Abstract
IMPORTANCE
Attention-deficit/hyperactivity disorder (ADHD) research has long focused on the dopaminergic system’s contribution to pathogenesis, although the results have been inconclusive. However, a case has been made for the involvement of the noradrenergic system, which modulates cognitive processes, such as arousal, working memory, and response inhibition, all of which are typically affected in ADHD. Furthermore, the norepinephrine transporter (NET) is an important target for frequently prescribed medication in ADHD. Therefore, the NET is suggested to play a critical role in ADHD.
OBJECTIVE
To explore the differences in NET nondisplaceable binding potential (NET BPND) using positron emission tomography and the highly selective radioligand (S,S)-[18F]FMeNER-D2 [(S,S)-2-(α-(2-[18F]fluoro[2H2]methoxyphenoxy)benzyl)morpholine] between adults with ADHD and healthy volunteers serving as controls.
DESIGN, SETTING, AND PARTICIPANTS
Twenty-two medication-free patients with ADHD (mean [SD] age, 30.7 [10.4] years; 15 [68%] men) without psychiatric comorbidities and 22 age- and sex-matched healthy controls (30.9 [10.6] years; 15 [68%] men) underwent positron emission tomography once. A linear mixed model was used to compare NET BPND between groups.
MAIN OUTCOMES AND MEASURES
The NET BPND in selected regions of interest relevant for ADHD, including the hippocampus, putamen, pallidum, thalamus, midbrain with pons (comprising a region of interest that includes the locus coeruleus), and cerebellum. In addition, the NET BPND was evaluated in thalamic subnuclei (13 atlas-based regions of interest).
RESULTS
We found no significant differences in NET availability or regional distribution between patients with ADHD and healthy controls in all investigated brain regions (F1,41 < 0.01; P = .96). Furthermore, we identified no significant association between ADHD symptom severity and regional NET availability. Neither sex nor smoking status influenced NET availability. We determined a significant negative correlation between age and NET availability in the thalamus (R2 = 0.29; P < .01 corrected) and midbrain with pons, including the locus coeruleus (R2 = 0.18; P < .01 corrected), which corroborates prior findings of a decrease in NET availability with aging in the human brain.
CONCLUSIONS AND RELEVANCE
Our results do not indicate involvement of changes in brain NET availability or distribution in the pathogenesis of ADHD. However, the noradrenergic transmitter system may be affected on a different level, such as in cortical regions, which cannot be reliably quantified with this positron emission tomography ligand. Alternatively, different key proteins of noradrenergic neurotransmission might be affected.
Attention-deficit/hyperactivity disorder (ADHD), which is characterized by inattention, impulsivity, and hyperactivity,1 affects between 8% and 12% of children,2 persists into adulthood in approximately 30% of cases,3 and exhibits rising prevalence rates.4 Attention-deficit/hyperactivity disorder is often associated with detrimental comorbidities5-7 as well as with a large personal and social burden.7 As a result, many individuals with ADHD routinely receive psychopharmacologic treatment.
Patients with ADHD often receive methylphenidate hydrochloride and amphetamine sulfate, which are stimulant medications that enhance dopaminergic and noradrenergic signaling. Alternatively, atomoxetine hydrochloride, which is a nonstimulant drug that blocks the norepinephrine transporter (NET), is used. By blocking the NET, atomoxetine affects noradrenergic signaling and, particularly in brain regions lacking the dopamine transporter, increases dopaminergic transmission.8,9 Treatment using methylphenidate, amphetamine, and atomoxetine is associated with improvement of clinical symptoms and performance in controlled tasks eliciting executive functions, such as inhibitory control, and of cognitive functions, such as working memory and attention.10-13
Although amphetamine and methylphenidate have been suggested14-16 to exert therapeutic efficacy via an increase in extracellular dopamine, they also have been shown16,17 to modulate noradrenergic neurotransmission, which may be therapeutically relevant. Methylphenidate may dose-dependently block the NET, thereby regulating noradrenergic and dopamine reuptake.18,19 In a similar manner, atomoxetine has been shown20 to facilitate therapeutic response by binding the NET. In addition, quetiapine fumarate, which is not used as an ADHD medication but has been shown21 to improve cognitive function in patients with psychosis, was shown22 to bind to the NET. Ultimately, facilitation of therapeutic response by catecholamines in general and the NET in particular suggests that these systems may be relevant to ADHD.
Furthermore, ADHD symptoms have long been attributed to abnormalities within the frontostriatal and frontoparietal networks implicated in executive functions23 modulated by catecholaminergic systems.24,25 The noradrenergic system, which originates in the locus coeruleus and exerts virtually ubiquitous influence within the brain, modulates, among other cortical regions, the prefrontal cortex through dynamic adaption of tonic and phasic firing.26 Studies27,28 displaying improvement of such symptoms by application of α2 agonists further link noradrenergic influence on prefrontal cortex–mediated cognitive functions to ADHD.
More assertive investigation of underlying neurobiological correlates is made possible through positron emission tomography (PET) imaging studies, which have focused on ADHD-related changes in the dopaminergic system. Although changes in dopamine transporter29-31 and dopamine D2 and D3 receptor levels and distribution29,32,33 as well as dopamine release34,35 have been investigated, the results remain inconclusive. However, the proposition that methylphenidate, amphetamine, and atomoxetine may induce therapeutic response via NET modulation suggests that noradrenergic factors, and more specifically changes in the NET, may play a role in ADHD pathogenesis.
Therefore, we proposed a thorough investigation of ADHD-related NET distribution, as has been performed for the serotonin transporter and dopamine transporter. To address this issue, we used the recently developed NET-specific radiotracer (S,S)-[18F]FMeNER-D2 [(S,S)-2-(α-(2-[18F]fluoro[2H2] methoxyphenoxy)benzyl)morpholine], which has been successfully applied in healthy control groups.36 To investigate the role of noradrenergic changes within ADHD, NET imaging was carried out in a region of interest (ROI) approach focusing on brain areas integral to the noradrenergic system.
Methods
Participants
Written informed consent was obtained from all participants after detailed explanation of the study protocol, and the participants received financial reimbursement. The study was approved by the ethics committee of the Medical University of Vienna and the General Hospital of Vienna (EK 552/2010).
Twenty-two adults with ADHD (mean [SD] age, 30.7 [10.4] years; 15 [68%] men) and 22 age- and sex-matched healthy individuals serving as controls (30.9 [10.6] years; 15 [68%] men) (Table 1) were recruited through an ADHD outpatient clinic at the Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria, and from the local community via advertisement. Patients had not received psychopharmacologic treatment for at least 6 months before the screening visit; all control participants were naive to all psychopharmacologic treatment. Of the original 51 study participants, 2 (4%) were excluded because of substance abuse, 2 (4%) because of somatic disorders, and 3 (6%) because of radiosynthesis difficulties.
Table 1.
Epidemiologic and Clinical Characteristics of Participants
| Characteristic | No. (%) | |
|---|---|---|
| ADHD Group (n = 22) |
Control Group (n = 22) |
|
| Age, mean (SD), y | 30.7 (10.4) | 30.9 (10.6) |
| Sex | ||
| Male | 15 (68) | 15 (68) |
| Female | 7 (32) | 7 (32) |
| Current smoker | 7 (32) | 11 (50) |
| Handedness | ||
| Right | 20 (91) | 17 (77) |
| Left | 2 (9) | 5 (23) |
| CAARS score, mean (SD)a | ||
| Inattentiveness | 18.8 (5.2) | 0.1 (0.4) |
| Hyperactivity/impulsivity | 19.6 (5.6) | 0.2 (0.6) |
| Past psychopharmacologic treatmentb | NA | |
| Stimulants | 4 (18) | |
| SNRIs | 2 (9) | |
| Stimulants and antidepressants | 1 (4) | |
| Past comorbidities | NA | |
| Depression, currently in remission | 7 (32) | |
| Drug abuse | 2 (9) | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CAARS, Conners Adult ADHD Rating Scale; NA, not applicable; SNRIs, selective norepinephrine reuptake inhibitors.
Differences between the patients with ADHD and the control participants were significant (P < .001).
The patients had received no psychopharmacologic drugs for at least 6 months before the investigation.
Medical Examination and Clinical Exploration
Participants underwent standard medical examination including general physical and neurologic status evaluation, electrocardiography, and routine laboratory tests at the screening and final visits to ensure their physical health. Women underwent a urine pregnancy test at the screening visit and before PET measurement. A multidrug urine test was performed at the screening visit to exclude current substance abuse. Participants were interviewed by experienced psychiatrists using the Conners Adult ADHD Diagnostic Interview for DSM-IV37 to evaluate current and childhood attentional and hyperactivity/impulsivity symptoms and confirm the ADHD diagnosis. The Conners Adult ADHD Rating Scale (CAARS)–Investigator Screening Version (Table 1) was applied to assess the presence and severity of inattentive and hyperactivity/impulsivity symptoms, and third-party–reported and self-reported symptoms were determined with the CAARS-Observer Screening Version and the CAARS-Self-Report Screening Version. The Structured Clinical Interview for DSM-IV Axis I and Axis II disorders was performed to exclude comorbid psychiatric disorders. Handedness was evaluated with the Edinburgh Inventory,38,39 and IQ was determined with the Viennese Matrices Test–2.40 Patients with ADHD did not differ significantly from the controls in IQ (ADHD, 92.86 [15.22]; controls, 98.77 [12.89]; P = .16; paired, 2-tailed t test). Participants were subdivided into groups best describing their smoking status according to the quantity of consumption, which was assessed in an open-question format (nonsmokers, 5 cigarettes/wk, 5 cigarettes/d, 5-10 cigarettes/d, 10 cigarettes/d, 10-15 cigarettes/d, 15 cigarettes/d, and 20 cigarettes/d; ranks were 1-8, respectively). The ADHD group did not significantly differ in smoking status compared with the control group (median rank: ADHD, 0; control, 0.5; z = −0.48, P = .63, Mann-Whitney test). Individuals with PET- or magnetic resonance imaging (MRI)–incompatible implants or in pregnancy or breastfeeding were not included in this study.
Data Acquisition
All PET was carried out at the Department of Biomedical Imaging and Image-Guided Therapy, Division of Nuclear Medicine, Medical University of Vienna, using a full-ring scanner (GE Advance; General Electric Medical Systems) in a 3-dimensional acquisition mode. We applied (S,S)-[18F]FMeNER-D2, which is among the most suitable PET tracers for in vivo NET quantification41,42 as described previously43 for the following reasons: (1) fluorine F 18–labeled reboxetine analogues enable specific binding equilibrium to be reached within a reasonable time frame for PET measurement owing to their 5-fold higher half-life44; (2) in vivo defluorination can be reduced considerably, and the interpretability of regions in proximity to bone thereby increased, through the use of deuterated homologues45; and (3) (S,S)-[18F]FMeNER-D2 has shown45 both high affinity and selectivity to the NET. A 5-minute transmission scan using retractable germanium Ge 68 rod sources for tissue attenuation correction was performed before the emission scan. Data acquisition started 120 minutes after a bolus intravenous injection of 4.7 MBq/kg of body weight (ADHD, 395.1 [98.7] MBq; controls, 379.0 [62.2] MBq; P = .53, 2-tailed, paired t test) of (S,S)-[18F]FMeNER-D2. Mean (SD) specific radioactivity of (S,S)-[18F]FMeNER-D2 was 589.4 (399.7) GBq/μmoL (ADHD) and 440.4 (233.7) GBq/μmoL (controls) (P = .15, 2-tailed, paired t test). Brain radioactivity was measured in a series of 6 consecutive time frames lasting 10 minutes each in the interval of 120 to 180 minutes after administration of the bolus. Acquired data were reconstructed in volumes consisting of 35 transaxial sections (128 × 128 matrix) using an iterative filtered back-projection algorithm46 with a spatial resolution of 4.36 mm full-width at half of the maximum 1 cm next to the center of the field of view. For coregistration, MRIs were acquired from all participants on a 3-T scanner (Achieva; Philips) using a 3-dimensional T1 fast field echo–weighted sequence, yielding 0.88-mm section thickness and in-plane resolution of 0.8 × 0.8 mm.
Data Quantification
Each time frame of the dynamic PET scan was realigned to the mean of frames with no head motion, identified by visual inspection. Subsequently, each summed image (PET integral image from realigned data) was coregistered (rigid body transformation) to each participant’s MRI using a mutual information algorithm implemented in SPM8 (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/). Parametric images of nondisplaceable binding potential (BPND) values were calculated using the caudate as the reference region because it was devoid of NET.44 According to nomenclature,47 the BPND values were defined as follows:
where Ctarget indicates radioactivity concentration of the target region and Creference, radioactivity concentration of the reference region.36 Caudate ROIs were delineated on MRIs in individual-participant space using image analysis software (PMOD, version 3.1; PMOD Technologies Ltd; http://www.pmod.com), which were subsequently transferred to coregistered summed PET images. Individual MRIs were spatially normalized to the T1-weighted MRI template provided in SPM8. Resulting transformation matrices were applied to the coregistered parametric images warping them into Montreal Neurological Institute (MNI) standard space.
Regions of Interest
The ROIs selected included NET-rich regions, based on postmortem and in vivo human brain studies,36,44 and show a good signal to noise ratio and an acceptable bone spillover due to (S,S)-[18F]FMeNER-D2 defluorination.48 Binding potential values were extracted from parametric maps from either atlas-generated ROIs or manually delineated ROIs. Atlas-generated ROIs were identified from the Hammers Maximum Probability Atlas49 including 6 regions: the hippocampus, putamen, pallidum, thalamus, midbrain with pons (including the locus coeruleus), and cerebellum. Since the NET concentration in the thalamus is not homogeneous,41 13 thalamic subnuclei were generated with the Wake Forest University Pickatlas Tool (Table 2).50 To verify atlas-generated ROIs, 4 atlas ROIs were delineated on the MNI T1 single-participant brain: the midbrain (dorsally located including raphe nuclei, excluding pons), locus coeruleus located according to Keren et al,51 claustrum, and hypothalamus. In addition to the above-mentioned atlas ROIs, further ROIs, specifically the locus coeruleus and thalamus, which are brain regions highest in NET concentration,41 were delineated manually for each participant for confirmatory purposes. Atlas ROIs match the MNI standard space.
Table 2.
Norepinephrine Transporter Binding Potential by ROIa
| Characteristic | Mean (SD) | |
|---|---|---|
| ADHD Group (n = 22) |
Control Group (n = 22) |
|
| Hammers Maximum Probability Atlas ROIs | ||
| Thalamus | 0.36 (0.08) | 0.37 (0.10) |
| Hippocampus | 0.12 (0.06) | 0.11 (0.06) |
| Midbrain with pons | 0.25 (0.11) | 0.26 (0.11) |
| Putamen | 0.18 (0.06) | 0.18 (0.05) |
| Pallidum | 0.23 (0.06) | 0.22 (0.06) |
| Cerebellum | 0.15 (0.10) | 0.16 (0.08) |
| MNI T1 single-participant brain-delineated ROIs | ||
| Midbrain without pons | 0.50 (0.12) | 0.46 (0.14) |
| Locus coeruleus | 0.41 (0.12) | 0.39 (0.13) |
| Claustrum | 0.18 (0.06) | 0.18 (0.05) |
| Hypothalamus | 0.29 (0.11) | 0.28 (0.10) |
| Manually delineated individual ROIs | ||
| Thalamus | 0.31 (0.13) | 0.50 (0.12) |
| Locus coeruleus | 0.35 (0.14) | 0.47 (0.10) |
| Thalamic subnuclei ROIs delineated with WFU Pickatlas Tool | ||
| Lateral | ||
| Dorsal nucleus | 0.16 (0.20) | 0.23 (0.17) |
| Geniculum body | 0.34 (0.13) | 0.31 (0.12) |
| Posterior nucleus | 0.37 (0.11) | 0.40 (0.12) |
| Mammillary body | 0.59 (0.14) | 0.55 (0.16) |
| Medial | ||
| Dorsal nucleus | 0.51 (0.41) | 0.53 (0.15) |
| Geniculum body | 0.52 (0.18) | 0.47 (0.16) |
| Midline nucleus | 0.06 (0.21) | 0.13 (0.17) |
| Pulvinar | 0.32 (0.13) | 0.33 (0.13) |
| Subthalamic nucleus | 0.40 (0.14) | 0.36 (0.12) |
| Ventral | ||
| Anterior nucleus | 0.12 (0.12) | 0.16 (0.12) |
| Lateral nucleus | 0.37 (0.10) | 0.39 (0.09) |
| Posterior lateral nucleus | 0.60 (0.15) | 0.59 (0.13) |
| Posterior medial nucleus | 0.75 (0.14) | 0.73 (0.16) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; MNI, Montreal Neurological Institute; ROI, region of interest; WFU, Wake Forest University.
No significant differences could be detected in the norepinephrine transporter nondisplaceable binding potential between the groups.
Statistical Analysis
Data were analyzed using linear mixed models for the outcome measure NET BPND with the ROI as a repeated factor; participant groups, sex, and ROI as fixed factors; and participants and matched participant pairs as random factors. A separate model was calculated for the 6 ROIs based on the Hammers Maximum Probability Atlas and for the 13 thalamic subnuclei. Likewise, manually delineated ROIs were assessed in 2 additional models: one using the 4 atlas-based ROIs and the other using the 2 individual-based ROIs. Fixed effects were included in the model in a multifactorial approach, whereas interaction effects were dropped in instances of nonsignificance. In cases of significant interactions or main effects, post hoc pairwise comparisons were computed and Bonferroni correction was performed for multiple comparisons. In a second exploratory approach to examine the effects of handedness, smoking status, and age, a mixed model was calculated using a stepwise procedure with backward elimination, starting with all candidate variables (including participant groups and ROIs) and followed by a stepwise deletion of interactions and variables with the largest P values. Finally, mixed-models analyses were also applied to investigate the effects of the clinical variables inattentiveness and hyperactivity/impulsivity, which were assessed with the CAARS-Investigator Screening Version. According to the Akaike information criterion,52 repeated measurements were modeled using a compound symmetric covariance structure. As an exploratory analysis, we also compared NET BPND between patients and controls at the voxel level using SPM8 (paired t test); SPSS, version 19.0 for Windows (SPSS Inc), was used for statistical computations. The 2-tailed significance level was set at P = .05. Region of interest and voxel-wise analysis results were corrected for multiple comparisons using Bonferroni and false discovery rate analysis, respectively.
Results
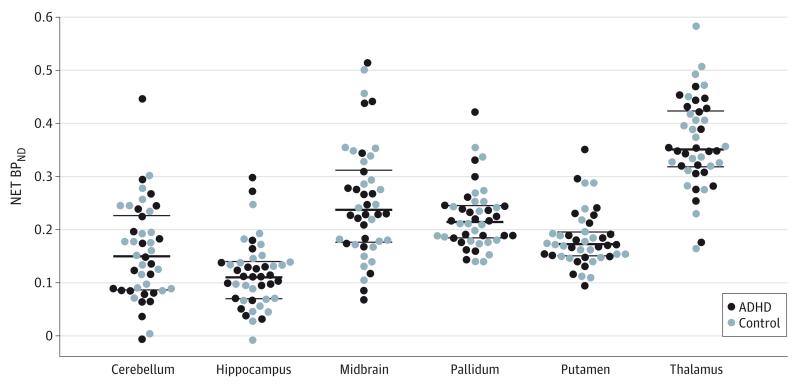
Linear mixed-models analysis revealed an expected main effect of ROI (F5,215 = 117.71; P < .001) but no main effects of participant group (F1,41 <0.01; P = .96) (Table 2 and Figure 1) or sex (F1,41<0.01; P = .98) and no interaction effects (all P > .10). Post hoc pairwise comparisons revealed significant NET BPND differences between the 6 tested brain regions (atlas-generated ROIs; P < .05, corrected) except for the comparisons of midbrain with pallidum and putamen with cerebellum, which had similar binding values (Table 2 and Figure 2). Analogous results were obtained from the 2 mixed models for the manually delineated ROIs, which showed main effects of ROI but no main effects of group and sex and no interaction effects. Similarly, the linear mixed model for NET binding within the thalamic subnuclei revealed a main effect of ROI (F12,516 = 105.53; P < .001) but no main effect of group (F1,41 = 0.08; P = .78) or sex (F1,41 = 0.39; P = .54) and no interaction effects (all P > .10). In addition, there was no significant difference in NET binding between patients with ADHD and the controls in any brain region at the voxel level (all P > .05, corrected).
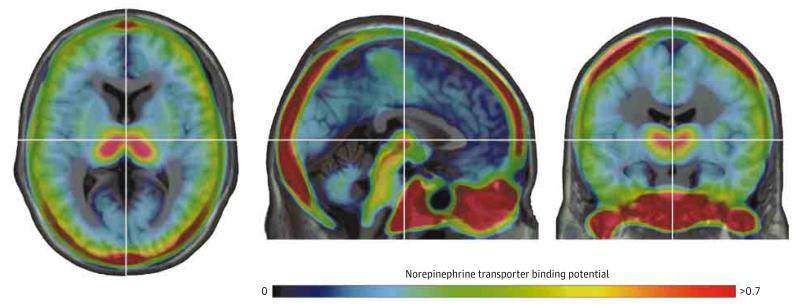
Figure 1. Mean (S,S)-[18F]FMeNER-D2 Distribution Normalized to the Montreal Neurological Institute T1 Template in 22 Healthy Control Participants.
High norepinephrine transporter nondisplaceable binding potential (NET BPND) was found in the thalamus and midbrain regions of interest, and the lowest was observed in the basal ganglia. The highest NET uptake occurred in bones, a phenomenon associated with tracer-specific defluorination. The color bar represents the BP at each voxel, with blue indicating the lowest and red the highest NET BPND (a unitless measure). The crosshair is set on the thalamus. (S,S)-[18F]FMeNER-D2 indicates (S,S)-2-(α-(2-[18F]fluoro[2H2]methoxyphenoxy)benzyl)morpholine.
Figure 2. Norepinephrine Transporter Nondisplaceable Binding Potential (NET BPND) in Selected Regions of Interest.
There were no significant differences between the ADHD and control groups in NET BPND (a unitless measure) in patients with attention-deficit/hyperactivity disorder (ADHD) and healthy control participants. The heavy rule within the scatterplots indicates the mean; thin rules, SD.
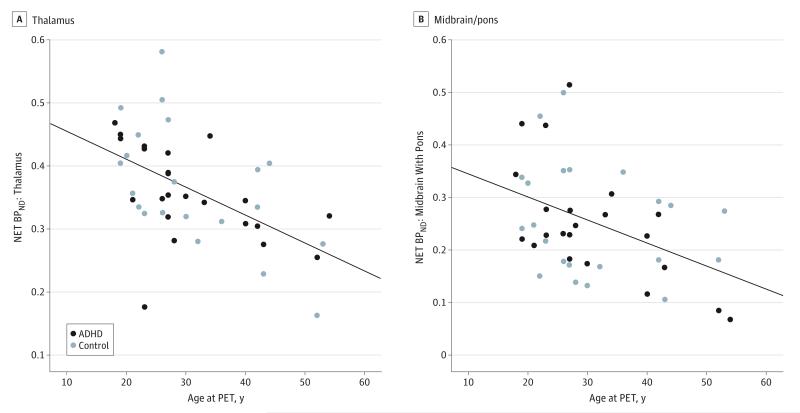
When investigating the potential effects of handedness, smoking status, and age, mixed-models analysis for ROI NET BPND based on the Hammers Maximum Probability Atlas revealed an interaction effect between ROI and age (F5,190 = 9.94; P < .001) in addition to a main effect of ROI but no main effect of age. Post hoc correlation analyses between regional NET BPND and age revealed strong negative correlations in the thalamus (R2 = 0.29; P < .01 corrected) and midbrain (R2 = 0.18 P < .01 corrected) (Figure 3), but these correlations did not differ significantly between the control and ADHD groups. Handedness and smoking status had no effect on NET BPND, nor did they lead to any significant interactions. Comparable results were observed for manually delineated ROIs, which showed strong negative correlations between NET BPND and age in the midbrain (R2 = 0.28; P < .01 corrected), locus coeruleus (R2 = 0.26; P < .01 corrected), and hypothalamus (R2 = 0.26; P < .01 corrected). In addition, no main or interaction effects were observed for clinical variables (CAARS-Inattentiveness and CAARS-Hyperactivity/Impulsivity) and ROI BPND. Finally, exclusion of 3 patients with previous methylphenidate intake in childhood (intake duration was 4, 5, and 7 years) and 2 patients with previous atomoxetine consumption in adulthood (intake duration was 5 and 6 months) did not change NET binding results. We further excluded 2 patients exhibiting predominantly inattentive symptoms and 1 exhibiting predominantly hyperactivity/impulsivity symptoms and, in a separate analysis, 2 patients with past drug abuse. Exclusion of these participants did not change the results.
Figure 3. Negative Correlation of Norepinephrine Transporter Nondisplaceable Binding Potential (NET BPND) and Age in the Thalamus and Midbrain/Pons.
A significant negative correlation existed between the NET BPND (a unitless measure) and age in the thalamus (R2 = 0.29; P < .01 corrected) (A) and midbrain/pons (R2 = 0.18; P < .01 corrected) (B). Regions of interest were extracted from Hammers Maximum Probability Atlas. The significance level was set at P < .05 and the results were Bonferroni corrected for multiple comparisons. ADHD indicates attention-deficit/hyperactivity disorder; PET, positron emission tomography. Please note the different NET BPND ranges on the y-axis.
Discussion
To our knowledge, this is the first PET study to investigate the differences in brain NET distribution and availability in adults with ADHD. We found no significant differences in the BPND of (S,S)-[18F]FMeNER-D2 between the patients with ADHD and the controls. Furthermore, exclusion of patients exhibiting either predominantly inattentive or predominantly hyperactivity/impulsivity subtypes and patients with previous ADHD pharmacotherapy or past drug abuse did not change the results. Our findings validate previous studies53 showing an age-related decrease in brain NET availability in the healthy human brain and show an age-related decrease in brain NET availability in adults with ADHD.
Randomized placebo-controlled studies54-56 have repeatedly shown that methylphenidate, amphetamine, and atom-oxetine significantly decrease symptoms in adult ADHD patient cohorts. The clinical efficacy of a pharmaceutical agent implies that the mechanism of action through which it attains a response is relevant to the neurobiology and resulting symptoms of a particular disease. Therefore, modulation of the noradrenergic system by these 3 drugs suggests noradrenergic abnormalities in ADHD.
Executive functions, such as response inhibition, vigilance, working memory, and planning, are typically impaired in ADHD.57,58 The association of these functions with the prefrontal cortex, which exhibits pronounced noradrenergic innervation, once again implicates, more generally, the noradrenergic system in ADHD.59
However, investigations into the involvement of other neurotransmitter systems in ADHD are similarly inconclusive. First, current data available on the dopaminergic contribution to ADHD are wrought with inconsistency. As is the case with the NET, therapeutic doses of methylphenidate have been shown60,61 using PET to reduce radiotracer striatal dopamine transporter binding in a dose-dependent manner in healthy individuals. Methylphenidate-induced dopamine transporter blockade has been causally linked to an increase in striatal extracellular dopamine in the human brain,14 and this effect has been associated with therapeutic responses to methylphenidate in ADHD.62 Moreover, striatal dopamine transporter availability in patients with ADHD was correlated with improvement of clinical symptoms after methylphenidate treatment.63 Brain imaging studies,31,63-65 however, have reported an array of partially contradictory results ranging from dopamine transporter increases to a lack of change66 to decreases29,67 in the brain of adults with ADHD. Although methodologic factors (eg, tracer choice) and patient characteristics (including the presence of prior medication, comorbidities, and differing sample sizes) have been suggested29,30 to account for this variability in results, investigations of other components of the dopaminergic system, such as the D2 and D3 receptors, are similarly inconsistent.29,32 In addition, serotonergic alterations have been discussed in the context of ADHD68 and are primarily based on the relationship between serotonergic innervation and impulsivity and hyperactivity, which are 2 core ADHD symptoms.69 However, serotonergic involvement in ADHD is contradicted by data showing the limited clinical efficacy of selective serotonin reuptake inhibitors in the improvement of ADHD symptoms. Furthermore, serotonin transporter imaging studies67,70 showed no difference in serotonin transporter distribution between patients with ADHD and healthy controls. Therefore, although existing evidence neither affirms nor disproves the neurotransmitter systems discussed above to be involved in ADHD, background pharmacologic evidence supporting, in particular, dopaminergic and noradrenergic contribution, is strong. It was recently suggested by del Campo et al32 that ADHD-related dopaminergic changes may reflect associated symptoms rather than a disease-specific endophenotype. Therefore, approaches that step away from the concept of endophenotypical noradrenergic changes in ADHD and focus on changes associated with ADHD symptoms may prove to be valuable. However, exclusion of patients exhibiting the predominantly inattentive subtype and predominantly hyperactivity/impulsivity subtype of ADHD did not change our main findings, strongly suggesting that our results reflect a lack of changes in the brain NET level in ADHD in general rather than a subtype-specific phenomenon. In this context, future studies may profit from incorporating cognitive tests and genetic data into analysis for further symptom-oriented and phenotypical classification of participants.
Despite the well-established link between modulation of the NET and improvement of ADHD symptoms, supported by recent genetic studies71 implicating the NET gene in ADHD, our study did not reveal differences in NET distribution between patients with ADHD and the controls. Atomoxetine, methylphenidate, and amphetamine modulation of the NET has yet to be investigated in individuals with ADHD. Therefore, one cannot exclude the possibility that pharmacologic mechanisms of stimulants and nonstimulants in patients with ADHD differ from those in healthy individuals, as has been proposed to be the case by some investigators,72 although not by others.73 However, the results of the present study may also be interpreted to suggest that, despite the proposed involvement in the efficacy of ADHD pharmaceuticals, the NET may not be integral to ADHD. Nevertheless, the missing difference in the NET between groups would not necessarily exclude the involvement of other components of the noradrenergic system in ADHD. In fact, guanfacine hydrochloride, an α2 adrenoceptor agonist and novel ADHD treatment option, appears to be a good treatment alternative to stimulant and nonstimulant medications.74 Although this finding does not necessarily imply that α2 adrenoceptors are integral to ADHD, it again underlines the link between noradrenergic innervation and ADHD symptoms while proposing that ADHD symptoms may also be modulated by other noradrenergic elements.
However, several characteristics attributed to the transporter limit PET investigations into the role of the NET in ADHD and therefore must be considered. First, although cortical and subcortical regions express NET, the levels of expression are generally considered to be low,36,75,76 particularly in frontal cortical regions. Therefore, comparability between participant groups is limited in these areas. Second, evaluation of NET levels in lateral cortical regions, including frontal regions, is made challenging by skull-bound radioactivity, which spills into adjacent regions and has been associated with (S,S)-[18F]FMeNER-D2.45,48 Therefore, owing to generally low frontal cortex NET levels, together with image contamination as a result of spillover from bone uptake, NET levels in lateral frontal cortical regions cannot be evaluated with (S,S)-[18F]FMeNER-D2. Thus, we cannot exclude the possibility of NET differences between patients with ADHD and healthy controls in these cortical regions.
Neuroanatomic traits intrinsic to the noradrenergic system further limit interpretability of the present study’s results. Partial volume effects resulting from the small size of the locus coeruleus together with current standards of PET spatial resolution may result in an underestimation of NET levels within this region.36 Accordingly, autoradiography studies44 have shown locus coeruleus NET values to be 10 times higher than those of other cortical and subcortical regions, including the thalamus. However, our findings confirm those of PET studies36,41 applying (S,S)-[18F]FMeNER-D2, showing only slight differences between the locus coeruleus and thalamus. These method-dependent differences speak for distortion of locus coeruleus values through partial volume effects. In addition, we cannot exclude the possibility that similar effects may influence NET values measured in the small thalamic subnuclei evaluated.
Conclusions
The lack of differences observed in NET distribution between patients with ADHD and control participants does not exclude noradrenergic abnormalities in ADHD, since only one molecular aspect and not all regional aspects of the noradrenergic system were investigated. To further clarify NET involvement in ADHD, cortical brain regions must be investigated and occupancy studies must be carried out to solidify the relationship between pharmacologically induced clinical improvement and noradrenergic changes.
Acknowledgments
Funding/Support: This research was supported by grant P22981 from the Austrian Science Fund (FWF) (Dr Lanzenberger).
Role of the Funder/Sponsor: The Austrian Science Fund (FWF) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
Dr Lanzenberger had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vanicek, Kranz, Kutzelnigg, Mitterhauser, Volkow, Kasper, Lanzenberger.
Acquisition, analysis, or interpretation of data: Vanicek, Spies, Rami-Mark, Savli, Höflich, Kranz, Hahn, Kutzelnigg, Traub-Weidinger, Wadsak, Hacker, Lanzenberger.
Drafting of the manuscript: Vanicek, Spies, Savli, Traub-Weidinger, Volkow, Lanzenberger.
Critical revision of the manuscript for important intellectual content: Vanicek, Spies, Rami-Mark, Savli, Höflich, Kranz, Hahn, Kutzelnigg, Mitterhauser, Wadsak, Hacker, Kasper, Lanzenberger.
Statistical analysis: Savli, Kranz.
Obtained funding: Kranz, Kutzelnigg, Volkow, Lanzenberger.
Administrative, technical, or material support: Vanicek, Spies, Rami-Mark, Höflich, Hahn, Kutzelnigg, Traub-Weidinger, Mitterhauser, Wadsak, Hacker, Kasper, Lanzenberger.
Study supervision: Kutzelnigg, Mitterhauser, Wadsak, Volkow, Kasper, Lanzenberger.
Conflict of Interest Disclosures
Without any relevance to this work, Dr Vanicek has received a travel grant from Eli Lilly and Company and Sanova and compensation for workshop participation by Eli Lilly and Company. Dr Spies has received travel grants from AOP Orphan Pharmaceuticals and Eli Lilly and Company and compensation for workshop participation from Eli Lilly and Company. Dr Kranz has received travel grants from AOP Orphan Pharmaceuticals and Roche. Dr Kutzelnigg has received travel grants from Affiris AG, AstraZeneca, Eli Lilly and Company, and Novartis Pharmaceuticals; payment for lectures, including service on the speakers' bureaus of Affiris AG, AstraZeneca, Eli Lilly and Company, and Novartis Pharmaceuticals Corp; and has served as a consultant and as a member of the advisory boards for the Austrian Federal Ministry of Health, Biogen-Idec, Eli Lilly and Company, and Medice Arzneimittel Pütter GmbH. Dr Wadsak has received research support from ABX, Advion, Iason GmbH, Raytest Austria GmbH, and Rotem GmbH and has served as a consultant/trainer for Bayer and THP Pharma. Dr Hacker has received conference speaker honoraria from Covidian, Endocyte, GE Healthcare, and IBA and consults for the advisory board of Endocyte. Dr Kasper has received grant/research support from the Austrian National Bank, Bristol-Myers Squibb, Dr Willmar Schwabe GmbH & Co KG, Eli Lilly and Company, Fonds für wissenschaftliche Förderung, GlaxoSmithKline, Lundbeck A/S, Organon, Servier, and Sunovion Pharmaceuticals; has served as a consultant for or on the advisory boards of AOP Orphan Pharmaceuticals, AstraZeneca, Austrian National Bank, Austrian Sick Foundation, Bristol-Myers Squibb, Eli Lilly and Company, German Research Foundation (Deutsche Forschungsgemeinschaft), Generali Insurance Company, GlaxoSmithKline, Janssen, Lundbeck A/S, Novartis, Organon, Pfizer, and Sepracor; and has served on speakers’ bureaus for AOP Orphan Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Janssen, Lundbeck A/S, Neuraxpharm, Servier, and Sunovion Pharmaceuticals. Dr Lanzenberger has received travel grants and conference speaker honoraria from AstraZeneca, Lundbeck A/S, and Roche Austria GmbH. No other disclosures were reported.
Additional Contributions
Medical support was provided by Mara Stamenkovic, MD (Department of Psychiatry and Psychotherapy, Medical University of Vienna), Claudia Klier, MD (Department of Child and Adolescence Medicine, Medical University of Vienna), Brigitte Hackenberg, MD (Department of Child and Adolescence Medicine, Medical University of Vienna), Anastasios Konstantinidis, MD (Department of Psychiatry and Psychotherapy, Medical University of Vienna), Pia Baldinger, MD (Department of Psychiatry and Psychotherapy, Medical University of Vienna), Diana Meshkat, MD (Department of Psychiatry and Psychotherapy, Medical University of Vienna), Jan Losak, MD (Department of Psychiatry and Psychotherapy, Medical University of Vienna), and Ralf Gößler, MD (Department of Child and Adolescence Psychiatry, Neurological Centre Rosenhügel, Vienna, Austria). Technical support was provided by the PET team, especially Georgios Karanikas, MD, Lucas Nics, MSc, PhD, Daniela Häusler, MSc, PhD, and Cecile Philippe, MSc, PhD (Department of Biomedical Imaging and Image-guided Therapy, Division of Nuclear Medicine, Medical University of Vienna). Administrative support was provided by Gregor Gryglewski, Marian Cotten, Jakob Unterholzner, and Mathis Godber Godbersen (Department of Psychiatry and Psychotherapy, Medical University of Vienna). These individuals received no financial compensation.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Health Disorders. 5th ed American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- 2.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 3.Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, Katusic SK. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637–644. doi: 10.1542/peds.2012-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG. Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA Pediatr. 2013;167(3):282–288. doi: 10.1001/2013.jamapediatrics.401. [DOI] [PubMed] [Google Scholar]
- 5.Goldman LS, Genel M, Bezman RJ, Slanetz PJ, Council on Scientific Affairs, American Medical Association Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA. 1998;279(14):1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 6.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155(4):493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 9.Carboni E, Silvagni A, Vacca C, Di Chiara G. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem. 2006;96(2):473–481. doi: 10.1111/j.1471-4159.2005.03556.x. [DOI] [PubMed] [Google Scholar]
- 10.Castells X, Ramos-Quiroga JA, Rigau D, et al. Efficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysis. CNS Drugs. 2011;25(2):157–169. doi: 10.2165/11539440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain SR, Hampshire A, Müller U, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(7):550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain SR, Robbins TW, Winder-Rhodes S, et al. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiatry. 2011;69(12):1192–1203. doi: 10.1016/j.biopsych.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2005;178(2-3):286–295. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145–e157. doi: 10.1016/j.biopsych.2011.02.036. doi:10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Berridge CW, Devilbiss DM, Andrzejewski ME, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Hannestad J, Gallezot JD, Planeta-Wilson B, et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68(9):854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentini V, Frau R, Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem. 2004;88(4):917–927. doi: 10.1046/j.1471-4159.2003.02238.x. [DOI] [PubMed] [Google Scholar]
- 20.Logan J, Wang GJ, Telang F, et al. Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nucl Med Biol. 2007;34(6):667–679. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Urben S, Baumann P, Barcellona S, et al. Cognitive efficacy of quetiapine in early-onset first-episode psychosis: a 12-week open label trial. Psychiatr Q. 2012;83(3):311–324. doi: 10.1007/s11126-011-9201-3. [DOI] [PubMed] [Google Scholar]
- 22.Nyberg S, Jucaite A, Takano A, et al. Norepinephrine transporter occupancy in the human brain after oral administration of quetiapine XR. Int J Neuropsychopharmacol. 2013;16(10):2235–2244. doi: 10.1017/S1461145713000680. [DOI] [PubMed] [Google Scholar]
- 23.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 24.Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35(1):278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 27.Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J, SPD503 STUDY GROUP Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 28.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, Wang GJ, Newcorn J, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34(3):1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Spencer TJ, Biederman J, Madras BK, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Campo N, Fryer TD, Hong YT, et al. A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: implications for ADHD and its treatment. Brain. 2013;136(pt 11):3252–3270. doi: 10.1093/brain/awt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry. 2005;57(3):229–238. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Newcorn J, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 35.Cherkasova MV, Faridi N, Casey KF, et al. Amphetamine-induced dopamine release and neurocognitive function in treatment-naive adults with ADHD. Neuropsychopharmacology. 2014;39(6):1498–1507. doi: 10.1038/npp.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arakawa R, Okumura M, Ito H, et al. Quantitative analysis of norepinephrine transporter in the human brain using PET with (S,S)-18F-FMeNER-D2. J Nucl Med. 2008;49(8):1270–1276. doi: 10.2967/jnumed.108.051292. [DOI] [PubMed] [Google Scholar]
- 37.Conners CK. Clinical use of rating scales in diagnosis and treatment of attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 1999;46(5):857–870. doi: 10.1016/s0031-3955(05)70159-0. [DOI] [PubMed] [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 39.Salmaso D, Longoni AM. Problems in the assessment of hand preference. Cortex. 1985;21(4):533–549. doi: 10.1016/s0010-9452(58)80003-9. [DOI] [PubMed] [Google Scholar]
- 40.Formann AK, Waldherr K, Piswanger K, editors. Wiener Matrizen–Test 2 Manual. Beltz Test GmbH; Göttingen, Germany: 2011. [Google Scholar]
- 41.Takano A, Varrone A, Gulyás B, Karlsson P, Tauscher J, Halldin C. Mapping of the norepinephrine transporter in the human brain using PET with (S,S)-[18F]FMeNER-D2. Neuroimage. 2008;42(2):474–482. doi: 10.1016/j.neuroimage.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 42.Seneca N, Gulyás B, Varrone A, et al. Atomoxetine occupies the norepinephrine transporter in a dose-dependent fashion: a PET study in nonhuman primate brain using (S,S)-[18F]FMeNER-D2. Psychopharmacology (Berl) 2006;188(1):119–127. doi: 10.1007/s00213-006-0483-3. [DOI] [PubMed] [Google Scholar]
- 43.Rami-Mark C, Zhang MR, Mitterhauser M, Lanzenberger R, Hacker M, Wadsak W. [18F]FMeNER-D2: reliable fully-automated synthesis for visualization of the norepinephrine transporter. Nucl Med Biol. 2013;40(8):1049–1054. doi: 10.1016/j.nucmedbio.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schou M, Halldin C, Pike VW, et al. Post-mortem human brain autoradiography of the norepinephrine transporter using (S,S)-[18F]FMeNER-D2. Eur Neuropsychopharmacol. 2005;15(5):517–520. doi: 10.1016/j.euroneuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Schou M, Halldin C, Sóvágó J, et al. PET evaluation of novel radiofluorinated reboxetine analogs as norepinephrine transporter probes in the monkey brain. Synapse. 2004;53(2):57–67. doi: 10.1002/syn.20031. [DOI] [PubMed] [Google Scholar]
- 46.Defrise M, Kinahan PE, Townsend DW, Michel C, Sibomana M, Newport DF. Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging. 1997;16(2):145–158. doi: 10.1109/42.563660. [DOI] [PubMed] [Google Scholar]
- 47.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 48.Takano A, Gulyás B, Varrone A, et al. Imaging the norepinephrine transporter with positron emission tomography: initial human studies with (S,S)-[18F]FMeNER-D2. Eur J Nucl Med Mol Imaging. 2008;35(1):153–157. doi: 10.1007/s00259-007-0598-8. [DOI] [PubMed] [Google Scholar]
- 49.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas–based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 51.Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47(4):1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akaike H. A new look at the statistical model identification: system identification and time-series analysis. IEEE Trans Automat Contr. 1974;AC-19:716–723. [Google Scholar]
- 53.Ding Y-S, Singhal T, Planeta-Wilson B, et al. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[11C]O-methylreboxetine and HRRT. Synapse. 2010;64(1):30–38. doi: 10.1002/syn.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkow ND, Swanson JM. Adult attention deficit-hyperactivity disorder. N Engl J Med. 2013;369(20):1935–1944. doi: 10.1056/NEJMcp1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004;24(1):24–29. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- 56.Adler LA, Spencer T, Brown TE, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29(1):44–50. doi: 10.1097/JCP.0b013e318192e4a0. [DOI] [PubMed] [Google Scholar]
- 57.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114(2):216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 59.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Wang GJ, Fowler JS, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43(3):181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 62.Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dresel S, Krause J, Krause KH, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518–1524. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- 64.Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354(9196):2132–2133. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- 65.Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285(2):107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- 66.van Dyck CH, Quinlan DM, Cretella LM, et al. Unaltered dopamine transporter availability in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2002;159(2):309–312. doi: 10.1176/appi.ajp.159.2.309. [DOI] [PubMed] [Google Scholar]
- 67.Hesse S, Ballaschke O, Barthel H, Sabri O. Dopamine transporter imaging in adult patients with attention-deficit/hyperactivity disorder. Psychiatry Res. 2009;171(2):120–128. doi: 10.1016/j.pscychresns.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Kranz GS, Mitterhauser M, Kutzelnigg A, et al. Reduced serotonin transporter binding in adult ADHD investigated by PET and [11C]DASB: 26th European College of Neuropsychopharmacology (ECNP) Congress, 5-9 October 2013, Barcelona, Spain, European. Neuropsychopharmacology. 2013;23(2):590–591. [Google Scholar]
- 69.Castellanos FX, Elia J, Kruesi MJ, et al. Cerebrospinal fluid monoamine metabolites in boys with attention-deficit hyperactivity disorder. Psychiatry Res. 1994;52(3):305–316. doi: 10.1016/0165-1781(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 70.Karlsson L, Tuominen L, Huotarinen A, et al. Serotonin transporter in attention-deficit hyperactivity disorder—preliminary results from a positron emission tomography study. Psychiatry Res. 2013;212(2):164–165. doi: 10.1016/j.pscychresns.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Thakur GA, Sengupta SM, Grizenko N, Choudhry Z, Joober R. Comprehensive phenotype/genotype analyses of the norepinephrine transporter gene (SLC6A2) in ADHD: relation to maternal smoking during pregnancy. PLoS One. 2012;7(11):e49616. doi: 10.1371/journal.pone.0049616. doi:10.1371/journal.pone.0049616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Millard WJ, Standish LJ. The paradoxical effect of central nervous system stimulants on hyperactivity: a paradox unexplained by the rate-dependent effect. J Nerv Ment Dis. 1982;170(8):499–501. doi: 10.1097/00005053-198208000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Rapoport JL, Inoff-Germain G. Responses to methylphenidate in attention-deficit/hyperactivity disorder and normal children: update 2002. J Atten Disord. 2002;6(suppl 1):S57–S60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- 74.Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53(2):153–173. doi: 10.1016/j.jaac.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138(2):703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 76.Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of [3H]desmethylimipramine binding in the human brain postmortem. Brain Res. 1988;456(1):120–126. doi: 10.1016/0006-8993(88)90353-8. [DOI] [PubMed] [Google Scholar]