Abstract
Disrupted-in-Schizophrenia 1 (DISC1) is a risk factor for schizophrenia and affective disorders. The full-length DISC1 protein consists of an N-terminal ‘head’ domain and a C-terminal tail domain that contains several predicted coiled-coils, structural motifs involved in protein-protein interactions. To probe the in vivo effects of missense mutation of DISC1’s C-terminal tail, we tested mice carrying mutation D453G within a predicted α-helical coiled-coil region. We report that, relative to wild-type littermates, female DISC1D453G mice exhibited novelty-induced hyperlocomotion, an anxiogenic profile in the elevated plus-maze and open field tests, and reduced social exploration of unfamiliar mice. Male DISC1D453G mice displayed a deficit in passive avoidance, while neither males nor females exhibited any impairment in startle reactivity or prepulse inhibition. Whole brain homogenates showed normal levels of DISC1 protein, but decreased binding of DISC1 to GSK3β, decreased phospho-inhibition of GSK3β at serine 9, and decreased levels of β-catenin in DISC1D453G mice of either sex. Interrupted GSK3β signaling may thus be part of the mechanism underlying the behavioral phenotype associated with D453G, in common with the previously described N-terminal domain mutations Q31L and L100P in mice, and the schizophrenia risk-conferring variant R264Q in humans.
Schizophrenia is a severe psychiatric condition characterized by three clusters of symptoms: positive symptoms (psychosis and thought disorder), negative symptoms (social and emotional deficits), and cognitive symptoms1,2. It is well established from family, twin and adoption studies that genetic factors play an important role in the risk of developing schizophrenia3. Disrupted-in-Schizophrenia-1 (DISC1) is a genetic susceptibility locus for schizophrenia and related psychiatric disorders4. The gene was first identified in a large Scottish family in which a balanced chromosomal translocation t(1;11) disrupting the DISC1 gene in the middle of its ORF co-segregates with schizophrenia and affective disorders5,6. The t(1;11) translocation is believed to be unique, but subsequent studies have supported DISC1’s candidacy as a susceptibility gene for these conditions.
Numerous common and rare DISC1 missense variants have been associated with the increased risk of psychiatric illness, altered brain morphology or cognitive deficits7,8. For instance, the major S704 allele of the common S704C variant (rs821616) is associated with increased risk of schizophrenia9 and increased severity of positive symptoms at the onset of psychosis10, while the minor 264Q allele of the common R264Q variant (rs3738401) is associated with increased risk of treatment-refractory schizophrenia11. Case-control mutation studies of DISC1 have identified rare missense mutations that confer an estimated attributable risk of about 2% in schizophrenia12 and 0.5% in bipolar disorder13, including R418H that was found in both disorders. Other studies have reported an increased burden of rare missense mutations in a Swedish schizophrenia cohort14, and an excess of exon 11 rare missense mutations in schizoaffective disorder15. Understanding how relatively subtle changes in the composition of the DISC1 protein may confer behavioral abnormalities is thus important in further elucidating the role of DISC1 in schizophrenia and related mental disorders.
The full-length DISC1 protein (854 amino acids) is predicted to consist of an N-terminal globular ‘head’ domain (residues 1–350) encoded by exons 1–2 and an α-helical coiled-coil-containing C-terminal tail domain (residues 351–854), encoded by exons 3–1316. The DISC1 protein acts as a scaffold, binding over 200 interacting molecules – the so-called ‘DISC1 interactome’17 – including PDE4B (phosphodiesterase 4B)18, GSK3β (glycogen synthase kinase 3 beta)19 and dopamine receptor D220. DISC1 carries out multiple functions in the nervous system by interacting with various proteins in different cell compartments from embryonic development until adulthood16. Several risk-conferring missense variants in DISC1 are located within known binding sites16, so missense variation in DISC1 has the potential to affect a variety of neural processes via disturbed interactions with critical binding partners. Indeed, the major S704 allele of S704C (rs821616) has shown decreased binding to NDEL1 (nuclear distribution gene E-like homolog 1)21 but increased binding to NDE1 (nuclear distribution gene E homolog 1)22 compared with the minor 704C allele; while the minor 264Q allele of R264Q (rs3738401) has shown decreased binding to GSK3β, increased Y216 autophosphorylation-induced activation of GSK3β, and decreased levels of the GSK3β-specific substrate β-catenin compared with the major R264 allele23, thus lending support to a putative pathogenic role.
The linked hypotheses that different DISC1 variants will predispose to different phenotypes and that the phenotype of any given DISC1 variant will be modulated by components within, upstream and downstream of the DISC1 scaffold complex are amenable to experimental testing. Two ethylnitrosourea (ENU)-derived mutant Disc1 mouse lines have previously been described, each with a different missense mutation in the N-terminal head domain of DISC1: Q31L and L100P24. Both mutants have inhibited cortical neuronal proliferation, aberrant neuronal migration, reduced dendritic spine density25, reduced brain volumes and deficits in spatial working memory24 compared with wild-type mice. Furthermore, the L100P mutants show behaviors, including profound prepulse inhibition (PPI) and latent inhibition (LI) deficits, akin to those seen in some with schizophrenia, which could be reversed by treatment with antipsychotic medications24. By contrast, the Q31L mice exhibit a more depressive-like behavior, with abnormalities in social behavior and the forced swim test, partially ameliorated by the antidepressant bupropion, but not by rolipram, a PDE4 inhibitor24.
Neither Q31 nor L100 is conserved between mouse and human – corresponding to R35 and S100, respectively, in human DISC1 – so their direct involvement in human conditions is unclear. However, given their potential for structural disruption and proximity to functional domains and protein binding regions, Q31L and L100P may still provide important mechanistic insights into the possible effects of DISC1 variants in humans. Mechanistically, both mutations reduce the interaction of DISC1 with PDE4B, but only Q31L mutant mice showed a decrease in PDE4B activity, consistent with the resistance of Q31L mutants and the contrasting sensitivity of L100P mutants and wild-type mice to treatment with rolipram24.
Mutations Q31L and L100P both reduce the interaction of DISC1 with GSK3β26,27, and via distinct routes lead to increased GSK3β activity26,27,28. L100P mice have decreased AKT-dependent phospho-inhibition of GSK3β at S9 in conjunction with increased DISC1 binding to dopamine receptor D2 (DRD2) in striatum28, but Y216 autophosphorylation-induced activation of GSK3β was unaltered in both striatum and hippocampus27. The same phenomenon was subsequently observed in postmortem striatum samples from schizophrenia patients of unknown DISC1 genotype28. Administration of either an interfering peptide that disrupts DISC1-DRD2 binding or the DRD2 antagonist haloperidol to L100P mice blocked the increase in DISC1-DRD2 complex formation and the decrease in phospho-inhibition of GSK3β, and successfully reversed their schizophrenia-related behavioral deficits28. By contrast, Q31L mice have shown increased Y216 autophosphorylation-induced activation of GSK3β in hippocampus, with no changes in phospho-inhibition of GSK3β at S926. The GSK3β inhibitor TDZD-8 ameliorated the behavioral deficits of both Q31L26 and L100P27 mutant lines, indicating that increased GSK3β activity is part of the mechanism underlying the pathophysiology of their abnormal behavior.
Given the insights provided by the Q31L and L100P mutant lines, and the scope for expanding the Disc1 allelic series to further refine genotype-phenotype correlations and elucidate DISC1 function in the mouse, we extended the ENU mutagenesis screen to a different part of the Disc1 locus. Herein, we report the initial analysis of the behavioral effects of a novel ENU-derived mutant Disc1 mouse line, which has a missense mutation (D453G) in a predicted α-helical coiled-coil region within the C-terminal tail domain of DISC1. D453G occurs within a known GSK3β binding region on DISC1 (residues 356–595)19, in which mutation R418H has been found in schizophrenia and bipolar disorder patients12,13. We report that DISC1D453G mutant mice show altered GSK3β signaling in the brain and sex-dependent behavioral deficits.
Results
Identification of missense mutation in DISC1 C-terminal tail
The C-terminal tail domain of mouse full-length DISC1 (NP_777279; 852 residues) spans amino acid residues 351–852 and is encoded by Disc1 exons 3–1316. We screened exon 5 (121 bp; residues 423–464) of Disc1 (ENSMUSG00000043051) in 7,776 ENU-mutagenized mice in the MRC Harwell ENU DNA archive. In a single mouse (EMRCB/26.2e), we detected an adenine to guanine (A1358G) transition, corresponding to an Asp453 (GAC) → Gly (GGC) (D453G) exchange (Fig. 1A), which abolished a BseNI restriction site (Fig. 1B). The exon 5 sequences of the BALB/cAnN and C3H/HeH parental strains are identical (data not shown), indicating that the DISC1D453G mutation arose as a result of ENU administration. We propose that the new allele be designated Disc1enu1H. The aspartic acid at position 453 occurs within a predicted α-helical coiled-coil region, and is conserved across mammals and reptiles, but not birds or fish (Fig. 1C). We used three programs, PolyPhen-229, PMut30 and PROVEAN31, to predict the potential effect of D453G on the function and structure of the DISC1 protein. PolyPhen-2 predicted D453G to be ‘probably damaging’; PMut predicted D453G to be ‘pathological’, with a reliability value of 7 out of 9; and PROVEAN predicted D453G to be ‘deleterious’. Based on all three programs predicting D453G to affect DISC1 protein function, we bred homozygous mutant and wild-type littermates for assessment in a series of behavioral tests to evaluate the effects of D453G on different domains of the mouse behavioral repertoire.
Figure 1. D453G mutation in mouse DISC1.
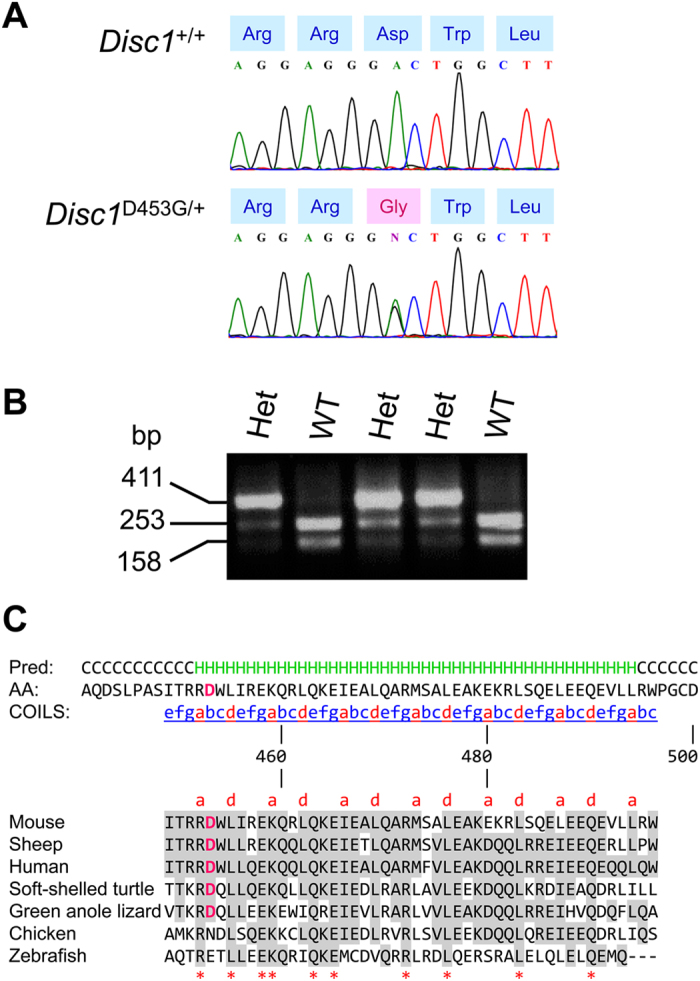
(A) DNA sequence chromatogram showing point mutation in Disc1 exon 5. Transition A1358G converts codon 453 from GAC aspartic acid (Asp) to GGC glycine (Gly). (B) BseNI restriction site (5′-ACTGGN*-3′) abolished by the A1358G mutation in Disc1. In wild-type (WT) mice, a 411-bp PCR amplicon is cleaved by BseNI into 253-bp and 158-bp fragments. (C) Top: PsiPred-predicted secondary structure and COILS server predicted coiled-coil within residues 441–500 of DISC1 are shown and colored accordingly: predicted helices: ‘H’ and colored green above the sequence; coiled-coil heptad repeats: ‘abcdefg’, and positions ‘a’ and ‘d’ shown in red text. Bottom: Alignment of predicted coiled-coil in mouse (Mus musculus), sheep (Ovis aries), human (Homo sapiens), soft-shelled turtle (Pelodiscus sinensis), green anole lizard (Anolis carolinensis), chicken (Gallus gallus) and zebrafish (Danio rerio), showing conservation of amino acid D453. Dashes indicate alignment gaps. Residues identical between human and the other species have a gray background, with highly conserved residues indicated by asterisks. D453 is shown in magenta text.
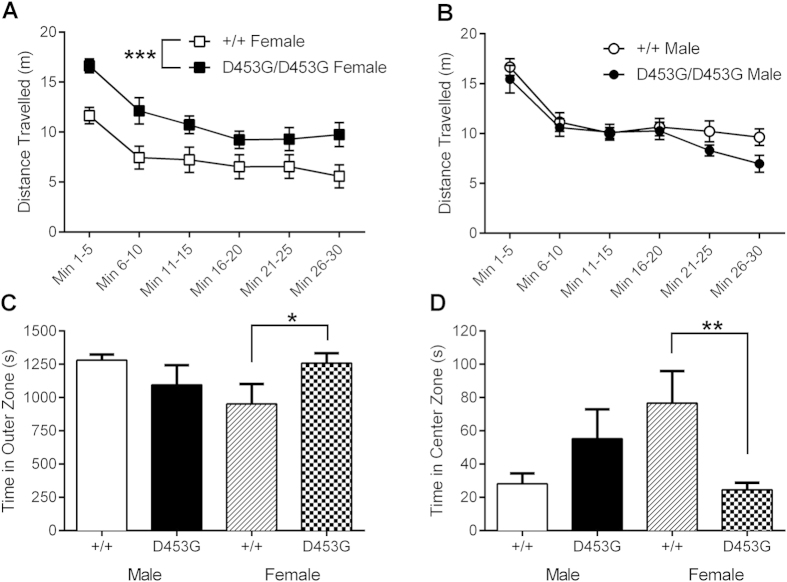
Female DISC1D453G mice show hyperlocomotion in the open field test
Hyperlocomotion in a novel open field is thought to be analogous to psychomotor agitation32, a positive symptom of schizophrenia, and has previously been shown by DISC1L100P mice24. Throughout the 30 min open field test, female DISC1D453G mice consistently travelled further than female wild-type littermates (Fig. 2A), while there was no obvious difference between male DISC1D453G mice and their wild-type counterparts (Fig. 2B). A repeated-measures three-way ANOVA found that, while there was no main effect of sex (F(1, 30) = 3.66, P > 0.05) or genotype (F(1, 30) = 3.15, P > 0.05), there was significant genotype x sex interaction (F(1, 30) = 10.52, P = 0.003). No other interactions were significant (F < 1). Following up on the significant genotype x sex interaction, a test of simple main effects (SME) revealed a significant difference between female DISC1D453G and wild-type mice (F(1, 30) = 12.59, P = 0.001) but not between male mutants and wild-types (F(1, 30) = 1.08, P > 0.05). Total distance travelled during the first min in the open field was not significantly different between genotypes or sexes (Supplementary Fig. S1; two-way ANOVA: genotype (F(1,30) = 3.37, P = 0.076), sex (F(1,30) < 1), genotype x sex (F(1,30) < 1)).
Figure 2. Female DISC1D453G mice show hyperlocomotion and thigmotaxis in the open field.
Wild-type (+/+; n = 17, 10 male and 7 female) and DISC1D453G (D453G/D453G; n = 17, 7 male and 10 female) mice were allowed 30 min for free exploration of the arena. (A) Female DISC1D453G mice consistently travelled further than female wild-type littermates over the 30 min test period, showing a significant genotypic difference in total distance travelled. (B) The distance travelled was not significantly different between male wild-type and DISC1D453G mice. When the arena was broken into outer, intermediate and center zones, female DISC1D453G mice spent significantly more time in the outer zone (C), and significantly less time in the center zone (D). There were no genotypic differences among males. * P < 0.05, **P < 0.01, ***P < 0.001 vs. female +/+.
The natural tendency of mice when first introduced into the open field is to remain close to the walls, and avoid the potentially dangerous open area. This behavior, termed “thigmotaxis”, has been used as an index of anxiety in mice, an interpretation supported by the fact that anxiolytic drugs decrease thigmotaxis33. To assess whether the female-specific difference in novelty-induced locomotion could reflect more subtle phenotypes such as anxiety, ambulation in the open field was divided into three different zones (outer, intermediate and center). Female DISC1D453G mice spent significantly more time in the outer zone compared with female wild-types (Fig. 2C; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 5.87, P = 0.022); SME revealed a significant difference between females (F(1, 30) = 4.56, P = 0.041) but not males (F(1, 30) = 1.67, P > 0.05)). Correspondingly, female DISC1D453G mice spent significantly less time in the center zone than female wild-types, with no genotypic differences among males (Fig. 2D; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 11.50, P = 0.002); SME reveals a significant different between females (F(1, 30) = 9.96, P = 0.004) but not males (F(1, 30) = 2.6, P > 0.05)). There was an almost significant difference between female mutant and wild-type mice for the time spent in the intermediate zone (Supplementary Fig. S1; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 4.95, P = 0.034); SME revealed an approaching significant difference between females (F(1, 30) = 3.76, P = 0.062) but not males (F(1, 30) = 1.46, P = 0.24)). The DISC1D453G mutation thus results in female-specific hyperlocomotion (as measured by distance travelled), thigmotaxis and reduced time spent in the center zone, a profile strongly suggestive of an anxiogenic phenotype.
As a final measure of exploration, time spent rearing in the open field was examined. In contrast to the above female DISC1D453G differences, male DISC1D453G mice spent significantly less time rearing, with no genotypic differences between female mice (Supplementary Fig. S1; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 5.43, p = 0.027); SME revealed a significant difference between males (F(1, 30) = 5.11, p = 0.031) but not females (F(1, 30) = 1.07, p = 0.31)). DISC1L100P mice have previously shown increased grooming34, but we found no significant differences for time spent grooming for either DISC1 genotype or sex (Supplementary Fig. S1; two-way ANOVA: all P > 0.05).
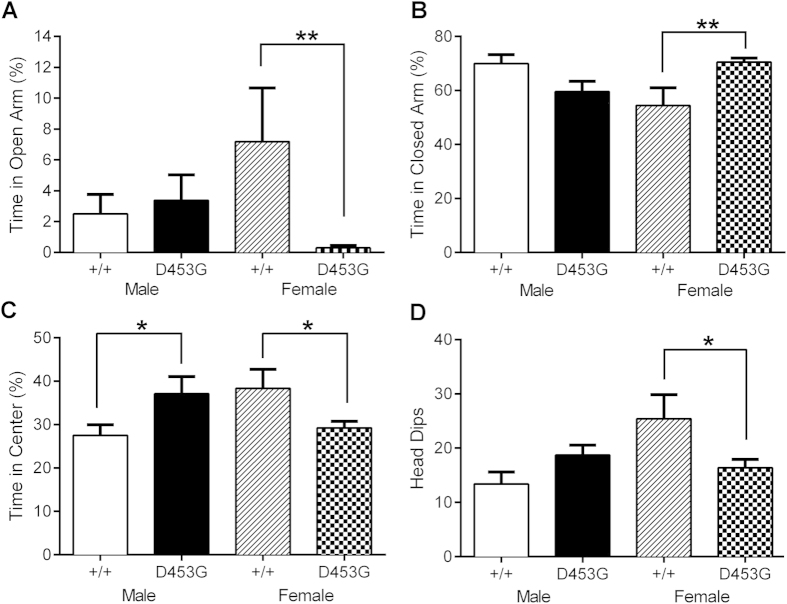
Female DISC1D453G mice exhibit a profile of anxiety but not depression
To further examine whether DISC1D453G results in an anxiety phenotype, as suggested by increased thigmotaxis and reduced center time in the open field, we measured the time spent exploring the open arms of an elevated plus-maze. DISC1D453G females spent significantly less time in the open arms than wild-type females, but there were no genotypic differences in open arm time among males (Fig. 3A; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 4.83, P = 0.036); SME revealed a significant difference between females (F(1, 30) = 7.61, P = 0.01) but not males (F(1, 30) < 1)). DISC1D453G females remained in the closed arms for significantly longer than wild-type females, whereas DISC1D453G males showed a non-significant trend for spending less time in the closed arms than wild-type males (Fig. 3B; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 11.98, P = 0.002); SME revealed a significant genotypic difference between females (F(1, 30) = 8.78, P = 0.006) but not males (F(1, 30) = 3.73, P = 0.063)). DISC1D453G males spent more time in the center square than wild-type males, but there were no genotypic differences in center time among females (Supplementary Fig. S2; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 9.68, P = 0.004); SME revealed a significant difference between females (F(1, 30) = 4.63, P = 0.04) and males (F(1, 30) = 5.06, P = 0.032)). Total distances travelled during the first min and throughout the 5 min elevated plus-maze test were not significantly different between genotypes or sexes (Supplementary Fig. S2, two-way ANOVA: all F(1, 30) < 1).
Figure 3. Female DISC1D453G mice exhibit an anxiogenic profile in the elevated plus-maze.
(A) Female DISC1D453G mice (D453G; n = 10) spent significantly less time than female wild-type littermates (+/+; n = 7) exploring the open arms. There were no genotypic differences among males (P > 0.05). (B) Female DISC1D453G mice spent significantly more time in the closed arms than female wild-types, whereas male DISC1D453G mice showed a non-significant trend for spending less time in the closed arms (P = 0.063). (C) The center of the elevated plus-maze is where the open and close arms intersect. Male DISC1D453G mice spent significantly more time at the center compared with wild-type males. Female DISC1D453G mice spent significantly less time at the center compared with wild-type females. (D) Head dips made on the open arms are indicative of exploratory behavior. Female DISC1D453G mice made significantly fewer head dips compared with wild-type females. *P < 0.05, **P < 0.01 versus +/+ of the same sex.
As a final measure of elevated plus-maze exploration, head dips (downward movement of mouse’s head toward the floor from the open arms) were counted35. Female DISC1D453G mice made significantly fewer head dips than female wild-types (Supplementary Fig. S2; two-way ANOVA: significant genotype x sex interaction (F(1, 30) = 7.89, P = 0.009); SME revealed a significant difference between females (F(1, 30) = 6.26, P = 0.018) but not males (F(1, 30) = 2.17, P = 0.15)).
The forced swim and tail suspension tests are established paradigms for the identification of depressive-like behaviors in rodents36, in which DISC1Q31L mice have previously shown increased immobility24. In the forced swim test, in which time spent floating within an inescapable cylinder of water is measured, there were no significant differences for either genotype or sex (Supplementary Fig. S2, two-way ANOVA, all factors P > 0.05). Similarly, there were no differences for either genotype or sex for the time spent immobile in the tail suspension test (Supplementary Fig. S2, two-way ANOVA, all factors P > 0.05). Taken together, the DISC1D453G mutation appears to result in an anxiogenic profile in females only, without an overtly depressive-like phenotype in either males or females.
Female DISC1D453G mice show reduced social interaction
Schizophrenia is associated with reduced sociability37, and DISC1Q31L mice have previously shown reduced social interaction24. To assess the sociability of DISC1D453G mice, we utilized the three-chambered social approach test38, which measures the preference of the mouse to either explore a novel adult male conspecific enclosed in a ventilated container (Stranger 1) versus an identical but otherwise empty container.
Notably, female wild-type mice spent significantly longer exploring the novel mouse compared to the empty container than any other group, including female DISC1D453G mice, although all groups showed a preference for exploring the novel mouse (Fig. 4A; RM three-way ANOVA, genotype x sex interaction (F(1, 29) = 7.73, P = 0.009); SME revealed a significant difference between female mice (F(1, 29) = 12.68, P = 0.001) but not male mice (F(1, 29) < 1)). The reduced social interaction of DISC1D453G females compared with wild-type females was not due to deficient ambulation within the arena, as there were no genotypic differences for distance travelled during the test (Supplementary Fig. S3; two-way ANOVA, all P > 0.05).
Figure 4. Female DISC1D453G mice show less social interaction.
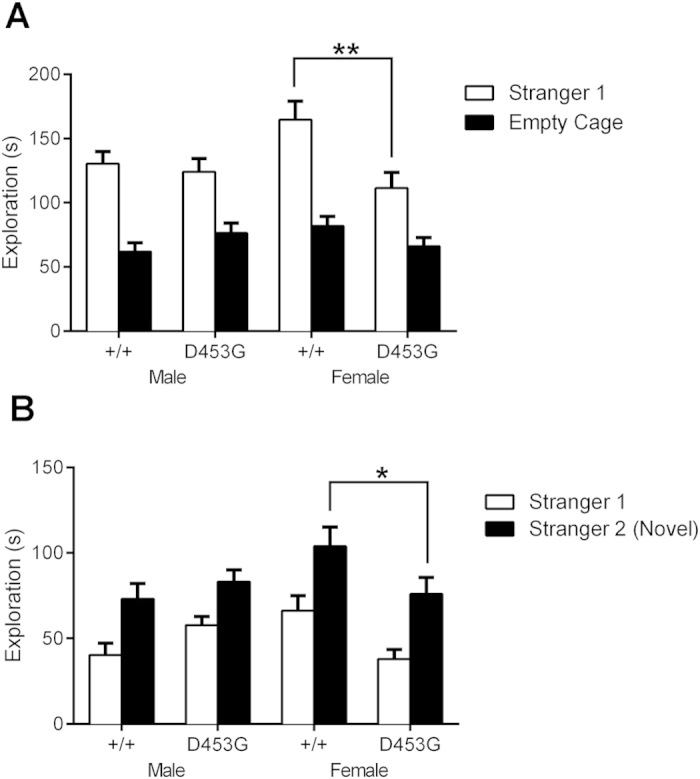
(A) Time spent by wild-type (+/+; n = 16, 9 male and 7 female) and DISC1D453G (D453G; n = 17, 7 male and 10 female) mice exploring a novel mouse or an empty container. All groups were able to discriminate between a novel mouse (Stranger 1) and an empty container, but female DISC1D453G mice spent significantly less time than wild-type females interacting with the novel mouse. There was no genotypic difference in time in contact with the novel mouse among males (P > 0.05). (B) The mice were then tasked with discriminating between the mouse previously explored and a second novel adult male mouse (Stranger 2). Again, both genotypes and sexes were able to correctly discriminate the second novel mouse, but female DISC1D453G mice spent significantly less time interacting with the novel male mouse. There were no male genotypic differences (P > 0.05). *P < 0.05, **P < 0.01 vs. female +/+.
To examine social recognition, the test mouse then encountered a second novel adult male mouse in the previously empty container (Stranger 2), as well as the now familiar conspecific. Once again, female DISC1D453G mice exhibited lower contact with the novel mouse and the previously introduced mouse than female wild-type littermates (Fig. 4B; RM three-way ANOVA, genotype x sex interaction (F(1, 29) = 9.86, P = 0.004); SME revealed a significant difference between female mice (F(1, 29) = 9.09, P = 0.005) but not male mice (F(1, 29) = 2.08, P = 0.16)), although they could clearly discriminate between Stranger 1 and Stranger 2. Again, this difference cannot be explained by reduced exploration of the arena, as there were no genotypic differences in the total distance travelled (Supplementary Fig. S3; two-way ANOVA, all P > 0.05). Taken together, the DISC1D453G mutation does not appear to disrupt the ability to discriminate between the two unfamiliar mice, a deficiency previously shown by DISC1Q31L mice24, but it does reduce total social interaction time in a female-specific manner.
Disruption of nesting behavior has been observed in a genetic mouse model of schizophrenia39 and offered as a murine measure of the negative symptom of self-neglect40,41. We found that nest-building ability was intact in DISC1D453G mice; both genotypes and sexes were able to make nests of similar quality (Supplementary Fig. S3; two-way ANOVA; no main effects or interactions (all F(1, 30) < 1)).
Male DISC1D453G mice show a deficit in passive avoidance
Sensory gating and information processing are frequently disrupted in schizophrenia, and are thought to reflect the cognitive impairments associated with the disorder42. Prepulse inhibition, whereby a preceding brief low-intensity stimulus decreases the response to a startle-eliciting stimulus, is disrupted in schizophrenia patients17 and in DISC1L100P mice24. We found that the DISC1D453G mutation did not alter the acoustic startle response for sound intensities ranging from 70 to 120 dB (Supplementary Fig. S4; three-way ANOVA, all main effects (F(1, 30) < 1), genotype x sex (F(1, 30) = 1.14, P > 0.05), dB intensity x genotype x sex (F(1, 30) = 1.14, P > 0.05)). There were no significant differences in PPI at the differing prepulse intensities between the groups (Fig. 5A; three-way ANOVA, main effects of genotype (F(1, 30) = 2.68, P > 0.05) and sex (F(1, 30) = 1.46, P > 0.05), genotype x sex (F(1, 30) < 1), dB intensity x genotype x sex (F(1, 30) < 1)).
Figure 5. DISC1D453G affects long-term memory of male mice in the passive avoidance test but has no effect on PPI.
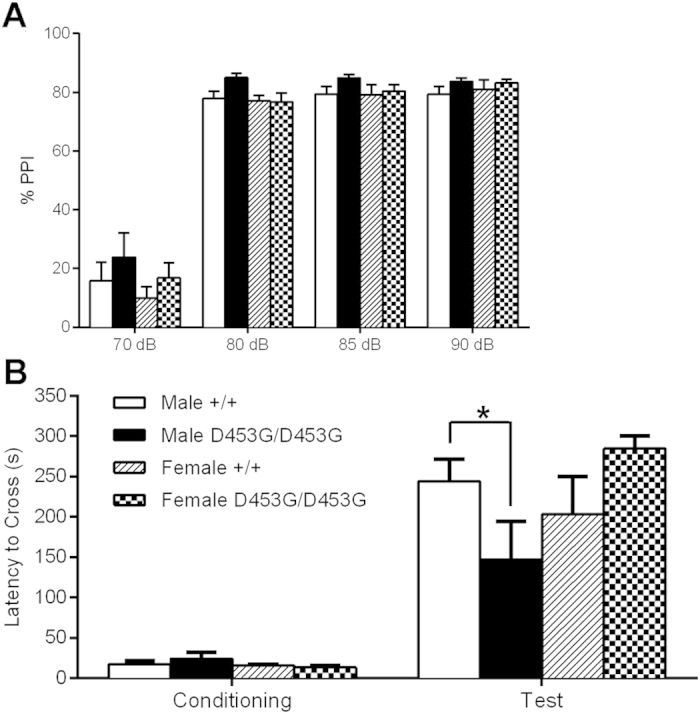
(A) The percentage of PPI at four different tones was measured. DISC1D453G mice (D453G/D453G; n = 17, 7 male and 10 female) showed similar PPI to wild-types (+/+; n = 17, 10 male and 7 female), with no significant differences (P > 0.05). (B) Long-term (24 hr) memory was assayed using the passive avoidance test, whereby the mouse must learn to suppress its innate preference for the dark chamber through footshock conditioning. Initially, mice readily cross to the dark chamber (left side, Conditioning). 24 hr later, the latency to cross was measured again (right side, Test). Compared with wild-type mice, DISC1D453G mice more readily crossed to the chamber in which the footshock was delivered. There were no genotypic differences among females (P > 0.05). *P < 0.05 vs. male +/+.
To assess long-term memory, we employed the passive avoidance test, which requires the mouse, via a single footshock conditioning trial, to subsequently inhibit its natural tendency to move from an illuminated chamber to a dark chamber (photophobia). Impairment in passive avoidance has previously been shown by a mouse model of psychosis induced by ketamine43,44. During the conditioning trial, DISC1D453G and wild-type mice of either sex showed similar latencies to cross to the dark chamber. During the probe trial 24 hours later, male DISC1D453G mice crossed to the dark chamber more readily than male wild-type mice. However, there were no significant genotypic differences between female mice (Fig. 5B; repeated-measures three-way ANOVA; genotype x sex interaction (F(1, 30) = 5.88, P = 0.022); SME revealed no significant genotypic differences between males (F(1, 30) = 1.27, P > 0.05) or females (F(1, 30) < 1) during conditioning, but there was a significant difference between male mice (F(1, 30) = 4.16, P = 0.050) but not female mice (F(1, 30) = 2.96, P = 0.096) during the test 24 hours post-conditioning).
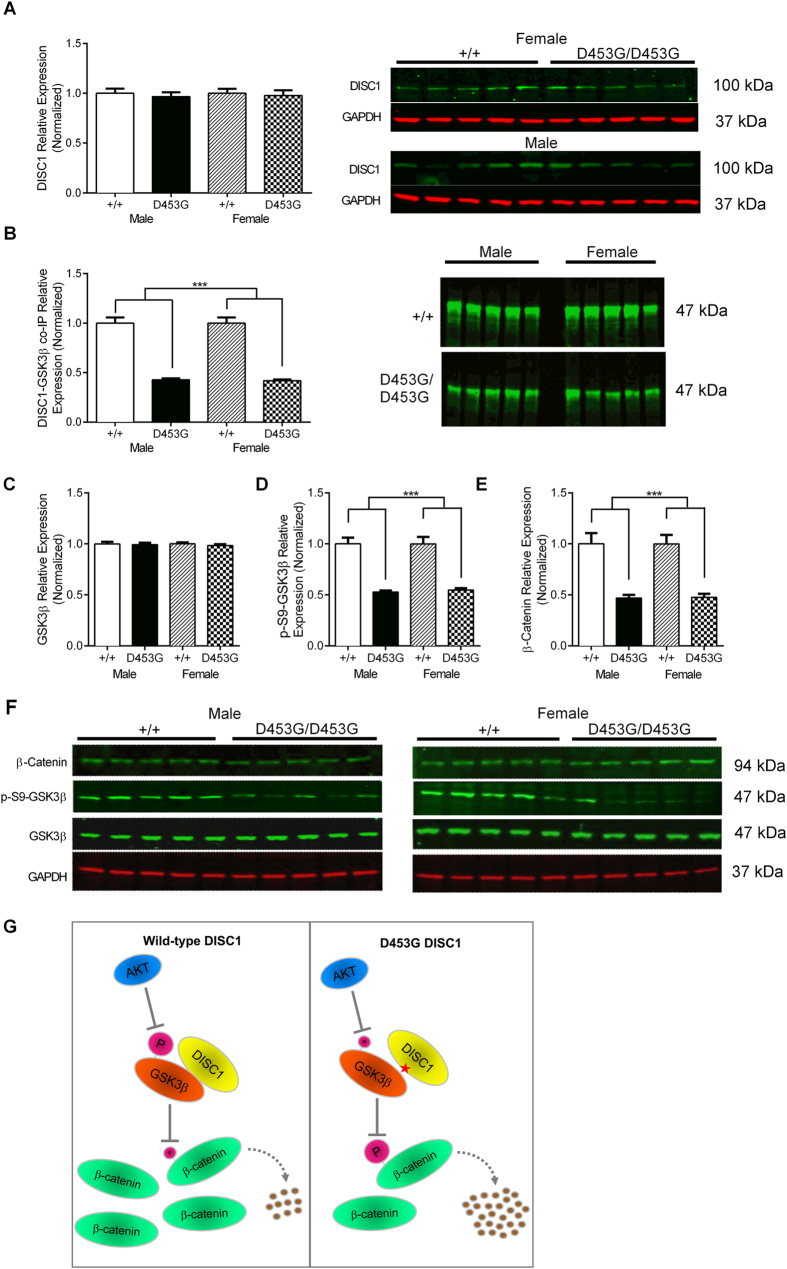
DISC1D453G mutation disrupts GSK3β signalling
To determine whether the D453G mutation affects expression of the DISC1 protein, whole brain homogenates were subjected to Western blot analysis. No differences were found in the abundance of DISC1 for either genotype or sex (Fig. 6A; two-way ANOVA: no main effects or interactions (all F(1, 60) < 1)). Previous studies have revealed DISC1’s involvement in regulation of the β-catenin/GSK3β pathway, whereby DISC1 inhibits GSK3β activity through direct physical interaction, which reduces β-catenin phosphorylation and stabilizes β-catenin19,23. However, the GSK3β binding sites on DISC1 have been localized with only limited precision: to a 220-residue region (1–220) at the N-terminus and a 240-residue region (356–595) that encompasses D453 on the C-terminal tail19. Missense variants within the N-terminal domain of DISC1 in mice (Q31L and L100P)26,27 and humans (R264Q)23 have been shown to reduce the interaction of DISC1 with GSK3β. To test whether the interaction of DISC1 with GSK3β is altered by the D453G mutation, DISC1-GSK3β binding was assessed in whole brain homogenates by co-immunoprecipitation. In DISC1D453G mice of either sex, DISC1-GSK3β binding was reduced by 57–58% compared with wild-type levels (Fig. 6B; two-way ANOVA: genotype (F(1, 60) = 184.5, P < 0.0001), sex (F(1, 60) < 1, P > 0.05), sex x genotype (F(1, 60) < 1, P > 0.05)). Such a marked reduction in DISC1-GSK3β binding identifies D453 as critical for this interaction.
Figure 6. DISC1D453G disrupts GSK3β signaling.
(A) Western blotting revealed comparable levels of DISC1 protein in whole brain homogenates from wild-type (+/+; n = 10, 5 male and 5 female) and DISC1D453G (D453G/D453G; n = 10, 5 male and 5 female) mice (left side; mean of three gels). Representative blots of DISC1 and GAPDH loading control for male and female DISC1D453G mice (right side). (B) The ability of DISC1 to complex with GSK3β was tested by co-immunoprecipitation (co-IP). DISC1D453G significantly reduced DISC1-GSK3β binding in both male and female DISC1D453G mice. (C) GSK3β protein levels were comparable between wild-type and DISC1D453G mice of either sex. (D) Levels of S9 phosphorylated GSK3β (p-S9-GSK3β) normalized to total GSK3β were significantly decreased in whole brain homogenates from both male and female DISC1D453G mice. (E) β-catenin protein levels were decreased in whole brain homogenates from DISC1D453G mice of either sex. (F) Representative blots for male (left side) and female (right side) wild-type and DISC1D453G mice. (G) Schematic model depicting the effects of D453G on GSK3β signaling. Mutation D453G reduces the interaction of DISC1 with GSK3β, and AKT-dependent phospho-inhibition of GSK3β at S9 is decreased. The less inhibited GSK3β phosphorylates more β-catenin, leading to more ubiquitin-mediated degradation of β-catenin, and reducing the cellular level of β-catenin. Red star: D453G mutation; T-shaped arrows: inhibition; magenta circles: phosphate group, size indicating degree of phospho-inhibition; curved broken arrow: ubiquitin-mediated degradation; brown circles: products of β-catenin proteolysis.
Decreased S9 phospho-inhibition of GSK3β has previously been observed in striatum from L100P mice28, and in striatum and frontal cortex samples from schizophrenia patients of unknown DISC1 genotype28,45. We found that the abundance of GSK3β in whole brain homogenates was unaffected by the D453G mutation, with males and females from both genotypes having comparable GSK3β protein levels (Fig. 6C; two-way ANOVA: no main effects or interactions (all F(1, 60) < 1)). However, levels of S9 phosphorylated GSK3β (p-S9-GSK3β), measured as the ratio of p-S9 density to total GSK3β density, were significantly decreased in whole brain homogenates from DISC1D453G mice, compared with wild-type (Fig. 6D; two-way ANOVA: genotype (F(1, 60) = 95.6, P < 0.0001), sex (F(1, 60) < 1, P > 0.05), sex x genotype (F(1, 60) < 1, P > 0.05)). Accumulation of β-catenin has been widely employed as an indirect assay of GSK3β activity on the basis that GSK3β phosphorylates β-catenin, thus targeting it for ubiquitin-mediated degradation by the proteosome, whereas inhibition of GSK3β by lithium causes accumulation of β-catenin in a dose-dependent manner46. The decrease in phospho-inhibition of GSK3β in DISC1D453G mice raised the possibility that the overall levels of β-catenin may be decreased. We found that whole brain homogenates from DISC1D453G mice indeed had significantly lower levels of β-catenin (Fig. 6E; two-way ANOVA: genotype (F(1, 60) = 52.0, P < 0.0001), sex (F(1, 60) < 1, P > 0.05), sex x genotype (F(1, 60) < 1, P > 0.05)), consistent with their reduced phospho-inhibition of GSK3β. Thus, the D453G mutation reduces β-catenin abundance (Fig. 6G).
Discussion
We have conducted an initial analysis of the behavioral effects of a novel missense mutation (D453G) within the C-terminal tail domain of DISC1 in mice with a predominantly C57BL/6N genetic background. The substitution of an aspartic acid (D; polar, hydrophilic, negative charge) by a glycine (G; aliphatic, neutral) at position 453 was predicted to have a harmful effect on the structure and function of DISC1 by all three bioinformatics algorithms utilized, consistent with D453 being conserved across mammals and reptiles. This conservation allows direct comparison across species, but the corresponding residue in human DISC1 (D456) is not the location of a known human disease-associated variant.
In the absence of a published crystal structure for DISC1, we used bioinformatics resources to predict the effects of D453G on DISC1 structure. The COILS program47 predicted a 48-residue coiled-coil region corresponding to residues 449-496 in mouse DISC1, encompassing D453. The PsiPred48 secondary structure prediction of α-helices corresponding to residues 452–494 overlaid appropriately with the region of coiled-coil helix propensity. This prediction closely matches a region of coiled-coil potential corresponding to residues 452–499 in human DISC116. The hallmark of most coiled-coils is a regular seven-residue sequence pattern known as a heptad repeat, which is often labeled ‘abcdefg’, where positions ‘a’ and ‘d’ are internalized, stabilizing the structure when two or more helices wind around each other to form ‘supercoils’, while the remaining positions are solvent exposed and usually provide surfaces for protein-protein interaction47. This is probably true in the case of D453, as it corresponds to position ‘b’ in the heptad repeat, and was found by co-immunoprecipitation in the present study to be critical for the interaction of DISC1 with GSK3β in whole brain homogenates. Among the seven vertebrate species examined for DISC1 protein conservation (Fig. 1C), none has a glycine (G) anywhere within the amino acid sequence of the predicted coiled-coil region corresponding to residues 449–496 in mouse DISC1, suggesting that D453G may indeed have a structural impact.
Among the known DISC1 binding partners, we focused specifically on GSK3β because D453G occurs within a known GSK3β binding region on DISC1 (residues 356–595)19. Moreover, mutations Q31L and L100P within another GSK3β binding region on DISC1 (residues 1–220) were both previously shown to reduce the interaction of DISC1 with GSK3β26,27 in the mouse brain. In the present study, we found that whole brain homogenates from DISC1D453G mice showed unaltered levels of DISC1 protein, but decreased binding of DISC1 to GSK3β, decreased S9 phospho-inhibition of GSK3β, and decreased levels of the GSK3β-specific substrate β-catenin (Fig. 6G).
Similarly reduced phospho-S9-GSK3β has previously been observed in striatum from L100P mice28, in striatum and frontal cortex samples from schizophrenia patients28,45, and in peripheral lymphocytes from schizophrenia patients45 and drug-free bipolar disorder patients49. Human lymphoblast cell lines endogenously expressing the schizophrenia risk variant R264Q have shown decreased levels of β-catenin compared with the major R264 allele23. β-catenin is known to regulate the self-renewal of neural progenitor cells and neuronal differentiation in the developing neocortical ventricular zone, subcortical areas of the telencephalon, and ventral midbrain50. Hence, interruption of GSK3β signaling could impair neurodevelopment, thus lending support to a putative pathogenic role in neurodevelopmental disorders.
In behavioral tests, we found that neither male nor female DISC1D453G mice exhibited impairment in startle reactivity or prepulse inhibition (Table 1). Female DISC1D453G mice did, however, exhibit novelty-induced hyperlocomotion in the open field, as well as an anxiogenic profile in the open field and the elevated plus-maze, and reduced social exploration of unfamiliar mice in the social approach test. The open arms of the elevated plus-maze were illuminated with an intensity of 200 lux, which is similar to the light levels used in elevated plus-maze experiments that detected an anxiolytic phenotype in Nos1 knockout mice51 and an anxiogenic phenotype in Alox5 knockout mice52. Another anxiety-related behavior that is sometimes measured in the elevated plus-maze is the frequency or duration of freezing (suppression of all movement except that required for respiration)35, but the overhead camera that we used with the elevated plus-maze was not of sufficiently high resolution to score freezing accurately.
Table 1. Summary of observed phenotypes of DISC1D453G mutant mice.
| Phenotype | Male DISC1D453G | Female DISC1D453G |
|---|---|---|
| Open Field | ||
| Distance travelled (m) | = | ↑ |
| Time spent in outer zone | = | ↑ |
| Time spent in center zone | = | ↓ |
| Rearing (s) | ↓ | = |
| Elevated Plus-Maze | ||
| Time spent in open arms (%) | = | ↓ |
| Open arm entries | = | ↓ |
| Time spent in closed arms (%) | = | ↑ |
| Time spent in center (%) | ↑ | = |
| Head dips | = | ↓ |
| Social Interaction | ||
| Novel mouse vs. empty container | = | ↓ |
| Stranger 2 vs. Stranger 1 | = | ↓ |
| Passive Avoidance | ||
| Latency to cross (24 hours) | ↓ | = |
| DISC1-GSK3β pathway | ||
| DISC1 protein level | = | = |
| DISC1-GSK3β binding | ↓ | ↓ |
| GSK3β protein level | = | = |
| Phospho-inhibition of GSK3β | ↓ | ↓ |
| β-catenin protein level | ↓ | ↓ |
= no significant difference, ↓ decreased, ↑ increased; versus wild-type of the same sex.
Male DISC1D453G mice appeared less anxious than DISC1D453G females. However, their decreased rearing in the open field and increased center time in the elevated plus-maze would be more suggestive of an increased anxiety state compared with wild-type males. Although these mice displayed a non-significant tendency to increased center time in the open field, which would normally be interpreted as mild anxiolysis, it should be noted that both the elevated plus-maze and open field tests commenced with the placement of mice at the center of the apparatus. The observed increase in center time in both novel environments may therefore have reflected an initial reluctance by the mice to move from their start position. However, this hypothesis was not supported by an analysis of total distance travelled in the first minute of each test, which failed to reveal any significant main effects or interactions. Male DISC1D453G mice also displayed a deficit in passive avoidance, a behavioral paradigm that reflects the cognitive ability of mice to associate the dark compartment with the aversive event (footshock) experienced in that compartment during training.
Consistent with the decreased inhibitory phosphorylation of GSK3β and the passive avoidance deficit exhibited by DISC1D453G males, mice with postnatal neuronal expression of the phosphorylation defective mutant GSK3β*S9A have also shown impaired long-term memory in passive avoidance53. However, the disruption of GSK3β signaling by DISC1D453G was observed in both sexes, so cannot readily account for the sex-dependent behavioral effects of the mutation. Assessing the behavioral effects of the GSK3β inhibitor TDZD-8 in DISC1D453G males and females would help to evaluate the contribution of alterations in GSK3β signaling to their respective behavioral phenotype, as done previously with the Q31L and L100P mutant lines26,27.
It is theoretically possible that disruption of GSK3β signaling could have different effects in males and females, as the female sex hormone and neuroprotectant estrogen regulates the stability of β-catenin, induces the translocation of β-catenin to the cell nucleus, and regulates β-catenin-mediated transcription in neurons54. However, as the current study has no data directly relevant to estrogen’s regulation of β-catenin, further study is needed to empirically test this hypothesis. Since D453 (human D456) occurs within a multi-protein interaction site16 and a self-association domain (residues 403–504)55, GSK3β is unlikely to be the only interaction partner affected by D453G. By virtue of the complexity of DISC1 interaction with proteins intimately involved in either neurodevelopment or neuromodulatory signaling17, other protein interactions and a variety of neural processes could be altered by D453G; their modulation by mechanisms for sex-dependent differences in gene expression56,57 could contribute to the sexually-dimorphic behavioral phenotype we observed.
Female DISC1D453G mice displayed an anxiogenic phenotype in the elevated plus-maze and open field, whereas males were less affected in these tests. It remains unclear why only female DISC1D453G mice exhibited increased anxiety. In human genetic studies, a single nucleotide polymorphism (SNP) in DISC1 intron 9 (rs821577) has shown female-specific associations with anxiety, depression and neuroticism in elderly Scottish subjects58, and with schizophrenia in a Japanese population59, while another DISC1 intron 9 SNP (rs2295959) has shown female-specific association with schizophrenia in Han Chinese subjects60. These findings suggest that variance in DISC1 has stronger effects in females than in males, which could potentially contribute to the sex differences found in the schizophrenia phenotype, such as age at onset, premorbid personality, clinical subtypes, psychosocial function, and responses to therapy61.
Transgenic mice with inducible expression of truncated human DISC1 (residues 1–597) under the control of the forebrain-specific Camk2a promoter also show sex-dependent behavioral abnormalities62. Male transgenics displayed enhanced spontaneous locomotor activity and alterations in social interaction, whereas female transgenics demonstrated deficient reference spatial memory in the Morris water maze62. In another mouse study, Disc1 knockdown specifically in adult-born dentate gyrus neurons resulted in increased mTOR signaling and several behavioral deficits, including enhanced anxiety in the elevated plus-maze, whereas Disc1 knockdown specifically in hippocampal CA1 neurons did not elicit any behavioral changes63. Treatment with rapamycin, an FDA-approved compound that inhibits mTOR, reversed the increases in mTOR signaling and both prevented and treated the behavioral deficits. Only male mice were used, so sex effects could not be detected63.
In the three-chambered social approach test38, DISC1D453G mice of either sex showed a preference for an unfamiliar mouse versus an empty container, and were clearly able to discriminate between the previously introduced mouse and a novel mouse. However, DISC1D453G females spent less time than wild-type females engaged in social exploration of the unfamiliar mice. We found that the significant difference was largely driven by the wild-type females (rather than any other group) spending more time socially approaching the unfamiliar mice. Given that we used male mice as the novel social stimulus, it is possible that a form of sexual attraction is responsible for the increased social exploration by the wild-type females, which was blunted in the DISC1D453G females. However, extensive validation using the same apparatus as currently employed found no detrimental effect of using male conspecifics as there were “no confounding sexual or aggressive behaviors, because the strangers were contained within the wire cages64”.
The DISC1D453G mice that we tested had a predominantly C57BL/6N background but descend from an ENU-mutagenized BALB/cAnN male. On average, by backcross generation N6, 0.78% of their genome would have been of BALB/cAnN origin. However, the significantly lower open field activity shown by BALB/c mice compared with C57BL/6 mice65 suggests that the observed behavioral phenotype of DISC1D453G mice is unlikely to reflect any residual contribution of the BALB/cAnN alleles surrounding Disc1 at 125,054,195-125,261,858 bp on chromosome 8.
In summary, we have generated and analyzed mice carrying mutation D453G in order to probe the in vivo effects of missense mutation of DISC1’s C-terminal tail. Our initial analysis revealed that the behavioral effects of D453G were sex dependent. While female DISC1D453G mice demonstrated a behavioral phenotype of novelty-induced hyperlocomotion, increased anxiety, and reduced social interaction of conspecifics, male DISC1D453G mice displayed a deficit in passive avoidance. Consistent with the location of D453G within a predicted α-helical coiled-coil and a known GSK3β binding region, whole brain homogenates from DISC1D453G mice of either sex showed evidence of interrupted GSK3β signaling, with decreased S9 phospho-inhibition of GSK3β, and decreased levels of β-catenin. Although D453G likely impacts binding to other proteins, our data suggest that interruption of GSK3β signaling may be part of the mechanism underlying the behavioral phenotype associated with D453G. This study thus adds to the converging evidence that GSK3β represents a pathogenic signaling pathway in schizophrenia and affective disorders.
Methods
Mutation Screen
Heteroduplex detection by high-resolution melting analysis in a LightScanner (Idaho Technology) was used to screen 7,776 male F1 progeny of ENU-mutagenized BALB/cAnN males and untreated C3H/HeH females in the MRC Harwell ENU DNA archive for mutations in exon 5 of Disc1, which was amplified using PCR primers F, 5′-TCT CCA CAC CTG TAC CAA TG-3′ and R, 5′-CCA CTC CCT TTC TGG AAG AT-3′. The A1358G mutation in Disc1 was confirmed by DNA sequencing using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). To predict the potential effect of D453G on the function and structure of the DISC1 protein, the following three programs were utilized: PolyPhen-2 (genetics.bwh.harvard.edu/pph2)29, PMut (mmb.pcb.ub.es/PMut)30 and PROVEAN (provean.jcvi.org/seq_submit.php)31. The secondary structure within mouse DISC1 (NP_777279) was analyzed and annotated using the coiled-coil predictor COILS with a 21-residue window (ch.embnet.org/software/COILS_form.html)46 and the secondary structure predictor PsiPred (bioinf.cs.ucl.ac.uk/psipred)47. ClustalW2 (ebi.ac.uk/Tools/msa/clustalw2)66 was used to align the DISC1 protein sequences of mouse (ENSMUSP00000112410), sheep (ENSOARP00000003502), human (ENSP00000355593), soft-shelled turtle (ENSPSIP00000005750), green anole lizard (ENSACAP00000002671), chicken (ENSGALP00000040765) and zebrafish (ENSDARP00000131863). DISC1D453G/+ frozen sperm is available from the MRC Mammalian Genetics Unit, UK (har.mrc.ac.uk).
Subjects
Heterozygous N2 backcross progeny of the founder DISC1D453G/+ (C3H/HeH × BALB/cAnN) F1 male (EMRCB/60.3d) and wild-type C57BL/6NTac females (Taconic) were backcrossed through the male and female lines to C57BL/6NCrl (Charles River) for four generations (N3-N6) before heterozygotes with a predominantly C57BL/6N genetic background (average of 98.4375% at N6) were intercrossed to generate homozygous DISC1D435G/D453G mutants for phenotypic testing, which were viable and grossly indistinguishable from their wild-type DISC1+/+ littermates. Mice were weaned at 3 weeks of age and grouped housed (2–4 mice/cage) with same-sex littermates under a 12 hour light/dark cycle (lights on at 06.00). Pelleted feed (CRM-P, SDS) and water were provided ad libitum.
DNA was extracted from ear biopsies taken at weaning. Mice were genotyped for the DISC1D453G mutation by the absence of a BseNI restriction site (5′-ACTGGN*-3′) in a 411-bp fragment amplified using PCR primers F, 5′-AAG CCC CAA CAG ATC CTA GT-3′ and R, 5′-AAA GGA GTG GCC GCT CTA C-3′ with HotShot Diamond (Clent Life Science) using the thermocycling program of: 95 °C for 5 min, followed by 34 cycles of 94 °C for 30 s, 59 °C for 60 s, 72 °C for 40 s, followed by 72 °C for 10 min. PCR products were subsequently incubated with BseNI (Fermentas) at 65 °C overnight and visualized by agarose gel electrophoresis, with a 253-bp band indicating the wild-type allele and a 411-bp band indicating the mutant allele. All procedures were approved by the University of Leeds Ethical Review Committee and conducted in accordance with the requirements of the Animals (Scientific Procedures) Act, 1986.
Behavioral Experiments
All behavioral experiments were conducted using young adults over 8 weeks of age. Mice were handled for one week prior to behavioral testing. Mice were transferred to the experimental room 30 mins prior to the start of testing. All apparatus was cleaned with 70% ethanol between each mouse. Unless stated otherwise, all experiments were recorded using the tracking software ANY-Maze (Stoelting). Experiments were conducted in the order described below. A 2–3 day inter-test interval was employed for less severe experiments, and an inter-test interval of 1–2 weeks was used for the more invasive tests (PPI, passive avoidance). For all experiments, except PPI and passive avoidance (that were illuminated by LEDs within the chambers), the apparatus was illuminated by standard fluorescent ceiling lights at an intensity of ~200 lux. Experiments were conducted using identical apparatus and protocols as described elsewhere67. Methods are described briefly below.
Open Field
Mice were placed into a 40 × 40 × 40 cm arena illuminated at ~200 lux at the center and were left to explore undisturbed for 30 mins. A webcam affixed to a tripod was positioned directly above the arena to record the trials. The tracking software divided the arena into the following zones, using the following dimensions: Outer Zone: 8 cm from the outer walls; Center Zone: 6.4 cm2 (16% of the total area); Intermediate Zone: the remaining area between the Outer Zone and the Center Zone. Time spent in each of the zones was measured, along with the distance travelled. The duration of rearing and grooming during the trial was measured by the experimenter.
Elevated Plus-Maze
The elevated plus-maze consisted of two closed arms with white opaque walls (15 cm high) and two open arms. All arms measured 30 cm long and 5 cm wide. There was a central square measuring 5 cm2 where all arms met. The open arms were illuminated with an intensity of 200 lux, the central square with 150 lux, and the closed arms with 100 lux. Sessions commenced by placing the mouse onto the central zone facing the open arm opposite from the experimenter. Mice were allowed to explore the maze for 5 min and were then returned to the home cage. Mouse movement was tracked using ANY-Maze and time in the open arms, closed arms and central zone was measured, in addition to the frequency of head dips (downward movement of mouse’s head toward the floor from the open arms). Entry into an arm was defined as when the hind legs crossed the boundary of the arm.
Forced Swim Test
A 5L glass beaker (17 × 27 cm) was filled with 3L of water at 25 ± 1 °C. The mouse was introduced into the water facing away from the experimenter and allowed to swim freely for 6 mins. Time spent floating was measured from 2 mins into the trial. Floating was defined as the lack of all movement except those required to keep the mouse afloat.
Tail Suspension Test
The mouse was suspended by electrical tape attached near the tip of the tail, which was affixed to a structure that allowed the mouse to be suspended 20 cm from the ground. The trial lasted for 6 mins, during which the time spent immobile was measured. Immobility was defined as the lack of all movement except that required for respiration.
Social Approach Test
Social approach was assessed using a three-chambered apparatus (60 × 40 cm), which had two doors to allow the mouse to access left and right chambers from a central compartment (each chamber being 40 × 20 cm). Following a 15-min habituation period, two cylindrical containers (10 × 10.5 cm, comprising vertical metal bars 9 mm apart) were placed into the left and right chambers, into one of which an unfamiliar adult male C57BL/6 mouse (age >10 weeks; ‘stranger 1’) was placed. Left/right placement was counterbalanced across groups. Time spent exploring each container was measured for 10 mins.
A second unfamiliar adult male C57BL/6 mouse (‘stranger 2’) was then placed into the empty cylinder and time spent exploring each container was measured for 10 mins. The time spent exploring stranger 1 or the empty cylinder in the first phase, and time spent exploring either stranger 1 or 2 in phase two were recorded. Due to a software recording error, only 9 male wild-type mice were included in the analysis of this experiment.
Passive Avoidance
Passive avoidance was assessed using the Med Associates Shuttle Box controlled by MedPC software. The shuttle box consisted of two chambers, divided by a remotely operated guillotine door, and housed within a sound attenuation box with a fan generating background noise. One half of the chamber was darkened using a black cloth cover (defined as the ‘dark chamber’). The other side of the chamber was illuminated by a light bulb. During the conditioning phase, latency to cross from the light chamber to the dark chamber was measured. When the mouse fully entered the dark chamber, the door closed and, 10 s later, a 0.45 mA footshock lasting 3 s was delivered. The mouse was removed 30 s after the footshock. 24 hours later, the latency to cross to the dark chamber was measured in a probe trial. The prove trial was stopped when either the mouse traversed to the dark chamber or if had not done so within 300 s.
Prepulse Inhibition
Mice were tested for acoustic startle reactivity (ASR) and prepulse inhibition (PPI) using the SR-LAB startle response system (San Diego Instruments), which consists of a sound attenuating box housing an animal enclosure platform, a fan and a speaker (all of which were lit from above by LEDs). Acoustic startle responses were measured at 70 dB, 80 dB, 85 dB, 90 dB and 120 dB. PPI was measured by the delivery of a tone at either 70 dB, 80 dB, 85 dB or 90 dB (prepulse) for 10 ms followed by a 100 ms gap with background noise and then a 120 dB ‘startle’ tone for 40 ms. Background noise at 65 dB was presented throughout the trials. 10 presentations of each ASR and PPI tone were given and the responses averaged. Inter-trial intervals averaged 25 s.
Nesting Behavior
This test was based on a published protocol68. Mice were individually housed before the onset of the dark phase of the light/dark cycle with no environmental enrichment apart from a 3 g ‘nestlet’ (Lillico) of pressed cotton. Nesting ability was assessed the following morning at 0800 h and was rated using the following scale:
1. Nestlet not noticeably touched (>90% intact); 2. Nestlet partially torn up (50–90% intact); 3. Nestlet mostly shredded but no identifiable nest site; 4. Identifiable nest, but flat; 5. A perfect or near-perfect nest.
Protein Analysis
Tissue Lysates
Using a dounce homogenizer, single hemispheres were homogenised in lysis buffer consisting of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and protease and phosphatase inhibitors (Roche), and incubated at 4 °C with constant agitation for 90 mins. Lysates were cleared by centrifugation at 14,000 rpm for 20 mins (4 °C) and protein concentration was determined using the Bradford assay. Protein concentration was equalized in all samples.
Antibodies
The following primary antibodies were used: anti-DISC-1 (1 in 1000, sc-47990, Santa Cruz), anti-GSK3β (1 in 1000, sc-9166, Santa Cruz), anti-phospho-GSK3β (Ser9) (1 in 1000, 9322, Cell Signaling), anti-β-catenin (1 in 1000, ab16051, Abcam), and anti-GAPDH (1 in 10,000, ab8245, Abcam).
Western Blotting
Samples were prepared by boiling in Laemmli buffer (10% SDS, 300 mM Tris-HCl, pH 7.2, 0.05% bromophenol blue, 50% glycerol, 10% β-mercaptoethanol) at 95 °C for 5 min, and resolved using the NuPAGE system with 4–12% pre-cast gels (Life Technologies) alongside protein standards (Precision Plus, BioRad). Proteins were then transferred onto nitrocellulose membranes (Amersham) at 30 V for 75 mins. The membranes were then blocked in 5% (w/v) Bovine Serum Albumin (BSA) in TBS-T (25 mM Tris-HCl; pH 7.6, 100 mM NaCl, 0.5% Tween-20) for 1 hour at room temperature. Primary antibodies were diluted in 1% (w/v) BSA in TBS-T, and blots were incubated overnight at 4 °C. Bound antibody was detected using fluorescently conjugated donkey anti-goat, anti-rabbit or anti-mouse secondary antibodies (1:10,000, Licor Biosciences) and visualized using the Licor Odyssey Imaging system (Licor Biosciences). Quantification of the band intensity was accomplished by densitometry using Image Studio Lite v.5 (Licor Biosciences). All experiments were run in triplicate.
Immunoprecipitation
For immunoprecipitation, a DISC1 antibody was used to immunoprecipitate endogenous GSK3β. The resulting immunocomplexes were captured using Protein G beads (GE Healthcare) and incubated on a rotating wheel overnight (4 °C). The immunocomplexes were then collected by centrifugation at 10,000 × g for 5 min and washed three times with lysis buffer. Isotype-matched IgG (Sigma-Aldrich) was used as a negative control to screen for non-specific binding. Bound proteins were then eluted in Laemmli sample buffer and subjected to Western blotting as described above.
Data Analysis
All data are expressed as mean ± standard error of the mean (SEM). All datasets were initially checked for normality and homogeneity of variance. To assess differences between the variables and their impact upon performance, analysis of variance (ANOVA) was conducted, with the factors of genotype and sex always examined. Performance across time bins or days was analyzed by repeated measures ANOVA. If there were significant interactions between variables, tests of simple main effects (SME) were performed (Bonferroni corrected for multiple comparisons), followed by post hoc analysis when necessary. The ranking of the nest building was transformed according to the mean scale, and a two-way ANOVA was performed. All analyses were performed using SPSS (version 20). In all cases, α was set at < 0.05. Graphs were prepared using GraphPad Prism version 6.
Additional Information
How to cite this article: Dachtler, J. et al. Missense mutation in DISC1 C-terminal coiled-coil has GSK3β signaling and sex-dependent behavioral effects in mice. Sci. Rep. 6, 18748; doi: 10.1038/srep18748 (2016).
Supplementary Material
Acknowledgments
We thank Deen Quwailid (MRC Mammalian Genetics Unit) for technical support. This work was supported by a UK Medical Research Council grant (G0900625) to S.J.C. and R.J.R., a University of Leeds Wellcome Trust ISSF Fellowship and a British Pharmacological Society grant to J.D., and a UK Medical Research Council grant (MR/J007412/1) to G.S.B.
Footnotes
Author Contributions S.J.C. identified the mutation; J.D. conducted the behavioral experiments and managed the Disc1D453G mouse colony; C.E. conducted the protein experiments; J.D. and C.E. conducted the statistical analyses; J.D. and S.J.C. prepared the figures; J.D., R.J.R., G.S.B. and S.J.C. designed the experiments. J.D., R.J.R. and S.J.C. wrote the paper. All authors reviewed and approved the manuscript.
References
- Lewis D. A. & Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med 12, 1016–1022 (2006). [DOI] [PubMed] [Google Scholar]
- Ross C. A., Margolis R. L., Reading S. A., Pletnikov M. & Coyle J. T. Neurobiology of schizophrenia. Neuron 52, 139–153 (2006). [DOI] [PubMed] [Google Scholar]
- Berrettini W. H. Genetics of psychiatric disease. Annu Rev Med 51, 465–479 (2000). [DOI] [PubMed] [Google Scholar]
- Porteous D. J. et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry 19, 141–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D. et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet 336, 13–16 (1990). [DOI] [PubMed] [Google Scholar]
- .Millar J. K. et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 9, 1415–1423 (2000). [DOI] [PubMed] [Google Scholar]
- Chubb J. E. et al. The DISC locus in psychiatric illness. Mol Psychiatry 13, 36–64 (2008). [DOI] [PubMed] [Google Scholar]
- Porteous D. J. et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry 19, 141–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott J. H. et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA 102, 8627–8632 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Bourgon J. et al. A Disrupted-in-Schizophrenia 1 Gene Variant is Associated with Clinical Symptomatology in Patients with First-Episode Psychosis. Psychiatry Investig 11, 186–191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouaffak F. et al. Association of Disrupted in Schizophrenia 1 (DISC1) missense variants with ultra-resistant schizophrenia. Pharmacogenomics J 11, 267–273 (2011). [DOI] [PubMed] [Google Scholar]
- Song W. et al. Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochem Biophys Res Commun 367, 700–706 (2008). [DOI] [PubMed] [Google Scholar]
- Song W. et al. Identification of high risk DISC1 protein structural variants in patients with bipolar spectrum disorder. Neurosci Lett 486, 136–140 (2010). [DOI] [PubMed] [Google Scholar]
- Moens L. N. et al. Sequencing of DISC1 pathway genes reveals increased burden of rare missense variants in schizophrenia patients from a northern Swedish population. PLoS One 6, e23450 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. K. et al. DISC1 exon 11 rare variants found more commonly in schizoaffective spectrum cases than controls. Am J Med Genet B Neuropsychiatr Genet 156B, 490–492 (2011). [DOI] [PubMed] [Google Scholar]
- Soares D. C. et al. DISC1: Structure, Function, and Therapeutic Potential for Major Mental Illness. ACS Chem Neurosci 2, 609–632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo L. M. et al. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry 12, 74–86 (2007). [DOI] [PubMed] [Google Scholar]
- Millar J. K. et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1187–1191 (2005). [DOI] [PubMed] [Google Scholar]
- Mao Y. et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136, 1017–1031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P. et al. A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron 84, 1302–1316 (2014). [DOI] [PubMed] [Google Scholar]
- Kamiya A. et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet 15, 3313–3323 (2006). [DOI] [PubMed] [Google Scholar]
- Burdick K. E. et al. Elucidating the relationship between DISC1, NDEL1 and NDE1 and the risk for schizophrenia: evidence of epistasis and competitive binding. Hum Mol Genet 17, 2462–2473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. K. et al. Common DISC1 polymorphisms disrupt Wnt/GSK3β signaling and brain development. Neuron 72, 545–558 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote S. J. et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402 (2007). [DOI] [PubMed] [Google Scholar]
- Lee F. H. F. et al. Disc1 Point Mutations in Mice Affect Development of the Cerebral Cortex. J Neurosci 31, 3197–3206 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina T. V., Wang M., Liu F. & Roder J. C. Synergistic interactions between PDE4B and GSK-3: DISC1 mutant mice. Neuropharmacology 62, 1252–1262 (2012). [DOI] [PubMed] [Google Scholar]
- Lipina T. V. et al. Genetic and pharmacological evidence for schizophrenia-related Disc1 interaction with GSK-3. Synapse 65, 234–248 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P. et al. A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron 84, 1302–1316 (2014). [DOI] [PubMed] [Google Scholar]
- Adzhubei I., Jordan D. M. & Sunyaev S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 7, 7.20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Costa C. et al. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics 21, 3176–3178 (2005). [DOI] [PubMed] [Google Scholar]
- Choi Y. & Chan A. P. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31, 2745–2747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H. Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res 179, 75–86 (2009). [DOI] [PubMed] [Google Scholar]
- Simon P., Dupuis R. & Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res 61, 59–64 (1994). [DOI] [PubMed] [Google Scholar]
- Walsh J., Desbonnet L., Clarke N., Waddington J. L. & O’Tuathaigh C. M. Disruption of exploratory and habituation behavior in mice with mutation of DISC1: an ethologically based analysis. J Neurosci Res 90, 1445–1453 (2012). [DOI] [PubMed] [Google Scholar]
- Walf A. A. & Frye C. A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2, 322–328 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V., Moser P., Roux S. & Porsolt R. D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 8, 8.10A (2011). [DOI] [PubMed] [Google Scholar]
- Goldberg J. O. & Schmidt L. A. Shyness, sociability, and social dysfunction in schizophrenia. Schizophr Res 48, 343–349 (2001). [DOI] [PubMed] [Google Scholar]
- Yang M., Silverman J. L. & Crawley J. N. Automated three-chambered social approach task for mice. Curr Protoc Neurosci 8, 8.26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton M. R., Blaiss C. A., Powell C. M. & Südhof T. C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA 106, 17998–18003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tuathaigh C. M., Kirby B. P., Moran P. M. & Waddington J. L. Mutant mouse models: genotype-phenotype relationships to negative symptoms in schizophrenia. Schizophr Bull 36, 271–288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T. et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci USA 100, 8987–8992 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W. & Braff D. L. Information-processing deficits and thought disorder in schizophrenia. Am J Psychiatry 151, 363–367 (1994). [DOI] [PubMed] [Google Scholar]
- Uchihashi Y., Kuribara H., Isa Y., Morita T. & Sato T. The disruptive effects of ketamine on passive avoidance learning in mice: involvement of dopaminergic mechanism. Psychopharmacology (Berl) 116, 40–44 (1994). [DOI] [PubMed] [Google Scholar]
- Farber N. B. The NMDA receptor hypofunction model of psychosis. Ann NY Acad Sci 1003, 119–130 (2003). [DOI] [PubMed] [Google Scholar]
- Emamian E. S. et al. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36, 131–137 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang F., Phiel C. J., Spece L., Gurvich N. & Klein P. S. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem 278, 33067–33077 (2003). [DOI] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M. & Stock J. Predicting Coiled Coils from Protein Sequences. Science 252, 1162–1164 (1991). [DOI] [PubMed] [Google Scholar]
- McGuffin L. J., Bryson K. & Jones D. T. The PSIPRED protein structure prediction server. Bioinformatics 16, 404–405 (2000). [DOI] [PubMed] [Google Scholar]
- Polter A. et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 35, 1761–1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska M. B. Physiological role of β-catenin/TCF signaling in neurons of the adult brain. Neurochem Res 38, 1144–1155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wultsch T. et al. Behavioural and expressional phenotyping of nitric oxide synthase-I knockdown animals. J Neural Transm Suppl 72, 69–85 (2007), 10.1007/978-3-211-73574-9_10. [DOI] [PubMed] [Google Scholar]
- Joshi Y. B. & Praticò D. Knockout of 5-lipoxygenase results in age-dependent anxiety-like behavior in female mice. PLoS One 6, e29448 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I. et al. GSK3beta, a centre-staged kinase in neuropsychiatric disorders, modulates long term memory by inhibitory phosphorylation at serine-9. Neurobiol Dis 35, 193–200 (2009). [DOI] [PubMed] [Google Scholar]
- Wandosell F., Varea O., Arevalo M. A. & Garcia-Segura L. M. Oestradiol regulates β-catenin-mediated transcription in neurones. J Neuroendocrinol 24, 191–194 (2012). [DOI] [PubMed] [Google Scholar]
- Kamiya A. et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol 7, 1167–1178 (2005). [DOI] [PubMed] [Google Scholar]
- Ostrer H. Genome and hormones: gender differences in physiology. Invited review: sex-based differences in gene expression. J Appl Physiol 91, 2384–2388 (2001). [DOI] [PubMed] [Google Scholar]
- Reik W. & Walter J. Evolution of imprinting mechanisms: the battle of the sexes begins in the zygote. Nat Genet 27, 255–256 (2001). [DOI] [PubMed] [Google Scholar]
- Harris S. E. et al. Variation in DISC1 is associated with anxiety, depression and emotional stability in elderly women. Mol Psychiatry 15, 232–234 (2010). [DOI] [PubMed] [Google Scholar]
- Hashimoto R. et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet 15, 3024–3033 (2006). [DOI] [PubMed] [Google Scholar]
- Chen Q. Y. et al. Case-control association study of Disrupted-in-Schizophrenia-1 (DISC1) gene and schizophrenia in the Chinese population. J Psychiatr Res 41, 428–434 (2007). [DOI] [PubMed] [Google Scholar]
- Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology 28 Suppl 2, 17–54 (2003). [DOI] [PubMed] [Google Scholar]
- Pletnikov M. V. et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry 13, 173–186 (2008). [DOI] [PubMed] [Google Scholar]
- Zhou M. et al. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron 20, 647–654 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy S. S. et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3, 287–302 (2004). [DOI] [PubMed] [Google Scholar]
- Carola V., D’Olimpio F., Brunamonti E., Mangia F. & Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134, 49–57 (2002). [DOI] [PubMed] [Google Scholar]
- Larkin M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007). [DOI] [PubMed] [Google Scholar]
- Dachtler J. et al. Deletion of α-neurexin II results in autism-related behaviors in mice. Transl Psychiatry 4, e484 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R. M. Assessing nest building in mice. Nat Protoc 1, 1117–1119 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.