Summary
mRNA is thought to predominantly reside in the cytoplasm, where it is translated and eventually degraded. Although nuclear retention of mRNA has a regulatory potential, it is considered extremely rare in mammals. Here, to explore the extent of mRNA retention in metabolic tissues, we combine deep sequencing of nuclear and cytoplasmic RNA fractions with single-molecule transcript imaging in mouse beta cells, liver, and gut. We identify a wide range of protein-coding genes for which the levels of spliced polyadenylated mRNA are higher in the nucleus than in the cytoplasm. These include genes such as the transcription factor ChREBP, Nlrp6, Glucokinase, and Glucagon receptor. We demonstrate that nuclear retention of mRNA can efficiently buffer cytoplasmic transcript levels from noise that emanates from transcriptional bursts. Our study challenges the view that transcripts predominantly reside in the cytoplasm and reveals a role of the nucleus in dampening gene expression noise.
Graphical Abstract
Highlights
-
•
Genome-wide catalog of nuclear and cytoplasmic mRNA in mouse tissues
-
•
Spliced, polyadenylated mRNA is retained in the nucleus for many protein-coding genes
-
•
Retained genes include ChREBP and liver Nlrp6, co-localized with nuclear speckles
-
•
Nuclear retention of mRNA reduces cytoplasmic gene expression noise
Bahar Halpern et al. combine whole-transcriptome and single-molecule approaches to demonstrate that a substantial fraction of genes have higher levels of spliced, polyadenylated mRNA in the nucleus compared to the cytoplasm in mammalian tissues. This nuclear retention reduces cytoplasmic gene expression noise created by transcriptional bursts.
Introduction
The life course of mRNA begins with transcription, splicing, and processing, which generally occur at the nuclear sites of transcription, and ends with cytoplasmic translation and degradation. Nuclear export of mRNA is considered a transient phase, lasting only a few minutes in mammalian cells (Oeffinger and Zenklusen, 2012, Shav-Tal et al., 2004, Vargas et al., 2005), a negligible time compared to the other phases. Recent studies applied deep sequencing of RNA from cellular fractions to identify RNA molecules that are retained in the nucleus (Bhatt et al., 2012, Djebali et al., 2012, Pandya-Jones et al., 2013, Tilgner et al., 2012). These, however, predominantly included long non-coding RNA (lncRNA), such as Xist, Malat1, and Neat1; hyper-edited mRNA (Chen and Carmichael, 2009); or incompletely spliced mRNA (Boutz et al., 2015, Shalgi et al., 2014) rather than mature protein-coding mRNA. Though rare examples exist for nuclearly retained transcripts (Prasanth et al., 2005), a global picture of mRNA nuclear retention in mammalian tissues is lacking.
Here, to explore the extent and possible roles of nuclear mRNA retention, we combined deep sequencing of RNA from nuclear and cytoplasmic fractions with single-molecule transcript imaging in intact mouse tissues. Surprisingly, we found a wide range of spliced polyadenylated protein-coding mRNA, which are nuclearly retained for the majority of their lifetime. These include Glucokinase and Glucagon receptor in beta cells; Nlrp6 in the liver; and, most strikingly, the transcription factor Mlxipl, also known as ChREBP, the transcripts of which are highly retained in nuclear speckles in liver, beta cells, and intestinal tissue. We developed a single-molecule in situ technique to quantify nuclear mRNA lifetimes and found that the transcripts of these genes can spend hours in the nucleus before being exported to the cytoplasm.
To study the potential role of nuclear retention, we analyzed its impact on fluctuations in cytoplasmic mRNA levels. Mammalian genes are transcribed in bursts (Larson et al., 2011, Darzacq et al., 2007, Suter et al., 2011, Bahar Halpern et al., 2015, Dar et al., 2012, Senecal et al., 2014), leading to temporal fluctuations in cellular mRNA levels and variability among identical cells (Blake et al., 2003, Eldar and Elowitz, 2010, Golding et al., 2005, Kaern et al., 2005, Maheshri and O’Shea, 2007, Paulsson, 2004, Raj and van Oudenaarden, 2008). We demonstrate theoretically and experimentally that nuclear retention can effectively buffer these fluctuations, facilitating lower variability in cytoplasmic mRNA.
Results
RNA Sequencing of Cell Fractions Reveals Broad Nuclear Localization of mRNA in Beta Cells and Liver
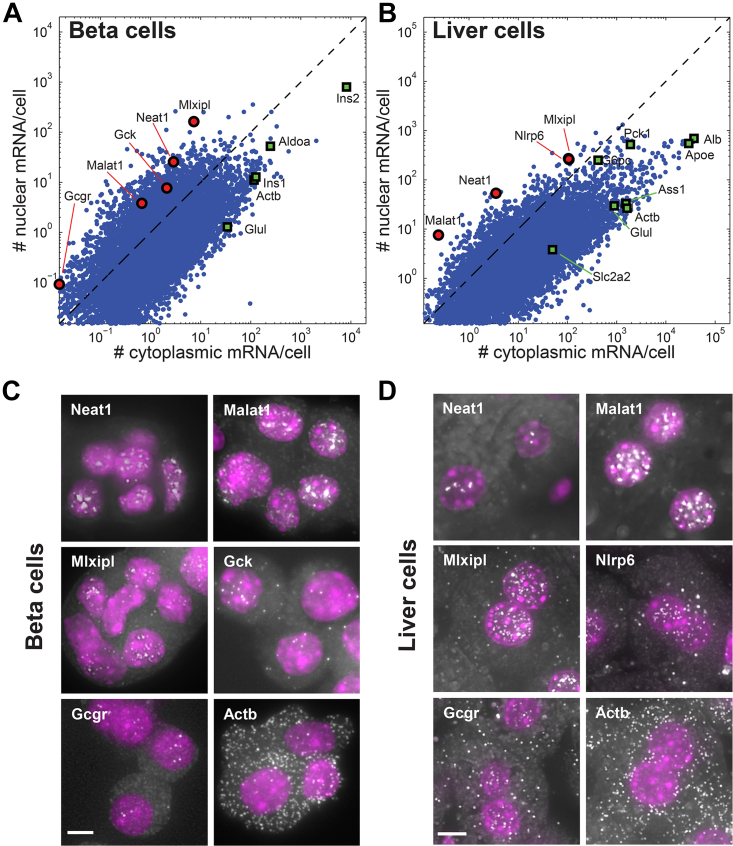
To obtain a genome-wide catalog of genes in mammalian metabolic tissues that are potentially nuclearly retained, we extracted nuclear and cytoplasmic fractions from MIN6 pancreatic beta cell line (Miyazaki et al., 1990) and mouse liver and performed whole-transcriptome RNA sequencing (RNA-seq). We used single-molecule fluorescence in situ hybridization (smFISH) (Bahar Halpern et al., 2015, Itzkovitz et al., 2011, Lyubimova et al., 2013) of representative genes to convert the number of reads to estimates of cytoplasmic and nuclear mRNA numbers per cell (Tables S1 and S2). Our analysis revealed that most genes had more transcripts in the cytoplasm compared to the nucleus in MIN6 cells (mean cytoplasm/nucleus = 3.8 ± 0.05, Figure 1A). Examples include the insulin genes Ins1 (cytoplasm/nucleus = 13.2 ± 4.6) and Ins2 (cytoplasm/nucleus = 10.2 ± 0.45), as well as housekeeping genes such as beta-actin (Actb, cytoplasm/nucleus = 10.6 ± 1.1).
Figure 1.
Deep Sequencing of Cellular Fractions Reveals Broad Nuclear Retention of mRNA
(A and B) RNA-seq of nuclear and cytoplasmic fractions of MIN6 cells (A) and mouse liver cells (B). Each dot represents a gene, x axis is the number of cytoplasmic mRNA molecules per cell, and y axis is the number of nuclear mRNA molecules. Dashed line represents the locus of genes that have equal numbers of nuclear and cytoplasmic mRNA copies. Green squares mark representative genes with higher cytoplasmic mRNA numbers, and red circles mark representative genes with higher nuclear mRNA numbers.
(C) Validation in primary pancreatic islet cells of some of the nuclear genes identified in (A) using smFISH. Images are maximum projections of 20 optical sections spaced 0.3 μm apart.
(D) Validation in intact liver frozen sections of some of the nuclear genes identified in (B) using smFISH.
Images are maximum projections of eight optical sections spaced 0.3 μm apart. Scale bars, 5 μm (C and D). See also Figures S1 and S2.
A substantial fraction (30%) of the genes in MIN6 cells, however, had equal or higher levels of mRNA in the nucleus. These genes included the lncRNAs Malat1 (cytoplasm/nucleus = 0.33 ± 0.27) and Neat1 (cytoplasm/nucleus = 0.11 ± 0.02), as well as small nucleolar RNA (snoRNA; Weinstein and Steitz, 1999; Figure S1C). Interestingly, they also included key protein-coding genes such as Glucokinase (Gck, cytoplasm/nucleus = 0.29 ± 0.12), Glucagon receptor (Gcgr, cytoplasm/nucleus = 0.53 ± 0.46), and the transcription factor Mlxipl, also known as ChREBP (cytoplasm/nucleus = 0.05 ± 0.004; Herman et al., 2012, Postic et al., 2007, Uyeda and Repa, 2006; Figure 1A).
We next performed RNA-seq of nuclear and cytoplasmic fractions of liver cells isolated from mice (Figure 1B). Here, as well, we found that the majority of genes had predominantly cytoplasmic mRNA (mean cytoplasm/nucleus = 6.5 ± 1.3). As in MIN6 cells, however, a non-negligible 13.1% of protein-coding genes had more mRNA in the nucleus compared to the cytoplasm. Notably, Mlxipl was nuclearly retained in this tissue as well (cytoplasm/nucleus = 0.38 ± 0.01; Figure 1B). Another notable nuclear gene was the inflammasome component nucleotide-binding oligomerization domain protein-like receptor 6 (Nlrp6; Anand et al., 2012, Elinav et al., 2011, Strowig et al., 2012; cytoplasm/nucleus = 0.41 ± 0.03; Figure 1B).
To validate the nuclear enrichment, we imaged individual mRNA molecules of representative genes in primary pancreatic islets and in liver frozen sections using smFISH (Figures 1C and 1D). This revealed the absolute numbers and intra-cellular localizations of the transcripts of interest, clearly demonstrating that transcripts of Gck, Gcgr, Nlrp6, and Mlxipl were indeed substantially more numerous in the nucleus compared to the cytoplasm.
We used the RBPmap tool (Paz et al., 2014) to identify several putative target sites for known RNA-binding proteins in the 3′ UTR of the most nuclearly retained genes in both liver and MIN6 (Figure S2A; Table S4). Moreover, the 3′ UTR sequences of the nuclearly retained genes exhibited common sequence motifs that were not identified in a size-controlled group of the most cytoplasmic genes (Figure S2B). While genes with higher nuclear mRNA were enriched in lncRNA and snoRNA (Figures S1B and S1C; p < 0.001), the vast majority of genes with nuclear mRNA were protein-coding genes (Figure S1A). The median intron splicing efficiency for the nuclear mRNAs was >95% (Figure S1D; Table S3), and only 20% of the nuclear liver genes we identified have been shown to have intron detention (Boutz et al., 2015). Thus, our analysis revealed that a substantial fraction of genes in liver and MIN6 cells have higher levels of spliced, polyadenlyated mRNA in the nucleus than in the cytoplasm.
Single-Molecule Transcript Imaging Reveals Increased Nuclear Retention for Key Protein-Coding Transcripts
Nuclear localization of mRNA does not necessarily imply increased nuclear lifetime, namely, low export rate of mRNA. High transcription rates combined with high cytoplasmic mRNA degradation rates can give rise to large numbers of nuclear mRNA and low numbers of cytoplasmic mRNA, even when nuclear export rate is high. To understand this effect, we considered a simple mathematical model describing the dynamics of nuclear and cytoplasmic mRNAs. In this model, nuclear mRNA is produced at rate , exported from the nucleus at rate , and degraded in the cytoplasm at rate (we considered only properly spliced mRNA for which nuclear degradation rate is negligible; Garneau et al., 2007).
| (Equation 1) |
| (Equation 2) |
Equations 1 and 2 yield the following results for the levels of nuclear and cytoplasmic mRNAs at steady state :
| (Equation 3) |
| (Equation 4) |
Equations 3 and 4 indicate that the ratio between the amount of mRNA in the nucleus and that in the cytoplasm equals the ratio of the rates of cytoplasmic degradation and nuclear export . The amount of nuclear mRNA at steady state increases with increasing transcription rates and decreases with increasing export rate . The ratio of transcription rate and total nuclear mRNA levels can thus be used to estimate nuclear export rates .
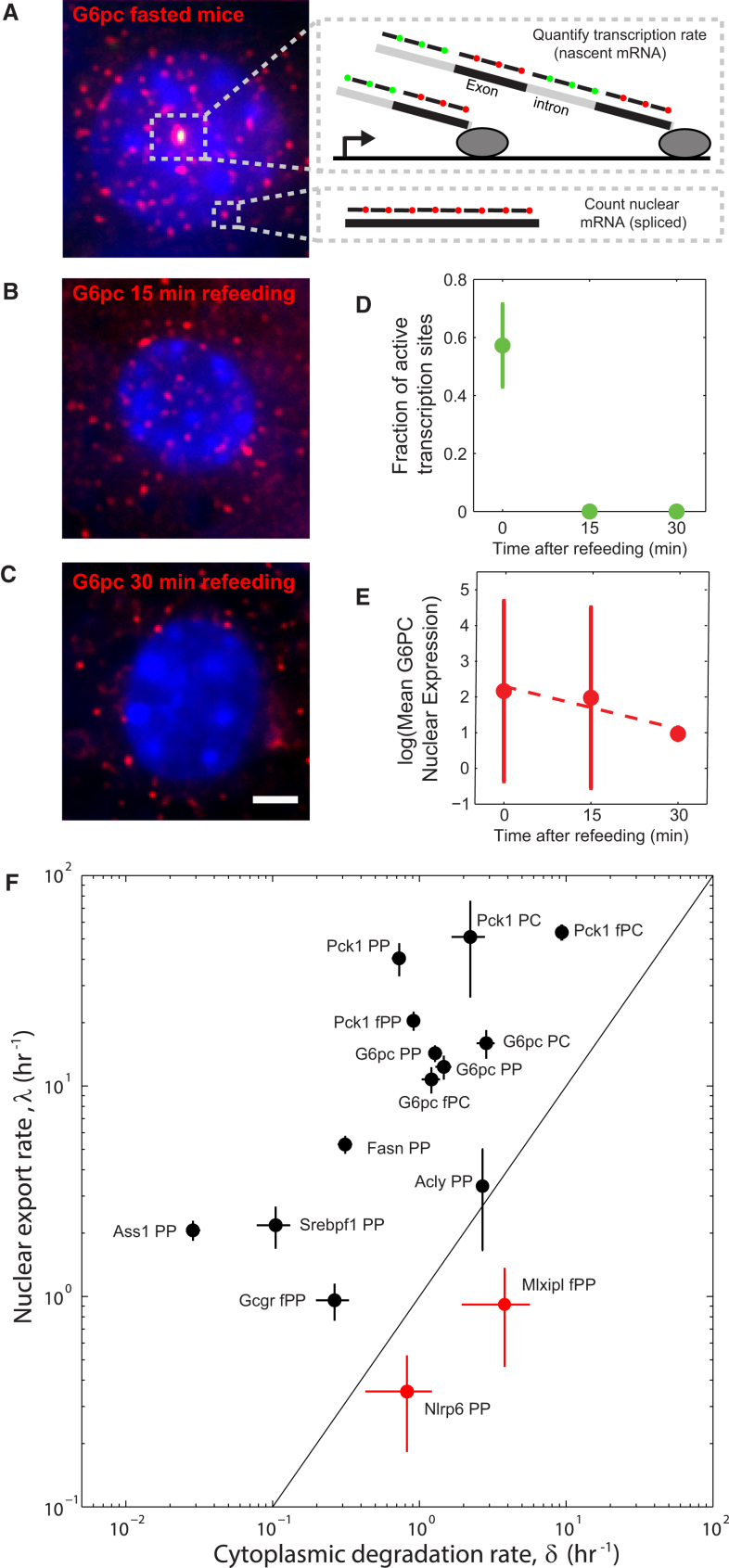
To quantify nuclear export rates in situ, we developed a method to jointly quantify transcription rates and total nuclear mRNA (Figure 2A). We designed pairs of smFISH probe libraries targeting the introns and exons of the genes of interest and coupled them to two spectrally resolvable fluorophores, enabling quantification of the transcription rates, (Figure 2A; Supplemental Experimental Procedures). We also counted the total number of mRNA molecules in the nucleus and used Equation 3 to extract the nuclear export rate . Similarly, we counted the number of cytoplasmic mRNA and used Equation 4 to extract the cytoplasmic degradation rate .
Figure 2.
Single-Molecule Approach for Measuring Nuclear Export Rate and Cytoplasmic Lifetime
(A) Example shows identification of active transcription site of G6pc in liver cryosection from a fasting mouse using dual-color labeling of introns (green) and exons (red).
(B and C) G6pc nuclear levels rapidly decline 15 (B) and 30 min (C) after refeeding of fasting mice.
Images in (A)–(C) are single optical sections. Scale bar, 2 μm.
(D) Active transcription sites disappear 15 and 30 min after refeeding, indicating a complete shutdown of transcription.
(E) Quantification of the number of nuclear transcripts of G6pc at 5 hr fasting (time 0), as well as 15 and 30 min after refeeding. Data were divided by the expression at time 0 (145 mRNA per nucleus). Nuclear mRNA declined at a rate of 5.3 ± 1.24 hr−1, compatible with the in situ estimation based on the fasting state of 4.99 ± 0.99 hr−1 (n = 2 mice per time point).
(F) Degradation and nuclear export rates of liver genes estimated from in situ measurements in intact liver tissue. Solid line represents the locus of genes with equal rates of nuclear export and cytoplasmic degradation. Nlrp6 and Mlxipl (marked in red) have significantly lower nuclear export rates. PC, pericentral; PP, periportal; f, fast. Error bars represent SEM.
To validate our estimates of nuclear export rates, we sought to measure the temporal decline in nuclear mRNA following cessation of transcription. In such cases, nuclear mRNA should exponentially decline at rate . Since actinomycin D treatment on primary hepatocytes caused extensive perturbation to cellular physiology, we reverted to measure G6pc, a gene that is highly expressed in fasting mice but rapidly shuts down upon refeeding (Bahar Halpern et al., 2015). Indeed, we observed complete shutdown of transcription upon refeeding, as evident by the lack of double-labeled intronic-exonic nuclear dots at 15 and 30 min (Figures 2B and 2D). Nuclear mRNA diluted at a rate of 5.3 ± 1.24 hr−1, consistent with our estimates of = 4.99 ± 0.99 hr−1 obtained from in situ measurements of mice at the fasting state (Figure 2E). Similarly, our estimates of degradation rates were within 15% error of the validated values (Bahar Halpern et al., 2015).
We next applied this methodology to representative liver genes (Figure 2F; Table S5). Most export rates were higher than the cytoplasmic degradation rates, and they conformed to previous estimates of nuclear lifetimes of a few minutes. Notably, however, Nlrp6 and Mlxipl had substantially longer nuclear lifetimes of 1.98 ± 0.96 hr for Nlrp6 and 0.75 ± 0.37 hr for Mlxipl. The nuclear export rates of Mlxipl and Nlrp6 were also substantially lower than their cytoplasmic degradation rates (cytoplasmic lifetimes were 0.85 ± 0.4 hr for Nlrp6 and 0.18 ± 0.09 hr for Mlxipl). For these genes, mRNA spends more time in the nucleus than in the cytoplasm.
Nuclear Localization of Mlxipl and Nlrp6 mRNA in Diverse Tissues and Metabolic Conditions
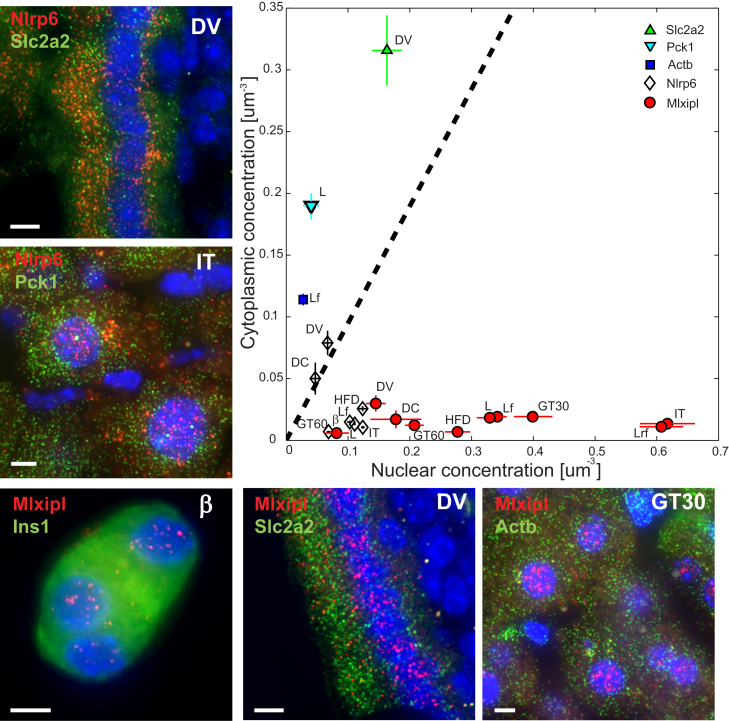
Next, we turned to characterize the patterns of nuclear mRNA localization for Mlxipl and Nlrp6, two of the most prominent nuclear genes we uncovered. Mlxipl encodes the ChREBP transcription factor, a key regulator of lipogenic and glycolytic genes in metabolic tissues (Herman et al., 2012, Postic et al., 2007, Uyeda and Repa, 2006). We found that Mlxipl mRNA was predominantly nuclear in liver, intestine, and beta cells (Figure 3), as well as in different metabolic conditions, such as after intraperitoneal (i.p.) injection of glucose or insulin and following a high-fat diet (HFD) (Figure S3A). As controls, we measured the genes Pck1 and Actb in the liver and the gene Slc2a2 (also known as Glut2) in the intestinal epithelium. Unlike Mlxipl, these genes had substantially higher mRNA concentrations in the cytoplasm compared to the nucleus (Figure 3).
Figure 3.
Mlxipl and Nlrp6 Are Highly Nuclearly Retained in Diverse Metabolic Tissues and Conditions
In contrast, Pck1, Actb, and Slc2a2 are highly enriched in the cytoplasm. Shown are the nuclear and cytoplasmic concentrations, as well as example images. DV, Duodenum, Villus; DC, Duodenum, Crypt; L, liver, ad libitum; Lf, liver, fasting; Lrf, liver, re-fed; IT, liver, insulin tolerance; GT30 (GT60), liver from mice sacrificed 30 min (60 min) after glucose injection; HFD, liver, high-fat diet; β, pancreatic beta cell. Images are maximum projections of 15–20 optical sections spaced 0.3 μm apart, respectively. Scale bar, 5 μm. Error bars represent SEM. See also Figures S3 and S4.
To examine whether nuclear retention of Mlxipl could be regulated by external conditions, we applied diverse stimuli on MIN6 cells and used smFISH to examine the patterns of Mlxipl mRNA nuclear localization. We found that Mlxipl remains nuclearly enriched following glucose challenges, insulin stimulation, heat shock, and serum starvation (Figure S3B).
Nlrp6, encoding a component of the inflammasome, which orchestrates diverse functions during homeostasis and inflammation including steady-state regulation of the composition and function of the intestinal microbiome (Anand et al., 2012, Elinav et al., 2011, Strowig et al., 2012), is expressed in both the liver and the intestinal epithelium. We found that Nlrp6 transcripts were predominantly nuclear in the livers of mice fed a normal diet (Figure 3) or an HFD (Figure S3A). Unlike Mlxipl, which was nuclear in all tissues we examined, Nlrp6 was cytoplasmic in the intestinal epithelium (Figure 3). To assess whether the cytoplasmic localization of intestinal Nlrp6 mRNA is regulated by the intestinal microbiota, we examined germ-free (GF) mice, as well as colonized mice treated with wide-spectrum antibiotics for 4 weeks. Intestinal Nlrp6 mRNA remained cytoplasmic in these conditions (Figure S3C). Thus, exposure to bacteria does not seem to be a cue for regulating nuclear export of intestinal Nlrp6 mRNA.
Nuclear mRNA Co-localizes with Nuclear Speckles
Spector (2001) have shown that CTN-RNA is retained in the nucleus through sequestration to nuclear paraspeckles (Prasanth et al., 2005), sites of active RNA editing (Chen and Carmichael, 2009). To explore whether the retained genes found in our study are spatially correlated with nuclear domains, we performed dual-color smFISH of our nuclear genes and lncRNA markers of speckles (Malat1) and paraspeckles (Neat1). We used particle image cross-correlation spectroscopy (PICCS) (Semrau et al., 2011) to assess the spatial correlation, α, between the nuclear transcripts and either Malat1 or Neat1 foci (Figure S4). We found a highly significant spatial correlation between Malat1 foci and both Mlxipl (α = 0.178, p < 1e−15) and Nlrp6 (α = 0.175 ± 0.012, p < 1e−15; Figure S4). Interestingly, Malat1 and Nlrp6 were not significantly correlated in the intestine, tissue in which Nlrp6 mRNA exhibited cytoplasmic localization (α = −0.026 ± 0.037, p = 0.71; Figure S4C). Mlxipl was also significantly co-localized with Malat1 in beta cells (α = 0.12, p = 0.003) and in the intestine (α = 0.121, p = 0.002). In contrast, mRNA of ATP citrate lyase (Acly), which was not nuclearly retained (Figure 2F), was not co-localized with speckles and none of the genes tested were spatially correlated with paraspeckles in liver tissue (Figure S4). These results indicate that preferential binding or detention of mRNA of Mlxipl and Nlrp6 in nuclear speckles could facilitate their nuclear retention.
Nuclear mRNA Retention Can Reduce Cytoplasmic Gene Expression Noise
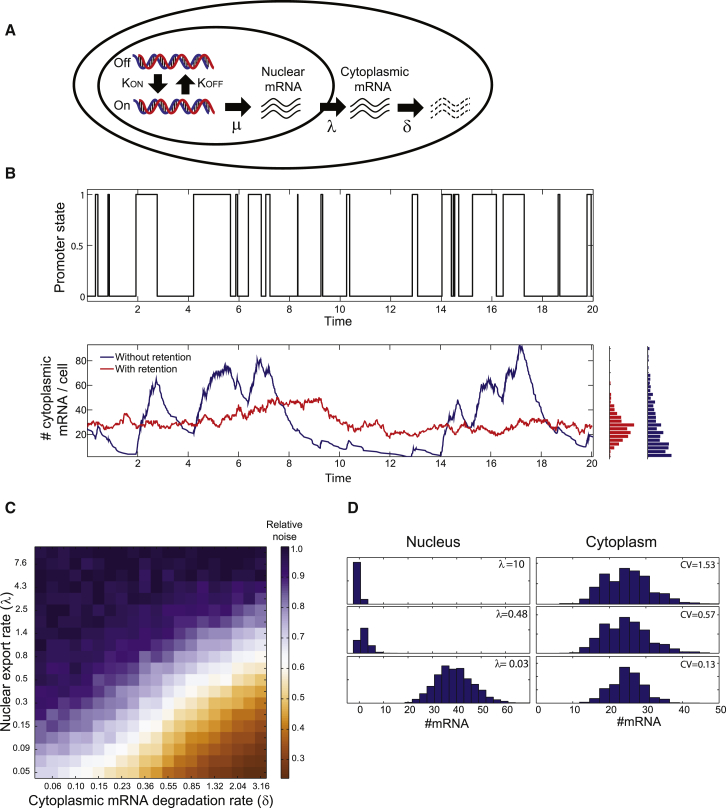
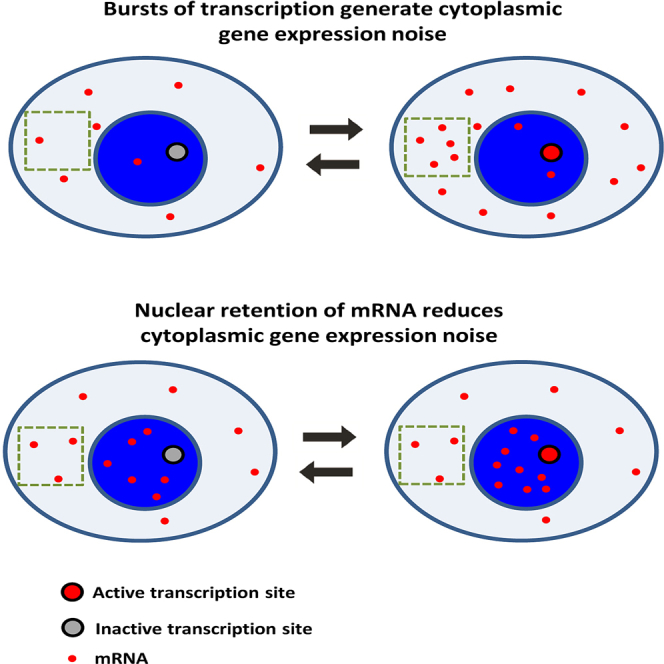
What could be the role of nuclear retention of mature mRNA? At first glance nuclear retention seems like an inefficient strategy for regulating gene expression, as most of the RNA molecules do not reside in the cytoplasmic compartment where they should function. A possible advantage of nuclear retention could involve robustness to noise generated by stochastic mRNA production (Battich et al., 2015, Singh and Bokes, 2012, Xiong et al., 2010). Transcription in a wide range of organisms, including mammals, has been shown to be a pulsatile process, consisting of stochastic bursts of production followed by periods of promoter quiescence (Figure 4A; Larson et al., 2011, Darzacq et al., 2007, Suter et al., 2011, Bahar Halpern et al., 2015, Dar et al., 2012, Senecal et al., 2014). Bursty transcription can lead to profound variations in cellular mRNA content, a phenomenon termed gene expression noise. When promoters are in a transcriptionally active state, the cell accumulates mRNA, whereas when the promoters switch to an off state, mRNA levels decline (Figure 4B). Compartmentalization of mRNA could potentially reduce these burst-associated fluctuations in cytoplasmic mRNA concentrations, the fluctuations that eventually propagate to protein levels.
Figure 4.
Nuclear Retention Can Buffer Gene Expression Noise
(A) Schematic diagram of a two-state burst model that includes a nuclear retention phase. Promoters switch between off and on states at rates and , generating mRNA from the on state at rate .
(B) Nuclear retention significantly reduces cytoplasmic variability. Stochastic simulation of a bursty promoter with = 1 hr−1, = 3 hr−1, = 100 mRNA/hr, and = 1 hr−1 is shown. (Top) Plot of promoter state versus time is shown. (Bottom) Time course of mRNA levels in the cytoplasm without (blue) and with (red) nuclear retention ( = 0.2 hr−1) is shown. Side histograms demonstrate a substantially lower cytoplasmic variability of CV = 0.33 with retention compared to CV = 0.91 without retention (CV, coefficient of variation).
(C) Cytoplasmic noise decreases with increased nuclear retention (decreased export rate ). Heatmap of CVs for different combinations of degradation rates and nuclear export rates is shown. Values are normalized to the maximal CV for each degradation rate. Values are averages of 2,000 stochastic simulations of a bursty promoter with = 1 hr−1 and = 3 hr−1. Transcription rate was set to = 100∗ so that average cytoplasmic levels were 25 mRNA for all combinations.
(D) Histograms of nuclear (left) and cytoplasmic (right) mRNA levels. As nuclear retention increases, average cytoplasmic levels remain identical but noise is decreased. Cytoplasmic degradation was = 3.16 for all simulations.
To assess the potential noise-reduction feature of low nuclear export rate on cytoplasmic variability, we performed Gillespie simulations (Gillespie, 1977) of a bursty promoter that stochastically transitions between on and off states at rates and , producing transcripts at rate μ only when the promoter is on (Raj et al., 2006). While nuclear mRNA levels fluctuated in line with the promoter dynamics, cytoplasmic levels exhibited damped fluctuations compared to those expected when nuclear export was immediate (Figures 4A and 4B). The coefficient of variation (CV) of cytoplasmic transcripts reduced substantially when nuclear export rates were lower than the cytoplasmic mRNA degradation rates (Figures 4B–4D). Thus, reduced nuclear export rate can decrease cytoplasmic variability without changing the average cytoplasmic mRNA level (as evident by Equation 4), at the expense of accumulating more nuclear transcripts (Figure 4D).
Nuclear Retention of Mlxipl and Nlrp6 mRNAs Reduces Their Cytoplasmic Gene Expression Noise
Assessing whether nuclear retention buffers cytoplasmic gene expression noise requires comparing the observed single-cell distribution of cytoplasmic mRNA for a nuclearly retained gene with the distribution that would be expected if nuclear export were immediate. To this end, we used our previously reported method (Bahar Halpern et al., 2015, Bahar Halpern and Itzkovitz, 2015) to identify transcription sites and quantify their bursting dynamics in the intact liver lobule (Figure 2) for the nuclearly retained genes Mlxipl and Nlrp6. We found that both genes were expressed in a bursty manner; 43% of Mlxipl sites were actively transcribing at any given moment and had on average M = 38 ± 6 polymerase molecules, a number that was too high to be compatible with a non-bursty transcription model (Figures 5 and S5). Similarly, Nlrp6 exhibited rare transcription sites with only 17% of sites transcriptionally active at any given moment and an average occupancy of M = 5 ± 2 polymerase molecules.
Figure 5.
Nuclear Retention of Nlrp6 and Mlxipl Reduces Cytoplasmic Gene Expression Noise
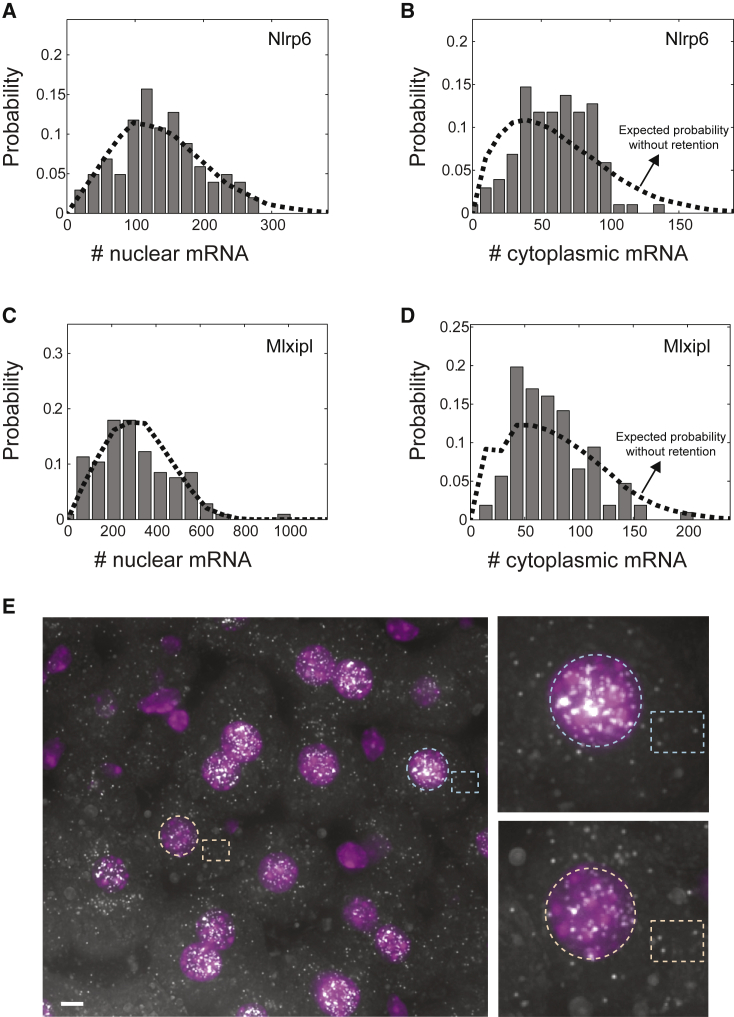
(A–D) Probability distributions of mRNA levels in the nucleus (A and C) and cytoplasm (B and D) of hepatocytes measured in the intact mouse liver. Dashed lines are the fits of a two-state bursty model to the nucleus (A and C) and indicate the expected probability distributions of cytoplasmic mRNA if export was immediate (B and D). Fitted burst parameters were = 0.48 hr−1 and = 2.34 hr−1 for Nlrp6 and = 0.17 hr−1 and = 0.23 hr−1 for Mlxipl. While the two-state model fits the nuclear mRNA distributions (A and C), the measured cytoplasmic distributions are significantly narrower compared to the distributions expected based on the promoter bursting dynamics and no nuclear retention (B, D, and E).
(E) Example of Mlxipl expression in liver section from fasting mouse. Dashed yellow and blue circles label nuclei of two tetraploid hepatocytes with variable mRNA content, and dashed yellow and cyan boxes label their cytoplasmic areas, demonstrating the low variability in cytoplasmic concentration. Scale bar, 5 μm. Image is maximum projection of 15 optical sections spaced 0.3 μm apart.
See also Figure S5.
Next, we fitted the model of Raj et al., 2006, Bahar Halpern et al., 2015) to the nuclear mRNA distributions to extract the rates of promoter opening and closing, and . The distributions of nuclear mRNA for both genes were well fitted by a two-state bursty model (Figures 5A and 5C). In contrast, cytoplasmic mRNA levels for both Mlxipl and Nlrp6 were significantly narrower, compared with the distribution expected based on the same burst parameters but immediate export (Figures 5B and 5D; CV = 0.46 versus CV = 0.56 for Mlxipl, p < 0.002; CV = 0.42 versus CV = 0.55 for Nlrp6, p < 0.0001; Supplemental Experimental Procedures). Cytoplasmic mRNA noise level of Pck1, for which export rate was substantially higher than the cytoplasmic degradation rate (Figure 2F), was identical to the noise predicted based on the fitted two-state bursty model (CV = 0.62 versus 0.56, p = 0.91; Figure S5F). Thus, nuclear retention of mRNA decreases cytoplasmic gene expression noise emanating from promoter bursts, when the mRNA is retained in the nucleus for time periods that exceed its cytoplasmic lifetime.
Discussion
Our experiments revealed a surprisingly wide range of genes in metabolic tissues for which fully spliced, polyadenylated mRNA molecules are retained in the nucleus for time periods that exceed their cytoplasmic lifetimes. Since mRNAs are transcribed and processed at the sites of transcription and translated in the cytoplasm, this lengthy retention period raises the intriguing possibility that the nucleus may have previously overlooked roles.
The nuclear retention of the genes we followed up on in our study (Mlxipl and Nlrp6) appeared to be constitutive, rather than regulated, at least for the stimuli we applied. These included acute exposure to glucose, a condition that has been shown to elicit a potent response from the ChREBP protein (Herman et al., 2012, Postic et al., 2007, Uyeda and Repa, 2006), but which did not yield higher cytoplasmic mRNA levels. In addition, exposure to the intestinal microbiota, a potential regulator of Nlrp6, does not seem to be the stimulus responsible for the tissue-specific cytoplasmic localization of Nlrp6 mRNA in the intestine, but not the liver. It would be important to test additional stimuli that might give rise to differential nuclear retention for other genes we identified in our study.
The ubiquitous nuclear enrichment of transcripts of Mlxipl and Nlrp6 under diverse conditions prompted us to consider additional roles for lengthy mRNA nuclear retention periods. Gene expression in unicellular organisms, as well as in mammalian tissues, consists of transcriptional bursts that can generate profound variability in mRNA levels among identical cells and in a given cell over time. While several papers demonstrated the advantage of this variability as a bet-hedging strategy in unicellular organisms (Chalancon et al., 2012, Eldar and Elowitz, 2010), it is yet unclear if variability is advantageous in mammalian tissues or simply a by-product of the promoter bursting dynamics. Fundamental processes in gene expression can either reduce or amplify burst-associated noise. Lifetimes of mRNA and proteins are key in modulating this variability. Long-lived transcripts render the cell insensitive to the fluctuations in mRNA production by temporally averaging several burst events. Extended protein lifetimes also can achieve a similar effect of time-averaging of fluctuations in mRNA levels, even when cytoplasmic mRNA lifetimes are short (Raj et al., 2006). Nuclear retention has a similar effect, since the nucleus averages the stochastic promoter bursts. An attractive feature of nuclear retention is that it can decrease cytoplasmic noise without affecting the average steady-state levels (Equation 4). In contrast, noise reduction through lengthened mRNA or protein lifetimes requires fine-tuning of the rates of transcription or translation, respectively, to maintain the same steady-state levels.
Given the wide range of nuclearly retained mRNAs described here (13% and 30% in liver and beta cells, respectively), it seems that nuclear retention of mRNA is a meaningful, previously underappreciated step in the mRNA life cycle. Nuclear retention likely has diverse mechanisms and roles. Our study opens the way to exploring this unique mode of gene regulation in diverse physiological and pathological states.
Experimental Procedures
Mice and Tissues
C57bl6 male mice (5 months old) were fed normal chow ad libitum, fasted, or re-fed for the indicated times. HFD was applied to 2-month-old mice for a duration of 8 weeks. Mice were stimulated with insulin and glucose by i.p. injection 30 or 60 min prior to sacrifice. GF C57bl6 mice were housed in sterile isolators. For the antibiotic treatment, mice were given a combination of antibiotics in their drinking water (Supplemental Experimental Procedures). Tissues were harvested and fixed as described previously (Bahar Halpern et al., 2015) and in the Supplemental Experimental Procedures. Primary pancreatic islets were isolated from 6- to 8-week-old mice, cultured up to 1 day, and fixed in 4% paraformaldehyde for 15 min (Supplemental Experimental Procedures). At least two mice were analyzed for each time point and condition.
Cell Fractionation and RNA-Seq
Fractionation of nuclear and cytoplasmic liver RNAs was performed according to Menet et al. (2012), except for minor modifications (Supplemental Experimental Procedures). Fractionation of nuclear and cytoplasmic RNAs from MIN6 cell line (passage 30) is described in the Supplemental Experimental Procedures. RNA-seq was performed using Illumina HiSeq 2500. Read analysis is described in detail in the Supplemental Experimental Procedures. MIN6 RNA-seq results were based on RNA extractions in two independent experiments. Liver RNA-seq was performed on two fasting mice independently processed and analyzed.
Hybridization and Imaging
Probe library constructions, hybridization procedures, and imaging conditions were described previously (Itzkovitz et al., 2011, Lyubimova et al., 2013).
Computational Procedures
To assess the nuclear export rates, cytoplasmic degradation rates, and burst parameters, we used our previously reported method (Bahar Halpern et al., 2015, Bahar Halpern and Itzkovitz, 2015). We detected active transcription sites of the genes of interest based on dots that appeared in both the intronic and exonic channels. The burst fraction f, transcription rate from active transcription sites , and overall transcription rate per cell β were calculated as described in the Supplemental Experimental Procedures. The bursting rates and were computed by fitting the model of Raj et al. (2006; Supplemental Experimental Procedures). To assess the noise that would be observed without nuclear retention, we used Equation S1 (Supplemental Experimental Procedures) with and our inferred and .
Author Contributions
Conceptualization, K.B.H., I.C., D.L., and S.I.; Methodology, K.B.H., D.L., I.C., I.U., M.L., S.L., E.E., and S.I.; Software, D.L., I.U., and S.I.; Validation, K.B.H., I.C., and M.L.; Formal Analysis, D.L., I.U., and S.I.; Investigation, K.B.H. and I.C.; Data Curation, D.L., I.U., and S.I.; Writing – Original Draft, K.B.H, D.L., and S.I.; Writing – Review & Editing, K.B.H, D.L., and S.I.; Funding Acquisition, S.I.; Supervision, S.I.
Acknowledgments
We thank Yuval Dor, Agnes Klochendler, and Jerome Menet for help with reagents and experimental protocols and Phillip Sharp and Paul Boutz for the liver intron detention data. We thank Shlomit Gilad and Inbal Paz for technical help and Nir Drayman and all members of our lab for valuable comments on the manuscript. E.E. is supported by Yael and Rami Ungar, Leona M. and Harry B. Helmsley Charitable Trust, the Gurwin Family Fund for Scientific Research, Crown Endowment Fund for Immunological Research, estate of Jack Gitlitz, estate of Lydia Hershkovich, the Benoziyo Endowment Fund for the Advancement of Science, Adelis Foundation, John L. and Vera Schwartz, Alan Markovitz, Cynthia Adelson, Centre National de la Recherche Scientifique (CNRS), estate of Samuel and Alwyn J. Weber, and Mr. and Mrs. Donald L. Schwarz. E.E. is the incumbent of the Rina Gudinski Career Development Chair. I.U. is supported by the Sygnet Career Development Chair for Bioinformatics by grants from the Israeli Science Foundation (1242/14 and 1984/14), the I-CORE Program of the Planning and Budgeting Committee and the Israel Science Foundation (grant 1796/12), the Minerva Foundation, the Fritz-Thyssen Foundation, the Rising Tide Foundation, and by a research grant from The Abramson Family Center for Young Scientists. S.I. is supported by the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics, The Leir Charitable Foundations, Richard Jakubskind Laboratory of Systems Biology, Cymerman-Jakubskind Prize, The Lord Sieff of Brimpton Memorial Fund, The Human Frontiers Science Program, the I-CORE program of the Planning and Budgeting Committee and the Israel Science Foundation (grants 1902/12 and 1796/12), and the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement 335122. S.I. is the incumbent of the Philip Harris and Gerald Ronson Career Development Chair.
Published: December 17, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, five figures, and five tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.11.036.
Accession Numbers
The accession number for all sequencing data reported in this paper is GEO: GSE73977.
Supplemental Information
Reads were normalized to estimated numbers per cell based on the calibration factors of Table S1.
Additional probe libraries are described in Bahar Halpern et al. (2015). W is the probe library weight factor and L is the gene length. Probe weight factors were computed as described in Bahar Halpern et al. (2015). These depend on the physical location of the smFISH probes along the genes of interest, and are used to convert the intensities of the exon channel to number of Pol2 molecules per transcription site. The factors for the additional genes studied appear in Bahar Halpern et al. (2015).
References
- Anand P.K., Malireddi R.K.S., Lukens J.R., Vogel P., Bertin J., Lamkanfi M., Kanneganti T.-D. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar Halpern K., Tanami S., Landen S., Chapal M., Szlak L., Hutzler A., Nizhberg A., Itzkovitz S. Bursty gene expression in the intact mammalian liver. Mol. Cell. 2015;58:147–156. doi: 10.1016/j.molcel.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar Halpern K., Itzkovitz S. Single molecule approaches for quantifying transcription and degradation rates in intact mammalian tissues. Methods. 2015;15:30155–30159. doi: 10.1016/j.ymeth.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Battich N., Stoeger T., Pelkmans L. Control of transcript variability in single mammalian cells. Cell. 2015;163:1596–1610. doi: 10.1016/j.cell.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Bhatt D.M., Pandya-Jones A., Tong A.-J., Barozzi I., Lissner M.M., Natoli G., Black D.L., Smale S.T. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell. 2012;150:279–290. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake W.J., Kaern M., Cantor C.R., Collins J.J. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- Boutz P.L., Bhutkar A., Sharp P.A. Detained introns are a novel, widespread class of post-transcriptionally spliced introns. Genes Dev. 2015;29:63–80. doi: 10.1101/gad.247361.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalancon G., Ravarani C.N.J., Balaji S., Martinez-Arias A., Aravind L., Jothi R., Babu M.M. Interplay between gene expression noise and regulatory network architecture. Trends Genet. 2012;28:221–232. doi: 10.1016/j.tig.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-L., Carmichael G.G. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol. Cell. 2009;35:467–478. doi: 10.1016/j.molcel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar R.D., Razooky B.S., Singh A., Trimeloni T.V., McCollum J.M., Cox C.D., Simpson M.L., Weinberger L.S. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc. Natl. Acad. Sci. USA. 2012;109:17454–17459. doi: 10.1073/pnas.1213530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X., Shav-Tal Y., de Turris V., Brody Y., Shenoy S.M., Phair R.D., Singer R.H. In vivo dynamics of RNA polymerase II transcription. Nat. Struct. Mol. Biol. 2007;14:796–806. doi: 10.1038/nsmb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar A., Elowitz M.B. Functional roles for noise in genetic circuits. Nature. 2010;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E., Strowig T., Kau A.L., Henao-Mejia J., Thaiss C.A., Booth C.J., Peaper D.R., Bertin J., Eisenbarth S.C., Gordon J.I., Flavell R.A. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau N.L., Wilusz J., Wilusz C.J. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- Gillespie D.T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977;81:2340–2361. [Google Scholar]
- Golding I., Paulsson J., Zawilski S.M., Cox E.C. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Herman M.A., Peroni O.D., Villoria J., Schön M.R., Abumrad N.A., Blüher M., Klein S., Kahn B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkovitz S., Lyubimova A., Blat I.C., Maynard M., van Es J., Lees J., Jacks T., Clevers H., van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 2011;14:106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaern M., Elston T.C., Blake W.J., Collins J.J. Stochasticity in gene expression: from theories to phenotypes. Nat. Rev. Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- Larson D.R., Zenklusen D., Wu B., Chao J.A., Singer R.H. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science. 2011;332:475–478. doi: 10.1126/science.1202142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimova A., Itzkovitz S., Junker J.P., Fan Z.P., Wu X., van Oudenaarden A. Single-molecule mRNA detection and counting in mammalian tissue. Nat. Protoc. 2013;8:1743–1758. doi: 10.1038/nprot.2013.109. [DOI] [PubMed] [Google Scholar]
- Maheshri N., O’Shea E.K. Living with noisy genes: how cells function reliably with inherent variability in gene expression. Annu. Rev. Biophys. Biomol. Struct. 2007;36:413–434. doi: 10.1146/annurev.biophys.36.040306.132705. [DOI] [PubMed] [Google Scholar]
- Menet J.S., Rodriguez J., Abruzzi K.C., Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife. 2012;1:e00011. doi: 10.7554/eLife.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Oeffinger M., Zenklusen D. To the pore and through the pore: a story of mRNA export kinetics. Biochim. Biophys. Acta. 2012;1819:494–506. doi: 10.1016/j.bbagrm.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya-Jones A., Bhatt D.M., Lin C.-H., Tong A.-J., Smale S.T., Black D.L. Splicing kinetics and transcript release from the chromatin compartment limit the rate of Lipid A-induced gene expression. RNA. 2013;19:811–827. doi: 10.1261/rna.039081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson J. Summing up the noise in gene networks. Nature. 2004;427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- Paz I., Kosti I., Ares M., Jr., Cline M., Mandel-Gutfreund Y. RBPmap: a web server for mapping binding sites of RNA-binding proteins. Nucleic Acids Res. 2014;42:W361–W367. doi: 10.1093/nar/gku406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C., Dentin R., Denechaud P.-D., Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu. Rev. Nutr. 2007;27:179–192. doi: 10.1146/annurev.nutr.27.061406.093618. [DOI] [PubMed] [Google Scholar]
- Prasanth K.V., Prasanth S.G., Xuan Z., Hearn S., Freier S.M., Bennett C.F., Zhang M.Q., Spector D.L. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–263. doi: 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Raj A., van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A., Peskin C.S., Tranchina D., Vargas D.Y., Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrau S., Holtzer L., González-Gaitán M., Schmidt T. Quantification of biological interactions with particle image cross-correlation spectroscopy (PICCS) Biophys. J. 2011;100:1810–1818. doi: 10.1016/j.bpj.2010.12.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senecal A., Munsky B., Proux F., Ly N., Braye F.E., Zimmer C., Mueller F., Darzacq X. Transcription factors modulate c-Fos transcriptional bursts. Cell Rep. 2014;8:75–83. doi: 10.1016/j.celrep.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi R., Hurt J.A., Lindquist S., Burge C.B. Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep. 2014;7:1362–1370. doi: 10.1016/j.celrep.2014.04.044. [DOI] [PubMed] [Google Scholar]
- Shav-Tal Y., Darzacq X., Shenoy S.M., Fusco D., Janicki S.M., Spector D.L., Singer R.H. Dynamics of single mRNPs in nuclei of living cells. Science. 2004;304:1797–1800. doi: 10.1126/science.1099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Bokes P. Consequences of mRNA transport on stochastic variability in protein levels. Biophys. J. 2012;103:1087–1096. doi: 10.1016/j.bpj.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D.L. Nuclear domains. J. Cell Sci. 2001;114:2891–2893. doi: 10.1242/jcs.114.16.2891. [DOI] [PubMed] [Google Scholar]
- Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Suter D.M., Molina N., Gatfield D., Schneider K., Schibler U., Naef F. Mammalian genes are transcribed with widely different bursting kinetics. Science. 2011;332:472–474. doi: 10.1126/science.1198817. [DOI] [PubMed] [Google Scholar]
- Tilgner H., Knowles D.G., Johnson R., Davis C.A., Chakrabortty S., Djebali S., Curado J., Snyder M., Gingeras T.R., Guigó R. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 2012;22:1616–1625. doi: 10.1101/gr.134445.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Repa J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Vargas D.Y., Raj A., Marras S.A.E., Kramer F.R., Tyagi S. Mechanism of mRNA transport in the nucleus. Proc. Natl. Acad. Sci. USA. 2005;102:17008–17013. doi: 10.1073/pnas.0505580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein L.B., Steitz J.A. Guided tours: from precursor snoRNA to functional snoRNP. Curr. Opin. Cell Biol. 1999;11:378–384. doi: 10.1016/S0955-0674(99)80053-2. [DOI] [PubMed] [Google Scholar]
- Xiong L.P., Ma Y.Q., Tang L.H. Attenuation of transcriptional bursting in mRNA transport. Phys. Biol. 2010;7:016005. doi: 10.1088/1478-3975/7/1/016005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reads were normalized to estimated numbers per cell based on the calibration factors of Table S1.
Additional probe libraries are described in Bahar Halpern et al. (2015). W is the probe library weight factor and L is the gene length. Probe weight factors were computed as described in Bahar Halpern et al. (2015). These depend on the physical location of the smFISH probes along the genes of interest, and are used to convert the intensities of the exon channel to number of Pol2 molecules per transcription site. The factors for the additional genes studied appear in Bahar Halpern et al. (2015).