SUMMARY
Therapeutic antibodies targeting programmed cell death-1 (PD-1) activate tumor-specific immunity and have shown remarkable efficacy in the treatment of melanoma. Yet, little is known about tumor cell-intrinsic PD-1 pathway effects. Here we show that murine and human melanomas contain PD-1-expressing cancer subpopulations and demonstrate that melanoma cell-intrinsic PD-1 promotes tumorigenesis, even in mice lacking adaptive immunity. PD-1 inhibition on melanoma cells by RNA interference, blocking antibodies, or mutagenesis of melanoma-PD-1 signaling motifs suppresses tumor growth in immunocompetent, immunocompromised and PD-1-deficient tumor graft recipient mice. Conversely, melanoma-specific PD-1 overexpression enhances tumorigenicity, as does engagement of melanoma-PD-1 by its ligand, PD-L1, whereas melanoma-PD-L1 inhibition or knockout of host-PD-L1 attenuate growth of PD-1-positive melanomas. Mechanistically, the melanoma-PD-1 receptor modulates downstream effectors of mTOR signaling. Our results identify melanoma cell-intrinsic functions of the PD-1:PD-L1 axis in tumor growth and suggest that blocking melanoma-PD-1 might contribute to the striking clinical efficacy of anti-PD-1 therapy.
Keywords: Melanoma, programmed cell death-1, PD-1, immune checkpoint, blockade, antibody, therapy, PD-L1, S6 ribosomal protein, p-S6, mTOR signaling
INTRODUCTION
Immune checkpoints are crucial regulatory pathways that maintain immune homeostasis by modulating the amplitude and quality of several adaptive and innate effector mechanisms in favor of immunogenic tolerance (Pardoll, 2012). Using various strategies, such as triggering functional exhaustion of tumor-reactive cytotoxic T-lymphocytes (CTLs), cancers exploit immune checkpoints to evade antitumor immunity. Programmed cell death 1 (PD-1) is a prominent checkpoint receptor that, upon engagement by its ligands, PD-L1 (also known as B7-H1) or PD-L2 (also known as B7-DC), dampens T-effector functions by inhibiting signaling downstream of the T-cell receptor (TCR) (Topalian et al., 2012a). Thus, expression of PD-1 ligands, and particularly PD-L1, in the tumor microenvironment (TME) protects cancers from immune-mediated rejection (Dong et al., 2002; Topalian et al., 2012a). Consequently, a number of antibody-based therapeutics targeting the PD-1:PD-L1 axis have entered clinical development or have been approved for melanoma therapy (Postow et al., 2015).
In phase I trials (Hamid et al., 2013; Herbst et al., 2014; Topalian et al., 2012b; Wolchok et al., 2013), PD-1 pathway blockade demonstrated unprecedented response rates and encouraging toxicity profiles in patients with advanced-stage cancers of various etiologies, including malignant melanoma. On the basis of recent phase III data demonstrating improved overall survival in melanoma patients receiving PD-1 inhibitors compared to those treated with chemotherapy, the FDA approved two anti-PD-1 antibodies, nivolumab and pembrolizumab, for the treatment of patients with advanced melanoma who are no longer responding to other drugs (Postow et al., 2015; Weber et al., 2015). PD-L1 expression by cancer cells and tumor-infiltrating lymphocytes (TILs) (Herbst et al., 2014; Topalian et al., 2012b; Tumeh et al., 2014), the presence of type 1 T-helper cell (Th1)-associated inflammatory mediators (Herbst et al., 2014; Tumeh et al., 2014), increased density and proliferation and decreased diversity in antigen-specificity of CD8+ T-cells (Tumeh et al., 2014), and the frequency of tumor-associated neo-antigens within the TME (Gubin et al., 2014; Rizvi et al., 2015; Yadav et al., 2014) are associated with clinical response to PD-1 pathway interference. These findings established that optimal anti-PD-1 cancer therapeutic efficacy requires the activation and expansion of tumor-specific T-cell immunity.
However, in addition to benefiting patients afflicted with immunogenic cancers, such as malignant melanoma (Hamid et al., 2013; Herbst et al., 2014; Topalian et al., 2012b; Wolchok et al., 2013), PD-1 pathway blockade has also yielded meaningful clinical activity in patients with lesser immunogenic cancers that have hitherto not typically responded to immunotherapy (Herbst et al., 2014; Topalian et al., 2012b). Moreover, patients with advanced melanoma refractory to treatment with ipilimumab, an FDA-approved antibody targeting the immune checkpoint protein, cytotoxic T-lymphocyte antigen (CTLA)-4, showed marked clinical response to anti-PD-1 therapy (Hamid et al., 2013; Weber et al., 2015; Wolchok et al., 2013). While the presence of neo-antigens and an immune-active TME are similarly associated with favorable outcome in melanoma patients treated with either PD-1- (Gubin et al., 2014; Rizvi et al., 2015; Yadav et al., 2014) or CTLA-4-directed checkpoint blockade (Snyder et al., 2014), current evidence suggests that PD-1 inhibitors produce greater anticancer activity and fewer immune-related adverse events than ipilimumab (Postow et al., 2015). Taken together, these observations raise the possibility that anti-PD-1 therapy, in addition to deregulating T-cell specific immune checkpoint functions, may also inhibit complementary protumorigenic mechanisms, thereby contributing to its superior clinical efficacy compared to CTLA-4 blockade. Because PD-1 is not only expressed by immune cells, but also by melanoma subpopulations with enhanced tumorigenicity, even in highly immunocompromised tumor xenograft recipient mice (Schatton et al., 2010), we hypothesized that the growth-suppressive effects of PD-1 therapy might also partially result from the direct inhibition of this protein on melanoma cells.
Here we report that established human and murine melanoma cell lines as well as clinical melanomas frequently contain PD-1-expressing cancer subpopulations, and that enforced melanoma-PD-1 expression enhances melanoma growth, even in the absence of adaptive immunity. Conversely, antibody-mediated melanoma-PD-1 blockade, melanoma-specific PD-1 knockdown, as well as mutagenesis of melanoma-PD-1 signaling motifs inhibit tumor growth, independently of adaptive immunity. Efficient melanoma-PD-1-driven tumorigenesis requires melanoma-PD-1 interactions with its predominant ligand, PD-L1, which activate effectors of the mTOR signaling pathway downstream of the melanoma-PD-1 receptor. Our results expand our current understanding of PD-1 pathway functions in melanoma, and suggest that cancer cell-intrinsic PD-1 targeting might significantly contribute to the therapeutic efficacy of PD-1 antibodies, rendering PD-1 inhibition, in conjunction with its demonstrated effect on immune checkpoint blockade, superior to alternative therapies that target immune checkpoints alone.
RESULTS
Melanomas frequently contain PD-1-expressing cancer subpopulations
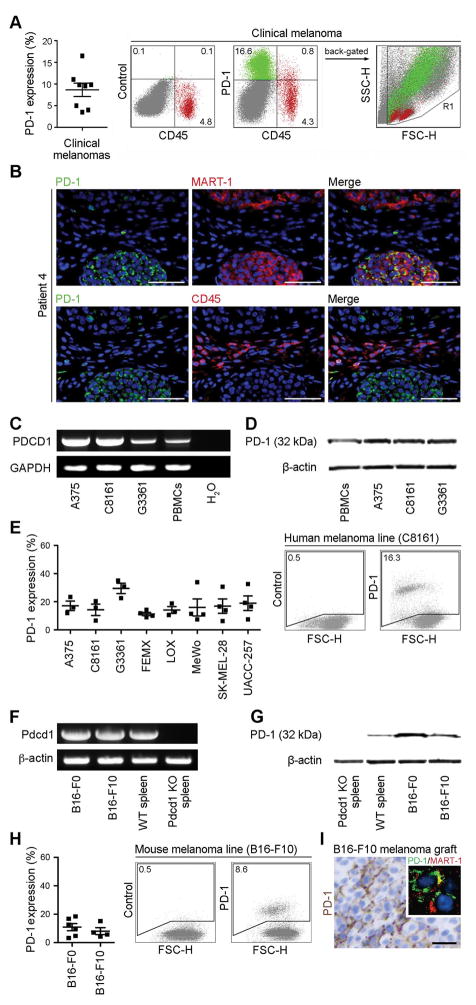
We first examined PD-1 expression in a series of melanoma patient samples and established melanoma cell lines, to further expand upon the potential clinical significance of our previous demonstration that melanoma cells can express PD-1 (Schatton et al., 2010). Flow cytometric analysis of single cell suspensions derived from clinical tumor specimens (n=8 patients) revealed PD-1 surface protein expression by melanoma subpopulations negative for the pan-lymphocyte marker, CD45, and the endothelial marker, CD31, in 8/8 melanoma specimens examined, with tumor cell frequencies ranging from 3.5% to 16.5% (cell frequency 8.7%, 1.5%, mean + SEMs, Fig. 1A and Fig. S1A). Immunofluorescence double-labeling of clinical melanoma biopsies (n=50) for PD-1 and the melanoma antigen recognized by T-cells (MART)-1 further confirmed PD-1 protein expression by subpopulations of MART-1+ melanoma cells that were cytologically distinct from CD45+ lymphocytes (Fig.1B), with n=22/36 melanoma patients demonstrating melanoma-PD-1 positivity in at least one of their tumor lesions (Table S1).
Figure 1. PD-1 expression by melanoma cells.
(A) Percentages (mean±s.e.m.) (left) and representative flow cytometry plots (right) of PD-1 surface protein expression by clinical tumor biopsy-derived melanoma cells (green) from n=8 distinct melanoma patients. These cells are negative for the CD45 lymphocyte common antigen (red) and the CD31 endothelial marker (see also Fig. S1A). (B) Representative immunofluorescence double staining of a clinical melanoma biopsy for co-expression of PD-1 (green) and MART-1 (red) or of PD-1 (green) and CD45 (red) on a serial tissue section. Nuclei were counterstained with DAPI (blue). Size bars, 100μm. Representative of n=22/36 melanoma patients demonstrating melanoma-PD-1 positivity. A patient was considered melanoma-PD-1 positive if any tumor biopsy (total of n=50) showed expression of PD-1 by MART-1+ and/or CD45− cells. See also Table S1. (C) RT-PCR expression analysis of full-length PD-1 (PDCD1) mRNA and (D) immunoblot of PD-1 protein expression by human melanoma lines and PBMCs. (E) Percentages (mean±s.e.m., left) and representative flow cytometry plots (right) of PD-1 surface protein expression by human melanoma lines (n=3–4 independent experiments, respectively). (F) RT-PCR expression analysis of full-length PD-1 (Pdcd1) mRNA and (G) immunoblot of PD-1 protein expression by murine B16-F0 and B16-F10 melanoma cells, wildtype (WT) and Pdcd1 knockout (KO) C57BL/6-derived splenocytes. (H) Percentages (mean±s.e.m., left) and representative flow cytometry plots (right) of PD-1 surface protein expression by B16 cells (n=4–6 independent experiments, respectively). (I) Representative PD-1 immunohistochemistry and immunofluorescence double staining for co-expression of PD-1 (green) with MART-1 (red) (inset photomicrograph) of a B16-F10 melanoma graft grown in NSG mice (size bar, 50μm). See also Figures S1 and S2, and Table S1.
Based on our intention to mechanistically dissect the role of melanoma-expressed PD-1 in experimental tumor growth, we next characterized PD-1 expression in established human and murine melanoma cell lines. RT-PCR amplification and sequencing of the full coding sequence (CDS) of the human PD-1 (PDCD1) gene revealed PDCD1 mRNA expression (Fig.1C), and immunoblot analysis demonstrated PD-1 protein expression by human A375, C8161 and G3361 melanoma cells (Fig.1D). Flow cytometric analyses showed PD-1 surface protein expression by 8/8 melanoma lines tested, with PD-1+ tumor cell frequencies ranging from 11.3%±1.2% to 29.5%±3.7% (mean±SEM Fig. 1E), and revealed preferential PD-1 expression by melanoma cell subsets positive for the tumor-initiating cell determinant (Schatton et al., 2008), ABCB5 (Fig. S2A–C), consistent with our previous demonstration of preferential PD-1 expression by melanoma-initiating cells (Schatton et al., 2010). Human melanoma lines also demonstrated positivity for both PD-1 ligands, PD-L1 and PD-L2, ranging from 2.4%±0.1% to 99.2%±0.1% and 0.6%±0.1% to 88.9%±2.6% of cells (mean±SEM), respectively (Fig. S1B), and PD-1 co-expression with its ligands (not shown). Murine B16-F0 and B16-F10 cultures also expressed both PD-1 (Pdcd1) mRNA, as determined by amplification and sequencing of the full Pdcd1 CDS (Fig. 1F), and PD-1 protein as determined by immunoblotting (Fig. 1G). Flow cytometric analysis revealed PD-1 (cell frequency 9.4%±2.5% and 6.6%±2.4%, mean±SEM, Fig. 1H) and PD-L1 (43.4%±9.4% and 37.5%±2.3%), but not PD-L2 surface protein expression by B16-F0 and B16-F10 melanoma cells (Fig. S1C). B16 melanoma grafts grown in non-obese diabetic severely combined immunodeficient (NOD/SCID) interleukin-2 receptor (IL-2R) γ-chain(-/-) null (NSG) mice lacking adaptive immunity also demonstrated PD-1 expression by MART-1+ melanoma cells (Fig. 1).
Melanoma-expressed PD-1 promotes murine tumor growth
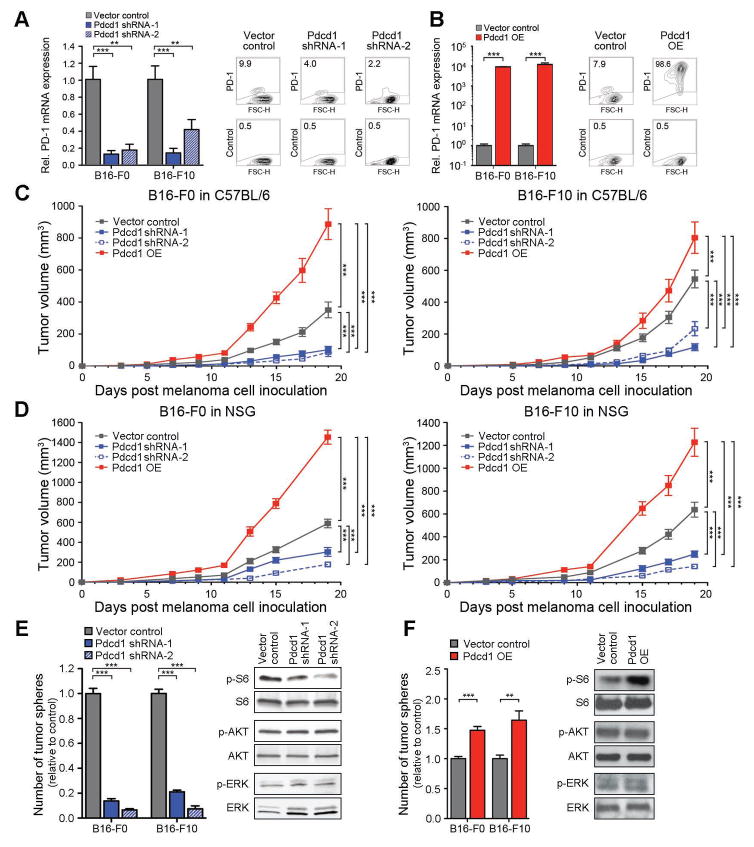
To functionally dissect the potential role of melanoma-expressed PD-1 in tumor growth, we generated stable Pdcd1 knockdown (KD) and Pdcd1-overexpressing (OE) B16 melanoma lines. Transduction of B16-F0 and B16-F10 cells with two distinct short hairpin (sh) RNAs targeting Pdcd1 inhibited murine PD-1 mRNA expression by ≥59% and significantly blocked PD-1 protein expression compared to controls (Fig.2A), but did not significantly alter expression of PD-L1 or PD-L2, respectively (not shown). Conversely, transduction of B16 cells with Pdcd1-encoding constructs resulted in upregulation of PD-1, both at the mRNA and protein level (Fig.2B). Melanoma-specific Pdcd1-KD resulted in decreased and Pdcd1-OE in increased B16-F0 and B16-F10 melanoma growth in immunocompetent C57BL/6 mice compared to that of vector controls (Fig.2C). Pdcd1-KD melanoma grafts demonstrated diminished (Fig. S3A) and Pdcd1-OE melanomas significantly enhanced Pdcd1 mRNA and PD-1 protein expression compared to control tumors at the experimental endpoint (Fig. S3B). We next compared the tumorigenic ability of native PD-1+- vs. PD-1−-sorted B16-F0 and B16-F10 melanoma cells and found that PD-1+ subpopulations demonstrated significantly increased growth in C57BL/6 mice compared to PD-1− cells (Fig. S2C). Together, these findings identify melanoma-expressed PD-1 as a protumorigenic mechanism.
Figure 2. Melanoma-expressed PD-1 promotes tumorigenicity in murine melanoma models.
(A) PD-1 expression by Pdcd1-shRNA-1 and Pdcd1-shRNA-2 vs. vector control and by (B) Pdcd1-overexpressing (OE) vs. vector-control B16-F0 or B16-F10 melanoma cells. Representative flow cytometry plots show PD-1 expression in B16-F10 melanoma variants. (C) Tumor growth kinetics (mean±s.d.) of Pdcd1-shRNA-1/-2 vs. Pdcd1-OE vs. vector control B16-F0 or B16-F10 melanomas in C57BL/6 mice (n=10–30 each) or (D) NSG mice (n=10–20 each). (E) Mean number of tumor spheres±s.e.m (left) and immunoblot analysis of phosphorylated (p) and total S6, AKT, and ERK (right) in Pdcd1-shRNA-1 and Pdcd1-shRNA-2 vs. control and (F) Pdcd1-OE vs. vector-control B16 melanoma variants. Results are representative of n=2–3 independent experiments (**P<0.01, ***P<0.001). See also Figures S3 and S4.
PD-1 expressed by cells of the adaptive immune system has been established as a modulator of tumor-specific immunity (Topalian et al., 2012a). To determine whether the observed tumor growth-accelerating effects of melanoma-expressed PD-1 depend on melanoma-PD-1:lymphocyte interactions, we compared the abilities of Pdcd1-KD and Pdcd1-OE vs. control B16 melanomas to initiate tumor growth in immunocompromised, T- and B-cell-deficient NSG mice. We found that Pdcd1-KD inhibited and Pdcd1 overexpression increased tumorigenicity of B16-F0 and B16-F10 melanomas in NSG mice compared to controls (Fig.2D), suggesting lymphocyte-independent roles of melanoma-PD-1 in tumorigenesis. Significant Pdcd1-KD (Fig. S4A) and overexpression (Fig. S4B) in B16 melanoma grafts was confirmed after in vivo growth. Consistent with our findings using Pdccd1-overexpressing melanoma variants, we found that PD-1+ melanoma subpopulations purified from native B16-F0 and B16-F10 lines demonstrated increased tumorigenicity in NSG mice compared to PD-1− cell isolates (Fig. S3C). We next examined whether melanoma-specific Pdcd1 silencing or overexpression affects melanoma cell growth in vitro, in the complete absence of immune cells, using an established culture system designed for the study of tumorigenic minority populations (Aceto et al., 2012; Civenni et al., 2011). Consistent with our in vivo findings, Pdcd1-KD impaired (Fig.2E) and Pdcd1-OE promoted in vitro three-dimensional B16-F0 and B16-F10 culture growth compared to respective controls (Fig.2F). Because PD-1 receptor signaling in T-cells modulates several downstream pathways (Riley, 2009) that also serve critical roles in melanomagenesis (Flaherty et al., 2012), such as MAPK/ERK, PI3K/AKT, and mTOR signaling, we next examined melanoma-Pdcd1-specific changes in phospho (p)-ERK1/2, p-AKT, and p-S6 ribosomal protein levels. Pdcd1-KD reduced (Fig. 2E) and Pdcd1-OE increased phosphorylation of the mTOR effector molecule, S6, compared to control B16 melanoma cells (Fig. 2F), indicating melanoma cell-intrinsic, PD-1-mediated induction of protumorigenic mTOR pathway activity. Together, these in vitro findings suggest lymphocyte-independent, cancer cell-intrinsic functions of melanoma-expressed PD-1 in tumor growth.
Melanoma-PD-1:PD-L1 interactions promote murine melanoma growth
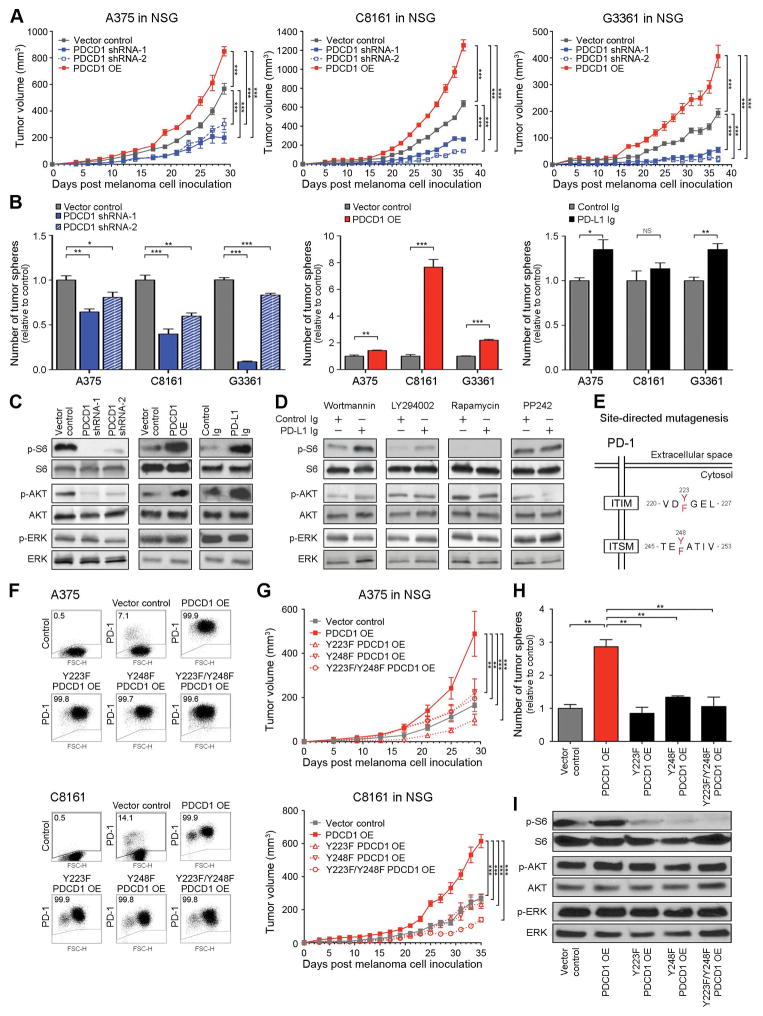
We next examined whether ligation of melanoma-PD-1 to its predominant ligand, PD-L1, is required for PD-1-driven tumorigenesis. To test whether melanoma-PD-1:host-PD-L1 interactions promote tumor growth in the absence of adaptive immunity, we grafted Pdcd1-OE vs. control B16-F10 cells to wildtype Rag(−/−) vs. PD-L1(−/−) KO Rag(−/−) mice (Francisco et al., 2009). We found that the growth of Pdcd1-OE melanomas was attenuated in PD-L1(−/−) KO Rag(−/−) compared to PD-L1(+/+) Rag(−/−) recipients (Fig. S5A). To examine if PD-L1 expressed by melanoma cells (Fig. S1) also contributes to melanoma-PD-1-dependent tumorigenesis, we treated PD-L1(−/−) KO Rag(−/−) vs. wildtype Rag(−/−) mice grafted with Pdcd1-OE melanomas with a PD-L1 blocking antibody. We found that PD-L1 blockade inhibited Pdcd1-OE B16-F10 melanoma growth compared to isotype control antibody treatment in PD-L1(−/−) KO mice (Fig.3A). Additionally, PD-L1 antibody treatment resulted in significantly reduced tumor growth of Pdcd1-OE melanomas in PD-L1(−/−) KO Rag(−/−) compared to wildtype Rag(−/−) mice (Fig.3A). These findings suggest growth-accelerating functions not only of host-PD-L1:melanoma-PD-1, but also of melanoma-PD-L1:melanoma-PD-1 interactions.
Figure 3. Tumor cell-intrinsic PD-1 signaling promotes murine melanoma growth.
(A) Growth kinetics (mean±s.d.) of Pdcd1-OE vs. vector control B16-F10 melanomas in PD-L1(−/−) KO Rag(−/−) KO (n=14 vs. 20 vs. 10) vs. wildtype Rag(−/−) KO recipients (n=14 vs. 14 vs. 8) treated with anti-PD-L1- vs. isotype control monoclonal antibody (mAb). (B) Growth kinetics (mean±s.d.) in C57BL/6 (left) and NSG mice (right) of Pdcd1-OE B16-F10 cells co-transduced with PD-L1 (Cd274, also known as Pdcd1lg1)-shRNA vs. control-shRNA compared to vector controls (n=10 each). (C) Mean number of tumor spheres±s.e.m (left), and immunoblot analysis of p- and total S6, AKT, and ERK in PD-L1 Ig vs. control Ig-treated B16 cultures (right). (D) Immunoblot analysis of p- and total S6, AKT, and ERK in PD-L1 Ig vs. control Ig-treated B16-F10 melanoma cells cultured in the presence of the pharmacologic PI3K inhibitors, wortmannin or LY294002, or the mTOR pathway inhibitors, rapamycin or PP242. (E) Schematic diagram illustrating the introduction of tyrosine to phenylalanine mutations to murine PD-1 signaling motifs via site-directed mutagenesis. (F) Relative Pdcd1 mRNA expression (top, mean±s.d.) and representative flow cytometry plots of PD-1 surface protein expression (bottom) by wildtype Pdcd1-OE vs. Y225F-Pdcd1-OE, Y248F-Pdcd1-OE, Y225F/Y248F-Pdcd1-OE, and vector-control B16-F10 variants. (G) Tumor growth kinetics in C57BL/6 (top, n=10–14 each) and NSG mice (bottom, n=8–10 each), (H) mean number of tumor spheres±s.e.m, and (I) immunoblot analysis of p- and total S6, AKT, and ERK in B16-F10 melanoma variants as in (F). Immunoblot results are representative of n=2 independent experiments, respectively (*P<0.05, **P<0.01, ***P<0.001). See also Figure S5.
To further demonstrate protumorigenic melanoma-PD-L1 effects in the absence of adaptive immunity, we generated PD-L1 gene (Cd274, also known as Pdcd1lg1)-KD B16-F10 melanoma cells (Fig. S5B) and tested their ability to maintain culture growth and form tumors. Compared to vector controls, Pdcd1lg1 silencing impaired three-dimensional B16 melanoma growth in vitro (Fig. S5C) and in vivo tumorigenesis in both immunocompetent C57BL/6 and immunocompromised NSG mice (Fig. S5D). Moreover, Pdcd1lg1-KD reversed the significant increase in tumorigenicity of Pdcd1-OE vs. vector control B16-F10 melanoma cells in C57BL/6 and NSG mice (Fig.3B). To further demonstrate that PD-L1 interactions with melanoma-PD-1 promote melanoma growth, we treated native B16-F0 and B16-F10 cultures with a recombinant PD-L1 Fc-fusion protein (PD-L1 Ig), known to elicit changes in PD-1 receptor signaling in T-cells (Francisco et al., 2009). Compared to control Ig treatment, addition of PD-L1 Ig to B16 cultures significantly augmented three-dimensional growth and phosphorylation of S6 ribosomal protein (Fig.3C). Because both PI3K/AKT and mTOR signaling are known to feed into downstream S6 phosphorylation, we examined whether pharmacologic inhibition of either pathway can reverse the observed increase in p-S6 expression. We found that mTOR pathway blockade (via rapamycin or PP242) but not PI3K inhibition (via wortmannin or LY294002) suppressed the PD-L1 Ig-dependent phosphorylation of S6 in murine B16-F10 melanoma cells (Fig. 3D). Together, these findings demonstrate that interactions between melanoma-expressed PD-1 with its ligand, PD-L1, promote tumor growth and activate mTOR signaling.
Tumor cell-intrinsic PD-1 signaling is required for efficient murine melanoma growth
To determine whether melanoma cell-intrinsic PD-1 signaling is required for efficient tumor growth, we generated Pdcd1-OE B16 variants containing tyrosine to phenylalanine single point mutations of two PD-1 signaling motifs, the immunoreceptor tyrosine-based inhibitory motif (ITIM, disrupted by Y225F mutation) and the immunoreceptor tyrosine-based switch motif (ITSM, disrupted by Y248F mutation), within the cytoplasmic tail of melanoma-PD-1 (Fig.3E). A construct containing point mutations of both tyrosines (Y225F/Y248F) was also created. In immune cells, ITIM and ITSM play pivotal roles in PD-1 signaling (Riley, 2009). Transduction of wildtype vs. mutant Pdcd1-constructs into B16-F0 or B16-F10 melanoma cells resulted in similarly high expression levels of PD-1 (Fig.3F and Fig. S5E), permitting a direct comparison between wildtype Pdcd1-OE and each of the mutant variants. Strikingly, mutation of either one (Y225F or Y248F) or both (Y225F/Y248F) melanoma-PD-1 signaling motifs significantly abrogated the increased tumor growth observed in both C57BL/6 and NSG mice grafted with wildtype Pdcd1-OE vs. vector-control B16 melanoma variants (Fig.3G and Fig. S5F), suppressed three-dimensional tumor growth in vitro (Fig.3H and Fig. S5G), and phosphorylation of S6 ribosomal protein (Fig. 3I) compared to enforced expression of wildtype Pdcd1, respectively.
Melanoma cell-intrinsic PD-1 enhances human tumor xenograft growth
We next analyzed the effects of melanoma-specific PD-1 knockdown vs. PD-1 overexpression on human melanoma xenograft growth. Transduction of human A375, C8161, or G3361 melanoma cells with two distinct PDCD1-shRNAs significantly inhibited PD-1 mRNA expression and blocked PD-1 protein expression between 53–75% (Fig. S6A), and infection with PDCD1-OE constructs resulted in marked upregulation of PD-1, both at the mRNA and protein level (>90% positivity), respectively (Fig. S6B). PDCD1-KD significantly inhibited and PDCD1-OE markedly increased human melanoma xenograft growth in NSG mice compared to vector control-transduced A375, C8161, or G3361 tumors (Fig.4A). Preservation of PDCD1 silencing and overexpression were confirmed for all melanoma xenografts at the experimental endpoint, respectively (Fig. S6C and S6D). Moreover, PD-1+ cancer cell subsets purified from native C8161 cultures showed significantly increased tumorigenicity in NSG mice, compared to PD-1− C8161 cells (Fig. S6E). Consistent with our in vivo findings, PDCD1-KD impaired and PDCD1-OE promoted three-dimensional A375, C8161, and G3361 culture growth compared to controls (Fig. 4B). Furthermore, relative to control Ig treatment, addition of human PD-L1 Ig augmented tumor sphere formation of A375 and G3361, but not C8161 melanoma cultures (Fig. 4B), the latter of which express greater than 3-fold higher endogenous PD-L1 levels than A375 and G3361 cells (Fig. S1B). Similar to our findings in murine B16 cells, human PDCD1-KD lines showed a reduction and PDCD1-OE and PD-L1 Ig-treated human G3361 melanoma cells an increase in p-S6 levels compared to respective controls (Fig.4C). Additionally, pharmacologic inhibition of mTOR but not PI3K signaling blocked the increase in p-S6 expression in PD-L1 Ig compared to control Ig-treated human G3361 melanoma cells (Fig.4D), indicating mTOR pathway dependence of S6 phosphorylation downstream of the melanoma-PD-1 receptor, consistent with our findings in murine B16 melanoma cells (Fig.3D).
Figure 4. PD-1 expression by human melanoma cells promotes experimental tumor growth.
(A) Tumor growth kinetics (mean±s.d.) of PDCD1-shRNA-, PDCD1-shRNA-2, and PDCD1-OE vs. vector control human A375 (left), C8161 (center), and G3361 melanoma cells (right) grafted to NSG mice (n=8–20 each). (B) Mean number of tumor spheres±s.e.m and (C) immunoblot analysis (G3361) of phosphorylated (p) and total ribosomal protein S6, AKT, and ERK in PDCD1-shRNA-1/-2 vs. vector control, PDCD1-OE vs. vector-control, and PD-L1 Ig- vs. control Ig-treated human A375, C8161, and G3361 melanoma cultures. (D) Immunoblot analysis of p- and total S6, AKT, and ERK in PD-L1 Ig vs. control Ig-treated G3361 melanoma cells cultured in the presence of the pharmacologic PI3K inhibitors, wortmannin or LY294002, or the mTOR pathway inhibitors, rapamycin or PP242. (E) Schematic diagram illustrating the introduction of tyrosine to phenylalanine mutations to human PD-1 signaling motifs via site-directed mutagenesis. (F) Representative flow cytometry plots of PD-1 surface protein expression and (G) tumor growth kinetics (mean±s.d.) of PDCD1-OE vs. Y225F-PDCD1-OE, Y248F-PDCD1-OE, Y225F/Y248F-PDCD1-OE, and vector-control human A375 (top, n=10–24) and C8161 melanomas (bottom, n=10–12) in NSG mice, respectively. (H) Mean number of tumor spheres±s.e.m and (I) immunoblot analysis of p- and total S6, AKT, and ERK in C8161 melanoma variants as in (D). Immunoblot results are representative of n=2–3 independent experiments (*P<0.05, **P<0.01, ***P<0.001). See also Figure S6.
Furthermore, generation of human PDCD1-OE ITIM (Y223F) and/or ITSM (Y248F) mutant A375 and C8161 cell lines (Fig.4E) with similarly high expression levels of human PD-1 (Fig.4F) revealed that mutation of either one (Y223F or Y248F) or both (Y223F/Y248F) signaling motifs within the melanoma-PD-1 cytoplasmic tail abrogated the increased tumor growth observed in mice grafted with wildtype PDCD1-OE vs. vector-control A375 or C8161 variants (Fig.4G). Additionally, Y223F-, Y248F-, and Y223F/Y248F-mutant PDCD1-OE C8161 melanoma cells demonstrated significantly impaired three-dimensional culture growth (Fig.4H) and reduced p-S6 levels compared to wildtype PDCD1-OE C8161 cells (Fig.4I). Together, these findings identify PD-1 expressed by human melanoma cells as a lymphocyte-independent tumor growth-accelerating mechanism.
Antibody-mediated blockade of PD-1 on melanoma cells inhibits murine melanoma growth
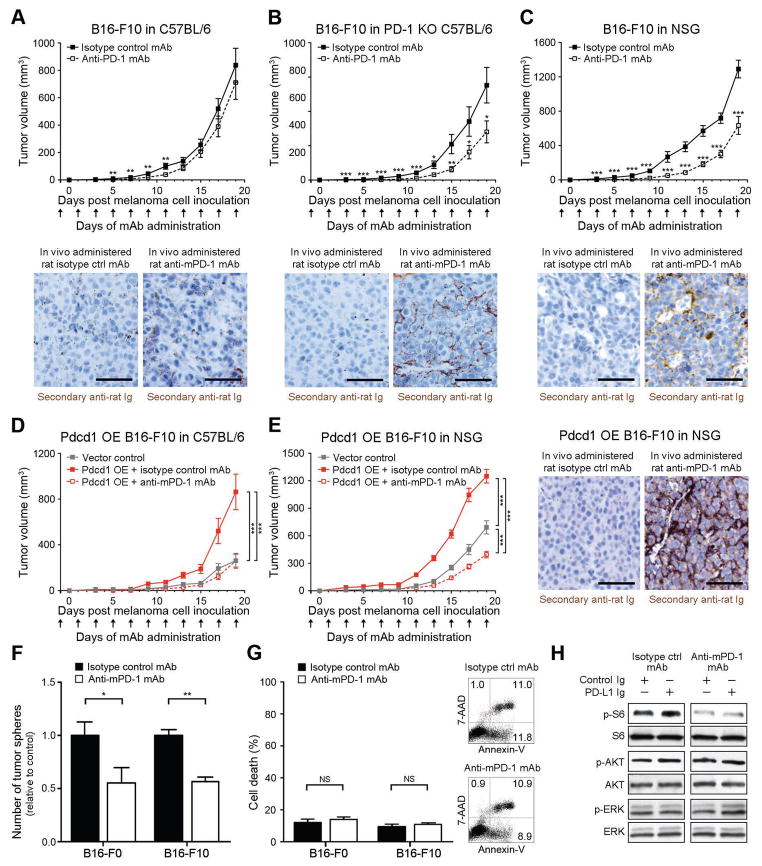
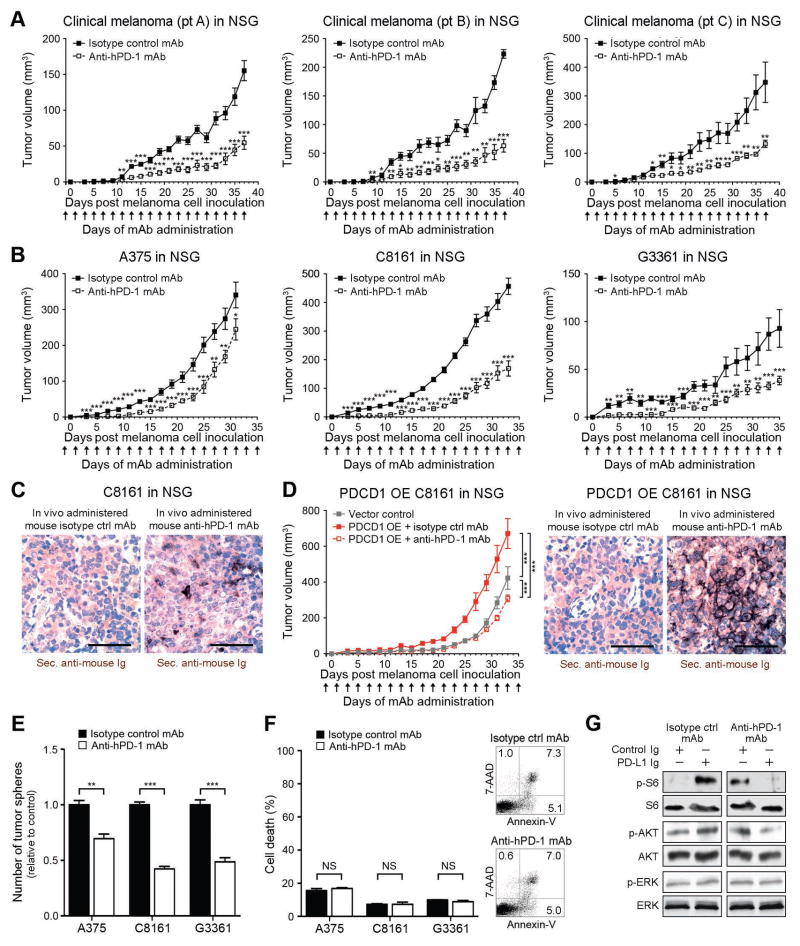
We next examined whether antibody-mediated melanoma-PD-1 blockade significantly inhibits tumor growth, even in immunocompromised NSG hosts, as would be expected based upon the herein demonstrated melanoma cell-intrinsic, protumorigenic PD-1 receptor functions. First, administration of a PD-1 blocking antibody to immunocompetent, C57BL/6 recipients starting one day before inoculation with B16-F10 cells resulted in modest inhibition of melanoma growth between days 5 and 11 post inoculation (P<0.01), but showed no significant differences in tumorigenicity compared to isotype control antibody-treatment at later time points (Fig.5A), consistent with previous studies (Peng et al., 2012; Woo et al., 2012). However, we found that antibody-mediated PD-1 blockade significantly (P<0.05) inhibited B16-F10 melanoma growth in PD-1(−/−) KO C57BL/6 mice compared to controls, for the entire duration of the experiment (Fig.5B). Immunohistochemical examination of melanoma grafts harvested at the experimental endpoint revealed >5-fold increased binding (P<0.05) of in vivo-administered anti-PD-1 antibody to B16 melanoma target tissue in PD-1(−/−) KO (Fig.5B) compared to wildtype C57BL/6 hosts (Fig.5A), supporting the notion of a more pronounced PD-1 antibody effect on melanoma cells in PD-1(−/−) KO mice. A >20% increase in PD-1 antibody titer in the serum of PD-1(−/−) KO vs. PD-1(+/+) C57BL/6 hosts, as determined by rat-IgG2a-specific ELISA (Fig. S7A), further indicated that increased PD-1 antibody availability might, at least in part, contribute to the growth-inhibitory effect of PD-1 blockade in PD-1(−/−) KO hosts. Anti-PD-1 antibody administration to NSG mice also significantly (P<0.001) diminished B16-F10 melanoma growth compared to isotype control antibody treatment (Fig.5C). Interestingly, while compared to B16 melanoma grafts grown in C57BL/6 mice, B16 melanomas grafted to NSG mice tended to show increased anti-PD-1 antibody binding, antibody titers were not increased in NSG mouse serum (Fig. S7A), suggesting strain-specific differences in PD-1 antibody kinetics. To control for the possibility that the observed growth-inhibitory effects might result from antibody-mediated blockade of PD-1-expressing innate immune cell subtypes present in NSG mice, we administered anti-PD-1 antibody to NK cell-, macrophage-, and neutrophil-depleted NSG recipients of B16-F10 melanoma cells (Fig. S7B). We also generated NSG mice depleted of all three innate immune effector subsets. Anti-PD-1 antibody treatment inhibited B16 melanoma growth compared to isotype control antibody in all innate immune cell-depleted NSG hosts (Fig. S7C).
Figure 5. Anti-PD-1 blocking antibody inhibits murine melanoma growth in immunocompetent, immunocompromised and PD-1-deficient tumor graft recipient mice.
(A) Tumor growth kinetics (mean±s.d.) of B16-F10 melanomas in wildtype C57BL/6 (n=32 vs. 34), (B) PD-1(−/−) knockout (KO) C57BL/6 (n=20 vs. 16), and (C) NSG (n=20 vs. 18), and of (D) Pdcd1-overexpressing (OE) vs. vector control B16-F10 melanomas in C57BL/6 (n=10 each) or (E) NSG mice (n=10 each) treated with anti-PD-1- vs. isotype control antibody. Representative immunohistochemical images illustrate binding of in vivo-administered rat anti-mouse PD-1 blocking but not isotype control antibody to the respective B16-F10 melanoma grafts (size bars, 50μm). (F) Mean number of tumor spheres±s.e.m. and (G) flow cytometric assessment of cell death (percent AnnexinV+/7AAD+ cells, mean±s.e.m. (left) and representative flow cytometry plots (right) of anti-PD-1- vs. isotype control mAb-treated murine B16-F0 and B16-F10 melanoma cultures. (H) Immunoblot analysis (representative of n=2 independent experiments) of phosphorylated (p) and total ribosomal protein S6, AKT, and ERK in B16 cultures concurrently treated with PD-L1 Ig vs. control Ig and/or anti-PD-1- vs. isotype control mAb NS: not significant, *P<0.05, **P<0.01, ***P<0.001). See also Figure S7.
To further confirm melanoma-specific PD-1 inhibition of the PD-1 blocking antibody, we administered anti-PD-1 antibody to C57BL/6 and NSG mice grafted with Pdcd1-OE vs. vector control B16-F10 melanoma cells. Anti-PD-1 antibody treatment reversed the increase in tumor growth of isotype control-treated Pdcd1-OE compared to vector-control B16 melanomas in both C57BL/6 (Fig.5D) and NSG mice, concomitant with binding of in vivo-administered anti-PD-1 antibody to B16 melanomas (Fig.5E), thereby confirming recognition of melanoma-PD-1 by the PD-1 blocking antibody. Antibody-mediated PD-1 blockade also reduced three-dimensional B16-F0 and B16-F10 melanoma growth in vitro (Fig.5F), but did not induce significant cell death compared to isotype control antibody-treatment (Fig.5G). Moreover, treatment of B16 melanoma cultures with anti-PD-1 but not isotype control antibody inhibited phosphorylation of S6 ribosomal protein (Fig.5H). Together, these findings show that antibody-mediated PD-1 blockade directly on melanoma cells inhibits tumor cell-intrinsic, protumorigenic PD-1 functions, including in the absence of adaptive immunity.
Antibody-mediated PD-1 blockade inhibits human melanoma xenograft growth in immunodeficient mice
We next examined whether antibody-mediated PD-1 blockade can also inhibit human melanoma growth in NSG mice. To assess the translational relevance of targeting melanoma cell-intrinsic PD-1 to impede tumor growth, we first administered anti-PD-1 antibody to NSG mice grafted with patient-derived melanoma cells. Consistent with our findings in murine B16 models (Fig.5C), in vivo anti-PD-1 antibody administration to NSG mice significantly inhibited mean tumor volumes of clinical melanoma xenografts derived from three distinct melanoma patients (Fig.6A). Anti-PD-1 antibody treatment also significantly inhibited the growth of human A375, C8161, and G3361 melanoma xenografts in NSG mice compared to that of the respective control antibody-treated melanomas (Fig.6B). Immunohistochemical analysis revealed binding of in vivo-administered anti-human PD-1 antibody to melanoma xenografts (Fig.6C). Administration of anti-PD-1 antibody to NSG mice also abrogated the increased melanoma xenograft growth of isotype control-treated human PDCD1-OE compared to vector-control C8161 xenografts (Fig.6D). Marked melanoma binding of in vivo-administered anti-PD-1 antibody to PDCD1-OE C8161 melanomas (Fig.6D) further confirmed melanoma-PD-1 reactivity of the human anti-PD-1 blocking antibody. Compared to isotype control antibody-treatment, PD-1 blockade also decreased three-dimensional melanoma growth in vitro (Fig.6E), but did not significantly induce apoptosis in human A375, C8161, or G3361 melanoma cultures (Fig.6F). Finally, treatment of G3361 melanoma cells with anti-PD-1 but not isotype control antibody inhibited PD-L1 Ig-dependent phosphorylation of S6 ribosomal protein (Fig.6G). Together, our findings in NSG mice indicate that anti-PD-1-mediated melanoma growth inhibition results from direct interference with melanoma-expressed PD-1 and is not necessarily dependent on adaptive immunity.
Figure 6. Anti-PD-1 blocking antibody inhibits human melanoma xenograft growth in immunocompromised mice.
(A) Kinetics (mean±s.d.) of clinical melanoma xenograft growth in NSG mice treated with anti-human PD-1 or isotype control antibody (patient A, n=7 each; patient B, n=5 vs. 4; patient C: n=10 each). (B) Tumor growth kinetics (mean±s.d.) and (C) representative secondary antibody staining (size bars, 50μm) of mouse anti-human PD-1 vs. isotype control antibody-treated human A375 (n=14 each), C8161 (n=14 each), or G3361 melanoma xenografts (n=16 vs. 12) or of (D) human PDCD1-OE vs. vector control-transduced C8161 xenografts in NSG mice (n=10 each). Immunohistochemical images illustrate binding of in vivo-administered mouse anti-human PD-1 blocking but not isotype control antibody to the respective human melanoma xenograft (size bars, 50μm). (E) Mean number of tumor spheres±s.e.m. and (F) flow cytometric assessment of cell death (percent AnnexinV+/7AAD+ cells, mean±s.e.m. (left) and representative flow cytometry plots (right) of anti-PD-1- vs. isotype control mAb-treated human A375, C8161, and G3361 melanoma cultures. (H) Immunoblot analysis (representative of n=3 independent experiments) of phosphorylated (p) and total ribosomal protein S6, AKT, and ERK in G3361 cultures concurrently treated with PD-L1 Ig vs. control Ig and/or anti-PD-1- vs. isotype control mAb (NS: not significant, *P<0.05, **P<0.01, ***P<0.001).
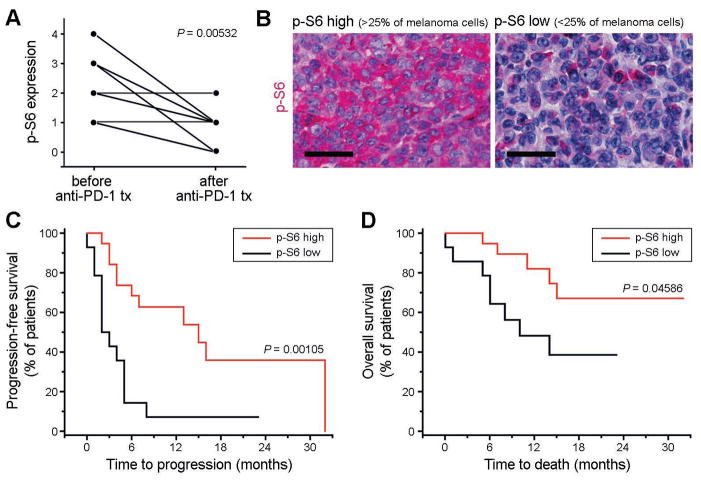
Melanoma cell expression of the PD-1 effector molecule, p-S6, correlates with response to PD-1 therapy in cancer patients
To further assess the translational relevance of melanoma cell-intrinsic PD-1 receptor signaling, we performed p-S6 staining and quantitatively assessed melanoma-p-S6 positivity in pre-treatment vs. post-treatment tumor biopsies obtained from n=11 melanoma patients undergoing anti-PD-1 therapy. We found that melanoma biospecimens sampled post PD-1 therapy demonstrated significantly (P=0.005) decreased p-S6 expression compared to patient-matched pre-treatment biopsies (Fig.7A), consistent with our findings in PD-1 antibody-treated melanoma cell lines (Figs.5H,6G). Additionally, in a cohort of n=34 melanoma patients where pre-treatment tumor tissue was available for analysis, we found that patients with high p-S6 expression (>25% of melanoma cells, Fig.7B) prior to treatment showed a >3-fold increase in progression-free survival (mean progression-free survival: 17.0 vs. 4.5 months, P=0.001, Fig.7C) and significantly (P<0.05) enhanced overall survival (mean overall survival: 25.1 vs. 13.0 months, Fig.7D) compared to melanoma patients with low p-S6 levels (<25% of melanoma cells, Fig.7B) in pre-treatment tumor biospecimens (Table S2). These findings suggest a relationship between p-S6 and response to PD-1 pathway blockade, thereby indicating the potential translational relevance of melanoma cell-intrinsic PD-1 receptor functions.
Figure 7. Analysis of p-S6 expression in tumor biospecimens obtained from patients with advanced-stage melanoma undergoing anti-PD-1 antibody therapy.
(A) Expression of phospho (p)-S6 ribosomal protein by melanoma cells in tumor biospecimens obtained from n=11 patients with stage IV melanoma before treatment start compared to that in patient-matched progressive lesions sampled after initiation of anti-PD-1 antibody therapy. p-S6 expression by melanoma cells was determined by immunohistochemical analysis and graded by three independent investigators blinded to the study outcome on a scale of 0–4 (0: no p-S6 expression by melanoma cells; 1: p-S6 expression in 1–25%; 2: 26–50%; 3: 51–75%; 4: >75% of melanoma cells). (B) Representative p-S6 immunohistochemistry of tumor biospecimens obtained from melanoma patients before initiation with systemic anti-PD-1 antibody therapy showing low (<25%) vs. high (>25%) melanoma cell expression of p-S6. Size bars, 50μm. (C) Kaplan-Meier estimates of progression-free survival and (D) of overall survival probability in stage IV melanoma patients (n=34) demonstrating low (<25%, n=14 patients) vs. high melanoma cell-expression of p-S6 (>25%, n=20 patients) in tumor biospecimens obtained before initiation of systemic anti-PD-1 antibody treatment. See also Table S2.
DISCUSSION
Our study provides several insights into PD-1 pathway functions in melanoma. First, we have conducted a comprehensive characterization of PD-1 transcript and protein expression by cancer cells in clinical tumor biopsies and established melanoma lines. Until now, PD-1 expression has been mainly reported in immune-competent cells of the hematopoietic lineage (Topalian et al., 2012a). We found that both melanoma cell lines and surgical specimens frequently harbor PD-1-expressing cancer cells. However, PD-1 is not uniformly present on all melanoma cells among heterogeneous tumor samples. Rather, it is restricted to small melanoma subpopulations that are nonetheless critically important for tumor growth, consistent with our previous findings demonstrating preferential PD-1 expression by melanoma-initiating cells (Schatton et al., 2010). In our current study, RT-PCR, immunoblot, and flow cytometric analyses revealed PD-1 on melanoma cells in all cell lines and clinical tumor samples examined. Furthermore, immunofluorescence double labeling similarly showed PD-1 expression by melanoma subpopulations in clinical biopsy specimens obtained from >60% of melanoma patients. Thus, using various independent methods, our work clearly establishes that melanomas frequently contain PD-1+ tumor cell fractions. Comparably, melanoma cell expression of the PD-1 ligand, PD-L1, is often confined to small subsets of cancer cells within clinical tumor specimens (Herbst et al., 2014; Topalian et al., 2012b).
Second, this study demonstrates PD-1 receptor signaling in a non-immune cell type, i.e. melanoma cells. To date, PD-1 immunobiology has been mainly studied in T-cells (Topalian et al., 2012a). Binding of T-cell-expressed PD-1 to its ligands mediates inhibitory signals that downmodulate T-effector functions. For example, in the cancer context, PD-1 expression by tumor-reactive CTLs results in their exhaustion or functional impairment (Fourcade et al., 2010; Sakuishi et al., 2010), which represents a key mechanism underlying tumor immune evasion (Pardoll, 2012). Similar to the protumorigenic effects of T-cell-expressed PD-1, our findings establish PD-1 expressed by melanoma cells as a tumor growth-promoting mechanism in multiple independent experimental in vitro and in vivo systems. However, while T-cell-PD-1 promotes cancer progression by dampening antitumor immune responses, melanoma-PD-1 promotes tumor growth, even in the absence of a functional adaptive immune system.
Our results further indicate that efficient, PD-1-driven tumorigenesis requires melanoma-PD-1 interactions with host- and/or melanoma-expressed PD-L1, because both PD-L1-deficient and PD-L1 antibody-treated mice grafted with Pdcd1-OE melanomas demonstrate decreased tumor growth compared to respective controls. Additionally, PD-L1 (Pdcd1lg1) silencing reversed melanoma-PD-1-driven tumorigenesis and recombinant PD-L1 Ig treatment promoted melanoma spheroid growth. PD-L1 expression by melanoma cells has established roles in tumor immune evasion (Dong et al., 2002). Beyond promoting cancer progression by engaging with TIL-expressed PD-1, our study indicates that melanoma-expressed PD-L1 may also promote tumor growth via paracrine or autocrine interactions with the melanoma-PD-1 receptor.
In T-cells, PD-1 engagement by its ligands modulates signaling networks downstream of the TCR, including mTOR and PI3K/AKT (Riley, 2009). Consistent with the interrelationship of PD-1 and PI3K/AKT/mTOR signaling in T-cells, the important role of these pathways in melanoma proliferation (Flaherty et al., 2012), and the herein described protumorigenic effects of melanoma-PD-1, we found that PDCD1-KD, antibody-mediated PD-1 blockade, and mutagenesis of melanoma-PD-1 signaling motifs decreased, while PDCD1-OE and PD-L1 Ig-treatment increased phosphorylation of the mTOR effector molecule (Corcoran et al., 2013), ribosomal protein S6. Melanoma-PD-1-dependent S6 phosphorylation was reversed via pharmacologic inhibition of mTOR but not PI3K, suggesting that the PD-1 receptor on melanoma cells activates downstream mTOR signaling through a PI3K/AKT-independent pathway. However, whereas PD-1 activation augments p-S6 levels in melanoma cells and enhances tumor growth, it dampens mTOR signaling in T-cells, leading to diminished proliferation (Riley, 2009). Because S6 phosphorylation represents a point of convergence that integrates multiple upstream signaling networks (Corcoran et al., 2013; Flaherty et al., 2012) it is possible that melanoma-PD-1 might also modulate several alternative signaling networks, in addition to the mTOR pathway.
PD-1 ligation in T-lymphocytes is known to recruit phosphatases SHP-1 and SHP-2 to its ITIM and ITSM cytosolic loci, which induces dephosphorylation of proximal TCR signaling intermediaries and subsequent suppression of several pathways downstream of the TCR, including mTOR (Riley, 2009). SHP-2 is also expressed by melanoma cells (Ostman et al., 2006) and tumor-initiating cell subsets in other cancers (Aceto et al., 2012; Liu et al., 2011), paralleling our previous findings of preferential PD-1 expression by melanoma-initiating cells (Schatton et al., 2010). In cancer cells, SHP-2-dependent signaling promotes activation of protumorigenic pathways, including mTOR (Liu et al., 2011; Ostman et al., 2006). The divergent effects of PD-1 ligation on mTOR signaling in melanoma cells vs. T-cells are thus entirely consistent with the opposing, protumorigenic vs. growth-inhibitory roles of SHP-2 in the respective tissues.
Finally, our work reveals that PD-1 pathway interference exerts tumor growth-inhibitory effects, not only in mice with fully intact immunity, but also in melanoma cultures devoid of immune cells and in severely immunocompromised, T-cell-, B-cell-, and innate immune cell-deficient hosts. Together, these results show that antibody-mediated blockade of PD-1 at the level of the melanoma cell inhibits tumor growth. Because PD-1 pathway inhibitors have produced unprecedented response rates in otherwise treatment-refractory patients with advanced cancers, including malignant melanoma (Hamid et al., 2013; Herbst et al., 2014; Topalian et al., 2012b; Weber et al., 2015; Wolchok et al., 2013), our findings are of potential translational importance. It has been well established that PD-1 blockade reverses cancer antigen-specific T-cell exhaustion, thereby restoring antitumor immunity (Fourcade et al., 2010; Sakuishi et al., 2010). Nevertheless, our data suggest that blockade of PD-1 directly on melanoma cells might represent an important additional, tumor cell-intrinsic mechanism that could contribute to the clinical effectiveness of PD-1 cancer therapy. In support of this possibility, our data obtained in a small cohort of patients with stage IV melanoma suggests that tumoral expression of the PD-1 receptor signaling mediator, p-S6, appears to correlate with response to anti-PD-1 antibodies. However, the possible utility of p-S6 as a potential biomarker of PD-1 inhibitor sensitivity will require independent validation in larger patient cohorts, including prospective cohort studies.
Consistent with our findings of protumorigenic effects of the melanoma-PD-1:PD-L1 axis, clinical trial data suggest a correlation between melanoma-PD-L1 and TIL-PD-L1 expression and objective response to PD-1 checkpoint blockade (Herbst et al., 2014; Topalian et al., 2012b; Tumeh et al., 2014). Interestingly, both elevated PD-L1 (Jiang et al., 2013) and p-S6 expression levels (Corcoran et al., 2013) have evolved as potential biomarkers of resistance to melanoma therapies targeting oncogenic BRAF mutations. Therefore, our data suggest that combination of therapies targeting the MAPK pathway (Flaherty et al., 2012) with PD-1 inhibitors may be effective, not only because they activate tumor-specific immunity while concurrently blocking the MAPK oncogenic pathway, but also because PD-1/PD-L1 blockade might additionally suppress mTOR-associated protumorigenic signals. Furthermore, in light of our findings, the superior clinical activity and safety profile of anti-PD-1- compared to anti-CTLA-4 therapy (Hamid et al., 2013; Pardoll, 2012; Postow et al., 2015; Weber et al., 2015) might, at least in part, relate to the fact that the latter merely interferes with T-cell function, whereas PD-1 antibody treatment may also directly target other PD-1-expressing immune cell types (Topalian et al., 2012a) or the tumor itself, as suggested by our data. Finally, robust clinical response to anti-PD-1 therapy in patients with cancers that have hitherto not typically responded to immunotherapy (Herbst et al., 2014; Topalian et al., 2012b) could at least be partially explained by direct PD-1 inhibition on tumor cells in the respective malignancies.
In summary, our findings identify PD-1 expressed by melanoma cells as a tumor growth receptor and molecular mediator of melanoma cell-intrinsic mTOR signaling, serving to promote tumorigenesis in addition to its protumorigenic role when expressed by immune cells. Recognition of melanoma-PD-1 receptor-driven tumorigenesis critically enhances our understanding of the mechanisms underlying melanoma progression and could contribute to the further refinement of PD-1-targeted therapies, for improved outcomes in patient with advanced stage cancer.
EXPERIMENTAL PROCEDURES
Melanoma cell lines, culture methods, and clinical specimens
Authenticated melanoma cell lines were cultured as described (Schatton et al., 2008). Human PBMCs were obtained from healthy volunteers and clinical tumor biospecimens were obtained from melanoma patients in accordance with protocols approved by the IRBs of Partners Health Care Management, the Dana-Farber Cancer Institute, the University of Zurich, Switzerland, and the University of Bern, Switzerland. Informed consent was obtained from all subjects and all studies were conducted in accordance with the Declaration of Helsinki. PD-1+ and PD-1− melanoma subpopulations were generated as described (Schatton et al., 2008; Schatton et al., 2010).
RT-PCR, real-time quantitative PCR, and flow cytometry
Full-length PDCD1 was amplified and sequenced following reverse transcription of total mRNA using PDCD1-specific primer pairs. Relative PDCD1, PDCD1LG1 (CD274) and PDCD1LG2 (CD273) transcript levels were determined by real-time qRT-PCR and calculated using the 2(−∆∆Ct) method (Schatton et al., 2008; Schatton et al., 2010). PD-1 surface protein expression by established melanoma lines and patient-derived melanoma single cell suspensions was analyzed by flow cytometry (Schatton et al., 2008; Schatton et al., 2010).
Western blot analysis
Cells were lysed, total protein separated by SDS/PAGE and transferred to a PVDF membrane by electroblotting (Posch et al., 2013). Expression levels of human and murine PD-1, and of phosphorylated vs. total ERK1/2, AKT and S6 proteins were determined using enhanced chemiluminescence (Posch et al., 2013) or the Odyssey CLx imaging system (LI-COR Biosciences).
Immunohistochemistry and immunofluorescence staining
Immunofluorescence double-labeling for PD-1, MART-1 and/or CD45, and immunohistochemical analysis of PD-1 expression in experimental tumors and of p-S6 expression in tumor biospecimens obtained from melanoma patients undergoing anti-PD-1 antibody therapy were carried out as described (Schatton et al., 2008; Schatton et al., 2010). p-S6 immunoreactivity by melanoma cells was graded by three independent investigators blinded to the study outcome on a scale of 0–4 (0: no p-S6 expression by melanoma cells; 1: p-S6 expression in 1–25%; 2: 26–50%; 3: 51–75%; 4: >75% of melanoma cells).
Generation of stable PD-1 or PD-L1 knockdown and PD-1-overexpressing melanoma cell line variants
Stable PD-1 or PD-L1 knockdown melanoma lines were generated using lentiviral transduction particles containing shRNAs against human PDCD1, murine Pdcd1, or murine Pdcd1lg1 (Cd274), and PD-1-overexpressing melanoma lines by infection with viral particles containing the full length murine Pdcd1 or human PDCD1 CDS. PDCD1-OE melanoma variants containing tyrosine to phenylalanine single-point mutations within PD-1 signaling motifs were generated by site-directed mutagenesis followed by enforced expression, as above.
Three-dimensional melanoma culture
Melanoma tumor sphere cultures of native or melanoma-PD-1 variant lines were maintained, as described (Aceto et al., 2012; Civenni et al., 2011), in standard culture medium, as above, in the presence or absence of anti-PD-1 or isotype control mAb, recombinant PD-L1 Ig or control Ig.
Murine melanoma induction and human melanoma xenotransplantation
C57BL/6, PD-1(−/−) KO C57BL/6, NSG, Rag(−/−), and PD-L1(−/−) KO Rag(−/−) mice (Francisco et al., 2009) were maintained and experiments performed in accordance with IACUC approved experimental protocols. For tumorigenicity studies, melanoma cells were injected subcutaneously into flanks of recipient mice (Schatton et al., 2008). For PD-1 and PD-L1 targeting experiments melanoma cells were grafted, mice intraperitoneally injected with anti-PD-1, anti-PD-L1 or isotype control mAbs (200μg, respectively) every other day starting one day before melanoma inoculation, and tumor formation/growth assessed as described (Schatton et al., 2008).
Statistical analysis
Gene and protein expression levels, tumor spheroid and in vivo melanoma growth were compared statistically using the unpaired Student’s t test, the nonparametric Mann-Whitney test (comparison of two experimental groups) or repeated measures two-way ANOVA followed by the Bonferroni correction (comparison of three or more experimental groups). Kaplan-Meier estimates and the log-rank test were used to analyze statistical differences in progression-free and overall survival between melanoma patients treated with anti-PD-1 antibody therapy, whose pre-treatment tumor biopsies showed low vs. high melanoma cell expression of p-S6. Differences in p-S6 expression in patient-matched tumor biospecimens obtained before and after PD-1 therapy were statistically compared using the paired Student’s t test. Data was tested for normal distribution using the D'Agostino and Pearson omnibus normality test. A two-sided value of P<0.05 was considered statistically significant.
See also the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank M. Joubert, I. Portugal, C. Correia, and C. Lee for technical assistance. This work was supported by an Innovative Research Grant from the Melanoma International Foundation, a Fund to Sustain Research Excellence from the Brigham Research Institute, Brigham and Women’s Hospital, Department of Dermatology funding for new investigators (to T.S.), a Swiss National Science Foundation grant PMDPP3_151326 (to E.G.), and NIH/NCI grants 1R01CA158467 (to M.H.F. and G.F.M.) and U54 CA163125 (to A.H.S.). T.S. is the recipient of a Research Career Development award from the Dermatology Foundation. C.P. and H.M. are recipients of a Klaus Wolff Fellowship by the Austrian Society of Dermatology and Venereology and received salary support from the Fondation René Touraine. C.P. was awarded a Melanoma Research Scholar Award from the Outrun the Sun Melanoma Foundation. N.L. is the recipient of a Medical Student Grant from the American Skin Association. V.R.J. is supported by the Department of Defense through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program. The authors declare that there are no conflicts of interest.
Footnotes
AUTHOR CONTRIBUTIONS
S.K. and T.S. planned the project. S.K., C.P., S.R.B., H.M., C.S., E.G., C.P.E., N.L., V.R.J., Q.Z., R.T., W.H., and T.S. carried out experimental work. S.K., C.P., S.R.B., H.M., C.S., E.G., C.P.E., N.L., V.R.J., Q.Z., W.H., A.C., R.D., M.C.M., K.T.F., M.H.F., G.F.M., A.H.S., T.S.K., and T.S. analyzed data. S.K. and T.S. wrote the paper. All authors discussed the results and commented on the manuscript.
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two table and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2015.08.052.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G, Confalonieri S, Quarto M, Hu G, Balwierz PJ, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med. 2012;18:529–537. doi: 10.1038/nm.2645. [DOI] [PubMed] [Google Scholar]
- Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Rothenberg SM, Hata AN, Faber AC, Piris A, Nazarian RM, Brown RD, Godfrey JT, Winokur D, Walsh J, et al. TORC1 suppression predicts responsiveness to RAF and MEK inhibition in BRAF-mutant melanoma. Sci Transl Med. 2013;5:196ra198. doi: 10.1126/scitranslmed.3005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: targeted strategies for melanoma. Nat Rev Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- Liu KW, Feng H, Bachoo R, Kazlauskas A, Smith EM, Symes K, Hamilton RL, Nagane M, Nishikawa R, Hu B, et al. SHP-2/PTPN11 mediates gliomagenesis driven by PDGFRA and INK4A/ARF aberrations in mice and humans. J Clin Invest. 2011;121:905–917. doi: 10.1172/JCI43690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizee G, Radvanyi L, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, Chong K, Peng L, Dimon MT, Phillips T, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci. 2013;110:4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012a;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012b;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment: a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.