Abstract
Chronic low-grade inflammation is associated with obesity. This study investigated effect of high hydrostatic pressure extract of red ginseng (HRG) on inflammation in rats with high-fat (HF) diet induced obesity. Male, Sprague-Dawley rats (80~110 g) were randomly divided into two groups, and fed a 45% HF diet (HF) and a 45% HF diet containing 1.5% HRG (HF+HRG) for 14 weeks. At the end of the experiment, the serum leptin level was reduced by the HRG supplementation. The mRNA expression of genes related to adipogenesis including peroxisome proliferator-activated receptor-gamma and adipocyte protein 2 was down-regulated in the white adipose tissue (WAT). The mRNA levels of major inflammatory cytokines such as tumor necrosis factor-α, monocyte chemoattractant protein 1, and interleukin-6 were remarkably down-regulated by the HRG in WAT. These results suggest that HRG might be beneficial in ameliorating the inflammation-associated health complications by suppressing adipogenic and pro-inflammatory gene expression.
Keywords: red ginseng, high hydrostatic pressure, high-fat diet, inflammation, gene expression
INTRODUCTION
Chronic low-grade inflammation is a common feature of obesity (1). Adipose tissue expansion results in inflammation in white adipose tissue (WAT), which is followed by obesity-related health complications including insulin resistance, type 2 diabetes, and other metabolic complications (1,2). Therefore, targeting the occurrence and progression of adipose tissue inflammation during the development of obesity has been regarded as a therapeutic tool for preventing/treating obesity-related metabolic disorders.
Korean red ginseng has been widely used as a traditional herbal medicine in Asia (3). Numerous studies have reported that Korean red ginseng exerts several beneficial properties such as anti-aging, anti-fatigue, anti-stress, anti-atherosclerosis, anti-diabetic, and anti-cancer activities (3). Moreover, a recently published animal study indicates that the consumption of Korean red ginseng is associated with anti-inflammatory effects in high-fat (HF) diet induced atherosclerosis (4). In addition, supplementation of Korean red ginseng reduces adipose tissue mass and prevents obesity in diet-induced obese mice (5). However, to the best of our knowledge, information on how Korean red ginseng affects inflammation in WAT during the progression of obesity has been elusive.
The beneficial effects of red ginseng have mainly been attributed to ginsenosides, which are major bioactive compounds found in ginseng. Up to date, approximately 40 ginsenosides have been identified in Korean ginseng (6). High hydrostatic pressure (HHP) is a non-thermal food-processing technique that has been used as an alternative to the high-heat processing in the food industry (7). Usage of low temperature (between −20 and 60°C) in the HHP process results in an increase of extraction efficiency without destroying heat-sensitive bio-active constituents (7). Lee et al. (8) reported that the amount of major ginsenosides found in high hydrostatic pressure extracts of Korea ginseng was 45% more compared to the extracts using conventional methods.
Therefore, we explored the anti-inflammatory effect of high hydrostatic pressure extract of red ginseng (HRG) in a HF diet induced obese rat model. We hypothesized that supplementation of HRG may decrease mRNA expression of genes related to adipogenesis and inflammation in WAT, which may have potential to ameliorate obesity-related complications.
MATERIALS AND METHODS
Preparation of HRG
HRG was kindly supplied by the Korea Food Research Institute (Seongnam, Korea). To obtain red ginseng, 6 year old Panax ginseng root from Gimpo-Paju Ginseng Agricultural Cooperative (Gimpo, Korea) was boiled and simmered for 5 h. After drying the surface of the ginseng at room temperature, the ginseng was incubated at 70°C overnight. For preparation of the HRG, a red ginseng root suspension was poured into plastic bags with 25 mL of each enzyme including Termamyl 120 L (Novo Nordisk, Bagsværd, Denmark), Celluclast 1.5 L (Novo Nordisk), and Viscozyme L (Novo Nordisk), and then transferred to a programmable high-pressure treatment apparatus (TFS-10L, Innoway Co., Bucheon, Korea) that was set at a pressure of 100 MPa for 24 h at 50°C. After incubation, the extract was heated at 100°C for 10 min to inactivate the enzyme. After cooling, the extract was centrifuged at 11,000 g for 10 min, and the supernatant was filtered using Whatman No. 4 filter paper. The filtrate was freeze-dried and used as HRG.
Analysis of total phenolics, total flavonoids, and uronic acid
Total phenolic content was determined using a method modified from the Folin-Denis method (9). Briefly, 0.1 mL of red ginseng extract was added to 3.5 mL of distilled water and 0.5 mL of 1 N Folin-Ciocalteu’s phenol reagent. Then, 1 mL of 20% sodium carbonate was added, and the resulting solution was shaken thoroughly. After being kept for 2 h in the dark room at room temperature, the absorbance was measured at 710 nm in a spectrophotometer using catechin as a standard. The total flavonoids were determined using a method modified from The Association of Official Analytical Chemists (AOAC) (10). To 1 mL of red ginseng extract, 10 mL of diethylene glycol and 1 mL of 1 N NaOH were added and mixed thoroughly. The mixed solution was incubated 1 h at 37°C, and the absorbance was measured at 420 nm in a spectrophotometer (V-550 UV/VIS Spectrophotometer, JASCO Inc., Tokyo, Japan) using rutin as a standard. Uronic acid was determined by the Carbazole reaction (11) with D-galacturonic acid as a standard.
Analysis of ginsenosides
Ginsenosides of the HRG were quantified by high-performance liquid chromatography (HPLC) using the method described by Lee et al. (12) with slight modifications (13).
Animals and diets
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Ewha Womans University, Korea (IACUC No. 2012-02-011). Male Sprague–Dawley rats (80~110 g) were divided into two groups (8 rats in each group): the control group was given a 45% HF diet, and the experimental group was given a HF diet containing 15 g/kg HRG. These experimental diets were based on the AIN-76 diet. The rats were allowed ad libitum access to food and water. For the last 3 days of the experiment, feces were collected and stored at −20°C. At the end of the 14-week period, the rats were scarified in a fasting state. Blood samples were collected by cardiac puncture, and serum was obtained by centrifugation. Liver and WAT (perirenal and epididymal) were excised, weighed, and immediately frozen in liquid nitrogen until analysis.
Serum biochemical measurements
Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), triacylglycerol (TG), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-C) were determined using commercial enzymatic kits (Asan Pharmaceutical, Seoul, Korea). Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula (14). Atherogenic index (AI), a factor for the diagnosis of atherosclerosis, was calculated using the Rosenfeld formula (15):
Hepatic and fecal lipids analysis
Hepatic and fecal lipids were extracted using the Bligh and Dyer method (16) with slight modifications (17). TC and TG concentrations were determined by enzymatic colorimetric methods using commercially available kits (Asan Pharmaceutical, Seoul, Korea) in accordance with the manufacturer’s instructions.
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from epididymal WAT using TRIzol® reagent (Life Technologies, Carlsbad, CA, USA). The corresponding cDNA was synthesized from 4 μg of RNA using M-MLV reverse transcriptase (Life Technologies). After cDNA synthesis, qRT-PCR was performed using Universal SYBR® Green PCR Master Mix (Qiagen, Valencia, CA, USA) on a fluorometric thermal cycler (Corbett Research, Mortlake, NSW, Australia). The sequences of the sense and anti-sense primers (Table 1) were designed using an online program (18). The Δ ΔCt method was used for relative quantification (19). The Δ ΔCt value for each sample was determined by calculating the difference between the Ct value of the target gene and the Ct value of the reference gene (β-actin or GAPDH). The normalized level of expression of the target gene in each sample was calculated using the formula 2−Δ ΔCt. Values were expressed as a fold-change over the control.
Table 1.
Primers used for quantitative real-time RT-PCR
| Name | Gene Bank No. | Sense | Anti-sense |
|---|---|---|---|
| PPAR-γ | NM_001145366 | TGTGGGGATAAAGCATCAGC | CAAGGCACTTCTGAAACCGA |
| aP2 | NM_053365 | TCACCCCAGATGACAGGAAA | CATGACACATTCCACCACCA |
| β-actin | NM_031144 | GGCACCACACTTTCTACAAT | AGGTCTCAAACATGATCTGG |
| IL-6 | NM_012589 | ATAGTCCTTCCTACCCCAAC | TGCCGAGTAGACCTCATAGT |
| MCP-1 | NM_031530 | ACTCACCTGCTGCTACTCAT | CTACAGCTTCTTTGGGACAC |
| TNF-α | NM_012675 | CCCCTTTATCGTCTACTCCT | ACTACTTCAGCGTCTCGTGT |
| GAPDH | NM_017008 | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Statistical analysis
Data are expressed as mean±standard error of the mean (SEM) and were compared by t-tests; P<0.05 was considered significant. Statistical analysis was performed using the using the SPSS version 20 software (IBM Corporation, Armonk, NY, USA).
RESULTS
Main bioactive compounds in HRG
Total phenolics, total flavonoids, and uronic acid contents in HRG are shown in Table 2. The total phenolics, total flavonoids, and uronic acids contents in the HRG were 6.37, 1.69, and 52.63 mg/g, respectively. The ginsenoside composition of the HRG is presented in Table 2. The total ginsenosides content in the HRG was 15.83 mg/g. Ginsenosides Rg1, Re, Rf, Rg2+Rh1, Rb1, Rc, Rb2, Rb3, Rd, Rg3 (S, R), and Rh2 were detected in order by measurement of HPLC, and the concentrations of were 2.19, 1.49, 0.64, 0.72, 3.84, 1.74, 2.30, 0.37, 2.14, 0.40, and 0.00 mg/g, respectively.
Table 2.
Main bioactive compounds found in HRG (mg/g)
| Contents | HRG |
|---|---|
| Total phenolics | 6.37±0.52 |
| Total flavonoids | 1.69±0.31 |
| Ginsenosides | |
| Rg1 | 2.19±0.01 |
| Re | 1.49±0.01 |
| Rf | 0.64±0.01 |
| Rg2+Rh1 | 0.72±0.00 |
| Rb1 | 3.84±0.02 |
| Rc | 1.74±0.01 |
| Rb2 | 2.30±0.01 |
| Rb3 | 0.37±0.00 |
| Rd | 2.14±0.01 |
| Rg3 (S, R) | 0.40±0.01 |
| Rh2 | 0.00±0.00 |
| Total | 15.83±0.06 |
| Uronic acid | 52.63±1.14 |
Results are expressed as mean±SEM.
HRG: High hydrostatic pressure extracts of red ginseng.
Body weight, energy intake, and fat accumulation
At the beginning of the experiment, the initial body weight was not statistically different between the two groups (Table 3). At 14 weeks of the experiment, the final body weight, body weight gain, and WAT mass (perirenal and epididymal) in the HRG group was not statistically different from that of the HF group (Table 3). Over the study period, the food intake and energy intake did not differ between the two groups (Table 3).
Table 3.
Effects of HRG on physiological variables
| Variable | Group | |
|---|---|---|
|
| ||
| HF | HF+HRG | |
| Initial body weight (g) | 98.95±2.32 | 99.21±2.21 |
| Final body weight (g) | 546.94±14.99 | 514.67±11.44 |
| Body weight gain (g/14 weeks) | 451.36±11.87 | 419.95±11.50 |
| Food intake (g/d) | 19.85±0.44 | 20.05±0.40 |
| Energy intake (kcal/d) | 94.81±2.12 | 95.77±1.92 |
| Energy efficiency (g/gain kcal consumed) | 0.05±0.00 | 0.05±0.00 |
| Liver weight (g/100 g body weight) | 2.36±0.05 | 2.35±0.04 |
| Adipose tissue weight (g/100 g body weight) | 6.93±0.26 | 6.57±0.12 |
| Perirenal | 3.74±0.20 | 3.68±0.12 |
| Epididymal | 3.37±0.10 | 3.12±0.13 |
Results are expressed as mean±SEM.
HF, high fat; HRG, high hydrostatic pressure extracts of red ginseng.
Serum, liver, and fecal metabolites
Dietary HRG did not affect the total lipid levels in serum, liver, and feces (Table 4). TG and cholesterol levels in the HRG group were not statistically different from those of the HF group (Table 4). Meanwhile, the serum leptin level in the HRG group was lowered by 26%, compared with the HF group (P<0.05) (Table 4).
Table 4.
Effects of HRG on serum, liver and fecal metabolites
| Variable | Group | |
|---|---|---|
|
| ||
| HF | HF+HRG | |
| Serum lipids | ||
| Triacylglycerol (mmol/L) | 0.96±0.12 | 0.81±0.05 |
| Total cholesterol (mmol/L) | 2.68±0.15 | 2.45±0.07 |
| LDL-cholesterol (mmol/L)1) | 1.60±0.11 | 1.37±0.06 |
| HDL-cholesterol (mmol/L) | 1.46±0.10 | 1.49±0.07 |
| Atherogenic index (AI)2) | 0.81±0.08 | 0.70±0.07 |
| HDL/TC ratio | 0.56±0.02 | 0.60±0.02 |
| Liver lipids | ||
| Triacylglycerol (μmol/g) | 9.71±0.40 | 9.20±0.65 |
| Total cholesterol (μmol/g) | 3.08±0.28 | 3.01±0.22 |
| Total lipid (mg/g) | 31.75±0.97 | 31.22±1.49 |
| Fecal lipids | ||
| Triacylglycerol (μmol/d) | 5.85±0.67 | 6.66±0.76 |
| Total cholesterol (μmol/d) | 17.36±0.84 | 16.49±0.73 |
| Total lipid (mg/d) | 78.59±5.55 | 85.18±4.51 |
| Serum AST (IU/L) | 49.67±2.38 | 50.16±3.02 |
| Serum ALT (IU/L) | 12.41±1.18 | 12.40±0.89 |
| Leptin (ng/mL) | 9.75±0.89 | 7.18±0.51* |
Results are expressed as mean±SEM.
HF, high fat; HRG, high hydrostatic pressure extracts of red ginseng; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TC, total cholesterol; AST, aspartate transaminase; ALT, alanine transaminase.
LDL-C=TC –HDL-C –(TG / 5).
AI=(TC –HDL-C) / HDL-C.
P<0.05.
Liver weight and serum AST and ALT activities
At the dose given, HRG did not cause increase in serum AST and ALT activities when compared with the HF group (Table 4). In addition, the liver weights of rats were unaffected by HRG supplementation (Table 3), meaning the dose of HRG was well tolerated by the rats.
mRNA levels for genes related to adipogenesis and inflammation
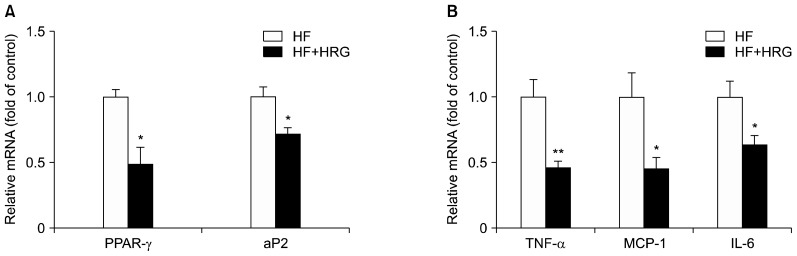
Analysis was carried out to examine the effect of HRG on mRNA expression of genes involved in adipogenesis and inflammation in WAT. In the HRG group, mRNA levels of adipogenic genes such as peroxisome proliferator-activated receptor-gamma (PPAR-γ) and adipocyte protein 2 (aP2) were down-regulated by 50 and 28%, respectively, compared with the HF group (Fig. 1A). Specifically, mRNA levels of pro-inflammatory genes including tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6) were down-regulated in the HRG group by 54, 54, and 36%, respectively, compared with the HF group (Fig. 1B).
Fig. 1.
Effects of HRG on mRNA levels of (A) adipogenic and (B) pro-inflammatory genes. Results are expressed as mean±SEM (each group, n=8). *P<0.05, **P<0.01 compared with the HF group.
DISCUSSION
Chronic low-grade inflammation and progressive macrophages infiltration are characteristics of obesity, which lead to obesity-related metabolic pathologies (20). Ginseng contains various bioactive compounds such as ginsenosides (saponins), polyacetylenes, polyphenolic compounds, and acidic polysaccharides (21). Many studies reported its physiological activities such as enhancement of immunity and prevention of hyperlipidemia and diabetes mellitus (22). Red ginseng, which is steam-dry processed fresh ginseng, has been reported to enhance the pharmacological properties compared with fresh ginseng (22). It has been suggested that processing is one of reasons that enhances the bioactivity of red ginseng (22). High hydrostatic pressure (HHP) is a non-thermal food processing technique, which is used for increasing extraction efficiency (23). Since HHP uses low temperature, heat damage and loss of volatile compound could be avoided (24). Therefore, we applied HHP to red ginseng extraction, and the anti-inflammatory properties of the HRG was evaluated in a rat model of diet-induced obesity.
We previously reported ginsenosides compositions of hot water extracts of fresh ginseng (WEG) (13). Compared to the WEG, the contents of ginsenosides Rb1, Rb2, Rd, and Rc were higher in the HRG. Specifically, the amounts of ginsenosides Rb2 and Rd in the HRG were highest among the ginseng extracts. Lee et al. (8) compared ginsenosides compositions between red ginseng under high hydrostatic pressure extraction (HHPE) and red ginseng under heat extraction (HE). Total ginsenoside and ginsenosides Rg1, Rg2+Rh1, Rb1, Rb2, Rd, and F2 were increased under HHPE compared to HE. Specifically, HHPE increased ginsenoside Rd by 48% compared to HE.
Several studies have reported beneficial effects of ginsenosides on obesity. Ginsenoside-Rb1 reduced body weight gain and body fat content in rats with HF diet-induced obesity (25). Administration of ginsenoside-Rb1 inhibited TG accumulation in the liver via cAMP-production (26). In our results, the final body weight, white adipose tissue mass, and serum lipid levels were not significantly different between the two groups. Meanwhile, the rats fed a HF+HRG diet had decreased serum leptin levels compared with the rats in the HF group. Leptin is an adipocyte-derived hormone and plays a pivotal role in the regulation of food intake and energy expenditure (27). Circulating leptin levels is directly correlated with adipose tissue mass, which suggests obesity might lead to increases in serum leptin level (27). Accordingly, it is assumed that HRG may be in part associated with regulation of adiposity with lowered leptin secretion, and ginsenoside-Rb1 might be partially associated with the beneficial effect.
To better understand the anti-adipogenic activity of the HRG, we evaluated the mRNA levels of adipogenic genes such as PPAR-γ and aP2. PPAR-γ serves as a ligand-activated transcription factor that plays a pivotal role in differentiation and proliferation of preadipocytes (28). aP2 is a carrier protein for fatty acids, and it is involved in fat accumulation via lipid biosynthesis pathways (29). A previous study reported that treatment of Korean red ginseng extract in 3T3-L1 adipocytes inhibited lipid accumulation and the expression of adipocyte-specific genes such as PPAR-γ, C/EBPα, aP2, and leptin (30). Similarly, our study showed that HRG down-regulated both PPAR-γ and aP2 expression compared with those of the HF group, which suggests that administration of the HRG may partially affect adipogenesis with adipogenic gene regulation.
Increase in adipose tissue leads to changes in paracrine function of the adipocytes (27). These changes in turn increase inflammation by an increased recruitment of macrophages that reflect systematic inflammation and health outcomes (1). Increased secretion of leptin by adipocytes enhances production of pro-inflammatory cytokines, such as IL-6 and TNF-α (31). In addition, leptin contributes to the macrophage accumulation by transporting macrophages to adipose tissue and facilitating adhesion of macrophages to endothelial cell (27). Once macrophages are present and activated, a vicious cycle of macrophage recruitment, production of inflammatory cytokines, and impairment of adipocyte function could be persisted in adipose tissue (27). In an animal study, orally administered ginsenoside-Rb1 lowered the serum levels of IL-6 and TNF-α (32). Murine and human macrophage cells treated with ginsenoside-Rb1 or-Rb2 suppressed TNF-α production (33). Accordingly, we hypothesized that reduced serum leptin levels might be partially associated with anti-inflammation by HRG in WAT of rats with obesity.
To investigate the effects of HRG on adipose tissue inflammation, we further analyzed the pro-inflammatory gene expression in WAT. TNF-α and IL-6 are pro-inflammatory cytokines synthesized when the lipid content increases in WAT and contribute to the pathogenesis of obesity-linked complications (34). MCP-1 is expressed by adipocytes and contributes to the monocyte recruitment into WAT (35). A previous study reported that Korean red ginseng extract inhibited the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells (36). In another study, the Korean red ginseng saponin fraction down-regulated the mRNA expressions of genes related to inflammation including inducible nitric oxide synthase, MCP-1, IL-1β, TNF-α, and IL-6 (37). Similar to these results, our study showed that HRG down-regulated mRNA levels of TNF-α, MCP-1, and IL-6, significantly. Accordingly, we hypothesized that HRG might have an anti-inflammatory effect in obese adipose tissue, and ginsenosides Rb1 and Rb2 might at least partially account for the beneficial effect of the HRG.
In conclusion, it is likely that the anti-inflammatory effect of HRG might be possible through down-regulating the mRNA expression of adipogenic and pro-inflammatory genes in rats with HF diet induced obesity, which might be beneficial in ameliorating obesity-linked pathogenesis.
ACKNOWLEDGEMENTS
This study was supported by the Food High Pressure Technology Development Project, the Korea Food Research Institute (No. 202007-03-03-WT011), the National Research Foundation of Korea (NRF) funded by the Korean Government (MOE) (No. 2013R1A1A2009522), and BK21 Plus (22A20130012143).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Yang WS, Yu T, Sung GH, Park KW, Yoon K, Son YJ, Hwang H, Kwak YS, Lee CM, Rhee MH, Kim JH, Cho JY. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Cho JY, Kim WK. Anti-inflammation effect of exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr Res Pract. 2014;8:284–291. doi: 10.4162/nrp.2014.8.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Park D, Yoon M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet-induced obese C57BL/6J mice. Food Chem Toxicol. 2013;53:402–408. doi: 10.1016/j.fct.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 7.Oey I, Lille M, Van Loey A, Hendrickx M. Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: a review. Trends Food Sci Technol. 2008;19:320–328. doi: 10.1016/j.tifs.2008.04.001. [DOI] [Google Scholar]
- 8.Lee HS, Lee HJ, Yu HJ, Ju DW, Kim Y, Kim CT, Kim CJ, Cho YJ, Kim N, Choi SY, Suh HJ. A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng (Panax ginseng CA Meyer) J Sci Food Agric. 2011;91:1466–1473. doi: 10.1002/jsfa.4334. [DOI] [PubMed] [Google Scholar]
- 9.Swain T, Hillis W. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- 10.AOAC. Official method of analysis. 15th ed. Association of Official Analytical Chemists; Washington, DC, USA: 1990. pp. 139–247. [Google Scholar]
- 11.Dische Z. A new specific color reaction of hexuronic acids. J Biol Chem. 1947;167:189–198. [PubMed] [Google Scholar]
- 12.Lee HJ, Jung EY, Lee HS, Kim BG, Kim JH, Yoon TJ, Oh SH, Suh HJ. Bioavailability of fermented Korean red ginseng. J Food Sci Nutr. 2009;14:201–207. doi: 10.3746/jfn.2009.14.3.201. [DOI] [Google Scholar]
- 13.Jung S, Lee MS, Shin Y, Kim CT, Kim IH, Kim YS, Kim Y. Anti-obesity and anti-inflammatory effects of high hydrostatic pressure extracts of ginseng in high-fat diet induced obese rats. J Funct Foods. 2014;10:169–177. doi: 10.1016/j.jff.2014.06.007. [DOI] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Rosenfeld L. Lipoprotein analysis. Early methods in the diagnosis of atherosclerosis. Arch Pathol Lab Med. 1989;113:1101–1110. [PubMed] [Google Scholar]
- 16.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 17.Lee MS, Kim IH, Kim CT, Kim Y. Reduction of body weight by dietary garlic is associated with an increase of uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141:1947–1953. doi: 10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- 18.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−Δ ΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BG, Choi SY, Kim MR, Suh HJ, Park HJ. Changes of ginsenosides in Korean red ginseng (Panax ginseng) fermented by Lactobacillus plantarum M1. Process Biochem. 2010;45:1319–1324. doi: 10.1016/j.procbio.2010.04.026. [DOI] [Google Scholar]
- 22.Kim HJ, Lee SG, Chae IG, Kim MJ, Im NK, Yu MH, Lee EJ, Lee IS. Antioxidant effects of fermented red ginseng extracts in streptozotocin-induced diabetic rats. J Ginseng Res. 2011;35:129–137. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemley RJ. Effects of high pressure on molecules. Annu Rev Phys Chem. 2000;51:763–800. doi: 10.1146/annurev.physchem.51.1.763. [DOI] [PubMed] [Google Scholar]
- 24.Iberl B, Winkler G, Müller B, Knobloch K. Quantitative determination of allicin and alliin from garlic by HPLC. Planta Med. 1990;56:320–326. doi: 10.1055/s-2006-960969. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Y, Shen L, Liu KJ, Tso P, Xiong Y, Wang G, Woods SC, Liu M. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010;59:2505–2512. doi: 10.2337/db10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KH, Shin HJ, Song YB, Hyun HC, Cho HJ, Ham HS, Yoo YB, Ko YC, Jun WT, Park HJ. Possible role of ginsenoside Rb1 on regulation of rat liver triglycerides. Biol Pharm Bull. 2002;25:457–460. doi: 10.1248/bpb.25.457. [DOI] [PubMed] [Google Scholar]
- 27.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 29.Duplus E, Forest C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem Pharmacol. 2002;64:893–901. doi: 10.1016/S0006-2952(02)01157-7. [DOI] [PubMed] [Google Scholar]
- 30.Oh J, Lee H, Park D, Ahn J, Shin SS, Yoon M. Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid Based Complement Alternat Med. 2012;2012:265023. doi: 10.1155/2012/265023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong ML, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metab. 2012;56:597–607. doi: 10.1590/S0004-27302012000900001. [DOI] [PubMed] [Google Scholar]
- 32.Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb1 (ginseng) and parthenolide (feverfew) Food Chem Toxicol. 2003;41:1381–1390. doi: 10.1016/S0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 33.Cho JY, Yoo ES, Baik KU, Park MH, Han BH. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-α production and its modulation by known TNF-α antagonists. Planta Med. 2001;67:213–218. doi: 10.1055/s-2001-12005. [DOI] [PubMed] [Google Scholar]
- 34.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 36.Cho SO, Lim JW, Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Yayeh T, Jung KH, Jeong HY, Park JH, Song YB, Kwak YS, Kang HS, Cho JY, Oh JW, Kim SK, Rhee MH. Korean red ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]