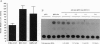Abstract
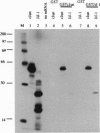
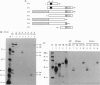
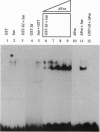
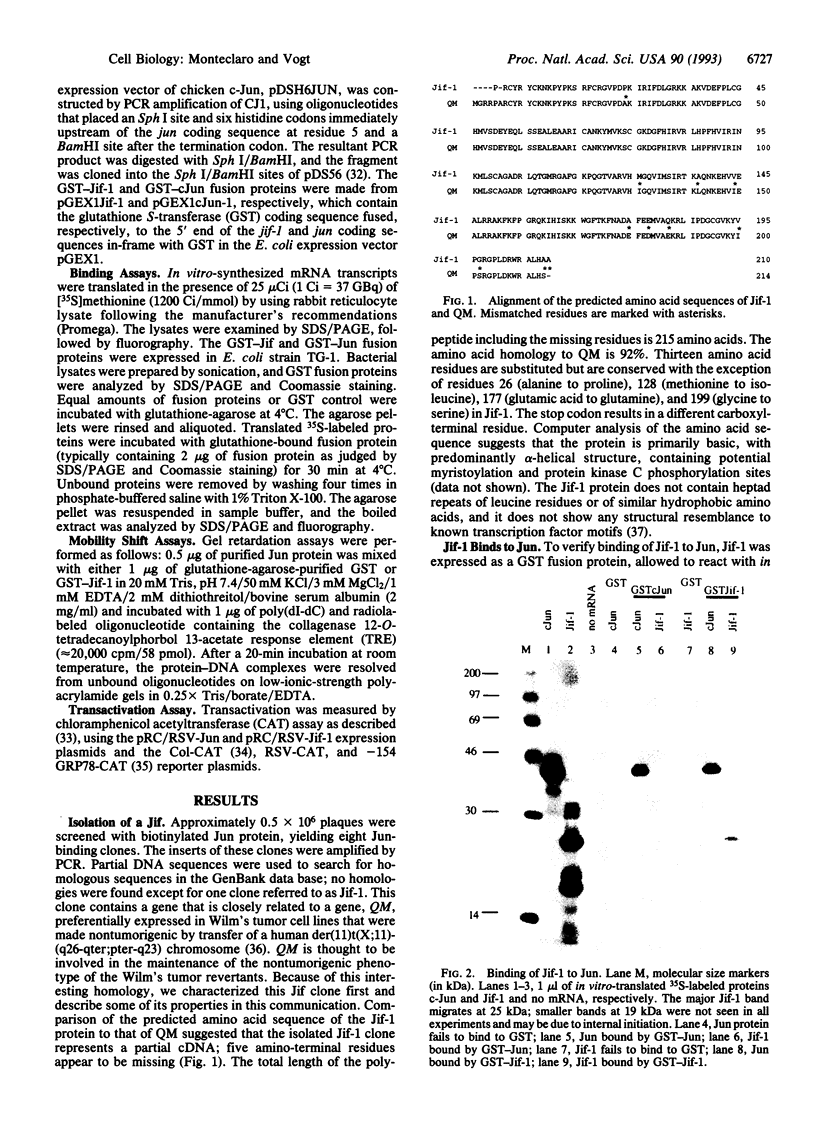
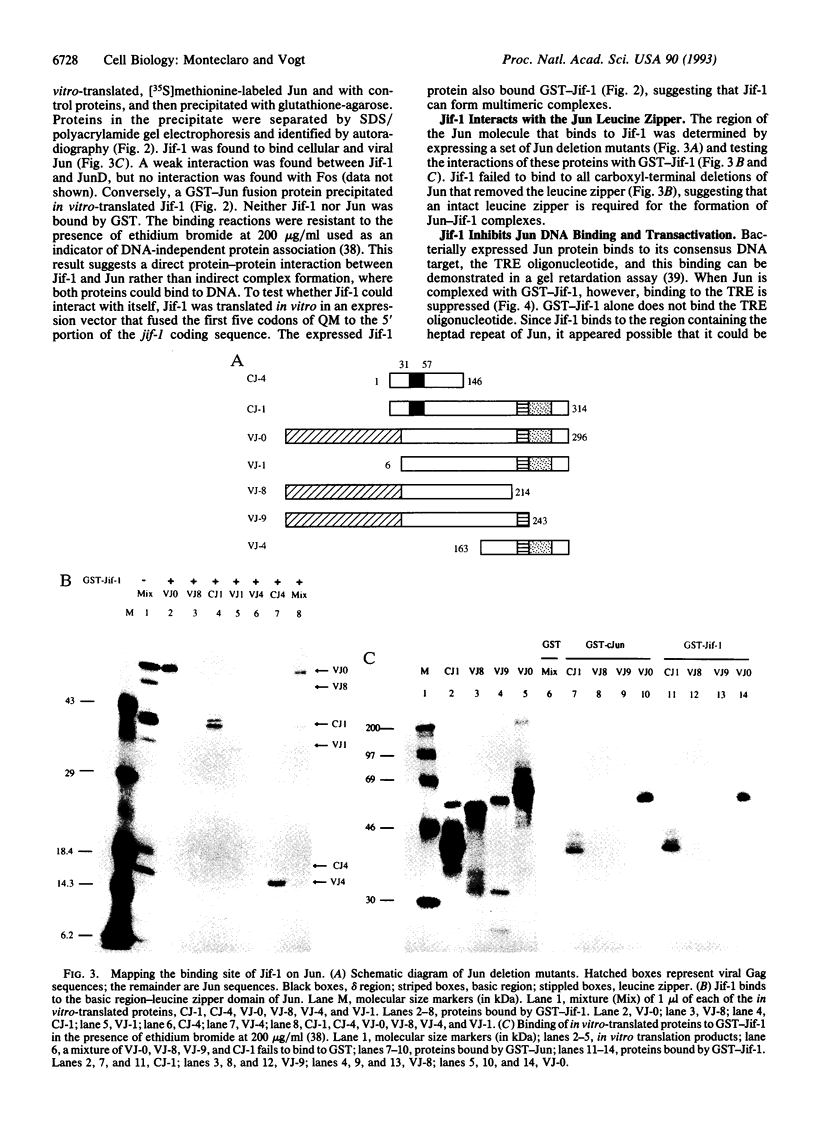
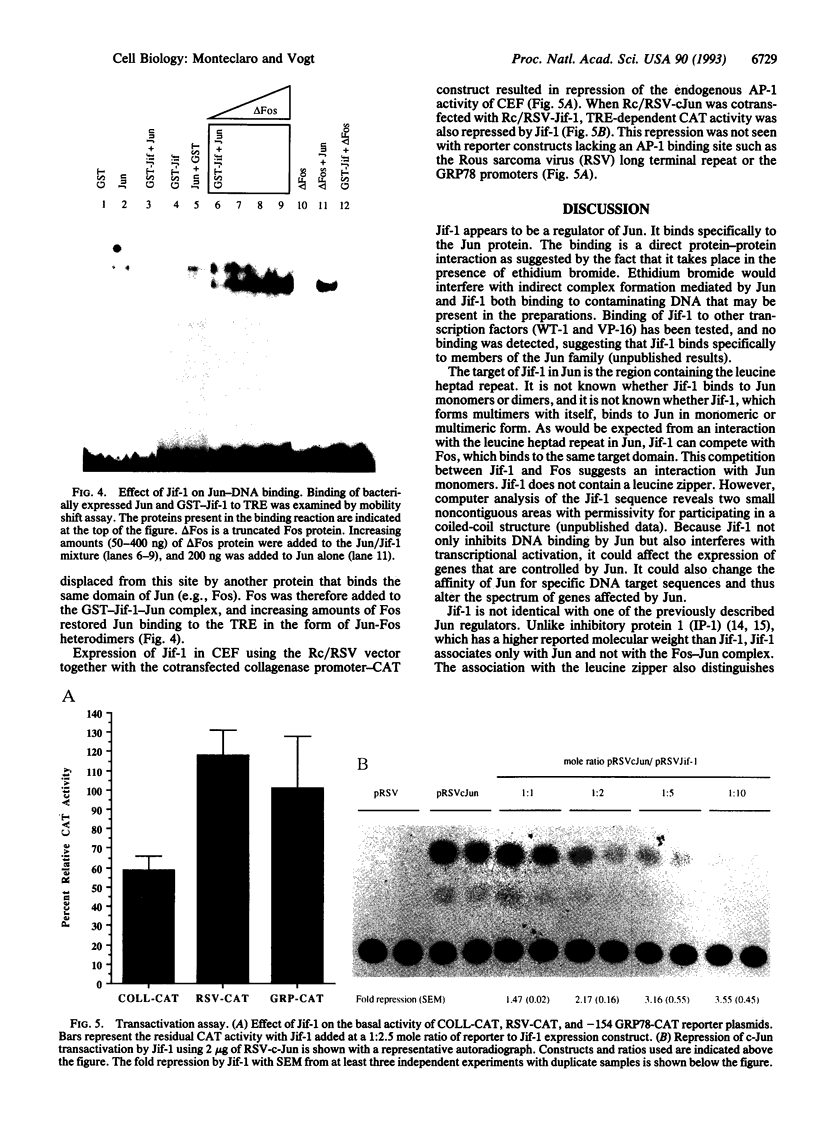
A lambda gt11 cDNA library of chicken embryo fibroblasts was screened with biotinylated Jun protein to identify Jun-binding clones. Eight such clones were isolated; one contains a gene referred to as jif-1 that is homologous to the putative tumor suppressor gene QM. jif-1 codes for a protein of 25 kDa that binds to the leucine zipper of viral and cellular Jun. The Jif-1 protein also binds to itself. Jif-1 does not contain a leucine zipper, and it does not bind to the 12-O-tetradecanoylphorbol 13-acetate response element DNA sequence. Complex formation of Jif-1 with Jun inhibits DNA binding and reduces transactivation by Jun. Addition of Fos protein to Jun-Jif-1 complexes restores DNA-binding activity. These observations suggest that Jif-1 is a negative regulator of Jun.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. A ubiquitous nuclear protein stimulates the DNA-binding activity of fos and jun indirectly. Cell Growth Differ. 1990 Oct;1(10):455–462. [PubMed] [Google Scholar]
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Adler V., Franklin C. C., Kraft A. S. Phorbol esters stimulate the phosphorylation of c-Jun but not v-Jun: regulation by the N-terminal delta domain. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5341–5345. doi: 10.1073/pnas.89.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alani R., Brown P., Binétruy B., Dosaka H., Rosenberg R. K., Angel P., Karin M., Birrer M. J. The transactivating domain of the c-Jun proto-oncoprotein is required for cotransformation of rat embryo cells. Mol Cell Biol. 1991 Dec;11(12):6286–6295. doi: 10.1128/mcb.11.12.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Smeal T., Meek J., Karin M. Jun and v-jun contain multiple regions that participate in transcriptional activation in an interdependent manner. New Biol. 1989 Oct;1(1):35–43. [PubMed] [Google Scholar]
- Auwerx J., Sassone-Corsi P. AP-1 (Fos-Jun) regulation by IP-1: effect of signal transduction pathways and cell growth. Oncogene. 1992 Nov;7(11):2271–2280. [PubMed] [Google Scholar]
- Auwerx J., Sassone-Corsi P. IP-1: a dominant inhibitor of Fos/Jun whose activity is modulated by phosphorylation. Cell. 1991 Mar 8;64(5):983–993. doi: 10.1016/0092-8674(91)90322-p. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Park A., Tjian R. The cell-type-specific activator region of c-Jun juxtaposes constitutive and negatively regulated domains. Genes Dev. 1992 Aug;6(8):1493–1502. doi: 10.1101/gad.6.8.1493. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell. 1990 Nov 16;63(4):815–825. doi: 10.1016/0092-8674(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Kerppola T. K., Luk D., Vandenberg M. T., Marshak D. R., Curran T., Abate C. Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts. Mol Cell Biol. 1992 Oct;12(10):4694–4705. doi: 10.1128/mcb.12.10.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Bengal E., Ransone L., Scharfmann R., Dwarki V. J., Tapscott S. J., Weintraub H., Verma I. M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992 Feb 7;68(3):507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Bignon C., Roux-Dosseto M., Zeigler M. E., Wicha M. S., Martin P. M. cDNA cloning and genomic analysis of a new multigene family sharing common phylogenetic and expression profiles with the laminin receptor gene. Biochem Biophys Res Commun. 1992 May 15;184(3):1165–1172. doi: 10.1016/s0006-291x(05)80005-1. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Black E. J., Street A. J., Gillespie D. A. Protein phosphatase 2A reverses phosphorylation of c-Jun specified by the delta domain in vitro: correlation with oncogenic activation and deregulated transactivation activity of v-Jun. Oncogene. 1991 Nov;6(11):1949–1958. [PubMed] [Google Scholar]
- Bohmann D., Tjian R. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell. 1989 Nov 17;59(4):709–717. doi: 10.1016/0092-8674(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Bohmann D. Transcription factor phosphorylation: a link between signal transduction and the regulation of gene expression. Cancer Cells. 1990 Nov;2(11):337–344. [PubMed] [Google Scholar]
- Bos T. J., Rauscher F. J., 3rd, Curran T., Vogt P. K. The carboxy terminus of the viral Jun oncoprotein is required for complex formation with the cellular Fos protein. Oncogene. 1989 Feb;4(2):123–126. [PubMed] [Google Scholar]
- Bujard H., Gentz R., Lanzer M., Stueber D., Mueller M., Ibrahimi I., Haeuptle M. T., Dobberstein B. A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 1987;155:416–433. doi: 10.1016/0076-6879(87)55028-5. [DOI] [PubMed] [Google Scholar]
- Chevray P. M., Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy S. F., Lai K. M., Weissman B. E., Matsui Y., Hogan B. L., Stanbridge E. J. The isolation and characterization of a novel cDNA demonstrating an altered mRNA level in nontumorigenic Wilms' microcell hybrid cells. Nucleic Acids Res. 1991 Oct 25;19(20):5763–5769. doi: 10.1093/nar/19.20.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Gentz R., Chen C. H., Rosen C. A. Bioassay for trans-activation using purified human immunodeficiency virus tat-encoded protein: trans-activation requires mRNA synthesis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):821–824. doi: 10.1073/pnas.86.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Håvarstein L. S., Morgan I. M., Wong W. Y., Vogt P. K. Mutations in the Jun delta region suggest an inverse correlation between transformation and transcriptional activation. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):618–622. doi: 10.1073/pnas.89.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. S., Herr W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6958–6962. doi: 10.1073/pnas.89.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chambard J. C., Karin M., Olson E. N. Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes Dev. 1992 Apr;6(4):676–689. doi: 10.1101/gad.6.4.676. [DOI] [PubMed] [Google Scholar]
- Lin A., Frost J., Deng T., Smeal T., al-Alawi N., Kikkawa U., Hunter T., Brenner D., Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell. 1992 Sep 4;70(5):777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Diamond M. I., Yamamoto K. R. Joints in the regulatory lattice: composite regulation by steroid receptor-AP1 complexes. Cell Growth Differ. 1991 Oct;2(10):525–530. [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Oemar B. S., Law N. M., Rosenzweig S. A. Insulin-like growth factor-1 induces tyrosyl phosphorylation of nuclear proteins. J Biol Chem. 1991 Dec 25;266(36):24241–24244. [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen R., Benvegnu D., O'Shea E. K., Kim P. S., Alber T. X-ray scattering indicates that the leucine zipper is a coiled coil. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):561–564. doi: 10.1073/pnas.88.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schwarz A., Wandrey C., Bayer E. A., Wilchek M. Enzymatic C-terminal biotinylation of proteins. Methods Enzymol. 1990;184:160–162. doi: 10.1016/0076-6879(90)84269-m. [DOI] [PubMed] [Google Scholar]
- Schüle R., Evans R. M. Cross-coupling of signal transduction pathways: zinc finger meets leucine zipper. Trends Genet. 1991 Nov-Dec;7(11-12):377–381. doi: 10.1016/0168-9525(91)90259-s. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. jun: oncogene and transcription factor. Adv Cancer Res. 1990;55:1–35. doi: 10.1016/s0065-230x(08)60466-2. [DOI] [PubMed] [Google Scholar]
- Wooden S. K., Li L. J., Navarro D., Qadri I., Pereira L., Lee A. S. Transactivation of the grp78 promoter by malfolded proteins, glycosylation block, and calcium ionophore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-I. Mol Cell Biol. 1991 Nov;11(11):5612–5623. doi: 10.1128/mcb.11.11.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ouweland A. M., Kioschis P., Verdijk M., Tamanini F., Toniolo D., Poustka A., van Oost B. A. Identification and characterization of a new gene in the human Xq28 region. Hum Mol Genet. 1992 Jul;1(4):269–273. doi: 10.1093/hmg/1.4.269. [DOI] [PubMed] [Google Scholar]