Abstract
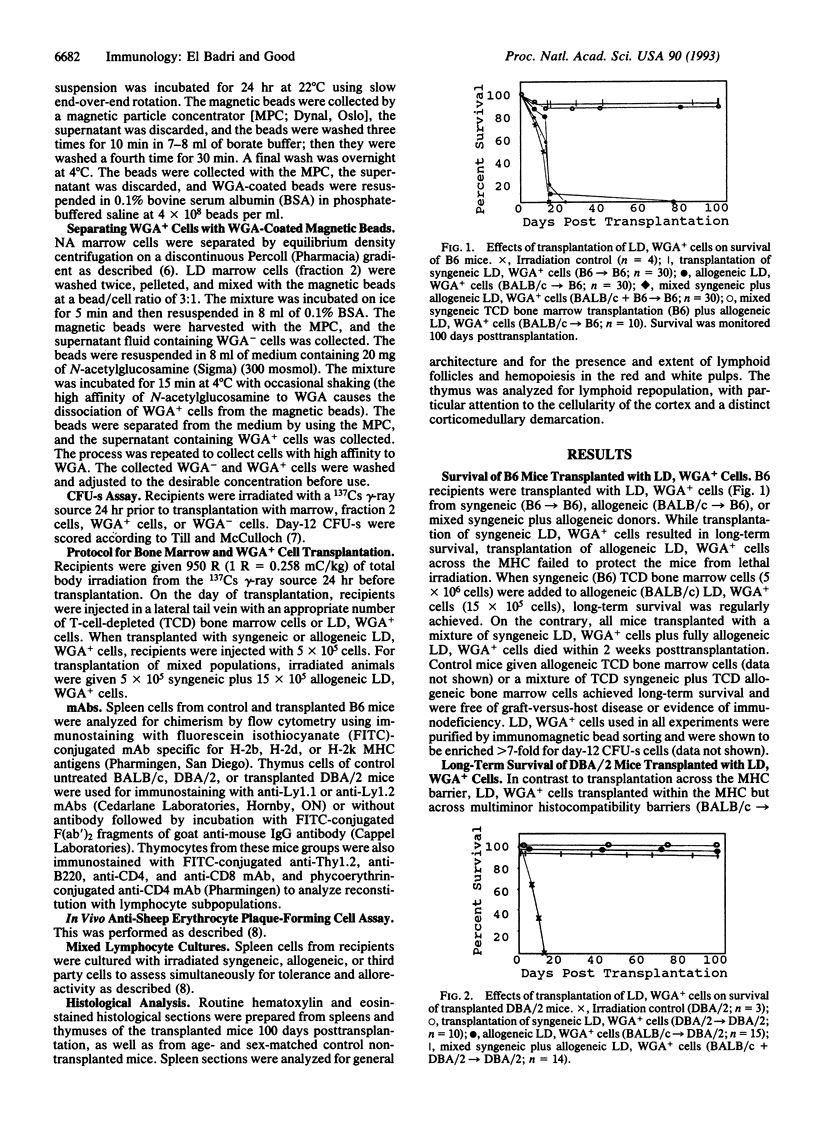
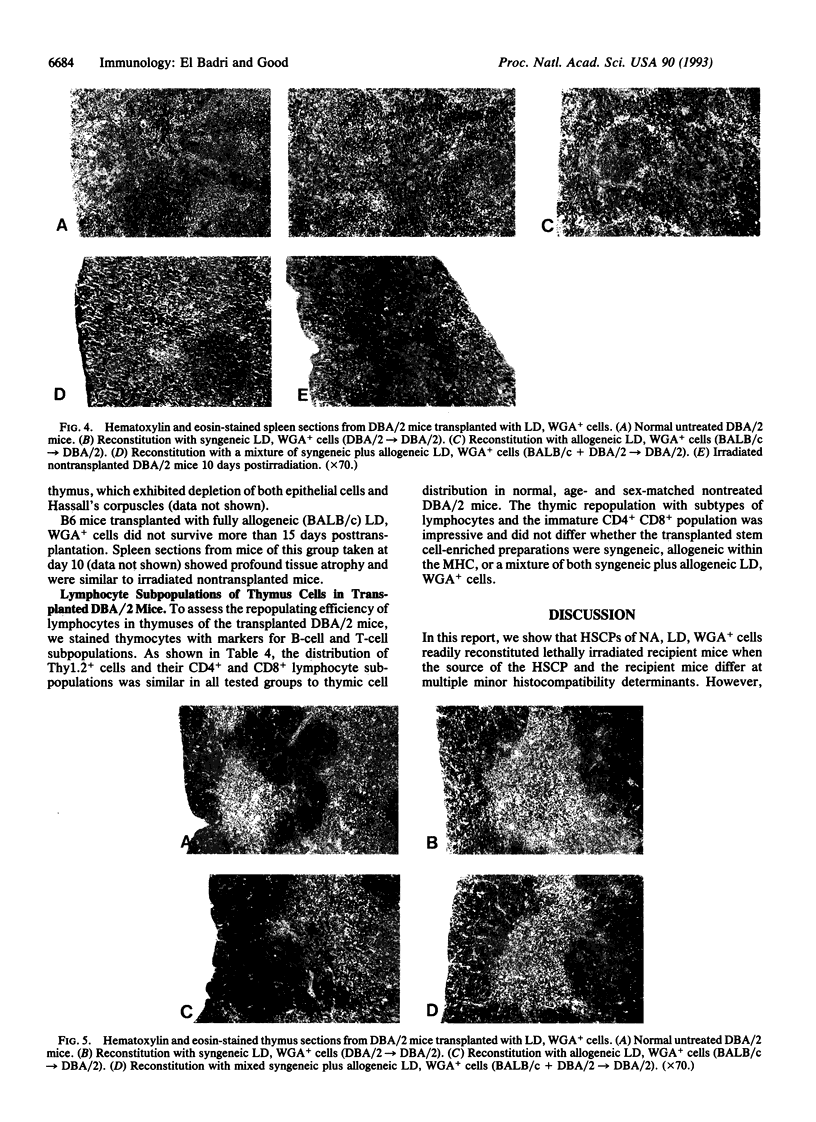
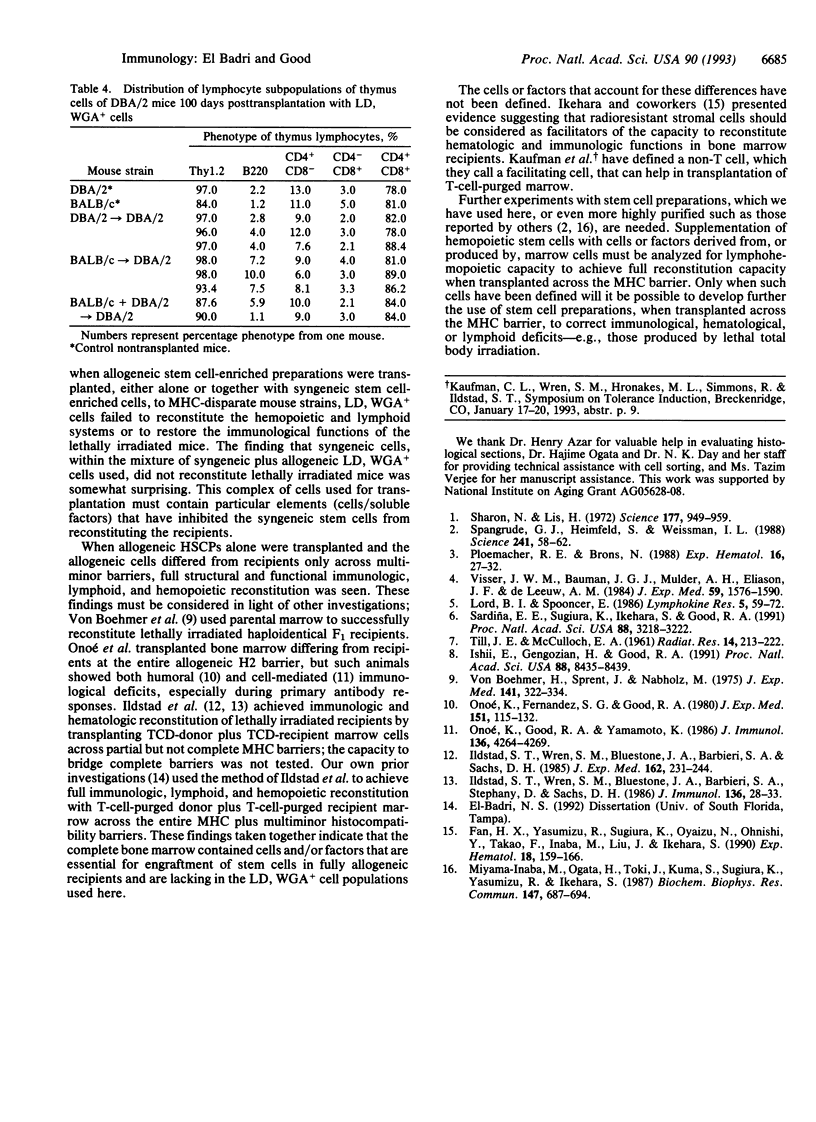
Nonadherent (NA), low density (LD), wheat germ agglutinin-positive (WGA+) murine hemopoietic stem cell-enriched preparations (HSCPs) were tested for the capability to reconstitute lymphohemopoietic elements in lethally irradiated mice. HSCPs from BALB/c mice reconstituted lethally irradiated, major histocompatibility complex (MHC)-matched DBA/2 mice to normal histology of the thymus and spleen and normal humoral and cellular immune functions. By contrast, lethally irradiated B6 mice could not be reconstituted after transplantation with NA, LD, WGA+ cells from MHC-mismatched BALB/c mice. We previously observed frequent survival, stable chimerism, and normally vigorous functioning immune systems in B6 mice transplanted with T-cell-depleted bone marrow from both BALB/c and B6 donors. To extend these findings to a stem cell transplantation system, lethally irradiated B6 mice were transplanted with NA, LD, WGA+ cells from both BALB/c and B6 mice. These mixed stem cell-enriched preparations did not reconstitute the lethally irradiated, MHC-mismatched mice. By contrast, such HSCPs from BALB/c plus DBA/2 into DBA/2 mice reconstituted the hematologic and lymphoid tissues and functional immune systems when the donor and the recipient pairs were matched at MHC and mismatched at multiminor histocompatibility barriers. These purified blood progenitors thus appear to lack certain cells/factors essential for engraftment and reconstituting recipients in a fully allogeneic environment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehmer H., Sprent J., Nabholz M. Tolerance to histocompatibility determinants in tetraparental bone marrow chimeras. J Exp Med. 1975 Feb 1;141(2):322–334. doi: 10.1084/jem.141.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. X., Yasumizu R., Sugiura K., Oyaizu N., Ohnishi Y., Takao F., Inaba M., Liu J., Ikehara S. Histogenesis of hemopoietic bone marrow in adult mice. Exp Hematol. 1990 Mar;18(3):159–166. [PubMed] [Google Scholar]
- Ildstad S. T., Wren S. M., Bluestone J. A., Barbieri S. A., Sachs D. H. Characterization of mixed allogeneic chimeras. Immunocompetence, in vitro reactivity, and genetic specificity of tolerance. J Exp Med. 1985 Jul 1;162(1):231–244. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildstad S. T., Wren S. M., Bluestone J. A., Barbieri S. A., Stephany D., Sachs D. H. Effect of selective T cell depletion of host and/or donor bone marrow on lymphopoietic repopulation, tolerance, and graft-vs-host disease in mixed allogeneic chimeras (B10 + B10.D2----B10). J Immunol. 1986 Jan;136(1):28–33. [PubMed] [Google Scholar]
- Ishii E., Gengozian N., Good R. A. Influence of dimethyl myleran on tolerance induction and immune function in major histocompatibility complex-haploidentical murine bone-marrow transplantation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8435–8439. doi: 10.1073/pnas.88.19.8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord B. I., Spooncer E. Isolation of haemopoietic spleen colony forming cells. Lymphokine Res. 1986 Winter;5(1):59–72. [PubMed] [Google Scholar]
- Miyama-Inaba M., Ogata H., Toki J., Kuma S., Sugiura K., Yasumizu R., Ikehara S. Isolation of murine pluripotent hemopoietic stem cells in the Go phase. Biochem Biophys Res Commun. 1987 Sep 15;147(2):687–694. doi: 10.1016/0006-291x(87)90985-5. [DOI] [PubMed] [Google Scholar]
- Onoé K., Fernandes G., Good R. A. Humoral and cell-mediated immune responses in fully allogeneic bone marrow chimera in mice. J Exp Med. 1980 Jan 1;151(1):115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoé K., Good R. A., Yamamoto K. Anti-bacterial immunity to Listeria monocytogenes in allogeneic bone marrow chimera in mice. J Immunol. 1986 Jun 1;136(11):4264–4269. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: II. Evidence for an early precursor of day-12 CFU-S and cells associated with radioprotective ability. Exp Hematol. 1988 Jan;16(1):27–32. [PubMed] [Google Scholar]
- Sardiña E. E., Sugiura K., Ikehara S., Good R. A. Transplantation of wheat germ agglutinin-positive hematopoietic cells to prevent or induce systemic autoimmune disease. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3218–3222. doi: 10.1073/pnas.88.8.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]




