Abstract
Human hepatocellular carcinoma (HCC) heavily endangers human heath worldwide. HCC is one of most frequent cancers in China because patients with liver disease, such as chronic hepatitis, have the highest cancer susceptibility. Traditional therapeutic approaches have limited efficacy in advanced liver cancer, and novel strategies are urgently needed to improve the limited treatment options for HCC. This review summarizes the basic knowledge, current advances, and future challenges and prospects of adeno-associated virus (AAV) and adenoviruses as vectors for gene therapy of HCC. This paper also reviews the clinical trials of gene therapy using adenovirus vectors, immunotherapy, toxicity and immunological barriers for AAV and adenoviruses, and proposes several alternative strategies to overcome the therapeutic barriers to using AAV and adenoviruses as vectors.
Keywords: Hepatocellular carcinoma, Adeno-associated virus, Adenovirus, Virus vectors
Core tip: This review summarizes the basic knowledge, current advances, and future challenges and prospects of adeno-associated virus (AAV) and adenoviruses as vectors for gene therapy of hepatocellular carcinoma. This paper also reviews the clinical trials of gene therapy using adenovirus vectors, immunotherapy, toxicity and immunological barriers for AAV and adenoviruses, and proposes several alternative strategies to overcome the therapeutic barriers to using AAV and adenoviruses as vectors.
INTRODUCTION
Human hepatocellular carcinoma (HCC) is the fifth leading cause of cancer death with an estimated > 33000 new cases per year in the United States, according to the American Cancer Society[1]. In China, HCC is also one of the five most common cancers, whose incidence rate ranks fourth and mortality ranks second, with 18.43 per 10000 among all types of cancers[2,3]. Surgery, chemotherapy, and radiotherapy are still the most common therapeutic options for HCC. Surgery is the first choice for early stage disease and offers a cure rate of 10%-20% in HCC patients. However, liver cancer recurrence rate remains high after tumor resection because of the aggressive traits of HCC, such as metastasis and chemo- or radiotherapy resistance[4,5]. Although tremendous efforts have been made to improve the anti-cancer effect for HCC, there are still no ideal therapeutic strategies. Gene therapy has attracted much interest as a novel promising therapeutic method since the first approved successful clinical trial for children with severe combined immunodeficiency (SCID) in 1990[6]. Henceforth, gene therapy has rapidly developed as a strategy for single gene hereditary diseases, infections, and cancer, using various viral or nonviral vectors[7,8]. Currently, most effective vectors are derived from recombinant viruses including adenovirus, adeno-associated virus (AAV), vaccinia virus, retrovirus, lentivirus, herpes simplex virus, Epstein-Barr virus, and chimeric viruses[8,9]. Since we proposed the Cancer Targeting Gene Virotherapy (CTGVT) strategy by combining cancer gene therapy and virotherapy using oncolytic adenoviral vector in 2001, numerous studies have been performed in HCC and other cancers using gene therapy[10-12]. Additionally, due to its distinct merits, AAV is considered as the prime candidate vector for clinical gene therapy for various liver diseases[13,14]. This review describes the advances, therapeutic mechanisms, and current challenges in using AAV and adenoviruses as vectors for gene therapy of HCC.
BIOLOGY OF AAV AND ITS APPLICATION AS A VECTOR FOR HCC
AAV, a member of the parvovirus family, has a single-stranded DNA genome of approximately 4.7 kb. The genome consists of two open reading frames (ORFs) rep and cap driven by three promoters (P5, P19 and P40), which are flanked by the 145 nucleotide long inverted terminal repeat sequences. The rep encodes four overlapping functional proteins (Rep78, Rep68, Rep52, and Rep40), which play a part in viral replication, transcriptional control, and accumulation of single-stranded progeny genomes, and the cap contains three capsid proteins (VP1, VP2, and VP3) functioning in the generation of infectious particles[15].
Essential properties of AAV vector
Since Hermonat et al[16] first used AAV as a vector for transgene delivery into cultured mammalian cells, AAV-based vectors have undergone rapid development in recent decades. Unlike the wild-type AAV that is able to integrate into the host genome, exogenous genes delivered by recombinant AAV vector can be persistently expressed in an episomal state[17,18]. AAV vectors have some others advantages, including a broad host spectrum that allows them to infect both non-dividing and dividing cells and low pathogenicity or no cytotoxicity[19].
Presently, there are more than 11 different serotypes of AAVs and over 100 new AAV variants to be identified and engineered into vectors. AAV2 serotype was the first to be used for the transfer of transgenes into host cells and has emerged as a promising carrier for clinical gene transfer in several single genetic disorders[20]. As the first clinical gene therapy medicine (AAV2-LPL) authorized by European Medicines Agency (EMA)[21], it largely promotes the development of AAV vectors for clinical transgene therapy. Besides AAV2, AAV1 and 3-9 serotypes were engineered as gene transfer vectors. AAV1 vector has a higher transfer rate in muscle than AAV2[22]. AAV1, 4, and 5 can transfer genes efficiently into muscles, lung, and central nervous system[8,23]. The serology of AAV6 is almost identical to that of AAV1, and the AAV6 vector was designed to transfer primary human hematopoietic stem cells into a mouse model[24]. AAV7 and AAV8 are two new members of the AAV family isolated from rhesus monkeys[25]. Furthermore, the efficiency of gene transfer to liver with AAV7 and 8 vectors was higher than that achieved using AAV2, although a variety of host factors may influence this important parameter, such as pre-existing antibodies, gender, and transgene immunity[25-27]. So far, the serological profiles of AAV10 and AAV11 are not well characterized[28,29]. AAV12 is a novel AAV serotype with transduction activity independent of sialic acid and heparan sulfate proteoglycan[30]. The AAV variants are not further identified and exploited as vectors due to the lack of serological profiling.
AAV vector development for HCC
In recent years, many important breakthroughs have been achieved in gene therapy in several disease types, including genetic diseases and cancer. Up to January 2015, there were 127 trials of gene therapy mediated by AAV vectors registered at the Journal of Gene Medicine Clinical Trial website[31]. These trials have been performed for the treatment of various diseases, including cancer and monogenic, neurological, ocular, cardiovascular, and infectious diseases. The landmark of AAV-mediated clinical studies is the development of inherited retinal diseases and anerythrochloropsia gene therapy. AAV-mediated PRE65 gene expression efficiently recovered visual function in patients with Leber’s congenital amaurosis with controlled safety and efficacy of gene transfer[32-34]. These encouraging results widened the clinical applications of AAV vectors, including gene therapy of cancer.
AAV vectors are engineered for delivery to patients suffering from liver diseases, including familial hypercholesterolemia, viral hepatitis, and hepatic malignancies. The first gene therapy experiment for HCC using AAV vectors was carried out by Su et al[35]. The constructed recombinant AAV virus, which carried the herpes simplex virus thymidine kinase (TK) gene driven by the human α-fetoprotein (AFP) enhancer and the albumin promoter, resulted in a selective killing effect on AFP-positive HCC cells but not non-hepatocyte tumor cells or AFP and albumin-negative hepatic tumor cells[35]. Moreover, the dose required to kill the cancer cells was inversely proportional to the level of AFP expression in the cells.
Actually, besides suicide-gene-directed enzyme/pro-drug therapy, gene therapy using AAV covers numerous therapeutic methods, such as inhibition of oncogenes and re-expression of tumor suppressor genes, immunotherapy, anti-angiogenesis therapy, and combination therapy[19]. There are a variety of targeting strategies for AAV application, including transcription-targeted, receptor-targeted, and conjugate-targeted strategies and various alternative AAV serotypes[8]. The application of human telomerase reverse transcriptase (hTERT) promoter was a good candidate for transcription-targeted cancer gene therapy[13]. In general, cancer cells have high telomerase activity, and hTERT, a catalytic subunit of the telomerase, is transcriptionally upregulated exclusively in about 90% of cancer cells[36]. Thus, telomerase is an excellent tumor marker. We first constructed a novel AAV vector targeting telomerase activity and investigated its targeting capability and anti-tumor potential though carrying a human interferon-β gene[13]. The recombinant virus AAV-hTERT-human interferon (hIFN)-β displayed cancer-specific hIFNβ expression and cytotoxicity in various human cancer cells in vitro and suppressed tumor growth in nude mice of lung cancer A549 and colorectal cancer SW620 xenografts[7]. In addition, we and others generated a recombinant AAV vector containing the tumor necrosis factor alpha related apoptosis inducing ligand (TRAIL) gene under the control of the hTERT promoter. The AAV-hTERT-TRAIL virus exhibited cancer-specific cytotoxicity and apoptosis that which significantly suppressed the growth of HCC xenograft tumors[37,38]. These results indicated that AAVs in combination with hTERT-mediated therapeutic gene expression provide a promising targeting approach for developing effective therapy for HCC (Figure 1).
Figure 1.
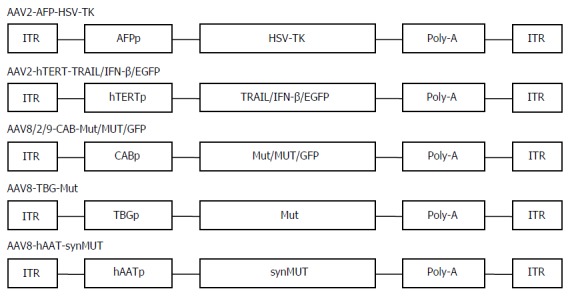
Schematic of adeno-associated virus vectors packaged into adeno-associated virus serotypes 2, 8, and 9 with different promoters used for gene delivery. AFPp: Human α-fetoprotein promoter; hTERTp: A truncated human telomerase; CBA: Chicken β-actin; TBG: The liver-specific thyroxine-binding globulin; hAAT: Human α1-antitrypsin.
AAV-mediated gene therapy of HCC has made great progress in other research areas. Intraportal injection of recombinant AAV carrying kallistatin gene suppressed hepatic and subcutaneous HCC tumors through antiangiogenic and antiproliferative activities[39]. Ling et al[40,41] validated that AAV serotype 3 is an excellent vector in efficiently transducing human liver cancer because AAV3 uses human hepatocyte growth factor receptor as a cellular co-receptor for binding and entry into these cells, which implies that AAV3 vectors can be applied to gene therapy of liver cancer. In addition, AAV8 may be the best liver-specific transfer vector and has good prospects for liver cancer gene therapy[42]. In particular, RNA interference (RNAi)-based approaches, such as antisense hypoxia-inducible factor-1α and microRNA (miRNA)-targeted therapy mediated by AAV have been recently exploited as new anti-cancer treatments for HCC[43-45]. Systemic administration of AAV-mediated miR-26a delivery efficiently inhibited HCC cell proliferation, induced tumor-specific apoptosis, and suppressed tumorigenesis in a murine liver cancer model[45]. Other than the general strategy for miRNA replacement therapies, inhibiting the oncogenic miR-221 by miRNA sponge was developed for therapy of HCC, in which AAVs were genetically modified to drive the expression of multiple binding sites for miR-221[44].
Combination therapy is an important tactic for clinical cancer therapy. AAV-mediated gene therapy is widely considered as a potential adjuvant of other therapies. We attempted combination therapy with AAV-hTERT-TRAIL and cisplatin, which could have a synergistic therapeutic effect on HCC. As expected, treatment with both AAV-hTERT-TRAIL and cisplatin exhibited a stronger inhibitory effect and induced more significant apoptosis compared with either agent alone in HCC cells and animal tumors[46]. Other studies showed that radiotherapy can enhance transduction of HCC cells by recombinant AAV in vitro and in vivo[47]. Even the cocktail viral gene therapy, combining the effect of AAV transducing hepatocyte growth factor dringle 1 and adenovirus transducing p53, significantly induced tumor cell death, inhibited tumor angiogenesis and tumor growth, and prevented tumor metastasis in HCC models[48]. Multigene-based combination therapy is an effective practice in cancer gene therapy. AAV-mediated coexpression of apoptin and interleukin (IL)-24 in HCC significantly suppressed the growth and induced apoptosis of HepG2 cells in vitro and in xenograft nude mice[49]. An AAV6 serotype designed for dendritic-cell-based cancer immunotherapy can be a useful targeting approach for efficient HCC therapy[50], which could lay the foundation for further development for AAV-mediated HCC immunotherapy.
THERAPEUTIC APPLICATION OF ADENOVIRUS IN HCC
Adenovirus has been the most common gene transfer vector for cancer gene therapy in past decades because of its unique advantages, such as broad tropism for infecting many human tissues including hepatocytes, capability of transducing nonreplicating cells and replicating cells, and easy acquisition of high titers, benefiting clinical use and efficient transgene expression[51,52]. Numerous studies have reported the potential application of adenovirus-mediated gene therapy for a wide variety of diseases and indicated their beneficial effects, tolerability, and safety. The following three aspects describe oncolytic adenovirus vector, adenovirus-mediated immune treatment, and clinical trials for HCC therapy, respectively.
Oncolytic adenovirus vector
Currently, oncolytic viruses represent a group of promising anti-cancer agents with the ability to lyse infected cancer cells but not normal cells. The oncolytic viruses include genetically modified adenovirus, vaccinia virus, herpes simplex virus, and reovirus[10]. ONYX-015, an oncolytic adenovirus designed with the E1B 55-kDa gene deletion, was engineered to replicate in and lyse cancer cells selectively[53]. Clinical trials using ONYX-015 alone or in combination with chemotherapy have been widely performed in patients with head and neck cancer, which achieved an obvious anti-cancer effect[54,55]. In addition, many targeted strategies based on oncolytic adenoviruses were conceived, and they exhibited potent anti-tumor activity in various preclinical studies[11,56,57]. Previously, our CTGVT, which combined the superiority of gene therapy and virotherapy, brought new hope for cancer therapy[10,58]. Similar to ONYX-015, the novel oncolytic adenovirus ZD55 was first engineered with E1B55-kD protein deletion based on CTGVT, and was combined with anti-cancer gene therapy to form the ZD55-gene system[58]. Our many experiments confirmed that the ZD55 gene exerted a potent anti-tumor effect in multiple tumor cell lines and mouse models through the synergetic mechanisms of the oncolytic action of the virus itself and overexpression of anti-tumor genes[11,52,59-63]. In particular, the novel oncolytic adenovirus ZD55-Smac increases anti-tumor activity of ZD55-TRAIL against HCC via a synergetic apoptotic effect[64]. In general, HCC frequently displays a high resistance to TRAIL-mediated cell death due to high expression of inhibitor of apoptosis proteins (IAPs), while Smac strongly inhibits IAPs and increases sensitivity of HCC cells to TRAIL[65]. Thus, combination treatment with ZD55-Smac and ZD55-TRAIL led to rapid and potent activation of apoptosis in HCC cells and eliminated completely tumor xenograft in all treated animals, which could provide a useful strategy for therapy of HCC[64].
Furthermore, numerous other combination strategies were adopted in HCC therapy based on the oncolytic adenovirus ZD55 system. Chu et al[62] found that adenoviral vector expressing cylindromatosis (CYLD) augments anti-tumor activity of ZD55-TRAIL by suppression of nuclear factor-κB survival signaling in HCC. Pan et al[66] constructed ZD55-mediated X-linked inhibitor of apoptosis protein (XIAP)-shRNA by RNAi to inhibit high XIAP of HCC cells and concluded that combination of ZD55-shXIAP and ZD55-TRAIL led to synergistic anti-tumor activity in experimental HCC. Moreover, the combination of XAF1, IL-24, or Smac-armed ZD55 and chemotherapy exhibited significantly enhanced suppression of HCC growth by a synergistic mechanism, especially in the induction of apoptosis[67-69].
However, traditional oncolytic adenovirus lacks the ability to target liver cancer. To improve the anti-cancer effect of oncolytic adenovirus in liver cancer, three main strategies have been developed in recent years. First, the transcription-targeted strategy is designed using cancer- or tissue-specific promoter to control the expression of viral genes essential for replication[70,71], which results in selective expression of viral genes, propagation of virus progeny, and tumoricidal activity[9,10]. We designed AFP-regulated oncolytic adenovirus AD and obtained a better anti-cancer effect in HCC than other types of cancer[72-76]. Golgi protein (GOLPH2) is an excellent HCC marker[77,78], therefore, we constructed a novel GOLPH2-regulated oncolytic adenovirus GD55 that targets HCC[79]. We showed that the novel GD55 had higher adenovirus replication ability and infectivity in liver cancer cells than the common oncolytic adenovirus ZD55. In addition, GD55 exerted a significant growth-suppressing effect on HCC cells or xenograft but caused little damage to normal liver cells, which may provide a promising oncolytic virus for future liver cancer treatment[79]. The second strategy targets the tumor signaling pathway through deletion or mutation of key adenovirus genes or some bases that are necessary for adenovirus replication in normal cells but not in tumor cells. The classical design of oncolytic adenovirus is ONYX-015 and ZD55, whose E1B 55 kDa gene is deleted by genetic engineering[53,58]. They are supposed to target and lyse p53-dysfunctinal tumor cells preferentially but not adjacent normal cells[80], but further study demonstrated that the adenovirus mutant enhanced the viral mRNA late nuclear transport and oncolysis[81]. We also noted inhibition of liposarcoma by histone deacetylase inhibitor occurs irrespective of p53 mutational status but via targeting of the MDM2-p53 signaling axis and phosphatase and tensin homolog[82]. Another modification was a 24 base pair deletion in the E1A region, such as oncolytic virus Ad5-E1A(Δ24); which is responsible for binding retinoblastoma (Rb) protein, and its replication is restricted in Rb-inactive arrested cells and exhibits tumor-selective capability[56,71,83]. The third approach is the receptor-targeted or capsid-modified strategy. Adenovirus can efficiently infect host cells by binding to specific receptors on the target cell surface with fibers on the capsid. Thus, modification of the adenovirus capsid may improve the binding ability of adenovirus to target cells. The adenovirus vector with genetically modified fibers (RGD-4C or chimera fibers of different serum types) demonstrated expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism[84,85]. Otherwise, efficient and selective gene transfer into primary human tumors using single-chain antibody-targeted adenoviral vectors with native tropism abolished the specific targeting ability[86]. In Table 1, we sum up Liver- or hepatocellular carcinoma- specific promoters and delivery gene in adeno-associated virus vectors for the past few years.
Table 1.
Liver- or hepatocellular carcinoma - specific promoters in adeno-associated virus vectors
| Promoter | Target | Delivery gene | Ref. |
| CBA | Liver | Reporter, Flk-1, Mut, MUT, GFP | [15,87-89] |
| TBG | Liver | Canine factor IX, IDS. Mut | [15,90,91] |
| hEF1α | Liver | IFN-α | [92] |
| hAAT | Liver | hFIX, synMUT | [15,93] |
| Mouse albumin | Liver | HGAA, tTA, EGFP | [94] |
| Albumin + α fetoprotein | Hepatocellular carcinoma | HSV-TK | [35,95] |
| hTERT | Hepatocellular carcinoma | TRAIL | [8] |
| survivin | Hepatocellular carcinoma | TSLC1 | [70] |
CBA: Chicken β-actin; TBG: The liver-specific thyroxine-binding globulin; hAAT: Human α1-antitrypsin.
Adenovirus-mediated immunotherapy
The three traditional anti-cancer therapies (surgery, radiation, and chemotherapy) often carry risks and/or cause adverse side effects and show limited efficacy, particularly for late-stage cancer. The fourth option is cancer biotherapy, including oncolytic viruses and immunotherapy, which has emerged as a promising therapy in preclinical trials and cancer patients. Immunotherapy was considered the Breakthrough of the Year for 2013 and 2014 because of the efficacy of antibodies against cytotoxic T-lymphocyte-associated protein (CTLA)-4, programmed death (PD)-1, ligand 1, and chimeric antigen receptor[96,97]. Encouragingly, CD19-targeted T cells achieved complete remission in children and adults with chemotherapy-refractory acute lymphoblastic leukemia[98]. These also brought inspiration and confidence to acquire the expected therapy effect either via oncolysis or antitumor immunity by recombinant oncolytic adenovirus.
Fortunately, people began to realize that oncolytic adenoviruses cause the immune system to stimulate an anti-tumor immune response[99]. A novel oncolytic adenovirus, Ad5D24-CpG, was engineered by inserting 18 immunostimulatory islands into Ad5D24. This virus showed increased anti-tumor activity via the stimulation of Toll-like receptor 9 and inactivation of myeloid-derived suppressor cells in modified virus-treated mice[100]. Moreover, to achieve superior anti-cancer immunity, oncolytic adenoviruses are often designed to express immunostimulatory molecules including CD40L, IL-2, IL-12, IL-24, and granulocyte-macrophage colony-stimulating factor (GM-CSF)[101]. Among these constructs, oncolytic adenovirus coding for GM-CSF (Ad5-D24-GMCSF) was a typical agent, and it induced anti-tumor immunity in cancer patients with advanced solid tumors refractory to standard therapies, indicating that the treatment was safe. The tumor completely disappeared in 2/20 patients[102].
Further modifications were made in the following approaches to improve clinical anti-tumor immunological benefit. The adenovirus serotype 5 capsid was replaced with serotype 3 fiber knob to form chimeric adenovirus vector Ad5/3-D24-GMCSF, avoiding the problem of coxsackie-adenovirus receptor downregulation in advanced tumors[99]. Another immunostimulatory molecule of interest is CD40L, a multifunctional protein. Oncolytic adenovirus encoding CD40L led to a strong anti-tumor effect[103]. Efficient targeted cancer immunotherapy was achieved with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4 (Ad5/3-Δ24aCTLA4), avoiding the severe immune-related adverse events by systemic administration of monoclonal antibodies ipilimumab or tremelimumab blocking CTLA-4[104]. It also suggests the feasibility of immunotherapy with oncolytic adenovirus-mediated CTLA4 antibody[105].
An alternative means of anti-tumor immunotherapy is oncolytic adenovirus-vector vaccines. Orally delivered oncolytic adenovirus vaccines have been utilized to prevent adenovirus-induced respiratory illness in military recruits, demonstrating safety and high efficacy[106]. The experience suggested that oral administration of live oncolytic adenoviruses holds promise for immunization against liver cancer and other infectious diseases because live adenoviruses can express intact tumor-associated antigens as transgenes in infected cells[107]. Another novel approach is incorporation of antigenic epitopes into adenovirus capsids, eliciting the strongest humoral and cell-mediated immune responses, both prior to and during virus replication, against cancer and infection[106,108,109].
Clinical trials for adenovirus-associated HCC therapy
The first gene transfer with recombinant replication-defective adenovirus was successfully implemented in HCC cells in 1995, which led to induction of sensitivity to ganciclovir in human HCC cells by adenovirus-mediated herpes simplex virus TK gene[110]. Since then, 293 papers have been published in the field of adenoviruses and HCC, and 317 gene therapy studies using adenovirus vectors have been registered at ClinicalTrials.gov. Adenoviruses are the most frequently used gene transfer vectors in clinical trials according to the Journal of Gene Medicine Clinical Trial site[31]. However, at the time of the preparation of this review (May 2015), official sources listed only eight clinical trials that described the status (Table 2), efficacy, and safety of adenovirus-associated therapy in HCC.
Table 2.
Overview of clinical trials that use adenovirus vector for hepatocellular carcinoma therapy
| Adenovirus | Phase | Trial | Status | Route | Notes |
| ADV-TK | II | NCT00300521 | Completed | it | As a single agent |
| TK99UN | I | NCT00844623 | Completed | it | As a single agent |
| AdVhAFP | I/II | NCT00669136 | Terminated | im | Combined with AFP and GM-CSF |
| AdVhAFP | II/IIIA/IIIB/IVA | NCT00093548 | Withdrawn | im and id | Combined with AFP and GM-CSF |
| ADV-TK | II | NCT02202564 | Completed | Intrahepatic | Double-dose |
| Ad5CMV-p53 | I | NCT00003147 | Terminated | Percutaneous injection | As a single agent |
| Adenovirus Type 5 | III | NCT01869088 | Recruiting | Arterial infusion | Combined with TACE |
| rAd-p53 | II | NCT02418988 | Recruiting | Arterial injection | Combined with TACE |
TACE: Transcatheter arterial chemoembolization; ADV-TK: Adenovirus thymidine kinase; AdVhAFP: α-fetoprotein adenoviral vector; GM-CSF: Granulocyte-macrophage colony-stimulating factor; TK99UN: Adenoviral vector containing thymidine kinase of herpes simplex virus; im: Intramuscularly; it: Intratumoral; id: Intradermally.
In particular, there are two studies that are currently recruiting participants. Recombinant human adenovirus type 5, with an E1B gene deletion, combining transcatheter arterial chemoembolization (TACE), is being tested as a stand-alone therapeutic intervention in a phase III trial in patients with advanced HCC not amenable to surgery or local ablative therapy (NCT01869088). A phase II trial of rAd-p53 artery injection combined with TACE in adults with HCC is being sponsored by Shenzhen Sibiono Genetech (NCT02418988). rAd-p53 was the first approved adenovirus agent worldwide for the treatment of head and neck cancer, and great success was achieved in these patients, especially when combined with chemotherapy or radiotherapy. In addition, three clinical trials have recently been completed using adenovirus vectors. The preliminary safety and efficacy of double-dose adenovirus-mediated adjuvant therapy (Adv-TK, adenovirus expressing TK) was evaluated in phase II trials, resulting in improved outcome of liver transplantation in patients with advanced HCC (NCT02202564). In addition, liver transplantation with Adv-TK gene therapy improved survival in patients with advanced HCC (NCT00300521). Intratumoral injection of TK99UN (an adenoviral vector containing TK) was assessed in a phase I clinical trial in adult HCC patients (NCT00844623). Two trials using adenovirus vectors were terminated for undisclosed reasons. A phase I trial of the effectiveness of gene therapy with Ad5CMV-p53 was also terminated in patients with liver cancer that could not be surgically removed (NCT00003147). A suspended phase I/II trial is testing immunization, safety, and toxicity of AFP plus GM-CSF plasmid prime and AFP-armed adenoviral vector (Adv-hAFP) in patients with locoregionally pretreated HCC (NCT00669136). A vaccine therapy study using Adv-hAFP was halted prior to enrollment for treating patients with stage II, IIIA, IIIB, or IVA liver cancer (NCT00093548).
EXISTING PROBLEMS AND CHALLENGE
Toxicity and immunological barriers for AAV
The nonpathogenic feature of AAV endows it as a promising gene therapy vector with little or no acute toxicity to the host. Results from gene therapy trials with AAV vectors, especially some exciting results from clinical studies of hemophilia B, congenital blindness, and familial lipoprotein lipase deficiency, have confirmed their therapeutic potential[110,111].
However, some of the limitations of in vivo AAV gene transfer have emerged. First, the host immune response to AAV capsid is an important obstacle to safety and efficacy of AAV-vector-mediated gene transfer in vivo[112,113]. AAV2-capsid-specific cytotoxic T cells were detectable following AAV2-mediated hepatic delivery of factor IX in hemophilia B patients, which resulted in killing and clearance of transduced hepatocytes and affected the therapeutic efficacy. It was hypothesized that this was caused by rejection of transduced hepatocytes by AAV-capsid-specific memory CD8+ T cells reactivated by AAV vectors, because these patients harbor a population of capsid-specific memory cytotoxic T cells formed during childhood infection with wild-type AAV2[114]. Second, the humoral immune response is a universal obstacle in virus-mediated gene transfer in vivo, largely affecting the therapeutic efficacy. Conceivably, both the transgene protein and AAV capsid can produce relevant antibodies. Anti-AAV capsid neutralizing antibodies are highly prevalent and detectable in two-thirds of the population. Even high titer AAV neutralizing antibodies can completely inhibit vector transduction to the target tissue, leading to lack of efficacy[113]. Although the integration potential of AAV into the host genome offers long-term transgene expression in animal experiments or clinical trials, there is a risk of insertional mutagenesis that may induce carcinogenesis[115]. In particular, a high incidence of HCC was observed in mice or other mouse models after systemic delivery of AAV gene therapy vector[115]. The hepatic genotoxicity may be caused by AAV integration into the RNA imprinted and accumulated in the nucleus (Rian) locus, resulting in overexpression of proximal miRNAs and retrotransposon-like 1 associated with HCC[15].
Problematic limitations for oncolytic adenovirus
Adenovirus has a broad range of vertebrate hosts, including humans, and commonly causes illnesses such as mild respiratory infections and cold-like syndrome in young children. Although wild-type adenovirus can kill some cancer cells, it also has many side effects. Numerous modified oncolytic viral constructs, such as H101, Ad-p53, and Ad5-D24-GMCSF, have indicated potent anti-tumor efficacy in patients with solid cancers refractory to standard therapeutics with limited or no toxicity to normal tissue[9]. However, there currently are some inevitable obstacles for clinical application of systemic adenovirus-mediated gene therapy. These are high prevalence of neutralizing antibodies, induction of immune and inflammatory responses, high promiscuity due to widespread expression of the coxsackie-adenovirus receptor, and adenovirus sequestration by the liver[116]. The approximately 36 kb genome of adenovirus comprises a variety of structural, replication, and regulatory genes, resulting in the complexity and uncertainty of the toxic effect induced by oncolytic adenovirus during systemic administration. The representative oncolytic adenovirus mutant is ONYX-015 (designed by Onyx Pharmaceuticals). Although phase I and II clinical trials of ONYX-015 were completed in patients with various solid tumors, a phase III trial for the treatment of recurrent head and neck cancer patients was suddenly repealed because of possible safety problems a decade ago. Therefore, improving anti-cancer efficacy and reducing toxic effects and immune response to adenovirus vectors remain potential challenges to successful HCC therapy.
FUTURE PROSPECTS
With the rapid development of HCC incidence and mortality in China, there is an urgent need for innovative, alternative therapies for HCC patients. Despite anti-tumor efficacy being achieved by AAV- or adenovirus-mediated gene therapy in experimental liver cancer models, researchers soon realized its limitations and ongoing efforts are being made to resolve these limitations. Efficient transfer of genes/small RNAs to the majority of cancer cells is still unrealistic for solid tumors[117]. To date, there are 17 trials using AAV vectors and 154 trials using adenoviruses for gene therapy of cancer registered at the Wiley Clinical Trial site[31], although none of these trials for AAV and only eight for adenoviruses are investigating liver cancer. Therefore, more efficient AAV or adenovirus vectors targeting HCC should be designed to achieve successful treatment of liver cancer. Current strategies are mainly aimed at chemical modification of the virus capsid, serotype substitution of different virus types, and hybrid vectors combining viral and synthetic vectors to improve therapeutic efficacy for HCC. These new strategies have gradually demonstrated that the modified vectors have the ability to escape neutralizing antivirus antibodies, to overcome liver tropism, and to reduce humoral and cellular immune responses and liver toxicity even after systemic virus administration, while maintaining their natural biological activity[118]. In addition, the various combination strategies between different virus vectors or gene therapy and conventional/cell therapy can optimize the efficacy of AAV or adenovirus-mediated therapy. Thus, we believe that optimal scheduled combinatorial regimens will likely have promising antineoplastic effects in the field of gene therapy with modified virus vectors.
Footnotes
Supported by National Natural Science Foundation of China. No. 81272687; Zhejiang Provincial Public Welfare Technology Application Research Projects, No. 2014C33275; Zhejiang Provincial Natural Science Foundation of China, No. LZ13H160004; and the Grant for 521 Talent Project of Zhejiang Sci-Tech University, Hangzhou, China.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 29, 2015
First decision: August 31, 2015
Article in press: September 30, 2015
P- Reviewer: Mizuguchi T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Scaggiante B, Kazemi M, Pozzato G, Dapas B, Farra R, Grassi M, Zanconati F, Grassi G. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol. 2014;20:1268–1288. doi: 10.3748/wjg.v20.i5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma L, Chua MS, Andrisani O, So S. Epigenetics in hepatocellular carcinoma: an update and future therapy perspectives. World J Gastroenterol. 2014;20:333–345. doi: 10.3748/wjg.v20.i2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011;26:28–35. doi: 10.1111/j.1440-1746.2010.06502.x. [DOI] [PubMed] [Google Scholar]
- 4.Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, Benvegnù L, Caturelli E, Zoli M, Borzio F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61:184–190. doi: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 5.Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TK. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand EW. SCID continues to point the way. N Engl J Med. 1990;322:1741–1743. doi: 10.1056/NEJM199006143222410. [DOI] [PubMed] [Google Scholar]
- 7.He LF, Wang YG, Xiao T, Zhang KJ, Li GC, Gu JF, Chu L, Tang WH, Tan WS, Liu XY. Suppression of cancer growth in mice by adeno-associated virus vector-mediated IFN-beta expression driven by hTERT promoter. Cancer Lett. 2009;286:196–205. doi: 10.1016/j.canlet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Wang YG, Huang F, Cai R, Qian C, Liu XY. Targeting strategies for adeno-associated viral vector. Zhongguo Kexue Tongbao. 2007;52:1590–1599. [Google Scholar]
- 9.Evans J. Recent deal highlights hopes for cancer-killing viruses. Nat Med. 2011;17:268–269. doi: 10.1038/nm0311-268b. [DOI] [PubMed] [Google Scholar]
- 10.Liu XY, Gu JF. Targeting gene-virotherapy of cancer. Cell Res. 2006;16:25–30. doi: 10.1038/sj.cr.7310005. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, Wang Y, Qian Q, Qian C, Wu J, et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–4298. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- 12.Jin H, Lv S, Yang J, Wang X, Hu H, Su C, Zhou C, Li J, Huang Y, Li L, et al. Use of microRNA Let-7 to control the replication specificity of oncolytic adenovirus in hepatocellular carcinoma cells. PLoS One. 2011;6:e21307. doi: 10.1371/journal.pone.0021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YG, Wang JH, Zhang YH, Gu Q, Liu XY. Antitumor effect of a novel adeno-associated virus vector targeting to telomerase activity in tumor cells. Acta Biochim Biophys Sin (Shanghai) 2004;36:492–500. doi: 10.1093/abbs/36.7.492. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Burt DR, Gao G. Adeno-associated virus-mediated microRNA delivery and therapeutics. Semin Liver Dis. 2015;35:81–88. doi: 10.1055/s-0034-1397352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler RJ, LaFave MC, Varshney GK, Trivedi NS, Carrillo-Carrasco N, Senac JS, Wu W, Hoffmann V, Elkahloun AG, Burgess SM, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest. 2015;125:870–880. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojala DS, Amara DP, Schaffer DV. Adeno-associated virus vectors and neurological gene therapy. Neuroscientist. 2015;21:84–98. doi: 10.1177/1073858414521870. [DOI] [PubMed] [Google Scholar]
- 18.Smith RH. Adeno-associated virus integration: virus versus vector. Gene Ther. 2008;15:817–822. doi: 10.1038/gt.2008.55. [DOI] [PubMed] [Google Scholar]
- 19.Park K, Kim WJ, Cho YH, Lee YI, Lee H, Jeong S, Cho ES, Chang SI, Moon SK, Kang BS, et al. Cancer gene therapy using adeno-associated virus vectors. Front Biosci. 2008;13:2653–2659. doi: 10.2741/2872. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi K, Nakai H, Couto LB, Kay MA. Modified infusion procedures affect recombinant adeno-associated virus vector type 2 transduction in the liver. Hum Gene Ther. 2005;16:299–306. doi: 10.1089/hum.2005.16.299. [DOI] [PubMed] [Google Scholar]
- 21.Miller N. Glybera and the future of gene therapy in the European Union. Nat Rev Drug Discov. 2012;11:419. doi: 10.1038/nrd3572-c1. [DOI] [PubMed] [Google Scholar]
- 22.Gerlach B, Kleinschmidt JA, Böttcher B. Conformational changes in adeno-associated virus type 1 induced by genome packaging. J Mol Biol. 2011;409:427–438. doi: 10.1016/j.jmb.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 23.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song L, Kauss MA, Kopin E, Chandra M, Ul-Hasan T, Miller E, Jayandharan GR, Rivers AE, Aslanidi GV, Ling C, et al. Optimizing the transduction efficiency of capsid-modified AAV6 serotype vectors in primary human hematopoietic stem cells in vitro and in a xenograft mouse model in vivo. Cytotherapy. 2013;15:986–998. doi: 10.1016/j.jcyt.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, Figueredo J, Lock M, Wilson JM. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori S, Takeuchi T, Enomoto Y, Kondo K, Sato K, Ono F, Sata T, Kanda T. Tissue distribution of cynomolgus adeno-associated viruses AAV10, AAV11, and AAVcy.7 in naturally infected monkeys. Arch Virol. 2008;153:375–380. doi: 10.1007/s00705-007-1097-8. [DOI] [PubMed] [Google Scholar]
- 29.Mori S, Wang L, Takeuchi T, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330:375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Journal of Gene Medicine. Gene Therapy Clinical Trials Worldwide. Available from: http://www.abedia.com/wiley/index.html.
- 32.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Chang JC, Xu SM, Kan YW. Selective killing of AFP-positive hepatocellular carcinoma cells by adeno-associated virus transfer of the herpes simplex virus thymidine kinase gene. Hum Gene Ther. 1996;7:463–470. doi: 10.1089/hum.1996.7.4-463. [DOI] [PubMed] [Google Scholar]
- 36.Murofushi Y, Nagano S, Kamizono J, Takahashi T, Fujiwara H, Komiya S, Matsuishi T, Kosai K. Cell cycle-specific changes in hTERT promoter activity in normal and cancerous cells in adenoviral gene therapy: a promising implication of telomerase-dependent targeted cancer gene therapy. Int J Oncol. 2006;29:681–688. [PubMed] [Google Scholar]
- 37.Wang Y, Huang F, Cai H, Zhong S, Liu X, Tan WS. Potent antitumor effect of TRAIL mediated by a novel adeno-associated viral vector targeting to telomerase activity for human hepatocellular carcinoma. J Gene Med. 2008;10:518–526. doi: 10.1002/jgm.1177. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Ma H, Zhang J, Liu S, Liu Y, Zheng D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life Sci. 2008;82:1154–1161. doi: 10.1016/j.lfs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Tse LY, Sun X, Jiang H, Dong X, Fung PW, Farzaneh F, Xu R. Adeno-associated virus-mediated expression of kallistatin suppresses local and remote hepatocellular carcinomas. J Gene Med. 2008;10:508–517. doi: 10.1002/jgm.1180. [DOI] [PubMed] [Google Scholar]
- 40.Ling C, Lu Y, Cheng B, McGoogan KE, Gee SW, Ma W, Li B, Aslanidi GV, Srivastava A. High-efficiency transduction of liver cancer cells by recombinant adeno-associated virus serotype 3 vectors. J Vis Exp. 2011;(49):pii: 2538. doi: 10.3791/2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling C, Wang Y, Zhang Y, Ejjigani A, Yin Z, Lu Y, Wang L, Wang M, Li J, Hu Z, et al. Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther. 2014;25:1023–1034. doi: 10.1089/hum.2014.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho KJ, Bass CE, Kroemer AH, Ma C, Terwilliger E, Karp SJ. Optimized adeno-associated virus 8 produces hepatocyte-specific Cre-mediated recombination without toxicity or affecting liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2008;295:G412–G419. doi: 10.1152/ajpgi.00590.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun X, Jiang H, Jiang X, Tan H, Meng Q, Sun B, Xu R, Krissansen GW. Antisense hypoxia-inducible factor-1alpha augments transcatheter arterial embolization in the treatment of hepatocellular carcinomas in rats. Hum Gene Ther. 2009;20:314–324. doi: 10.1089/hum.2008.164. [DOI] [PubMed] [Google Scholar]
- 44.Moshiri F, Callegari E, D’Abundo L, Corrà F, Lupini L, Sabbioni S, Negrini M. Inhibiting the oncogenic mir-221 by microRNA sponge: toward microRNA-based therapeutics for hepatocellular carcinoma. Gastroenterol Hepatol Bed Bench. 2014;7:43–54. [PMC free article] [PubMed] [Google Scholar]
- 45.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Huang F, Cai H, Wu Y, He G, Tan WS. The efficacy of combination therapy using adeno-associated virus-TRAIL targeting to telomerase activity and cisplatin in a mice model of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1827–1837. doi: 10.1007/s00432-010-0841-8. [DOI] [PubMed] [Google Scholar]
- 47.Peng D, Qian C, Sun Y, Barajas MA, Prieto J. Transduction of hepatocellular carcinoma (HCC) using recombinant adeno-associated virus (rAAV): in vitro and in vivo effects of genotoxic agents. J Hepatol. 2000;32:975–985. doi: 10.1016/s0168-8278(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 48.Shen Z, Wong OG, Yao RY, Liang J, Kung HF, Lin MC. A novel and effective hepatocyte growth factor kringle 1 domain and p53 cocktail viral gene therapy for the treatment of hepatocellular carcinoma. Cancer Lett. 2008;272:268–276. doi: 10.1016/j.canlet.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 49.Yuan L, Zhao H, Zhang L, Liu X. The efficacy of combination therapy using adeno-associated virus-mediated co-expression of apoptin and interleukin-24 on hepatocellular carcinoma. Tumour Biol. 2013;34:3027–3034. doi: 10.1007/s13277-013-0867-z. [DOI] [PubMed] [Google Scholar]
- 50.Pandya J, Ortiz L, Ling C, Rivers AE, Aslanidi G. Rationally designed capsid and transgene cassette of AAV6 vectors for dendritic cell-based cancer immunotherapy. Immunol Cell Biol. 2014;92:116–123. doi: 10.1038/icb.2013.74. [DOI] [PubMed] [Google Scholar]
- 51.Zou W, Luo C, Zhang Z, Liu J, Gu J, Pei Z, Qian C, Liu X. A novel oncolytic adenovirus targeting to telomerase activity in tumor cells with potent. Oncogene. 2004;23:457–464. doi: 10.1038/sj.onc.1207033. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, Wang Y, Zhang J, Zhang Z, Huiwang J, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther. 2005;16:845–858. doi: 10.1089/hum.2005.16.845. [DOI] [PubMed] [Google Scholar]
- 53.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 54.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–1342. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 55.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 56.Zhang KJ, Wang YG, Cao X, Zhong SY, Wei RC, Wu YM, Yue XT, Li GC, Liu XY. Potent antitumor effect of interleukin-24 gene in the survivin promoter and retinoblastoma double-regulated oncolytic adenovirus. Hum Gene Ther. 2009;20:818–830. doi: 10.1089/hum.2008.205. [DOI] [PubMed] [Google Scholar]
- 57.Yu de B, Zhong SY, Yang M, Wang YG, Qian QJ, Zheng S, Liu XY. Potent antitumor activity of double-regulated oncolytic adenovirus-mediated ST13 for colorectal cancer. Cancer Sci. 2009;100:678–683. doi: 10.1111/j.1349-7006.2009.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, Qian QJ, Liu XY. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13:481–489. doi: 10.1038/sj.cr.7290191. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B, Zhao L, Qian C, Qian Q, Liu X. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol Ther. 2005;11:553–562. doi: 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Li B, Liu X, Fan J, Qi R, Bo L, Gu J, Qian Q, Qian C, Liu X. A survivin-mediated oncolytic adenovirus induces non-apoptotic cell death in lung cancer cells and shows antitumoral potential in vivo. J Gene Med. 2006;8:1232–1242. doi: 10.1002/jgm.953. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L, Dong A, Gu J, Liu Z, Zhang Y, Zhang W, Wang Y, He L, Qian C, Qian Q, et al. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 62.Chu L, Gu J, Sun L, Qian Q, Qian C, Liu X. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene Ther. 2008;15:484–494. doi: 10.1038/gt.2008.6. [DOI] [PubMed] [Google Scholar]
- 63.He LF, Gu JF, Tang WH, Fan JK, Wei N, Zou WG, Zhang YH, Zhao LL, Liu XY. Significant antitumor activity of oncolytic adenovirus expressing human interferon-beta for hepatocellular carcinoma. J Gene Med. 2008;10:983–992. doi: 10.1002/jgm.1231. [DOI] [PubMed] [Google Scholar]
- 64.Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, Gu J, Qian C, Liu X. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371–1381. doi: 10.1002/hep.20203. [DOI] [PubMed] [Google Scholar]
- 65.Pan Q, Huang Y, Chen L, Gu J, Zhou X. SMAC-armed vaccinia virus induces both apoptosis and necroptosis and synergizes the efficiency of vinblastine in HCC. Hum Cell. 2014;27:162–171. doi: 10.1007/s13577-014-0093-z. [DOI] [PubMed] [Google Scholar]
- 66.Pan Q, Liu B, Liu J, Cai R, Liu X, Qian C. Synergistic antitumor activity of XIAP-shRNA and TRAIL expressed by oncolytic adenoviruses in experimental HCC. Acta Oncol. 2008;47:135–144. doi: 10.1080/02841860701403053. [DOI] [PubMed] [Google Scholar]
- 67.Ma B, Wang Y, Zhou X, Huang P, Zhang R, Liu T, Cui C, Liu X, Wang Y. Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol. 2015;141:419–429. doi: 10.1007/s00432-014-1835-8. [DOI] [PubMed] [Google Scholar]
- 68.Pan QW, Zhong SY, Liu BS, Liu J, Cai R, Wang YG, Liu XY, Qian C. Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus. Acta Pharmacol Sin. 2007;28:1996–2004. doi: 10.1111/j.1745-7254.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 69.Wu YM, Zhang KJ, Yue XT, Wang YQ, Yang Y, Li GC, Li N, Wang YG. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol Sin. 2009;30:467–477. doi: 10.1038/aps.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He G, Lei W, Wang S, Xiao R, Guo K, Xia Y, Zhou X, Zhang K, Liu X, Wang Y. Overexpression of tumor suppressor TSLC1 by a survivin-regulated oncolytic adenovirus significantly inhibits hepatocellular carcinoma growth. J Cancer Res Clin Oncol. 2012;138:657–670. doi: 10.1007/s00432-011-1138-2. [DOI] [PubMed] [Google Scholar]
- 71.Lei W, Liu HB, Wang SB, Zhou XM, Zheng SD, Guo KN, Ma BY, Xia YL, Tan WS, Liu XY, et al. Tumor suppressor in lung cancer-1 (TSLC1) mediated by dual-regulated oncolytic adenovirus exerts specific antitumor actions in a mouse model. Acta Pharmacol Sin. 2013;34:531–540. doi: 10.1038/aps.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X, Cao X, Wei R, Cai Y, Li H, Gui J, Zhong D, Liu XY, Huang K. Gene-viro-therapy targeting liver cancer by a dual-regulated oncolytic adenoviral vector harboring IL-24 and TRAIL. Cancer Gene Ther. 2012;19:49–57. doi: 10.1038/cgt.2011.67. [DOI] [PubMed] [Google Scholar]
- 73.Cao X, Yang M, Wei RC, Zeng Y, Gu JF, Huang WD, Yang DQ, Li HL, Ding M, Wei N, et al. Cancer targeting Gene-Viro-Therapy of liver carcinoma by dual-regulated oncolytic adenovirus armed with TRAIL gene. Gene Ther. 2011;18:765–777. doi: 10.1038/gt.2011.16. [DOI] [PubMed] [Google Scholar]
- 74.Wei RC, Cao X, Gui JH, Zhou XM, Zhong D, Yan QL, Huang WD, Qian QJ, Zhao FL, Liu XY. Augmenting the antitumor effect of TRAIL by SOCS3 with double-regulated replicating oncolytic adenovirus in hepatocellular carcinoma. Hum Gene Ther. 2011;22:1109–1119. doi: 10.1089/hum.2010.219. [DOI] [PubMed] [Google Scholar]
- 75.Zhang KJ, Qian J, Wang SB, Yang Y. Targeting Gene-Viro-Therapy with AFP driving Apoptin gene shows potent antitumor effect in hepatocarcinoma. J Biomed Sci. 2012;19:20. doi: 10.1186/1423-0127-19-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang F, Ma B, Wang Y, Xiao R, Kong Y, Zhou X, Xia D. Targeting gene-virus-mediated manganese superoxide dismutase effectively suppresses tumor growth in hepatocellular carcinoma in vitro and in vivo. Cancer Biother Radiopharm. 2014;29:403–411. doi: 10.1089/cbr.2014.1642. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Y, Yin X, Ying J, Zhang B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer. 2012;12:17. doi: 10.1186/1471-2407-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–1693. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Liu T, Huang P, Zhao H, Zhang R, Ma B, Chen K, Huang F, Zhou X, Cui C, et al. A novel Golgi protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor efficacy in hepatocellular carcinoma. Oncotarget. 2015;6:13564–13578. doi: 10.18632/oncotarget.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 81.O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Ou WB, Zhu J, Eilers G, Li X, Kuang Y, Liu L, Mariño-Enríquez A, Yan Z, Li H, Meng F, et al. HDACi inhibits liposarcoma via targeting of the MDM2-p53 signaling axis and PTEN, irrespective of p53 mutational status. Oncotarget. 2015;6:10510–10520. doi: 10.18632/oncotarget.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 84.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, Barnes MN, Alvarez RD, Siegal GP, Curiel DT, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 86.van Beusechem VW, Grill J, Mastenbroek DC, Wickham TJ, Roelvink PW, Haisma HJ, Lamfers ML, Dirven CM, Pinedo HM, Gerritsen WR. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J Virol. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shevtsova Z, Malik JM, Michel U, Bähr M, Kügler S. Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Exp Physiol. 2005;90:53–59. doi: 10.1113/expphysiol.2004.028159. [DOI] [PubMed] [Google Scholar]
- 88.Davidoff AM, Nathwani AC, Spurbeck WW, Ng CY, Zhou J, Vanin EF. rAAV-mediated long-term liver-generated expression of an angiogenesis inhibitor can restrict renal tumor growth in mice. Cancer Res. 2002;62:3077–3083. [PubMed] [Google Scholar]
- 89.Mori K, Gehlbach P, Yamamoto S, Duh E, Zack DJ, Li Q, Berns KI, Raisler BJ, Hauswirth WW, Campochiaro PA. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1994–2000. [PubMed] [Google Scholar]
- 90.Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardone M, Polito VA, Pepe S, Mann L, D’Azzo A, Auricchio A, Ballabio A, Cosma MP. Correction of Hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum Mol Genet. 2006;15:1225–1236. doi: 10.1093/hmg/ddl038. [DOI] [PubMed] [Google Scholar]
- 92.Berraondo P, Di Scala M, Korolowicz K, Thampi LM, Otano I, Suarez L, Fioravanti J, Aranda F, Ardaiz N, Yang J, et al. Liver-directed gene therapy of chronic hepadnavirus infection using interferon alpha tethered to apolipoprotein A-I. J Hepatol. 2015;63:329–336. doi: 10.1016/j.jhep.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, Tuddenham EG, Kemball-Cook G, McIntosh J, Boon-Spijker M, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raben N, Lu N, Nagaraju K, Rivera Y, Lee A, Yan B, Byrne B, Meikle PJ, Umapathysivam K, Hopwood JJ, et al. Conditional tissue-specific expression of the acid alpha-glucosidase (GAA) gene in the GAA knockout mice: implications for therapy. Hum Mol Genet. 2001;10:2039–2047. doi: 10.1093/hmg/10.19.2039. [DOI] [PubMed] [Google Scholar]
- 95.Guan M, Rodriguez-Madoz JR, Alzuguren P, Gomar C, Kramer MG, Kochanek S, Prieto J, Smerdou C, Qian C. Increased efficacy and safety in the treatment of experimental liver cancer with a novel adenovirus-alphavirus hybrid vector. Cancer Res. 2006;66:1620–1629. doi: 10.1158/0008-5472.CAN-05-0877. [DOI] [PubMed] [Google Scholar]
- 96.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 97.McNutt M. Cancer immunotherapy. Science. 2013;342:1417. doi: 10.1126/science.1249481. [DOI] [PubMed] [Google Scholar]
- 98.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hemminki A. Oncolytic immunotherapy: where are we clinically? Scientifica (Cairo) 2014;2014:862925. doi: 10.1155/2014/862925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cerullo V, Diaconu I, Romano V, Hirvinen M, Ugolini M, Escutenaire S, Holm SL, Kipar A, Kanerva A, Hemminki A. An oncolytic adenovirus enhanced for toll-like receptor 9 stimulation increases antitumor immune responses and tumor clearance. Mol Ther. 2012;20:2076–2086. doi: 10.1038/mt.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, Erbs P, Limacher JM, Preville X, Zitvogel L, et al. Trial Watch: Oncolytic viruses for cancer therapy. Oncoimmunology. 2014;3:e28694. doi: 10.4161/onci.28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, Nokisalmi P, Raki M, Laasonen L, Särkioja M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 103.Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, Bramante S, Parviainen S, Kanerva A, Loskog AS, et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 104.Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, Escutenaire S, Kanerva A, Pesonen S, Löskog A, et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19:988–998. doi: 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- 105.Du T, Shi G, Li YM, Zhang JF, Tian HW, Wei YQ, Deng H, Yu DC. Tumor-specific oncolytic adenoviruses expressing granulocyte macrophage colony-stimulating factor or anti-CTLA4 antibody for the treatment of cancers. Cancer Gene Ther. 2014;21:340–348. doi: 10.1038/cgt.2014.34. [DOI] [PubMed] [Google Scholar]
- 106.Deal C, Pekosz A, Ketner G. Prospects for oral replicating adenovirus-vectored vaccines. Vaccine. 2013;31:3236–3243. doi: 10.1016/j.vaccine.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E, Brunellière J, Bramson JL, Lichty BD, Wan Y. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther. 2010;18:1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kimball KJ, Rivera AA, Zinn KR, Icyuz M, Saini V, Li J, Zhu ZB, Siegal GP, Douglas JT, Curiel DT, et al. Novel infectivity-enhanced oncolytic adenovirus with a capsid-incorporated dual-imaging moiety for monitoring virotherapy in ovarian cancer. Mol Imaging. 2009;8:264–277. [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma A, Krause A, Xu Y, Sung B, Wu W, Worgall S. Adenovirus-based vaccine with epitopes incorporated in novel fiber sites to induce protective immunity against Pseudomonas aeruginosa. PLoS One. 2013;8:e56996. doi: 10.1371/journal.pone.0056996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qian C, Bilbao R, Bruña O, Prieto J. Induction of sensitivity to ganciclovir in human hepatocellular carcinoma cells by adenovirus-mediated gene transfer of herpes simplex virus thymidine kinase. Hepatology. 1995;22:118–123. [PubMed] [Google Scholar]
- 111.Ferreira V, Petry H, Salmon F. Immune Responses to AAV-Vectors, the Glybera Example from Bench to Bedside. Front Immunol. 2014;5:82. doi: 10.3389/fimmu.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salmon F, Grosios K, Petry H. Safety profile of recombinant adeno-associated viral vectors: focus on alipogene tiparvovec (Glybera®) Expert Rev Clin Pharmacol. 2014;7:53–65. doi: 10.1586/17512433.2014.852065. [DOI] [PubMed] [Google Scholar]
- 113.Masat E, Pavani G, Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov Med. 2013;15:379–389. [PubMed] [Google Scholar]
- 114.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 115.Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 116.Grünwald GK, Vetter A, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R, Schwaiger M, Zach C, Wagner E, Göke B, et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J Nucl Med. 2013;54:1450–1457. doi: 10.2967/jnumed.112.115493. [DOI] [PubMed] [Google Scholar]
- 117.van der Laan LJ, Wang Y, Tilanus HW, Janssen HL, Pan Q. AAV-mediated gene therapy for liver diseases: the prime candidate for clinical application? Expert Opin Biol Ther. 2011;11:315–327. doi: 10.1517/14712598.2011.548799. [DOI] [PubMed] [Google Scholar]
- 118.Laga R, Carlisle R, Tangney M, Ulbrich K, Seymour LW. Polymer coatings for delivery of nucleic acid therapeutics. J Control Release. 2012;161:537–553. doi: 10.1016/j.jconrel.2012.02.013. [DOI] [PubMed] [Google Scholar]


