773–777, 2008. [DOI] [PubMed] [Google Scholar]
- 96.Panahpour H, Dehghani GA, Bohlooli S. Enalapril attenuates ischaemic brain oedema and protects the blood-brain barrier in rats via an anti-oxidant action. Clin Exp Pharmacol Physiol 41: 220–226, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Panahpour H, Nekooeian AA, Dehghani GA. Candesartan attenuates ischemic brain edema and protects the blood-brain barrier integrity from ischemia/reperfusion injury in rats. Iranian Biomed J 18: 232–238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pelisch N, Hosomi N, Mori H, Masaki T, Nishiyama A. RAS inhibition attenuates cognitive impairment by reducing blood-brain barrier permeability in hypertensive subjects. Curr Hypertens Rev 9: 93–98, 2013. [DOI] [PubMed] [Google Scholar]
- 99.Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, Matsumoto M, Kohno M, Nishiyama A. Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens 24: 362–368, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pereira MG, Ferreira JC, Bueno CR Jr, Mattos KC, Rosa KT, Irigoyen MC, Oliveira EM, Krieger JE, Brum PC. Exercise training reduces cardiac angiotensin II levels and prevents cardiac dysfunction in a genetic model of sympathetic hyperactivity-induced heart failure in mice. Eur J Appl Physiol 105: 843–850, 2009. [DOI] [PubMed] [Google Scholar]
- 101.Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pittet P, Chappuis P, Acheson K, De Techtermann F, Jequier E. Thermic effect of glucose in obese subjects studied by direct and indirect calorimetry. Br J Nutr 35: 281–292, 1976. [DOI] [PubMed] [Google Scholar]
- 103.Pittet P, Gygax PH, Jequier E. Thermic effect of glucose and amino acids in man studied by direct and indirect calorimetry. Br J Nutr 31: 343–349, 1974. [DOI] [PubMed] [Google Scholar]
- 104.Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin II on body weight gain in young rats. Brain Res 959: 20–28, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol 287: R422–R428, 2004. [DOI] [PubMed] [Google Scholar]
- 106.Raghavendra V, Chopra K, Kulkarni SK. Brain renin angiotensin system (RAS) in stress-induced analgesia and impaired retention. Peptides 20: 335–342, 1999. [DOI] [PubMed] [Google Scholar]
- 107.Reisin E, Graves JW, Yamal JM, Barzilay JI, Pressel SL, Einhorn PT, Dart RA, Retta TM, Saklayen MG, Davis BR. Blood pressure control and cardiovascular outcomes in normal-weight, overweight, and obese hypertensive patients treated with three different antihypertensives in ALLHAT. J Hypertens 32: 1503–1513; discussion 1513, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rodrigues SF, Granger DN. Cerebral microvascular inflammation in DOCA salt-induced hypertension: role of angiotensin II and mitochondrial superoxide. J Cereb Blood Flow Metab 32: 368–375, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodrigues SF, Vital SA, Granger DN. Mild hypercholesterolemia blunts the proinflammatory and prothrombotic effects of hypertension on the cerebral microcirculation. J Cereb Blood Flow Metab 33: 483–489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rowland NE, Morien A, Fregly MJ. Losartan inhibition of angiotensin-related drinking and Fos immunoreactivity in hypertensive and hypotensive contexts. Brain Res 742: 253–259, 1996. [DOI] [PubMed] [Google Scholar]
- 111.Sabounchi NS, Rahmandad H, Ammerman A. Best-fitting prediction equations for basal metabolic rate: informing obesity interventions in diverse populations. Int J Obes (Lond) 37: 1364–1370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008. [DOI] [PubMed] [Google Scholar]
- 113.Santos EL, de Picoli Souza K, Guimaraes PB, Reis FC, Silva SM, Costa-Neto CM, Luz J, Pesquero JB. Effect of angiotensin converting enzyme inhibitor enalapril on body weight and composition in young rats. Int Immunopharmacol 8: 247–253, 2008. [DOI] [PubMed] [Google Scholar]
- 114.Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57: 340–347, 2008. [DOI] [PubMed] [Google Scholar]
- 115.Schenk J, McNeill JH. The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods 27: 161–170, 1992. [DOI] [PubMed] [Google Scholar]
- 116.Seale JL, Rumpler WV. Comparison of energy expenditure measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. Eur J Clin Nutr 51: 856–863, 1997. [DOI] [PubMed] [Google Scholar]
- 117.Seo MS, Fukamizu A, Saito T, Murakami K. Identification of a previously unrecognized production site of human renin. Biochim Biophys Acta 1129: 87–89, 1991. [DOI] [PubMed] [Google Scholar]
- 118.Sharma AM, Golay A. Effect of orlistat-induced weight loss on blood pressure and heart rate in obese patients with hypertension. J Hypertens 20: 1873–1878, 2002. [DOI] [PubMed] [Google Scholar]
- 119.Sharma HS. Effect of captopril (a converting enzyme inhibitor) on blood-brain barrier permeability and cerebral blood flow in normotensive rats. Neuropharmacology 26: 85–92, 1987. [DOI] [PubMed] [Google Scholar]
- 120.Shimabukuro M, Tanaka H, Shimabukuro T. Effects of telmisartan on fat distribution in individuals with the metabolic syndrome. J Hypertens 25: 841–848, 2007. [DOI] [PubMed] [Google Scholar]
- 121.Shorning BY, Jarde T, McCarthy A, Ashworth A, de Leng WW, Offerhaus GJ, Resta N, Dale T, Clarke AR. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut 61: 202–213, 2012. [DOI] [PubMed] [Google Scholar]
- 122.Spak E, Casselbrant A, Olbers T, Lonroth H, Fandriks L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf) 193: 181–190, 2008. [DOI] [PubMed] [Google Scholar]
- 123.Steig AJ, Jackman MR, Giles ED, Higgins JA, Johnson GC, Mahan C, Melanson EL, Wyatt HR, Eckel RH, Hill JO, MacLean PS. Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stucchi P, Cano V, Ruiz-Gayo M, Fernandez-Alfonso M. Aliskiren reduces body-weight gain, adiposity and plasma leptin during diet-induced obesity. Br J Pharmacol 158: 771–778, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tainter ML, Stockton AB, Cutting WC. Dinitrophenol in the treatment of obesity: Final report. JAMA 105: 332–337, 1935. [Google Scholar]
- 126.Takada S, Kinugawa S, Hirabayashi K, Suga T, Yokota T, Takahashi M, Fukushima A, Homma T, Ono T, Sobirin MA, Masaki Y, Mizushima W, Kadoguchi T, Okita K, Tsutsui H. Angiotensin II receptor blocker improves the lowered exercise capacity and impaired mitochondrial function of the skeletal muscle in type 2 diabetic mice. J Appl Physiol 114: 844–857, 2013. [DOI] [PubMed] [Google Scholar]
- 127.Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab 6: 506–512, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 121: 2126–2132, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. [DOI] [PubMed] [Google Scholar]
- 130.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27: 155–161, 2004. [DOI] [PubMed] [Google Scholar]
- 131.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci 69: 1212–1226, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Unger T. Inhibiting angiotensin receptors in the brain: possible therapeutic implications. Curr Med Res Opin 19: 449–451, 2003. [DOI] [PubMed] [Google Scholar]
- 133.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 134.Wang P, Holst C, Wodzig WK, Andersen MR, Astrup A, van Baak MA, Larsen TM, Jebb SA, Kafatos A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Viguerie N, Langin D, Saris WH, Mariman EC. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int J Obes (Lond) 36: 1545–1551, 2012. [DOI] [PubMed] [Google Scholar]
- 135.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, Claflin KE, Burnett CML, Pearson NA, Lutter ML, Grobe JL. Dietary sodium suppresses digestive efficiency via the renin-angiotensin system. Sci Rep 5: 11123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weisinger HS, Begg DP, Egan GF, Jayasooriya AP, Lie F, Mathai ML, Sinclair AJ, Wark JD, Weisinger RS. Angiotensin converting enzyme inhibition from birth reduces body weight and body fat in Sprague-Dawley rats. Physiol Behav 93: 820–825, 2008. [DOI] [PubMed] [Google Scholar]
- 137.Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiol Behav 98: 192–197, 2009. [DOI] [PubMed] [Google Scholar]
- 138.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 82: 222s–225s, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Wong TP, Debnam ES, Leung PS. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol 584: 613–623, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci 27: 9032–9042, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu J, Zhao D, Wu S, Wang D. Ang-(1–7) exerts protective role in blood-brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol 748: 30–36, 2015. [DOI] [PubMed] [Google Scholar]
- 143.Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, Fujikura J, Ebihara K, Hosoda K, Katsurada A, Ohashi N, Urushihara M, Kobori H, Morimoto N, Kawazoe T, Naitoh M, Okada M, Sakaue H, Suzuki S, Nakao K. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens 23: 425–431, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension 60: 1524–1530, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol 302: R244–R251, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology 153: 1411–1420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes 54: 991–999, 2005. [DOI] [PubMed] [Google Scholar]
- 148.Yvan-Charvet L, Massiera F, Lamande N, Ailhaud G, Teboul M, Moustaid-Moussa N, Gasc JM, Quignard-Boulange A. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology 150: 1421–1428, 2009. [DOI] [PubMed] [Google Scholar]
- 149.Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 171: 852–858, 2010. [DOI] [PubMed] [Google Scholar]
- 150.Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol 552: 112–122, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]