Abstract
Secreted and integral membrane proteins comprise up to one-third of the biological proteome. These proteins contain hydrophobic signals that direct their translocation across or insertion into the lipid bilayer by the Sec61 protein conducting channel. The molecular basis for how hydrophobic signals within a nascent polypeptide trigger channel opening is not understood. Here, we use electron cryo-microscopy to determine the structure of an active Sec61 channel that has been opened by a signal sequence. The signal supplants helix 2 of Sec61α, triggering a rotation that opens the central pore both axially across the membrane and laterally toward the lipid bilayer. Comparisons to structures of Sec61 in other states suggest a pathway for how hydrophobic signals engage the channel to gain access to the lipid bilayer.
The universally conserved Sec complex forms a gated protein translocation channel at the eukaryotic endoplasmic reticulum (ER) and bacterial plasma membrane [1]. The central component of this channel, SecY in bacteria and Sec61α in eukaryotes, contains ten transmembrane (TM) helices arranged around a central pore [2]. Two single-TM subunits in eukaryotes, Sec61β and Sec61γ, are peripheral to Sec61α. The central pore in the inactive Sec61α/SecY is occluded by a short “‘plug”’ helix that must be displaced to allow translocation. The interface where TM helices 2/3 contact helices 7/8 defines a “lateral gate” for membrane access of polypeptides [1-3].
Crystal structures of the Sec complex [2, 4-6] lack a translocating polypeptide and likely represent a range of inactive states. Depending on crystal contacts or translocation partners, the lateral gate and plug are in various states of opening and displacement. However, the biological relevance of these channel conformations has been difficult to interpret without a well-resolved and matched active structure. Previous structures of translocation or insertion intermediates of the ribosome-Sec complex determined by electron cryo-microscopy (cryo-EM) were of moderate resolution [7-9], contained heterogeneous substrates [9], required artificial stabilization [8], or were at an uncertain stage of insertion [7]. While these earlier structures were the first views of substrate-induced structural changes consistent with lateral gate opening, the data could not clearly resolve individual Sec61 TM helices or the nature of their interactions with the signal. Thus, a molecular understanding of how substrates open the channel for translocation or insertion is incomplete.
We devised a strategy to epitope-tag and purify the canine ribosome-Sec61 complex engaged by the first 86 residues of the secretory protein pre-prolactin (fig. S1). Translocation, protease-protection, and photo-crosslinking experiments verified that, like the well-characterized native 86-mer [10-15], our tagged complex represents a functional translocation intermediate engaged by Sec61 (fig. S2-S4). Importantly, the nascent polypeptide remains engaged with Sec61 during and after purification (Fig. S4), making it suitable for structure determination by single particle cryo-EM.
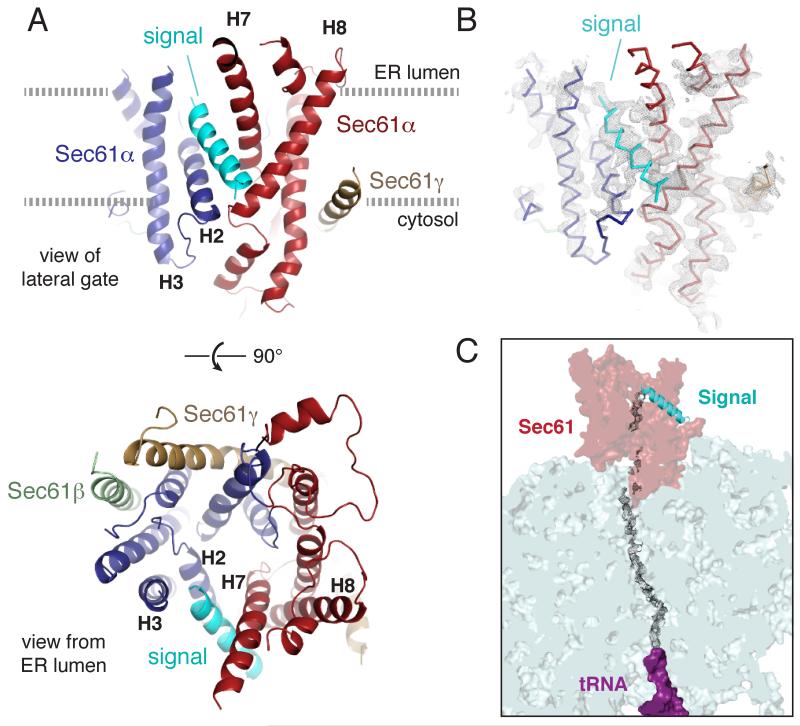
The structure of this engaged ribosome-Sec61 complex was reconstructed from 101,339 particles to an overall resolution of 3.6 Å (fig. S5, S6, and table S1). The local resolution of the Sec61 channel ranged from ~ 3.5 Å near the ribosome to ~7.0 Å at the lumenal loops. Most TM helices were at ~4.5-5.5 Å resolution (fig. S6), revealing clear helical pitch and many bulky side chains in sharpened maps (fig. S7). All twelve TM helices of the Sec61 complex could be unambiguously assigned, leaving a single helix we ascribed to the signal sequence (Fig. 1A, 1B and S8). Density visible throughout the ribosomal exit tunnel and in parts of the Sec61 channel (Fig. 1C) suggests a looped configuration for the nascent chain, consistent with earlier crosslinking studies [11].
Fig. 1. Structure of the signal peptide-engaged Sec61 complex.
(A) View of the lateral gate (top) or from the ER lumen of the Sec61 complex bound to the pre-prolactin signal peptide (cyan). The mobile regions of Sec61α are blue, while the comparatively immobile regions are red. The β and γ subunits are pale green and tan, respectively. Helices that comprise the lateral gate are labelled. (B) Experimental density for the structure (mesh, filtered to 4.5 Å resolution) superimposed on the backbone trace of the structural model. (C) Density observed for the nascent polypeptide through the ribosomal tunnel and parts of the Sec61channel.
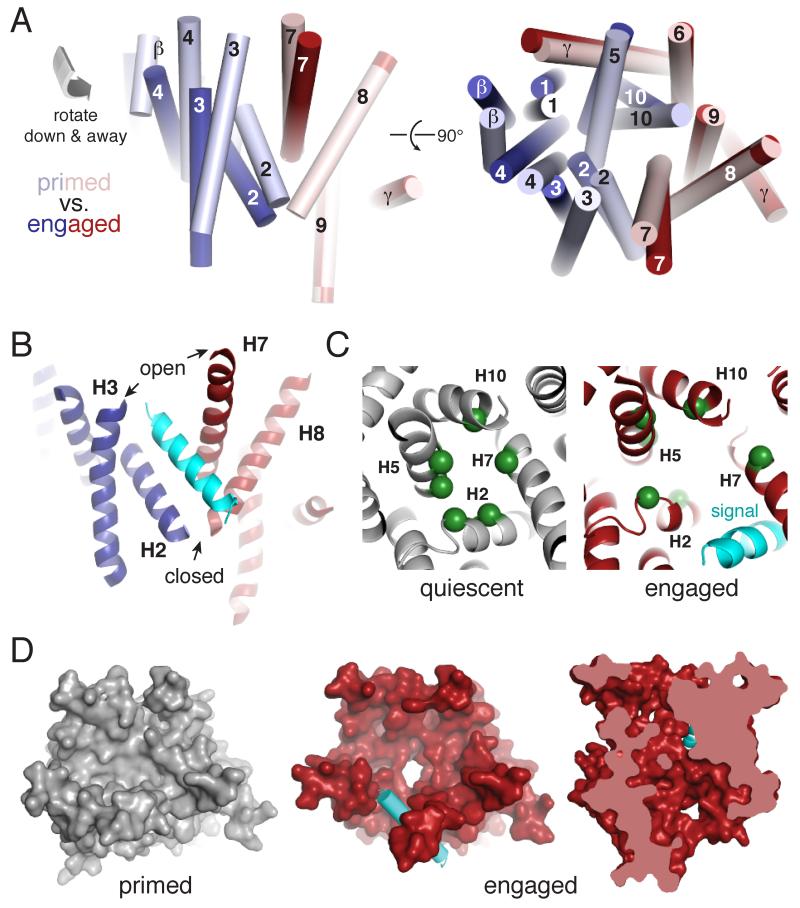
The well-resolved structure of a biochemically validated early translocation intermediate permitted detailed comparisons with other Sec61 states to gain insights into the conformational changes accompanying channel opening. A previous cryo-EM structure of the porcine ribosome-Sec61 complex lacking a nascent polypeptide [9] represents a “primed” state preceding nascent chain insertion. Relative to this primed structure, the engaged channel is open laterally toward the lipid bilayer and axially across the membrane (Fig. 2). The ribosome-Sec61 interaction remains fixed, with only minor movements of the associated Sec61γ and TM helices 6, 7, 8, and 9 of Sec61α. The other seven TM helices of the Sec61 complex rotate as a rigid body by ~22° (Fig. 2A and videos S1, S2), thereby creating space between helices 2 and 7 for intercalation of the signal peptide (Fig. 2B). Notably, cryo-tomography of the Sec61 complex in native ER microsomes shows a similar configuration [16]. Although the heterogeneous and uncertain functional state of the cryo-tomography structure complicates its placement within the translocation cycle [17], it does illustrate that our engaged structure is likely to be compatible with a membrane environment.
Fig. 2. Conformational changes to Sec61 upon engagement by a signal peptide.
(A) Positions of the transmembrane helices of the Sec61 complex in the ribosome-primed (pale colors, PDB 3J7Q) and signal-engaged (bright colors) states. The mobile regions of Sec61α are blue, while the comparatively immobile regions are red. Individual helices are labelled; the signal has been omitted for clarity. (B) View of the asymmetrically opened lateral gate. (C) View of the pore ring residue positions (green spheres) in the quiescent SecY crystal structure (grey, PDB 1RH5) and engaged Sec61 complex (red). The signal is cyan. (D) Left and middle: surface view of the primed (grey, PDB 3J7Q) and engaged (red) states of the Sec61 complex viewed from the ER lumen. The lumenal loops have been removed for clarity. The signal peptide is cyan. Right: cutaway view perpendicular to the membrane of the engaged Sec61 complex showing an open channel.
Because the 22° rotation is oblique relative to the plane of the membrane, lateral gate opening is asymmetric: the lumenal end of the gate parts by ~15 Å, while the cytosolic side remains closed (Fig. 2B). Oblique rotation of helices 2, 5, and 10 away from helix 7 displaces a set of six conserved “pore ring” residues (table S2) from their normally planar configuration (Fig. 2C). The plug is apparently destabilized by separation of the pore ring, atop which it ordinarily sits [2], leading to an unobstructed conduit across the membrane (Fig. 2D). Thus, opening of the Sec61 channel is directly coupled to successful signal sequence recognition via its intercalation in the lateral gate.
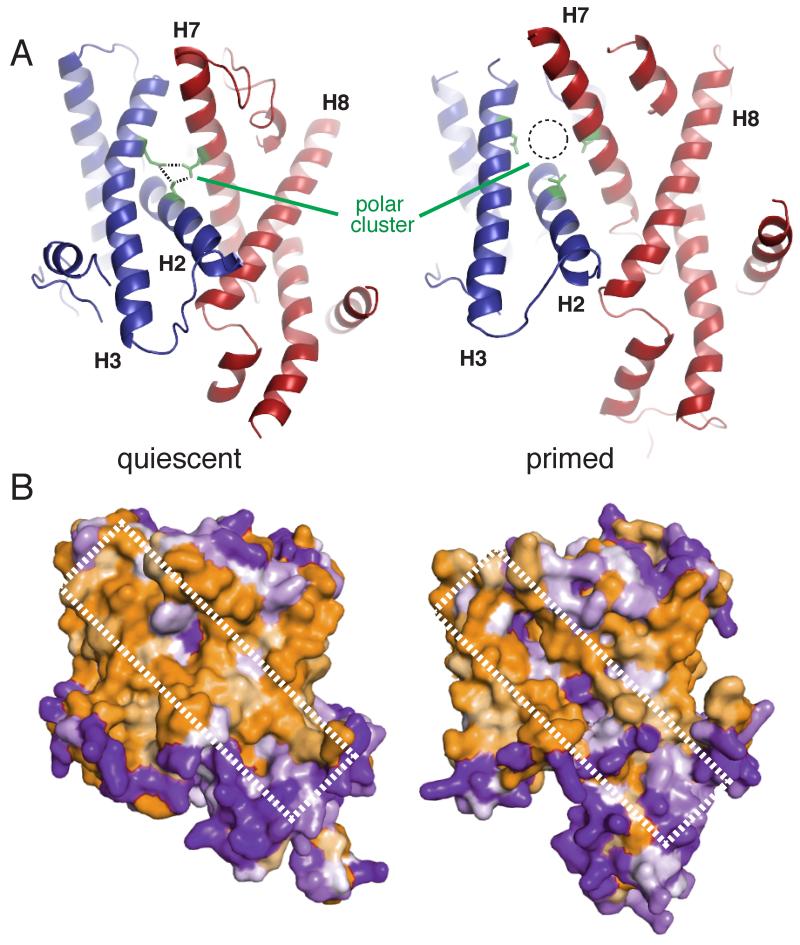
To understand how the signal sequence reaches this position, we asked whether Sec61 priming by the ribosome might favor subsequent signal sequence engagement. For comparison, we used the M. jannaschi x-ray structure of an isolated Sec complex in its “quiescent” state [2], whose overall architecture, TM helix interactions, and key functional motifs (table S2) are well conserved with mammals. Relative to the quiescent channel, the ribosome-primed Sec61 complex is partially destabilized in two ways. First, a “polar cluster” of three residues on helices 2 and 7 that form stabilizing hydrogen bonds is separated in the primed state (Fig. 3A). Hence, the lateral gate is partially cracked in the precise region eventually occupied by the signal peptide. Second, the external surface of the primed Sec61 complex (Fig. 3B) contains a hydrophilic seam that is energetically disfavored by its exposure to the hydrophobic interior of the lipid bilayer. A similar conformational change is seen in the bacterial SecY complex bound to the ATPase SecA [6]. Thus, two diverse translocation partners from different translocation pathways, the ribosome and SecA, induce similar priming events by binding to the cytosolic loops of the Sec61/SecY complex.
Fig. 3. The lateral gate is destabilized in the ribosome-primed Sec61 complex.
(A) Comparison of the quiescent SecY (left, PDB 1RH5) and primed Sec61 (right, PDB 3J7Q) complexes. Ribosome binding results in partial destabilization of the lateral gate by shifting helices 2 and 3 away from the midline, which disrupts the polar cluster (green). (B) Space filling model viewing the lateral gate of the quiescent (left) and primed (right) structures colored by hydrophobicity, in which orange is hydrophobic and purple is hydrophilic. The hydrophilic seam produced by ribosome binding is indicated.
When viewed from the ribosomal exit tunnel, nearly all of the surfaces on the primed Sec61 complex available to a nascent signal peptide are hydrophilic. The only substantive hydrophobic patch, deep within the channel pore, might serves as the initial interaction site for a hydrophobic signal (Fig. 4A). This hydrophobic patch is composed of residues from the lateral gate and pore ring (table S2), and is positioned adjacent to the region of the lateral gate weakened by ribosome priming (Fig. 4B). Packing of the hydrophobic signal at this destabilized region may facilitate its intercalation into the lateral gate, driven by the energetically favorable exposure of the signal to lipid, eventually adopting the conformation seen in our structure.
Fig. 4. Putative path of the signal peptide into the Sec61 lateral gate.
(A) Space-filling model of the cytosolic vestibule of Sec61 viewed from the ribosome, colored by hydrophobicity as in Fig. 3B. (B) Residues comprising the hydrophobic patch are at the lateral gate. (C) The eventual position of the signal peptide (cyan), relative to the stationary ribosome-bound regions of Sec61, is essentially identical to the position of helix 2 in the quiescent state (grey). (D) Positions of helix 2 in the quiescent (grey) and primed (yellow) states superimposed on the engaged Sec61 structure (red). The signal peptide is omitted for clarity, but would reside precisely in the position of the quiescent helix 2.
The position of the engaged signal perfectly supplants helix 2, replacing its interactions with helices 7 and 8 to stabilize the open channel conformation (Fig. 4C). Hydrophilic segments of polypeptide would not be energetically favored in this position, explaining why they cannot open the channel. The conserved hydrophobicity and length of helix 2 suggests that the biophysical properties of helix 2 may dictate what constitutes a functional signal for translocation, a property that is similar, but not identical across species. The concept of a hydrophobicity threshold set by an intramolecular “placeholder” is also used by the signal recognition particle at an earlier step of this pathway [18] and may be a general mechanism for increasing recognition fidelity of widely divergent sequences.
Our structure of the signal-engaged Sec61 translocon, together with earlier quiescent and primed structural states, leads to a molecular model for selective gating of the translocon by hydrophobic signals. Ribosome binding constrains the cytosolic loops of Sec61 to enforce a conformation in which key lateral gate contacts, including the conserved polar cluster, are weakened. A hydrophobic patch close to this site attracts hydrophobic signals to their point of initial engagement. If the signal is sufficiently hydrophobic, it can proceed further to displace the comparably hydrophobic helix 2, thereby accessing the lipid bilayer while simultaneously widening the central pore to destabilize the plug. Thus, polypeptides initially sample the cytosolic vestibule of Sec61, gaining access to the lipid bilayer via the channel interior contingent on both a suitably hydrophobic signal and an appropriately primed translocation channel.
This stepwise model for translocon opening is consistent with fluorescence and crosslinking studies showing that signals are initially in an aqueous environment [19] near Sec61α [10-13], and only access lipid after further elongation [13, 15]. Furthermore, mutants of the lateral gate, plug, and pore ring residues in the hydrophobic patch or polar cluster region allow promiscuous translocation of nonfunctional signal sequences [20-25]. Conversely, stabilization of lateral gate contacts increases the hydrophobic threshold for signal sequence-mediated translocation [24,25]. Similarly, inhibitors that impede Sec61 opening are thought to bind at the plug and lateral gate junction [26,27]. Thus, defining the location of a signal in the process of initiating translocation permits molecular interpretations of earlier functional data and provides a base for future studies of other stages in protein translocation. Analysis of signals and TM domains of different biophysical properties in both defined and native translocon complexes will be crucial for understanding how the Sec61 translocon is able to handle the remarkably diverse clientele transiting the membrane.
Supplementary Material
Acknowledgements
We thank F. de Haas, V. Ragunath, and C. Savva for help with data collection; S. Chen, G. McMullan, J. Grimmett and T. Darling for technical support; S. Shao for helpful discussions;. This work was supported by the UK Medical Research Council (MC_UP_A022_1007 to RSH) and a Wellcome Trust postdoctoral fellowship (RMV). The Cryo-EM map for the engaged ribosome-Sec61 complex has been deposited with the EMDataBank (EMDB- 3245). The Protein Data Bank accession number for the engaged Sec61 complex is 3JC2.
Footnotes
Materials and Methods
Figs. S1 to S8
Tables S1 to S2
Captions for Movies S1 to S2
References (28-46)
Movies S1 to S2
References and Notes
- 1.Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 3.Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell. 1998;94:795–807. doi: 10.1016/s0092-8674(00)81738-9. [DOI] [PubMed] [Google Scholar]
- 4.Egea PF, Stroud RM. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17182–17187. doi: 10.1073/pnas.1012556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukazaki T, et al. Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature. 2008;455:988–991. doi: 10.1038/nature07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmer J, Nam Y, Rapoport TA. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature. 2008;455:936–943. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogala M, et al. Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion. Nature. 2014;506:107–110. doi: 10.1038/nature12950. [DOI] [PubMed] [Google Scholar]
- 8.Park E, et al. Structure of the SecY channel during initiation of protein translocation. Nature. 2014;506:102–106. doi: 10.1038/nature12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voorhees RM, Fernandez IS, Scheres SHW, Hegde RS. Structure of the mammalian ribosome-Sec61 complex to 3.4 A resolution. Cell. 2014;157:1632–1643. doi: 10.1016/j.cell.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.High S, et al. Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane-inserted signal sequence. J. Biol. Chem. 1993;268:26745–26751. [PubMed] [Google Scholar]
- 11.Mothes W, Prehn S, Rapoport TA. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994;13:3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jungnickel B, Rapoport TA. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995;82:261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- 13.Mothes W, Jungnickel B, Brunner J, Rapoport TA. Signal sequence recognition in cotranslational translocation by protein components of the endoplasmic reticulum membrane. J. Cell Biol. 1998;142:355–364. doi: 10.1083/jcb.142.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994;78:461–471. doi: 10.1016/0092-8674(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 15.Martoglio B, Hofmann MW, Brunner J, Dobberstein B. The protein-conducting channel in the membrane of the endoplasmic reticulum is open laterally toward the lipid bilayer. Cell. 1995;81:207–214. doi: 10.1016/0092-8674(95)90330-5. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer S, et al. Structure of the native Sec61 protein-conducting channel. Nat. Comm. 2015:6. doi: 10.1038/ncomms9403. doi: 10.1038/ncomms9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Although P-site tRNA was absent from the majority of ribosomes used for the cryo-tomography structure [16], the presence or absence of heterogeneous endogenous nascent polypeptides within Sec61 could not be determined conclusively. Thus, the conclusion that the Sec61 complex is constitutively open upon ribosome binding, independent of substrate or functional state, may be premature. Indeed, biochemical studies in native microsomes show substrate-induced Sec61 opening [14], no translocation of Sec61-docked polypeptides with a mutant signal [12], and no lipid access to early translocation intermediates [12]. Genetic studies show that modulation of lateral gate interactions influences translocation [20-27]. Thus, multiple independent findings argue that substrates induce structural changes to the Sec61 complex that open it laterally.
- 18.Voorhees RM, Hegde RS. Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. Elife. 2015:4. doi: 10.7554/eLife.07975. doi:10.7554/eLife.07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley KS, Reinhart GD, Johnson AE. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell. 1993;73:1101–1115. doi: 10.1016/0092-8674(93)90640-c. [DOI] [PubMed] [Google Scholar]
- 20.Junne T, Schwede T, Goder V, Spiess M. Mutations in the Sec61p channel affecting signal sequence recognition and membrane protein topology. J. Biol. Chem. 2007;282:33201–33209. doi: 10.1074/jbc.M707219200. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA, Clemons WMJ, DeMars CJ, Flower AM. Modeling the effects of prl mutations on the Escherichia coli SecY complex. J Bacteriol. 2005;187:6454–6465. doi: 10.1128/JB.187.18.6454-6465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derman AI, Puziss JW, Bassford PJJ, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborne RS, Silhavy TJ. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 1993;12:3391–3398. doi: 10.1002/j.1460-2075.1993.tb06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maillard AP, Lalani S, Silva F, Belin D, Duong F. Deregulation of the SecYEG translocation channel upon removal of the plug domain. J. Biol. Chem. 2007;282:1281–1287. doi: 10.1074/jbc.M610060200. [DOI] [PubMed] [Google Scholar]
- 25.Trueman SF, Mandon EC, Gilmore R. A gating motif in the translocation channel sets the hydrophobicity threshold for signal sequence function. J. Cell Biol. 2012;199:907–918. doi: 10.1083/jcb.201207163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackinnon AL, Paavilainen VO, Sharma A, Hegde RS, Taunton J. An allosteric Sec61 inhibitor traps nascent transmembrane helices at the lateral gate. Elife. 2014;3:e01483. doi: 10.7554/eLife.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junne T, et al. Decatransin, a new natural product inhibiting protein translocation at the Sec61/SecYEG translocon. J Cell Sci. 2015;128:1217–1229. doi: 10.1242/jcs.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao S, von der Malsburg K, Hegde RS. Listerin-dependent nascent protein ubiquitination relies on ribosome subunit dissociation. Mol. Cell. 2013;50:637–648. doi: 10.1016/j.molcel.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao S, Hegde RS. Reconstitution of a minimal ribosome-associated ubiquitination pathway with purified factors. Mol. Cell. 2014;55:880–890. doi: 10.1016/j.molcel.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fons RD, Bogert BA, Hegde RS. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J. Cell Biol. 2003;160:529–539. doi: 10.1083/jcb.200210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma A, Mariappan M, Appathurai S, Hegde RS. In vitro dissection of protein translocation into the mammalian endoplasmic reticulum. Methods Mol. Biol. 2010;619:339–363. doi: 10.1007/978-1-60327-412-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter P, Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Hegde RS. Cotranslational partitioning of nascent prion protein into multiple populations at the translocation channel. Mol. Biol. Cell. 2002;13:3775–3786. doi: 10.1091/mbc.E02-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- 35.Bai X-C, Fernandez IS, McMullan G, Scheres SHW. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. Elife. 2013;2:e00461. doi: 10.7554/eLife.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 2003;142:334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 37.Scheres SHW. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheres SH. Beam-induced motion correction for sub-megadalton cryo-EM particles. Elife. 2014;3:e03665. doi: 10.7554/eLife.03665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheres SHW, Chen S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amunts A, et al. Structure of the yeast mitochondrial large ribosomal subunit. Science. 2014;343:1485–1489. doi: 10.1126/science.1249410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown A, et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D Biol. Crystallogr. 2015;71:136–153. doi: 10.1107/S1399004714021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholls RA, Long F, Murshudov GN. Low-resolution refinement tools in REFMAC5. Acta Crystallogr. D Biol. Crystallogr. 2012;68:404–417. doi: 10.1107/S090744491105606X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat. Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeLano WL. The PyMOL molecular graphics system. 2006 http://www.pymol.org.
- 46.Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 2007;157:281–287. doi: 10.1016/j.jsb.2006.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.