Abstract
The impact of mutations in four essential genes involved in dopamine (DA) synthesis and transport on longevity, motor behavior, and resistance to oxidative stress was monitored in Drosophila melanogaster. The fly lines used for this study were: (i) a loss of function mutation in Catecholamines up (Catsup26), which is a negative regulator of the rate limiting enzyme for DA synthesis, (ii) a mutant for the gene pale (ple2) that encodes for the rate limiting enzyme tyrosine hydroxylase (TH), (iii) a mutant for the gene Punch (PuZ22) that encodes guanosine triphosphate cyclohydrolase, required for TH activity, and (iv) a mutant in the vesicular monoamine transporter (VMATΔ14), which is required for packaging of DA as vesicles inside DA neurons. Median lifespans of ple2, PuZ22 and VMATΔ14 mutants were significantly decreased compared to Catsup26 and wild type controls that did not significantly differ between each other. Catsup26 flies survived longer when exposed to hydrogen peroxide (80 μM) or paraquat (10 mM) compared to ple2, PuZ22 or VMATΔ14 and controls. These flies also exhibited significantly higher negative geotaxis activity compared to ple2, PuZ22, VMATΔ14 and controls. All mutant flies demonstrated rhythmic circadian locomotor activity in general, albeit Catsup26 and VMATΔ14 flies had slightly weaker rhythms. Expression analysis of some key antioxidant genes revealed that glutathione S-transferase Omega-1 (GSTO1) expression was significantly up-regulated in all DA synthesis pathway mutants and especially in Catsup26 and VMATΔ14 flies at both mRNA and protein levels. Taken together, we hypothesize that DA could directly influence GSTO1 transcription and thus play a significant role in the regulation of response to oxidative stress. Additionally, perturbations in DA synthesis do not appear to have a significant impact on circadian locomotor activity rhythms per se, but do have an influence on general locomotor activity levels.
Keywords: Antioxidant, Catecholamine, Circadian, Dopamine, Drosophila, Glutathione-S-transferase, Hydrogen peroxide, Lifespan, Locomotor activity, Negative geotaxis, Oxidative stress, Paraquat, Vesicular monoamine transporter
1. Introduction
Dopamine (DA) is a catecholamine that modulates fast neurotransmission in the central nervous systems of both vertebrates and invertebrates. In insects such as the fruit fly, Drosophila melanogaster, DA has several roles in neural functions, from modulation of locomotor behaviors and arousal states, to appetitive and aversive learning and memory (Restifo and White, 1990; Barron et al., 2010; Waddell, 2013). The dopaminergic system in Drosophila is highly rhythmic, as supported by rhythmicity in responsiveness to DA agonists, and by the rhythmic transcription of the rate-limiting enzyme tyrosine hydroxylase (TH) encoded by the gene pale (ple) (Hirsh et al., 2010). pale mutants have been reported to show decreased locomotor activity (Pendleton et al., 2002). DA has been implicated in promoting arousal in Drosophila as well as promoting higher nocturnal activity in the ClkJrk clock mutant (Kumar et al., 2012; Kume et al., 2005). In rats, DA regulates the expression of the clock protein PER2 (Hood et al., 2010). However, precise links between DA synthesis levels and circadian locomotor activity behavior are unclear. It has been reported that disruptions in the circadian clock network result in increased sensitivity to stress as well as neurodegeneration (Krishnan et al., 2008; Krishnan et al., 2012; Wulff et al., 2010), whereas DA release is triggered from cells in response to diverse stressors (Abercrombie et al., 1989). In particular, oxidative stress is believed to cause neuronal degeneration, and the nigrostriatal DA system appears to be particularly sensitive (Ueda et al., 2000). This begs the question if there are reciprocal links between the two systems in their behavioral and stress responses or if they act independent of each other.
Components of DA biosynthesis are highly conserved across a divergent range of animal phyla and have been well described in mammalian and Drosophila systems (Barron et al., 2010). Therefore, investigations on the links between perturbations in DA biosynthesis and their physiological effects on behavioral and stress responsive pathways in Drosophila would likely inform its fundamental role in stress physiology in mammalian systems. DA synthesis requires closely regulated cooperation of two enzymatic pathways, and is highly sensitive to external cues. In D. melanogaster, tyrosine hydroxylase (TH), encoded by the gene pale, converts tyrosine to DA during catecholamine synthesis (Neckameyer and White, 1993). TH catalytic activity requires and is regulated by the cofactor, tetrahydrobiopterin (BH4). The enzyme GTP cyclohydrolase I (GTPCH) is the initiating and limiting component of BH4 biosynthesis and therefore also in DA production (Hsouna et al., 2007). Once catecholamines such as DA are produced, they can be transported by vesicular monoamine transporters (VMAT) from the cytoplasm into synaptic vesicles (Greer et al., 2005). Interestingly, DA is also a self-oxidizing catecholamine known to generate reactive oxygen species including H2O2, making catecholaminergic neurons extremely susceptible to higher oxidative stress and more free radical damage than other types of neurons (Graham, 1978; Hald and Lotharius, 2005). Catecholamines up (Catsup), works as a negative regulator of DA production that acts on TH and GTPCH, both of which are rate-limiting enzymes (Stathakis et al., 1999). Moreover, loss-of-function mutations in Catsup hyperactivate TH by a post-translational mechanism that also corresponds to increased catecholamine pool levels. Paradoxically, Catsup mutants are resistant to oxidative stress induced by paraquat (Chaudhuri et al., 2007) and have also been reported to cause dominant hyperactivation of both TH as well as GTPCH (Wang et al., 2011). The latter study also established that VMAT was also hyperactivated by Catsup, and the excess DA is both transported into synaptic vesicles and released into the synapse at higher rates in Catsup mutants. However, reasons behind the resistance of Catsup mutants to oxidative stress remains unclear.
Our objectives in this study were to investigate if perturbations in DA synthesis (by elevating DA pools or reducing DA synthesis) as well as DA transport could impact longevity, behavioral characteristics (circadian locomotor activity and negative geotactic response), and response to oxidative stress in D. melanogaster. Insights obtained from this investigation will contribute to a deeper understanding of DA involvement in response to oxidative stress and its link to the circadian clock network.
2. Material and Methods
2.1 Drosophila stocks and husbandry
D. melanogaster were reared on 1% agar, 6.25% cornmeal, 6.25% molasses, and 3.5% Red Star yeast at 25 °C in 12 h light:12 h dark (LD 12:12) cycles (with an average light intensity of ~2000 lux). Two different fly lines w1118 and Canton-S, which are wild type for the catecholaminergic pathway mutations employed in this study, were used as control strains. No significant differences in longevity, circadian rhythmicity, or response to oxidative stress was observed between these two control fly lines. Mutant fly lines used in this study were as follows: Catsup26/CyO (Stathakis et al., 1999), a deletion extending 600 bp into the gene from immediately upstream of the start codon produces no detectable protein and was derived from the mobilization of a 5’P-element insertion in CatsupKO5042. As Catsup mutant alleles are homozygous lethal, all experiments in this study were conducted using heterozygous strains. The Pu mutant allele employed in this study was generated in an ethylmethane sulfonate (EMS) mutagenesis screen and the genotype is dp cn PuZ22 a px sp/SM1. Genetic characteristics of this strain are reported elsewhere (Mackay et al., 1985; Reynolds and O'Donnell, 1988). The homozygous lethal ple2 is a loss-of-function allele recovered in an EMS screen and the heterozygous mutant w; ple2/TM3 Sb e was used (Neckameyer and White, 1993). For mutations in the transporter of DA, we used the VMAT loss of function mutant w; VMATΔ14/CyO, (Romero-Calderon et al., 2008). All behavioral studies were conducted on mutant heterozygotes crossed into the appropriate wild type background to eliminate balancers. Other stocks used in this study were the UAS-Catsup RNAi, w; TH-GAL4 (III) and w; GAL4-elav (II). Only male flies were used in this study, since female flies have altered physiological status because of the reproductive development.
2.2 Lifespan measurements
For lifespan measurement, 3 cohorts of 80 mated male flies of each genotype (n=240) were housed in 8 oz round bottom polypropylene bottles (Genesee Scientific, San Diego, CA, USA) inverted over 60 mm Falcon Primaria tissue culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ, USA) containing 15 mL of diet. Diet dishes were replaced daily without CO2, after tapping flies to the bottom of the bottle. Mortality was recorded daily. Median survival of flies was calculated using the Kaplan-Meier survival curve.
2.3 Survival in response to oxidative stress
To test the resistance of the fly lines with increased or decreased DA pools, or with impaired transporter function (along with their wild type controls), adult male flies (6-8 days old) were exposed to 80 μM hydrogen peroxide (Krishnan et al., 2008) or 10 mM paraquat in 5% sucrose (Lawal et al., 2010). Untreated controls were exposed to 5% sucrose. In all instances, flies were starved for 6 h before being transferred to vials containing a 22 mm filter paper disks soaked with 80 μM H2O2 (HP) or with 10 mM paraquat (PQ, methyl viologen, Sigma Chemical Co, St. Louis, MO) in a 5% sucrose solution. The number of dead flies was scored every 18 h, 24 h, and the experiment was terminated 72 h post exposure. The choice of scoring time was determined by the time of initiation of experiment as well as convenience of access to the incubators housed in the laboratory. HP or PQ was replenished once daily till the end of the experiment. Each vial contained 25 flies per genotype in 3 independent replicates. The percentage of flies that survived at the time of scoring were plotted for each genotype over the course of the experiment. For subsequent experiments related to oxidative stress exposure and gene expression and protein blot analysis, only HP treatment of 4 h was used, since it has been demonstrated earlier to be a potent elicitor of oxidative stress and the exposure time is optimal without leading to mortality (Krishnan et al., 2008).
2.4 Rapid iterative negative geotaxis assay (RING)
Genotype-specific negative geotaxis was tested using the RING assay at room temperature (25 ± 1°C) (Gargano et al., 2005). Three groups of 25 male flies (6-8 days old) of each genotype were transferred into empty narrow vials that were loaded onto the RING apparatus. Following a 3 min acclimatization, the apparatus was rapped sharply 3x to initiate a negative geotaxis response. The flies’ negative geotaxis climbing movement in tubes was recorded as a movie and digital images captured 4 s after initiating the behavior were used for data analyses. Five consecutive trials were interspersed with at least a 30 s rest. Thus, genotypes involved in the first trial had time to recover before the next trial which involved other genotypes and so on. The average height climbed by all flies in each vial during the 4 s interval was calculated, and the climbing performance was averaged for three vials of a given genotype. To preclude any difference between the groups in exhaustion in the behavioral response during consecutive trials, care was taken to randomize each trial as well as analysis among the replicate vials of each genotype.
2.5 Locomotor activity analysis
Flies were entrained in LD 12:12 at 25 °C to acclimatize them to the activity monitoring tubes in the Drosophila activity monitor. Locomotor activity of 6-8 days old males was recorded for 3 d in LD 12:12, followed by 10 d in constant darkness (DD) using the Trikinetics locomotor activity monitor (Waltham, MA, USA) as described by Pfeiffenberger et al., (2010). Locomotor activity, as counted by number of infrared beam crossings by the individual flies was collected in 15 min bins and represented as actograms. For daily activity profiles, the number of beam crossings in LD cycles was averaged for the 3 days in LD for all flies of a particular genotype. For a quantitative measure of circadian rhythmicity in DD, Fast Fourier Transform (FFT) analysis was conducted using CLOCKLAB software (Actimetrics; Coulbourn Instruments, Whitehall, PA, USA). Flies with FFT values <0.04 were classified as arrhythmic, ones with values of 0.04–0.08 were classified as weakly rhythmic, whereas flies with FFT values >0.08 were considered strongly rhythmic. Flies with both weak and strong rhythms that showed a single peak in the periodogram were included in the calculation of the free-running period using the CLOCKLAB software.
2.6 Quantitative real-time polymerase chain reaction
Three independent bio-replicates of male flies (6-8 days old) were collected following 4 h exposure to HP stress from each genotype. In parallel, flies from untreated groups were also collected in a similar manner. Total RNA was extracted from whole body homogenates of flies (25) using Tri Reagent (Sigma, St. Louis, MO, USA). The samples were purified and treated with Takara Recombinant DNase I (Clontech Laboratories Inc., Mountain View, CA, USA). Synthesis of cDNA was achieved with the iScript cDNA synthesis kit (BioRad, Hercules, CA, USA). Quantitative real-time PCR (qRT-PCR) was performed on the Eppendorf realplex2 Mastercycler (Eppendorf, USA) under default thermal cycling conditions, with a dissociation curve step. Every reaction contained Power SYBR Green (Applied Biosystems), 10 ng cDNA, and 400 nM primers. Primer sequences are given in Supplemental Table T1. Data were analyzed using the 2−ΔΔCT method with mRNA levels normalized to the gene rp49. Relative mRNA amplitude was calculated with respect to untreated control wild type w1118, or wild type Canton-S flies whose expression for a particular gene was set as 1.
2.7 Western blotting
Three independent bio-replicates of 6-8 days old males of different genotypes were collected following 4 h exposure to HP stress. In parallel, flies from untreated groups were also collected in a similar manner. About 20 flies were homogenized on ice in 50 mM phosphate buffer, sonicated, and centrifuged at 10,000 g for 10 min at 4 °C. The protein content was equalized using the bicinchoninic acid method (Smith et al., 1985) to ensure equal protein loading. Samples were then separated by polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% resolving gel (Laemmli, 1970) followed by transfer onto PVDF Immobilon membranes (Millipore Billerica, MA, USA) and incubated in 1x TBST (10 mM Tris, 0.15 M NaCl, 0.1% Tween-20, pH 7.5) + 5% milk for 2 h. Then the membranes were incubated overnight at 4 °C with primary antibody 1:10,000 for MnSOD (procured from Acris Antibodies, Inc.); and 1:1000 for GST Omega 1 (kind gift from Dr. K. Kim, Seoul National University, Korea), in blocking buffer. Membranes were treated for 2 h with 1:20,000 goat anti-rabbit IRDye680 (LI-COR Biosciences, Lincoln, NE, USA). Blots were scanned using the LI-COR Odyssey Infrared Imaging System (CLx) and imaging software Image Studio v. 3.0 (LI-COR Biosciences, Lincoln, NE, USA). Blots were quantified using ImageJ, v. 1.47 (National Institutes of Health, USA, http://imagej.nih.gov/ij).
2.8 Statistical analyses
Lifespan and survival curves were plotted using Kaplan-Meier survival curves and statistical significance of curves assessed using the Log-Rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests (GraphPad Prism v 5.01; GraphPad software Inc. San Diego, CA). For statistical analysis of locomotor activity, mortality to oxidative stress (the percentage data of surviving flies at each time point were transformed prior to statistical analysis), RING assay, gene expression and western blot analyses post-quantification, one-way ANOVA with Tukey’s post-hoc tests were conducted (GraphPad Instat v 3.0).
3. Results
3.1 Lifespan analysis
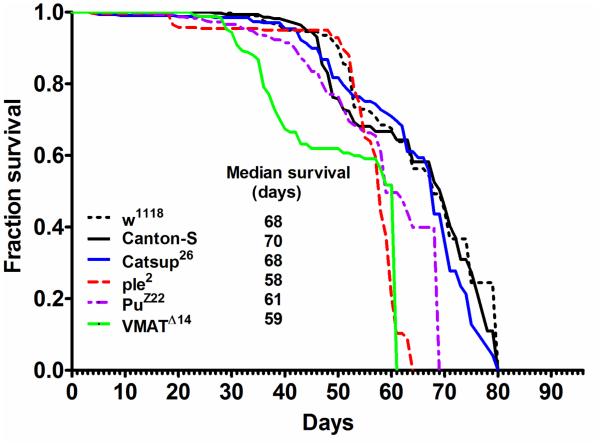
The median lifespan (MLS) of ple2, PuZ22 and VMATΔ14 mutants was significantly reduced to 58, 61 and 59 d respectively, when compared to wild type controls w1118 (68 d) and Canton-S flies (70 d) (Figure 1). Increased DA pools in Catsup26 mutants did not result in enhanced lifespan (MLS ~ 68 d) and the MLS was not significantly different from the control strains. However, this longevity was significantly greater (p<0.001) than the ple2, PuZ22 and VMATΔ14 mutants (Figure 1).
Figure 1.
Kaplan-Meier survival curves of male flies of w1118, Canton-S, Catsup26, ple2, PuZ22 and VMATΔ14 strains under 12h light: dark (LD) cycles and ad libitum feeding conditions. Data was obtained from three independent replicates of 80 flies each for each genotype (n=240).
3.2 Survival response to oxidative stress
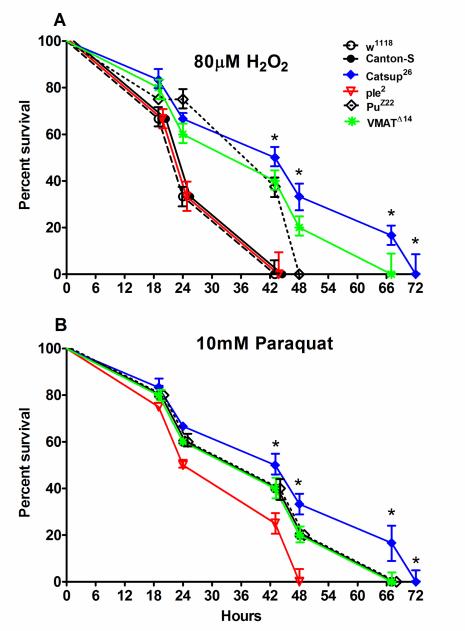
A differential survival response to oxidative stress induced by hydrogen peroxide (HP, 80 μM) or paraquat (PQ, 10 mM) was observed in the fly lines tested (Figure 2 A, B). Median survival of wild-type control flies (w1118 and Canton-S) was only 24 h when exposed to HP, which was similar to ple2 mutants (Figure 2 A). On the other hand, Catsup26 flies survived longer ~ 45.5 h upon exposure to HP which was not significantly different from PuZ22 or VMATΔ14 flies (43 h) (Figure 2 A).
Figure 2.
Mortality test of the experimental 6-8 days male flies exposed for 72 hours to (A) 80μM H2O2 (HP) or to (B) 10 mM paraquat (PQ). Mortality curves were plotted and values represent mean survival (%) ± SD at various hours post-exposure. Bars with * are significantly different at p<0.05 among genotypes.
In case of PQ exposure, median survival of control flies (w1118 and Canton-S) was 43 h which was significantly (p<0.05) more than the survival of ple2 flies (33.5 h) but not significantly different from Catsup26 (45.5 h), PuZ22, or VMATΔ14 flies (43 h) (Figure 2 B). Since HP was a more potent stressor in terms of average survival duration of control flies, all subsequent experiments were conducted following exposure to HP (see also Section 2.3 for choice of HP).
3.3 Negative geotaxis response
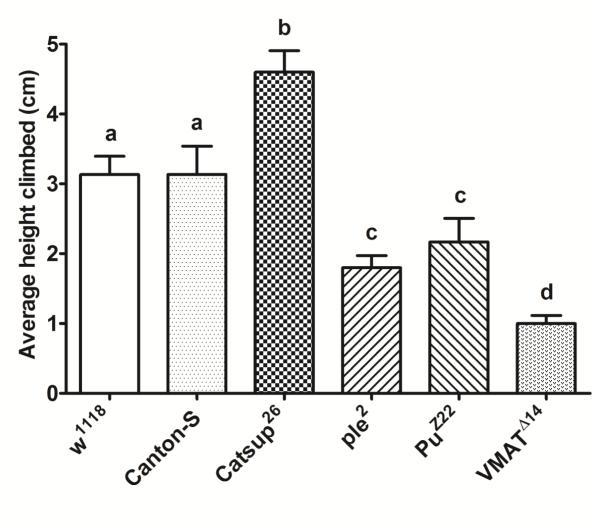
RING assay indicated that Catsup26 mutants exhibited enhanced climbing ability (p<0.001) compared to all other fly lines tested (Figure 3). No significant differences in the negative geotaxis response was recorded among control flies, which was however significantly more (p<0.001) than the climbing ability displayed by ple2, PuZ22, or VMATΔ14 mutants. ple2 and PuZ22 flies with impaired DA synthesis did not exhibit any difference in the negative geotactic response between each other, but recorded significantly less climbing ability than Catsup26 or the wild-type controls. The VMATΔ14 mutants recorded the least climbing ability (Figure 3).
Figure 3.
Rapid iterative negative geotaxis (RING) assay of experimental 6-8 days old male flies. Bar graphs represent the average height climbed by each genotype. Average values for each mutant and respective wild-type (w1118 and Canton-S) control were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Bars with different superscripts are significantly different at p<0.001.
3.4 Circadian locomotor activity rhythms
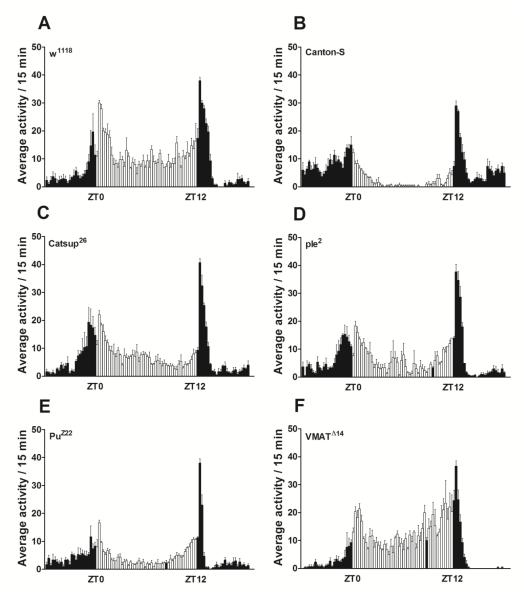
Average daily activity of the fly lines tested revealed that w1118, Catsup26 and VMATΔ14 flies showed the highest daily activity averaging ~830 horizontal beam crossings per day (Table 1, Figure 4 A, C, F). In contrast, PuZ22 flies recorded the least daily activity (415 ± 30) among all fly lines studied (Figure 4 E). ple2 mutants on the other hand showed higher average daily activity (629 ± 27) than PuZ22 flies but this was significantly less (p<0.05) than any of the other mutants or their controls. Interestingly, average daily activity of Canton-S flies was recorded to be significantly less (731 ± 43, p<0.05) than the w1118, Catsup26 or VMATΔ14 fly lines (Table 1, Figure 4 A, C, F).
Table 1.
Data representing the average daily activity per fly, the percentage of rhythmic flies, the strength of the rhythm exhibited by rhythmic flies, the period of rhythm (in constant darkness −DD) and the number of individuals of a genotype tested. Average daily activity was computed as beam crossings in 15 min bins over 3 days of LD cycles (see Materials and Methods Section 2.5 for more details). Values with different superscripts are significantly different at p<0.05 in a column (one-way ANOVA with Tukey’s multiple comparison test). Data are represented as mean ± SD.
| Genotype | Average daily activity/fly |
% Rhythmic |
FFT (strength of rhythm) |
Period of rhythm in DD (Hrs) |
Sample size (n) |
|---|---|---|---|---|---|
| w1118 | 836 ± 39a | 100 | 0.18 ± 0.02a | 23.7± 0.2 | 32 |
| Canton-S | 731 ± 43b | 100 | 0.13 ± 0.08b | 23.9 ± 0.2 | 32 |
| Catsup26 | 813 ± 29a | 70* | 0.055 ± 0.03c | 23.4 ± 0.2 | 48 |
| ple2 | 629 ± 27c | 67 | 0.12 ± 0.05b | 23.5 ± 0.2 | 48 |
| PuZ22 | 415 ± 30d | 94 | 0.16 ± 0.09a | 23.0 ± 0.1 | 48 |
| VMATΔ14 | 841 ± 43a | 75* | 0.052 ± 0.7c | 24.0 ± 0.7 | 48 |
weakly rhythmic
Figure 4.
Daily activity profiles (indicating a circadian rhythmic pattern) per 15 minutes of the experimental flies 6-8 days post-eclosion averaged over a period of 3 days. The open bars represent lights-on between 9:00 am (ZT0) and 9:00 pm (ZT12) while the filled bars represent averages of activity profiles under lights-off between 9:00 pm (ZT12) and 9:00 am (ZT0).
While control flies exhibited 100% rhythmicity in their circadian locomotor activity (Table 1), this was marginally reduced in PuZ22 mutants (94% rhythmic). Elevation of DA pools in Catsup26 flies or impaired transporter activity in VMATΔ14 flies resulted in decreased strength of rhythm (FFT~0.05) as well as a decrease in number of individuals displaying rhythmic locomotor behavior (Table 1). Interestingly PuZ22 mutants displayed strong rhythmic locomotor behavior comparable to w1118 wild type flies. There was no marked difference in the period length of rhythms in every fly line investigated.
3.5 Expression of antioxidant genes and levels of some key translated products
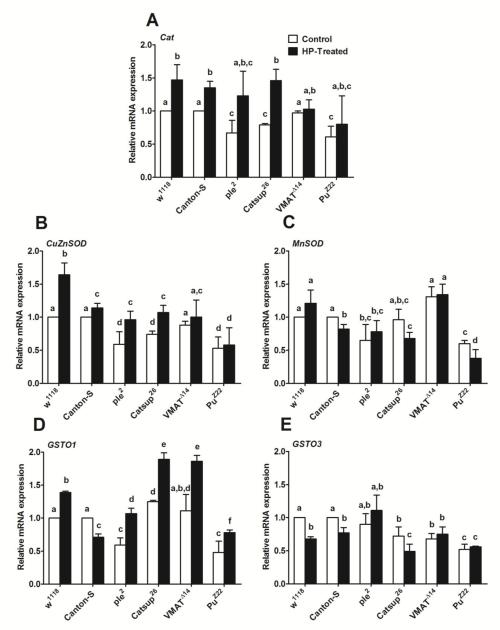
As mentioned in Section 3.2, for studying gene expression and the translated protein products of antioxidant enzymes, only HP exposure was employed, since it was a more potent stressor than PQ. A general up-regulation in Catalase (Cat) gene expression was recorded in all the fly lines upon exposure to HP (Figure 5 A). In general, Catsup26, ple2 and PuZ22 mutants showed markedly lower basal levels of Cat transcript under control unstimulated conditions compared to wild type controls as well as to the VMATΔ14 mutant. A similar pattern was observed in copper/zinc superoxide dismutase (Cu/Zn SOD) mRNA levels (Figure 5 B). mRNA levels of manganese superoxide dismutase (MnSOD) were either unchanged or decreased upon exposure to stress in all the fly lines. Interestingly, the MnSOD transcript markedly declined in PuZ22 mutants compared to all other fly lines tested upon exposure to HP (Figure 5 C). Glutathione S-transferase Omega 1 (GSTO1) transcript level was up-regulated in all DA mutant flies as well as w1118 control upon HP exposure with the exception of Canton-S where we observed a marked down-regulation of mRNA levels upon exposure to stressor (Figure 5 D). Interestingly, basal levels of GSTO1 were markedly higher in Catsup26 and VMATΔ14 compared to all other fly lines (Figure 5 D). Also, there is a significant up-regulation of GSTO1 mRNA levels in these two fly lines upon exposure to HP, which was the highest among all fly lines tested. PuZ22 showed the least amount of up-regulation of GSTO1 mRNA, which was only slightly higher than the expression observed in Canton-S. Glutathione S-transferase Omega 3 (GSTO3) transcript levels were either down-regulated or remained unchanged following exposure to oxidative stress by HP in all mutants and wild-type controls used in this study (Figure 5 E).
Figure 5.
Quantitative-Reverse Transcription PCR of some genes encoding key antioxidant enzymes: (A) Catalase, (B) CuZnSOD, (C) MnSOD, (D) GSTO1 and (E) GSTO3 after 4 hours exposure to 80 μM H2O2 (HP-treated). Relative mRNA expression of different fly lines was quantified using w1118 or Canton-S control lines (without H2O2 exposure-control) which was set as reference (= 1). Values represent mean ± SD of three independent biological replicates. One-way ANOVA with Tukey’s post-hoc test was conducted to separate out the means. Values with different superscripts are significantly different at p<0.05.
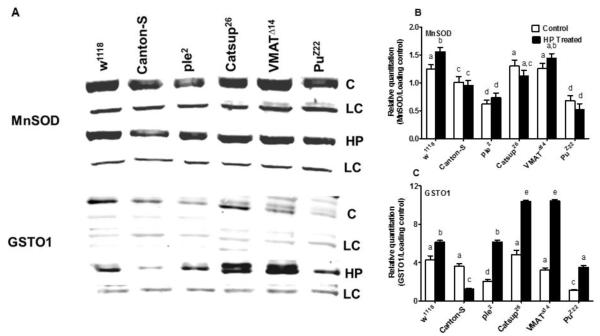
An investigation of the translated protein for MnSOD and GSTO1 (Figure 6) revealed a similar pattern as exhibited by gene expression (see Figure 5C and 5D). In case of MnSOD, only w1118 flies revealed a marked increase in protein levels upon exposure to HP. In all other fly lines tested, there was no significant difference in MnSOD between unexposed and exposed flies. However, we did detect differences in levels of MnSOD among the genotypes tested (Figure 6 A). In case of GSTO1, ple2 and PuZ22 flies showed significantly lower basal levels of GSTO1 compared to all other fly lines. Only Catsup26 showed higher basal levels of GSTO1 which was significantly up-regulated upon exposure to HP, followed by VMATΔ14 flies (Figure 6). While in general all genotypes revealed an increase of GSTO1 upon HP exposure, Canton-S flies were an exception and showed a marked decrease in GSTO1 levels following exposure to HP (Figure 6 B). This was exactly the pattern that was revealed in gene expression (Figure 5D).
Figure 6.
Western blots (A) and their quantifications (B, C) for two key proteins likely to be involved in response to OS in the experimental fly lines. Blots were performed for MnSOD, and for GSTO1, both with (HP) and without (C) exposure to hydrogen peroxide. LC represents the loading control, a non-specific protein band indicative of equal protein loading in both cases. Figures are representative of one of three independent blots. Blots were quantified by mean grey scale value relative to LC and bars are represented as mean ± SD of three independent blots. One-way ANOVA with Tukey’s post-hoc test was conducted to separate out the means. Values with different superscripts are significantly different at p<0.05.
4. Discussion
This study demonstrates that perturbations in DA synthesis or transport have a negative impact on the lifespan of fruit flies. However, elevation of DA pools as in the Catsup26 mutant does not appear to impact longevity, which is similar to the lifespan of wild type flies. DA has been shown to be a marker of neuronal senescence in Drosophila, since its levels decrease with increasing age, accompanied by deficits in dopaminergic modulated behaviors in aging flies (Neckameyer et al., 2000). It has been shown earlier that variation in the biosynthesis of biogenic amines such as DA could be a factor contributing to natural variation in lifespan of Drosophila (De Luca et al., 2003). The concept that lifespan is a function of the capacity to withstand extrinsic stress is well known. In agreement with this concept, one would expect that long-lived individuals are often empowered with an increased resistance against a variety of stresses throughout life. Therefore, genes that underlie stress response may have the ability to affect lifespan. The finding that Catsup26 mutants are more resistant to stress in general (Chaudhuri et al., 2007) would lead to the inference that these mutants should have significantly extended lifespan compared to control flies. However, this was not the case as observed in this study. It is plausible that stress resistance genes triggered by higher DA pools only get activated in response to certain stress situations and determine survival responses. Thus, the efficiency of stress responses will determine lifespan only under adverse circumstances. This is precisely what we observed in flies with DA synthesis or trafficking perturbed, in their response to oxidative stress. Catsup26 mutants were more resistant to both HP stress as well as PQ stress in general. Interestingly, it was pointed out in a previous study that Catsup26 mutants are resistant only to PQ stress and not to HP (Chaudhuri et al., 2007). In fact, Punch mutants were reported as more resistant to HP stress and less sensitive to PQ. We believe that this discrepancy in results could be due to differences in HP and PQ dose used in the present study. In fact, PuZ22 mutants showed lesser mortality at 24 h post exposure to HP compared to Catsup26 flies but eventually Catsup26 mutants survived the longest upon exposure to this stressor. The levels of DA are high in the heads of Catsup26 mutants whereas they are significantly depleted in ple2 and PuZ22 mutants, on the other hand BH4 levels are also elevated in Catsup mutants and depleted in PuZ22 flies but not in ple2 flies (Chaudhuri et al., 2007). In the case of VMATΔ14 flies ~65% reduction in DA levels is reported compared to controls (Simon et al., 2009). It is possible that the variation in the levels of resistance to oxidative stress that we observe in these flies is in part contributed by the variation in levels of DA pools. It was later demonstrated that Catsup mutants have elevated TH (3-fold) and GTPCH (7-fold) activity along with enhanced synaptic activity such that VMAT activity is enhanced with Catsup loss-of-function mutations (Wang et al., 2011).
The enhanced negative geotactic response in Catsup26 flies as demonstrated by the RING assay indicates that DA pools generated in excess are synaptically active. Other studies on polymorphisms in Catsup gene locus have also shown an association with locomotion (Carbone et al., 2006) as well as modulation of sleep (Harbison et al., 2009). Interestingly, in bees it was shown that exogenous injection of DA decreased walking behavior while increasing other behaviors (Mustard et al., 2010). This relationship between DA and mobility led us to investigate whether it is such elevated or depleted DA pools, or impaired synaptic trafficking, that leads to overt changes in locomotory rhythms. While we observe that average daily activity of flies with depleted DA levels was significantly decreased compared to Catsup26 mutants or wild type controls, yet, impairing DA’s trafficking (in VMATΔ14) did not result in any change in average daily activity compared to w1118 or Catsup26 flies. This implies that there are obviously other transporters of DA at work, such as the Drosophila dopamine transporter, encoded by the dDAT gene, which takes up DA from the extracellular space. It has been demonstrated that fumin (fmn) mutants with a mutation in dDAT gene, have abnormally high activity levels coupled with reduced rest (sleep) (Kume et al., 2005). dDAT transporters are expressed almost exclusively in the dopaminergic neurons (Porzgen et al., 2001) and function in the presynaptic membrane. They re-uptake the released DA, thereby diminishing DA signaling (Makos et al., 2009). Thus, a mutation in this transporter results in augmentation of DA signaling and causes an increased ratio in the duration of the active state, which is the enhanced negative geotaxis phenotype. Interestingly, the power-law property of the distribution of rest bouts is unchanged in these mutants though DA modulates the rest period length (Ueno et al., 2012). VMAT regulates cytosolic DA levels and impaired trafficking has a negative effect on the negative geotactic response but intriguingly has an opposite effect in the horizontal mobility response. It has been demonstrated previously that the dVMATP1 homozygous mutant have an impaired negative geotactic response but this response is potentiated in the heterozygote such that they demonstrate an enhanced escape response compared to their controls (Simon et al., 2009). The observed deviation from this result in our studies could be the effect of the specific mutation in this transporter (Romero-Calderon et al., 2008).
Flies with either elevated DA pools (Catsup26) or depleted DA levels (ple2 or PuZ22) or with impaired DA trafficking (VMATΔ14) were all rhythmic in their locomotor activity behavior (Figure S1). However, strength of the rhythms was affected in case of Catsup26 flies as well as in the flies with mutated VMAT, such that these flies were only “weakly” rhythmic. This is consistent with the idea that elevated DA pools would lead to enhanced arousal response (less sleep) (Kumar et al., 2012; Kume et al., 2005) and have an impact on the locomotor activity rhythms. On the other hand, ple2 and PuZ22 flies showed substantially increased rhythm strength comparable to wild type controls, despite depleted levels of DA. This could be due to the fact that these mutations in the heterozygous state would be still capable of producing sufficient critical levels of DA required to sustain robust circadian locomotor activity behavior. Interestingly, transgenic flies with Catsup gene impaired in all nervous tissues (elav-Gal4/UAS-Catsup-RNAi) resulted only in 7% rhythmic (weak, FFT 0.019 ± 0.01) flies (Figure S2). On the other hand, their average daily activity was 874 ± 57, marginally higher than the wide type controls. Using a Gal4 driver specific to TH (ple), we observed that RNAi of Catsup resulted in 13% rhythmic (weak, FFT 0.023 ± 0.01) flies (Figure S2) but the average daily activity plummeted to 474 ± 33. These results are in apparent agreement with the findings of Mustard et al., (2010) on honey bees. Thus, Catsup has pronounced impact on locomotor activity as well as its rhythms, when impaired throughout the nervous system. However, the neuronal and non-neuronal effects of DA is far from clear at this point and demand a more intensive investigation using the GAL4-UAS approach more extensively.
Despite being present in substantial levels in the heads, DA is equally rather more abundant in the body of Drosophila (Ream et al., 2003). This is probably due to the fact that DA is also functional in the cuticle, and immune response, in addition to its central role as a neurotransmitter. Thus, it would be reasonable to assume that mutations in Catsup, pale or Punch genes would reflect in stress tolerance signatures of the whole body. Therefore, we asked whether the primary antioxidant responsive systems such as catalase, superoxide dismutase, glutathione S-transferases would be affected by mutations that would result in enhanced or depleted DA levels or its impaired trafficking. Catalase gene expression was uniformly up-regulated in all mutant and control flies upon exposure to HP, indicating that all flies were responding to the stressor (catalase being involved in the decomposition of HP to water and oxygen). A differential response to oxidative stress was observed in the expression of Cu/Zn SOD, MnSOD, and GSTO3. Over-expression of Cu/Zn SOD has been demonstrated to protect dopaminergic neurons in Drosophila (Botella et al., 2008), however, in the present study, we did not observe any significant up-regulation of Cu/Zn SOD associated with increased tolerance to oxidative stress. While we quantified the gene expression patterns of five major antioxidant enzymes, we were able to target protein quantitation of only two antioxidative enzymes viz. MnSOD and GSTO1 since reliable antibodies were available only for them. Among the two, only glutathione S-transferase Omega 1 (GSTO1) gene expression and protein levels indicated that elevated DA pools could be triggering an up-regulation in the expression of this gene along with enhanced activity. In general, GSTs are evolutionarily conserved enzymes that are important in the detoxification of many xenobiotic compounds. These enzymes catalyze the conjugation of GSH to electrophile substrates, producing compounds that are generally less reactive and more soluble. This facilitates their removal from the cell via membrane based GSH conjugation pumps. The broad substrate specificity of GSTs allows them to protect cells against a range of toxic products (Salinas and Wong, 1999). DmGSTO1 has been demonstrated to be a novel genetic suppressor of the parkin dysfunction and has a protective role in a model of Parkinson’s disease (PD) (Kim et al., 2012). Active sites of GST Omega have a unique cysteine residue that can form a disulfide bond with glutathione (GSH) and facilitate the conjugation of lipid-derived carbonyls species. Cells respond to oxidative stress by inducing the expression of genes whose products protect the cell, and one such cellular defense mechanism is the antioxidant response element (ARE), a cis-acting enhancer element that is upstream of many phase II detoxification and antioxidant systems such as GSTs (Rushmore et al., 1991; Rushmore and Pickett, 1990). 6-hydroxydopamine (6-OHDA) a hydroxylated analog of DA has been shown to activate antioxidant response elements (Jakel et al., 2005) and we hypothesize that elevated DA levels in Catsup26 mutants could be acting in a similar manner potentiating an antioxidant response through GSTO1 and conferring the observed protective effect against oxidative stress. Taken together, these results add to an evolving picture of DA regulated defense against oxidative stress. Our blots confirm the validity of our real-time qRT-PCR results indicating a novel functional role of GSTO1 in this response. In this study we have not explored the role of DA receptors such as D1-like and D2-like and its likely interaction with the insulin signaling pathway as well as the circadian clock network (Gruntenko et al., 2012; Rauschenbach et al., 2014; Yujnovsky et al., 2006). There may be many such key players in the neuroendocrine regulation of stress response. Further analysis on the precise signaling pathways triggered by DA, as well as the balance between critical and toxic levels of DA would likely yield new insights on DA homeostasis and its relevance to stress physiology.
Supplementary Material
Acknowledgements
This research was supported by the start-up funds MSU#269110-151250 from NSF, EPSCOR and the MSU Schillig special teaching grant 365082 (NK), Grant No 140/2014/P from the Grant Agency of University of South Bohemia (AB). The stay of AB at MSU was partially covered from the grant KONTAKT No. LH 14047.
Abbreviations
- BH4
Tetrahydrobiopterin
- Cat
Catalase
- Clk
Clock
- Cu/Zn SOD
Copper-zinc superoxide dismutase
- DA
Dopamine
- DD
Constant darkness
- FFT
Fast fourier transform
- GSTO1
Glutathione S-transferase Omega-1
- GTPCH
Guanosine triphosphate cyclohydrolase
- HP
Hydrogen peroxide
- MLS
Median life span
- Mn SOD
Manganese superoxide dismutase
- ple
pale
- PQ
Paraquat
- Pu
Punch
- TH
Tyrosine Hydroxylase
- VMAT
Vesicular monoamine transporter
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Barron AB, Søvik E, Cornish JL. The roles of dopamine and related compounds in reward-seeking behavior across animal phyla. Front. Behav. Neurosci. 2010;4:163. doi: 10.3389/fnbeh.2010.00163. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella JA, Bayersdorfer F, Schneuwly S. Superoxide dismutase overexpression protects dopaminergic neurons in a Drosophila model of Parkinson’s disease. Neurobiol. Dis. 2008;30:65–73. doi: 10.1016/j.nbd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Carbone MA, Jordan KW, Lyman RF, Harbison ST, Leips J, Morgan TJ, De Luca M, Awadalla P, Mackay TF. Phenotypic variation and natural selection at Catsup, a pleiotropic quantitative trait gene in Drosophila. Curr. Biol. 2006;16:912–919. doi: 10.1016/j.cub.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O'Donnell JM. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu. Rev. Pharmacol. Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- De Luca M, Roshina NV, Geiger-Thornsberry GL, Lyman RF, Pasyukova EG, Mackay TF. Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nat. Genet. 2003;34:429–433. doi: 10.1038/ng1218. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang HY, Houshyar R, Bainton RJ, Diantonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J. Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Laukhina OV, Bogomolova EV, Karpova EK, Menshanov PN, Romanova IV, Rauschenbach I.Yu. Downregulation of the dopamine D2-like receptor in corpus allatum affects juvenile hormone synthesis in Drosophila melanogaster females. J. Insect Physiol. 2012;58:348–355. doi: 10.1016/j.jinsphys.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp. Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Harbison ST, Carbone MA, Ayroles JF, Stone EA, Lyman RF, Mackay TF. Co-regulated transcriptional networks contribute to natural genetic variation in Drosophila sleep. Nat. Genet. 2009;41:371–375. doi: 10.1038/ng.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J, Riemensperger T, Coulom H, Iche M, Coupar J, Birman S. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr. Biol. 2010;20:209–214. doi: 10.1016/j.cub.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J. Neurosci. 2010;30:14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsouna A, Lawal HO, Izevbaye I, Hsu T, O'Donnell JM. Drosophila dopamine synthesis pathway genes regulate tracheal morphogenesis. Dev. Biol. 2007;308:30–43. doi: 10.1016/j.ydbio.2007.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel RJ, Kern JT, Johnson DA, Johnson JA. Induction of the protective antioxidant response element pathway by 6-hydroxydopamine in vivo and in vitro. Toxicol. Sci. 2005;87:176–186. doi: 10.1093/toxsci/kfi241. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim S-H, Kim J, Kim H, Yim J. Glutathione S-transferase omega 1 activity is sufficient to suppress neurodegeneration in a Drosophila model of Parkinson disease. J. Biol. Chem. 2012;287:6628–6641. doi: 10.1074/jbc.M111.291179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Davis AJ, Giebultowicz JM. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem Biophys Res Commun. 2008;374:299–303. doi: 10.1016/j.bbrc.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Rakshit K, Chow ES, Wentzell JS, Kretzschmar. D. Giebultowicz. J.M. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012;45:1129–1135. doi: 10.1016/j.nbd.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Chen D, Sehgal A. Dopamine acts through cryptochrome to promote acute arousal in Drosophila. Genes. Dev. 2012;26:1224–1234. doi: 10.1101/gad.186338.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawal HO, Chang H-Y, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay W, Reynolds ER, O'Donnell JM. Tissue-specific and complex complementation patterns in the Punch locus of Drosophila melanogaster. Genetics. 1985;111:885–904. doi: 10.1093/genetics/111.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos MA, Kim YC, Han KA, Heien ML, Ewing AG. In vivo electrochemical measurements of exogenously applied dopamine in Drosophila melanogaster. Anal. Chem. 2009;81:1848–1854. doi: 10.1021/ac802297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard JA, Pham PM, Smith BH. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J. Insect Physiol. 2010;56:422–430. doi: 10.1016/j.jinsphys.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, White K. Drosophila tyrosine hydroxylase is encoded by the pale locus. J. Neurogenet. 1993;8:189–199. doi: 10.3109/01677069309083448. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiol. Aging. 2000;21:145–152. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Pendleton RG, Rasheed A, Sardina T, Tully T, Hillman R. Effects of tyrosine hydroxylase mutants on locomotor activity in Drosophila: a study in functional genomics. Behav. Genet. 2002;32:89–94. doi: 10.1023/a:1015279221600. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010;11:pdb.prot5518. doi: 10.1101/pdb.prot5518. [DOI] [PubMed] [Google Scholar]
- Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol. Pharmacol. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- Rauschenbach I.Yu., Karpova EK, Adonyeva NV, Andreenkova OV, Faddeeva NV, Burdina EV, Alekseev AA, Menshanov PN, Gruntenko NE. Disruption of insulin signaling affects the neuroendocrine stress reaction in Drosophila females. J.Exp. Biol. 2014 doi: 10.1242/jeb.106815. (In Press: doi: 10.1242/?jeb.106815) [DOI] [PubMed] [Google Scholar]
- Ream PJ, Suljak SW, Ewing AG, Han K-A. Micellar electro-kinetic capillary chromatography-electrochemical detection for analysis of biogenic amines in Drosophila melanogaster. Anal. Chem. 2003;75:3972–3978. doi: 10.1021/ac034219i. [DOI] [PubMed] [Google Scholar]
- Restifo L, White K. Molecular and genetic approaches to neurotransmitter and neuromodulator systems in Drosophila. Adv. Insect Physiol. 1990;22:116–219. [Google Scholar]
- Reynolds ER, O'Donnell JM. Characterization of new Punch mutations: identification of two additional mutant classes. Genetics. 1988;119:609–617. doi: 10.1093/genetics/119.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Calderon R, Uhlenbrock G, Borycz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann. B.T. Krantz, D.E. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4:e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- Salinas AE, Wong MG. Glutathione S-Transferases - A Review. Curr. Med. Chem. 1999;6:279–309. [PubMed] [Google Scholar]
- Simon AF, Daniels R, Romero-Calderon R, Grygoruk A, Chang H-Y, Najibi R, Shamouelian D, Salazar E, Solomon M, Ackerson LC, Maidment NT, DiAntonio A, Krantz DE. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 2009;181:525–541. doi: 10.1534/genetics.108.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchonininc acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stathakis DG, Burton Y, McIvor WE, Krishnakumar S, Wright TRF, O'Donnell JM. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Aikawa M, Ishizuya-Oka A, Yamaoka S, Koibuchi N, Yoshimoto K. Age-related dopamine deficiency in the mesostriatal dopamine system of zitter mutant rats: regional fiber vulnerability in the striatum and the olfactory tubercle. Neuroscience. 2000;95:389–398. doi: 10.1016/s0306-4522(99)00451-0. [DOI] [PubMed] [Google Scholar]
- Ueno T, Masuda N, Kume S, Kume K. Dopamine modulates the rest period length without perturbation of its power law distribution in Drosophila melanogaster. PloS One. 2012;7:e32007. doi: 10.1371/journal.pone.0032007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S. Reinforcement signaling in Drosophila; dopamine does it all after all. Curr. Opin. Neurobiol. 2013;23:324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferdousy F, Lawal H, Huang Z, Daigle JG, Izevbaye I, Doherty O, Thomas J, Stathakis DG, O'Donnell JM. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J. Neurochem. 2011;119:1294–1305. doi: 10.1111/j.1471-4159.2011.07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama. Dol, M., Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc. Natl. Acad. Sci. USA. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.