Summary
No country can afford to treat its way out of the cancer problem. This review charts the increasing burden, summarizes the causes and describes opportunities for prevention and early detection, including precision prevention based on recent advances in cancer biology.
Abstract
Cancer burden worldwide is projected to rise from 14 million new cases in 2012 to 24 million in 2035. Although the greatest increases will be in developing countries, where cancer services are already hard pressed, even the richest nations will struggle to meet demands of increasing patient numbers and spiralling treatment costs. No country can treat its way out of the cancer problem. Consequently, cancer control must combine improvements in treatment with greater emphasis on prevention and early detection. Cancer prevention is founded on describing the burden of cancer, identifying the causes and evaluating and implementing preventive interventions. Around 40–50% of cancers could be prevented if current knowledge about risk factors was translated into effective public health strategies. The benefits of prevention are attested to by major successes, for example, in tobacco control, vaccination against oncogenic viruses, reduced exposure to environmental and occupational carcinogens, and screening. Progress is still needed in areas such as weight control and physical activity. Fresh impetus for prevention and early detection will come through interdisciplinary approaches, encompassing knowledge and tools from advances in cancer biology. Examples include mutation profiles giving clues about aetiology and biomarkers for early detection, to stratify individuals for screening or for prognosis. However, cancer prevention requires a broad perspective stretching from the submicroscopic to the macropolitical, recognizing the importance of molecular profiling and multisectoral engagement across urban planning, transport, environment, agriculture, economics, etc., and applying interventions that may just as easily rely on a legislative measure as on a molecule.
Introduction
Valid debate on the role of environment and lifestyle, genes and chance in explaining variations in population-level cancer incidence rates have brought one risk of misinterpretation into sharp relief (1,2). Namely that emphasizing chance, or bad luck, may lead one to join erroneously with Estragon in the opening line of Samuel Beckett’s play, ‘Waiting for Godot’ and conclude, ‘Nothing to be done’.
The corollary of bad luck as ‘end of story’ warrants challenge because of the inviting prospects for understanding aetiology and intervening to reduce the cancer burden through prevention strategies. Indeed, in contrast to Estragon’s resigned air, there is already ‘plenty to be done’. Certain prevention imperatives, exemplified by smoking cessation and related lifestyle changes, have been established for decades, but the recent World Cancer Report 2014 (3) revealed the far broader scope of research findings and health initiatives offering pathways to reduced cancer incidence. Collins et al. (4) implied further opportunities for cancer prevention as a part of precision medicine, but this latter concept remains far clearer in relation to treatment. In this context, there is a need to better define the scope of cancer prevention, considering prevention efforts aimed at high-risk subgroups of individuals (the focus of precision) all the way through to interventions reaching the entire population and to evaluate how the most recent advances in molecular cancer genetics may translate to prevention measures at these different levels of population stratification (5).
Accordingly, we review the current successes and future opportunities for cancer prevention, including how prevention may find its place within precision medicine, by delineating key strategies based on a combination of identified risk factors and the underlying biology of cancer development (6). The scope is unprecedented, ranging from genomic-based discrimination between causative agents (7) to increasingly comprehensive global epidemiological data (8). This complementary approach to precision medicine, encompassing prevention, early detection and treatment, is vital as spiralling costs preclude even the richest countries from treating their way out of the cancer problem. This is particularly pertinent when the increasing cancer burden is considered on a global scale. In delineating such a broad perspective, citation of even a minor fraction of the relevant literature was not an option. We have therefore focused on summations or reviews published since World Cancer Report 2014 (3).
To underline the necessity of prevention, we begin with a description of the increasing number and changing patterns of cancer worldwide. This is followed by a summary of what is known about the causes of cancer, representing the foundation for preventive interventions. The paper goes on to outline the opportunities for prevention and early detection, including precision prevention approaches made feasible by advances in understanding cancer biology. Finally, the need to progress from evaluating the efficacy of interventions in clinical or community-based trials through to demonstrating effectiveness in programmes within health services is emphasized.
Background to preventive strategies
Making cancer data count
In 2012, the worldwide burden of cancer involved 14 million new cases per year, a figure expected to rise to 24 million annually within the next two decades. Over the same period, cancer deaths are predicted to rise from 8.2 million to 14.6 million annually. The most common cancers diagnosed in 2012 were those of the lung (1.8 million cases, 13.0% of the total), breast (1.7 million, 11.9%) and large bowel (1.4 million, 9.7%). The most common causes of cancer death were cancers of the lung (1.6 million, 19.4% of the total), liver (0.8 million, 9.1%) and stomach (0.7 million, 8.8%) (3).
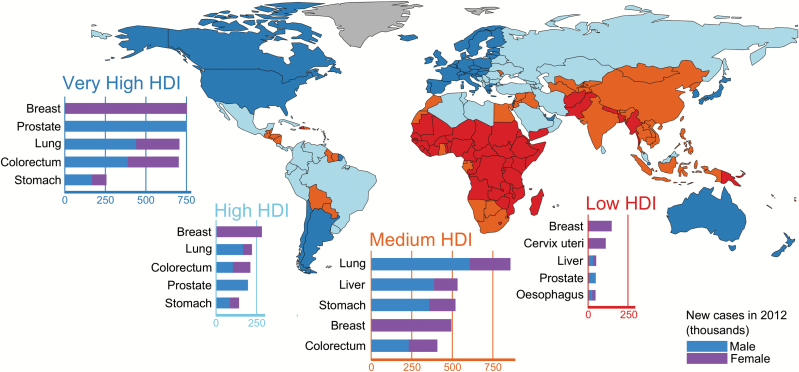
Low- and middle-income countries (LMICs) are disproportionately burdened by cancer: more than 60% of cases worldwide occur in Africa, Asia and Central and South America, leading to 70% of the world’s cancer deaths. The world’s two most populous countries, China and India, have rapidly rising cancer incidence and mortality rates with estimated all-cancer site mortality rates in China (122 per 100000) now exceeding those in the USA (105 per 100000) (8). The increases in the burden of cancer observed in LMICs reflect a combination of population growth, ageing and changes in the prevalence of underlying risk factors. At the same time, comparisons between nations based on the Human Development Index (Figure 1) reveal that countries undergoing economic transition are now exhibiting tumour types that are common in high income countries (HICs), including female breast, prostate, lung and colorectal cancer.
Figure 1.
The five most common cancers in 2012 according to levels of the Human Development Index (HDI) across the 184 countries included in GLOBOCAN. HDI is a composite measure of life expectancy, educational attainment, and command over the resources needed for a decent living (UNDP, 2013) and is used in this study to examine the cancer profiles according to four levels of societal development: low HDI (HDI < 0.53); medium HDI (0.53 ≤ HDI < 0.71); high HDI (0.71 ≤ HDI < 0.80) and very high HDI (HDI ≥ 0.80). [United Nations Development Programme (UNDP). Human Development Report 2013. The Rise of the South: Human Progress in a Diverse World (2013). New York: UNDP.]
Most cancers that are frequent in one population are relatively rare in another, and these patterns vary over time. For example, oesophageal cancer is common among men in East Africa but rare in West Africa. Colorectal cancer, once rare in Japan, increased fourfold in incidence in just two decades (3). Such observations are consistent with a major contribution of environmental and lifestyle exposures, providing the underlying rationale for cancer prevention.
Reliable information on cancer burden is a fundamental building block for cancer control and requires high quality cancer registries. Although the majority of HICs have at least partial population coverage by registries, they remain inadequate or nonexistent in many LMICs. Registries across China cover 13% of the population, whereas in India the figure is 7% and, in both cases, the rural population is under-represented. Such limitations acknowledged, cancer registration worldwide is improving (9). For example, recent registry data from Africa are providing a clearer picture of the most common cancers, notably prostate, liver and Kaposi sarcoma in men; and breast and cervix cancers in women (10).
Cancer registry data permit projections of overall cancer burden and changes in specific cancer sites, such as the increasing burden of thyroid, liver and pancreatic cancers in the USA by 2030 (11). Registry data may also be employed to highlight disease in identifiable subpopulations such as indigenous people (12). The inclusion of stage and treatment data will further improve the identification of populations at particular risk (13).
Elucidating aetiology
Estimates of attributable risk for cancer in HICs have identified up to a third being caused by smoking and, beyond the well-recognized tobacco-induced tumour types, breast and prostate cancer are now implicated (14). About 3.5% of the US cancer deaths, corresponding to 20000 cases, are attributable to alcohol consumption (15). Approximately 30% of cancer cases in Northern America and Western Europe are attributable to diet, overweight/obesity and/or lack of exercise (16). Sedentary behaviour increases risk of colon and endometrial cancer. Worldwide, the proportion of adults with a body mass index of 25kg/m2 or greater increased between 1980 and 2013 from 28.8% (28.4–29.3) to 36.9% (26.3–37.4) with 95% uncertainty intervals shown (17). Globally, 481000 cancers in 2012 (3.6% of all adult cancers) were attributable to excess body mass index (18).
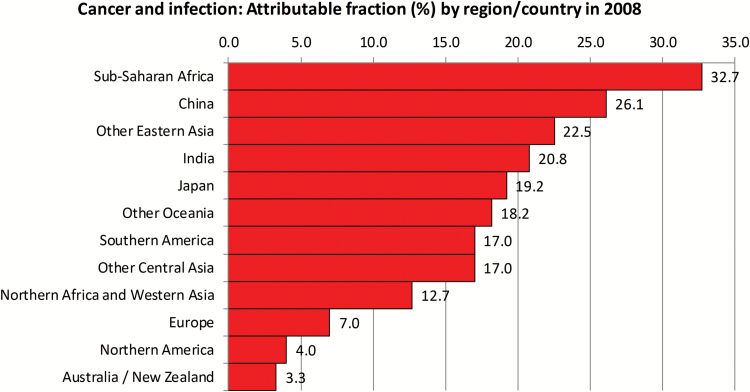
The proportion of cancer attributable to infectious agents varies markedly between regions and is greatest for some LMICs. Thus a third of cancer in sub-Saharan Africa is attributable to infectious agents, whereas for Australia and North America the proportion is around 3% (Figure 2). However, the scenario of a worsening burden of cancer in LMICs is not restricted to the impact of either an increasing, ageing population or the high prevalence of infectious agents. There is also increased prevalence of risk factors previously more common in industrialized countries.
Figure 2.
Regional variation in the burden of infection-related cancers. H. pylori, HBV and HCV, and HPV are responsible for 1.9 million cancer cases globally, including mainly gastric, liver and cervical cancer respectively. Infection with HIV substantially increases the risk of virus-associated cancers, through immunosuppression.
On a global scale, improving socioeconomic conditions is associated with increased risk of cancer through increased tobacco and alcohol usage, changes in diet toward high fat/sugar intake and decreased physical activity (16,19). Tobacco smoking now accounts for approximately a third of all male cancer deaths in China, where changes in dietary practice, physical activity and prevalence of obesity are also documented (20). Middle Eastern countries have obesity prevalence similar to that in the USA, attributable to diets now including more sugar, oils and animal protein (21).
Highest occupational exposures to carcinogens are recognized to occur in industrializing LMICs, often correlated with high rates of occupational cancer (22). The worst air pollution from industrial and vehicular sources, and water pollution by arsenic from natural and industrial sources, is evident in LMICs. Lung cancer unequivocally attributable to outdoor particulate matter is now recognized (23) with levels in Chinese cities among the highest observed (24).
Cancers prevalent in Africa, Asia, South America and the Middle East can also be attributable to cultural and environmental factors specific to a region, as exemplified by betel quid chewing, food contamination by aflatoxins and combustion of biomass in cooking stoves, though the latter is also relevant to the USA (25).
Evidence-based cancer prevention strategies
Primary cancer prevention is achieved through reduced exposure to recognized carcinogens and/or increased health promotion, whereas secondary prevention is exemplified by screening, earlier detection and chemoprevention. In some select cases, cancer prevention may be achieved by identification and monitoring individuals subject to heritable risk, but by far the major benefit comes from population-level interventions. That said, priorities for prevention often need to be tailored to the pattern of risk factors and cancers faced by a given country or region, taking account of more vulnerable subgroups in the community. Prevention of tobacco smoking is a priority that transcends these distinctions; the multiple interventions enumerated in the WHO Framework Convention on Tobacco Control (FCTC) are relevant to communities worldwide.
Minimizing cancer risk in HICs
Some HICs have a decreasing incidence of lung cancer in men as a result of decreased smoking rates over past decades, although this trend is less evident in women. Quitting is aided by the availability of pharmacological options addressing nicotine addiction. Higher prices, recognized as discouraging smoking (26), may also be used to deter alcohol drinking (27). Means of sun avoidance, as pertinent to skin cancer in some communities, are now established (28). Options to reduce or prevent overweight/obesity are emerging. In the USA, sugar-sweetened beverages have been subject to specific policies (29). A recognized focus is prevention of childhood obesity (30).
Individuals who stop smoking or drinking alcohol are at reduced risk of cancer by comparison with those who continue the respective behaviours (31). Adherence to guidelines on healthy diet, physical activity and weight management as defined by the World Cancer Research Fund/American Institute for Cancer Research is also associated with reduced likelihood of dying from cancer, circulatory and respiratory disease (32).
Primary prevention in LMICs
Priorities for cancer prevention in LMICs encompass options proven effective in HICs and measures evolved in light of local circumstances (33). LMIC-specific research is needed to formulate the best public policies on, for example, tobacco control (34). Likewise, research on local applicability is required concerning obesity (35). Despite possible nutritional deficiency and a high prevalence of underweight persisting in some low-income countries, obesity is considered an emerging priority.
Prevention of occupational cancers in LMICs necessitates increased awareness among workers (36). Interventions, from replacement of hazardous materials with safer ones through to using personal protective equipment, are then required. Reducing levels of industrial and vehicular air pollutants is a priority for countries with emerging economies (37). Demonstrable improvement in prospects for industrial emissions can be achieved if appropriate control measures are adopted (38).
Diverse means are available to prevent cancer caused by various agents particular to LMICs. Arsenic contamination of drinking water in south-east Asia is reduced by switching to deeper wells (39). Food policy reforms in China resulted in a dramatic decrease in aflatoxin exposure, reducing liver cancer risk (40), whereas reduced exposure in Africa has been achieved by improved grain storage (41). Indoor air pollution from cooking stoves may be reduced by installing flues, among other measures (42)
Currently available vaccines to prevent cancer have greatest potential benefits in LMICs (43). Efficacy of human papillomaviruses (HPVs) vaccines may now be inferred from reduced incidence of indicator lesions (44). Through hepatitis B virus (HBV) vaccination in Taiwan, China and Singapore initiated two decades ago, hepatitis B antigen carriers have dropped markedly and falls in incidence of hepatocellular carcinoma are becoming evident (45). HBV vaccination in Gambia has resulted in a fall in HBV chronic carriage rates in young children from 10 to 15% when the project was initiated, to less than 1% now (46).
Chronic infection with hepatitis C is curable with the new generation of drug treatments (47), albeit cost remains a barrier to access for a majority of populations. Eradication of Helicobacter pylori infection using antibiotic therapy to prevent stomach cancer has been limited by concerns regarding adverse consequences. Nonetheless, data from randomized control trials in Asia suggest that countries explore the possibility of introducing population-based H. pylori screening and treatment programmes in conjunction with a scientifically valid assessment (48).
Expanding opportunities for secondary prevention
Detection of premalignant lesions by Papanicolaou smear has dramatically decreased cervical cancer mortality, the procedure having global application. Moving forward, a negative high-risk HPV test may provide greater reassurance against development of precancerous lesions than a negative Pap test (49).
The three other most widely adopted population-based screening procedures are mammography for breast cancer, faecal occult blood testing for colorectal cancer and prostate-specific antigen testing for prostate cancer. In common with all screening procedures, the prospect of overdiagnosis is recognized as a major limitation (50). Despite controversy concerning mammography, there is the immediate prospect of benefit based on current screening programs (51). Notwithstanding some evidence for reduced mortality, population-based prostate-specific antigen screening remains precluded by three considerations: overdetection, treatment complications and disease progression (52).
A principle challenge for colorectal cancer screening is to engage the community at risk. Thus, in the USA, screening has been assessed as suboptimal, particularly among underserved populations such as the uninsured, recent immigrants and racial/ethnic minority groups (53). Even so, a decrease of 30% in the US colorectal cancer incidence between 2000 and 2010 among adults aged 50 and older has been attributed primarily to screening (54).
Distinct priorities for screening are evident in Asia. In India, a reduction in oral cancer mortality following oral visual screening in high-risk individuals (who chewed betel quid with tobacco or smoked or drank alcohol) was sustained when assessed 15 years after initiation of the study (55). In Japan, tests using des-γ-carboxyprothrombin and α-fetoprotein to detect liver cancer are adopted and have relevance to China (56).
Using pharmaceutical drugs such as tamoxifen to prevent second breast cancers exemplifies chemoprevention. Thus, clinical trial data demonstrate that the aromatase inhibitor anastrozole reduces breast cancer incidence by 53% in postmenopausal women who were at an increased risk of breast cancer upon entry into the study (57). In the context of genome-wide investigation, use of aspirin and/or non-steroidal anti-inflammatory drugs lowered risk of colorectal cancer, and this outcome differs according to genetic variation at two single-nucleotide polymorphisms on chromosomes 12 and 15 (58). Trials involving micronutrients have been largely unsuccessful (59).
Emerging strategies
The major cancer research development during recent years has involved tumour-specific genomics, leading to targeted therapy and hence, precision medicine. International collaborations have realized landmark publications through ‘The Cancer Genome Atlas Network’. Notably however, genomic and related analyses may also revolutionize cancer prevention and early diagnosis (60). For example, if the molecular genetics of a tumour are at least partially a reflection of the risk factors which led to the development of that tumour, this will provide new insights into prevention and molecular approaches to early diagnosis. Promising indications are arising, one example being a genomic characterization of head and neck squamous cell carcinoma which discriminated between cases caused by HPV and those attributable to tobacco (7).
New computational procedures may help identify high-risk individuals, providing an additional tool for precision prevention (61). For example, genome-wide association studies have already identified susceptibility loci for different tumour types, exemplified by colorectal and breast cancer (62). The promise of public health genomics and precision prevention are recognized for screening, through determinants of genetic susceptibility providing risk-stratified algorithms in the context of conventional screening tests (63).
Opportunities for identifying high-risk individuals may be joined by application of emerging genetic and genomic tests as an alternative or complementary approach to early diagnosis by detection of preclinical lesions (64). Thus for colorectal cancer, the development of diagnostic tests using biomarkers is envisaged. Alternative approaches for early diagnosis are being evaluated, such as immunosignatures (65). Circulating tumour cells and circulating tumour DNA are seen as having noninvasive diagnostic potential (66). Digital polymerase chain reaction-based technologies using specimens from patients with advanced disease detected circulating tumour DNA in >75% of cases (67). Such DNA is amenable to analysis for individual gene mutations. Increasing circulating tumour DNA concentration and its decreasing integrity have potential as diagnostic markers for primary and metastatic breast cancer (68).
New approaches to cancer aetiology
Genomic data for tumours attributable to ultraviolet radiation and tobacco smoke reveal mutations attributable to these carcinogens (69). Whole-genome sequencing of renal cell carcinomas in four populations revealed a mutational signature of aristolochic acid in one, the same population where this carcinogen had been previously associated with tumours of the upper urinary tract (70) further expanding the scope of mutational signatures (Table 1). Omic technology has provided the first integrated proteogenomic analysis, involving colorectal cancer, revealing tumour subtypes to be characterized by distinct mutation, methylation and protein expression patterns (71).
Table 1.
Sources of mutational signatures evident in particular tumour types
| Source | Mutation type | Relevant tumour(s) |
|---|---|---|
| Exogenous | ||
| Ultraviolet light | C to T, CC to TT | Skin cancer |
| Benzo[a]pyrene | G to T | Lung cancer |
| Aflatoxin B1 | AGG to AGT | Liver cancer |
| 4-Aminobiphenyl | G to C, G to A | Bladder cancer |
| Vinyl chloride | A to T | Liver angiosarcoma |
| Aristolochic acid | A:T to T:A | Renal cell carcinoma and upper urinary tract tumours |
| Endogenous | ||
| Spontaneous deamination | C to T | Stomach cancer |
| Apurinic site generation | C to T, G to T | Multiple cancers |
| Oxidative damage and ROS generation | G to T | Lung cancer |
| DNA polymerase error | G to T, T to C | Brain cancer, colon cancer |
| APOBEC | C to T | Cervical, breast, bladder, head and neck cancers |
Based on ref. (103) and references cited concerning respective agents in the main text.
APOBEC, apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; ROS, reactive oxygen species.
Elucidation of cancer aetiology will increasingly depend upon integration of environmental and genetic risk factors (72). Tumour-specific databases on the genome, transcriptome, epigenome and the like have the prospect of revealing aetiology through the discovery of patterns of genetic alteration indicative of particular carcinogens (73). More targeted biomarkers can assist in understanding complex mechanistic relationships between exposure, DNA damage, genetic susceptibility and cancer (74).
Determining carcinogenic risks
Carcinogenesis is increasingly described in molecular terms. Thus, key transcriptomic changes and genomic pathways are altered during initiation and progression of squamous cell carcinoma of the lung (75). Yet assigning mutations directly to exogenous carcinogens within the broad spectrum of mutations evident in malignant cells remains a challenge. Some insights are now available from experimental studies. In mouse models, for example, distinction can be made between carcinogen-induced lung cancers and those arising as a consequence of genetic manipulation (76). Applicability of such distinctions to human cancer remains to be established.
Multiple agents and risk factors will be examined by the IARC Monographs during 2015–2019 (77), and evaluations of carcinogenicity will increasingly draw on high-throughput biological data of mechanistic relevance (78). Such data, for example, indicate possible tumour-promoting activities of nicotine (79): findings relevant to e-cigarette usage. The recent Monograph evaluation concerning processed meat and red meat indicates the complexity of exposure that must be addressed as we go beyond cancer caused by single potent agents (80).
Otherwise unknown carcinogens do continue to be identified by singular circumstances of exposure giving rise to particular tumour types, exemplified by the causation of cholangiocarcinoma by 1,2-dichloropropane (81). However, more often, multiple and sometimes diverse exposures are implicated when individual cancers are assessed, and their elucidation requires new investigative approaches. The exposome, for example, attempts to allow such complexity to be addressed by encompassing life-course environmental exposures, including highlighting the possibility of critical windows of exposure in relation to risk (82). Increasingly, cancer preventive strategies must go beyond only a central tactic of avoiding exposure to listed carcinogens as applicable to everyone and encompass complex environmental circumstances as they impact upon individually determined susceptibility.
From strategies to outcomes: implementation
Granted validation of particular interventions, achieving large-scale prevention depends on the scope and circumstances of implementation. There is a need to translate efficacy (in trials) into effectiveness (in programmes). A global approach is possible. For example, the FCTC is a legally binding treaty obliging ratifying countries to implement the evidence-based measures specified for tobacco control. More often, however, demonstration of success has come through specific examples at national or regional level and had been achieved by a variety of methods.
National cancer prevention successes
For the USA, recognition in 2008 of ‘Cancer prevention efforts stalled’ was predicated on smoking rates, mammography and colorectal screening participation (83). However, a current assessment credits tobacco control with avoidance of 8 million premature deaths and an estimated extended mean life span of 19 years (84). The first federally funded US antismoking national media campaign starting in March 2012 was effective in increasing population-level quit attempts.
In Europe, decreases in alcohol consumption have occurred in France, Italy, Spain and other southern European countries (85). Mammographic screening, typically involving women aged 50 through to 66–69 years, is providing overall benefit. Twenty five of 28 European Union Member States have colorectal screening programs, though there are discrepancies in participation (86).
Prevention, as well as early detection of cancer, is central to cancer control in Asia. Across the continent, tobacco and alcohol control measures, HBV and HPV vaccination together with cancer screening are amongst the priorities. In Qidong County, China, liver cancer has decreased by nearly 50% in 40 years, due to marked reductions in dietary aflatoxins. At the same time, male lung cancer has trebled, driven by smoking prevalence recorded at 65% in 1991 (87). Scaling up from a successful pilot project for colorectal cancer screening in Lampang Province, Thailand exemplifies a critical stage of program development (88).
Legislation
Legislation concerning the promotion, presentation, availability, price and composition of tobacco products, as well as their circumstances of use, is common in HICs. In the USA, successful tobacco control has included passing of the Family Smoking Prevention and Tobacco Control Act (89). European Union actions to strengthen tobacco control include a ban on flavouring of tobacco: a measure recognized to enhance its appeal to young users (90). Commercial tanning services are banned across Australia from January 1, 2015 (91).
Particularly in relation to carcinogenic risk, air pollution is amenable to regulatory control. In 2012, the Chinese Government launched a National Plan on Air Pollution Control in Key Regions which requires that, by 2017, pollutant levels in specified cities must be markedly reduced (92).
Engagement with the community
Recently recognized achievements through advocacy include adoption by Tanzania of the FCTC (93). Module 6 of the WHO Guide for Effective Programmes—Cancer Control: Knowledge into Action is devoted to Policy and Advocacy. A tangential benefit of advocacy is data accrual exemplified by the survey of tobacco control activity in Europe (94).
Health authorities may partner with the communities they serve, specifically involving minority groups (95). Reference to the community is also required to evaluate the health-related impact that a program has achieved.
Determination of cost-effective options
Cost-effective cancer prevention and management are essential for tackling the growing burden of cancer in all resource settings (96). In HICs, economic analysis indicates that scant attention is paid to cancer prevention. For LMICs particularly, models that specifically include cost–effectiveness and affordability are proposed for assessing, for example, options for breast cancer control (97).
When constrained to a narrowly defined issue, such as expanding Norway’s HPV vaccination to include boys (98), or breast cancer control in Costa Rica and Mexico (99), clear determinations can be made on the basis of costs. Efforts to implement the FCTC in China are hindered by low funding assessment relative to the total available for all disease prevention (100).
Although cost–effectiveness can be a vital support to cancer control, it should not be given undue importance. The estimates themselves are often limited by the data available and can change rapidly with circumstances, for example, the rapid drop in cost of HPV vaccines. In addition, there is a strong moral basis to expenditure on health care for all societies.
Conclusion
Universally recognized principles of public health and medicine indicate strategies for cancer prevention that have been recognized for decades. However, there are many new opportunities, based on contemporary assessments and research in disciplines from epidemiology and genomics through to behavioural science, law making and economics. Precision prevention has been illustrated here as embracing the molecular sciences to characterize new etiologic risk factors, to detect cancer early and to target interventions to subgroups depending on risk profile. Thus the ‘precision’ of prevention may refer both to the refinement of measurement as well as to the refinement of target population and requires careful definition as the field develops through interdisciplinary cooperation (101).
Despite the promise, prevention remains the poor relation in terms of funding, attracting just 2–3% of cancer research funding in 2006–2011 in Australia, the USA and Canada (102). Consequently, two imperatives emerge for policy makers in all countries. Firstly, as addressing national and local circumstances, implement cancer prevention, including screening and early detection. Secondly, initiate and support research on cancer prevention, specifically engaging the latest biological technologies, where practicable. In this way, the full potential of precision medicine might be realized: supporting an integrated approach to prevention and treatment is the only viable strategy to avoid the increasing cancer burden worldwide.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- FCTC
Framework Convention on Tobacco Control
- HBV
hepatitis B virus
- HIC
high income country
- HPV
human papillomavirus
- LMIC
low- and middle-income country
References
- 1. Tomasetti C., et al. (2015) Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science, 347, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wild C., et al. (2015) Cancer risk: role of chance overstated. Science, 347, 728. [DOI] [PubMed] [Google Scholar]
- 3. Stewart B.W., et al. (2014) World Cancer Report 2014. International Agency for Research on Cancer, Lyon. [PubMed] [Google Scholar]
- 4. Collins F.S., et al. (2015) A new initiative on precision medicine. N. Engl. J. Med., 372, 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wild C.P., et al. (2015) Translational cancer research: balancing prevention and treatment to combat cancer globally. J. Natl Cancer Inst., 107, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vogelstein B., et al. (2013) Cancer genome landscapes. Science, 339, 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Cancer Genome Atlas Network (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, 517, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferlay J., et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 9. Bray F., et al. (2014) Planning and developing population-based cancer registration in low- and middle-income settings. IARC Technical Publication No. 43. IARC; Available from http://www.iarc.fr/en/publications/pdfs-online/treport-pub/treport-pub43/index.php, Lyon (27 November 2015, date last accessed). [PubMed] [Google Scholar]
- 10. Parkin D.M., et al. (2014) Cancer in Africa 2012. Cancer Epidemiol. Biomarkers Prev., 23, 953–966. [DOI] [PubMed] [Google Scholar]
- 11. Rahib L., et al. (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res., 74, 2913–2921. [DOI] [PubMed] [Google Scholar]
- 12. Arnold M., et al. (2014) The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut, 63, 64–71. [DOI] [PubMed] [Google Scholar]
- 13. Higashi T., et al. (2014) The national database of hospital-based cancer registries: a nationwide infrastructure to support evidence-based cancer care and cancer control policy in Japan. Jpn J. Clin. Oncol., 44, 2–8. [DOI] [PubMed] [Google Scholar]
- 14. Carter B.D., et al. (2015) Smoking and mortality–beyond established causes. N. Engl. J. Med., 372, 631–640. [DOI] [PubMed] [Google Scholar]
- 15. Nelson D.E., et al. (2013) Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am. J. Public Health, 103, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ng M., et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (published online 10 September 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scully T. (2014) Public health: society at large. Nature, 508, S50–S51. [DOI] [PubMed] [Google Scholar]
- 18. Arnold M., et al. (2015) Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet. Oncol., 16, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng M., et al. (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA, 311, 183–192. [DOI] [PubMed] [Google Scholar]
- 20. Adair L.S., et al. (2014) The emergence of cardiometabolic disease risk in Chinese children and adults: consequences of changes in diet, physical activity and obesity. Obes. Rev., 15(suppl. 1), 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klautzer L., et al. (2014) The curse of wealth—Middle Eastern countries need to address the rapidly rising burden of diabetes. Int. J. Health Policy Manag., 2, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raj P., et al. (2014) Recent trends in published occupational cancer epidemiology research: results from a comprehensive review of the literature. Am. J. Ind. Med., 57, 259–264. [DOI] [PubMed] [Google Scholar]
- 23. Hamra G.B., et al. (2014) Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ. Health Perspect., 122, 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loomis D., et al. (2014) The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: focus on China. Chin. J. Cancer, 33, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogalsky D.K., et al. (2014) Estimating the number of low-income Americans exposed to household air pollution from burning solid fuels. Environ. Health Perspect., 122, 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jha P., et al. (2014) Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med., 370, 60–68. [DOI] [PubMed] [Google Scholar]
- 27. Holmes J., et al. (2014) Effects of minimum unit pricing for alcohol on different income and socioeconomic groups: a modelling study. Lancet, 383, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walkosz B.J., et al. (2014) Dissemination of go sun smart in outdoor recreation: effect of program exposure on sun protection of guests at high-altitude ski areas. J. Health Commun., 19, 999–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kass N., et al. (2014) Ethics and obesity prevention: ethical considerations in 3 approaches to reducing consumption of sugar-sweetened beverages. Am. J. Public Health, 104, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young T., et al. (2014) Cochrane column. Interventions for preventing obesity in children. Int. J. Epidemiol., 43, 675–678. [DOI] [PubMed] [Google Scholar]
- 31. Jha P., et al. (2013) 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med., 368, 341–350. [DOI] [PubMed] [Google Scholar]
- 32. Vergnaud A.C., et al. (2013) Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study1,4. Am. J. Clin. Nutr., 97, 1107–1120. [DOI] [PubMed] [Google Scholar]
- 33. Sankaranarayanan R., et al. (2014) Managing the changing burden of cancer in Asia. BMC Med., 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mackay J., et al. (2013) Tobacco control in Asia. Lancet, 381, 1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Popkin B., et al. (2013) Overview: Bellagio Conference on Program and Policy options for preventing obesity in the low- and middle-income countries. Obes. Rev., 14(suppl. 2), 1–8. [DOI] [PubMed] [Google Scholar]
- 36. Zare Sakhvidi M.J., et al. (2014) Occupational cancer risk perception in Iranian workers. Arch. Environ. Occup. Health, 69, 167–171. [DOI] [PubMed] [Google Scholar]
- 37. Schluger N.W., et al. (2014) Lung disease in a global context. A call for public health action. Ann. Am. Thorac. Soc., 11, 407–416. [DOI] [PubMed] [Google Scholar]
- 38. Gu D., et al. (2013) Reduction in NO(x) emission trends over China: regional and seasonal variations. Environ. Sci. Technol., 47, 12912–12919. [DOI] [PubMed] [Google Scholar]
- 39. Johnston R., et al. (2014) Enhancing arsenic mitigation in Bangladesh: findings from institutional, psychological, and technical investigations. Sci. Total Environ., 488–489, 477–483. [DOI] [PubMed] [Google Scholar]
- 40. Chen J.G., et al. (2013) Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev. Res. (Phila)., 6, 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmidt C.W. (2013) Breaking the mold: new strategies for fighting aflatoxins. Environ. Health Perspect., 121, A270–A275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu W., et al. (2014) Personal and indoor PM2.5 exposure from burning solid fuels in vented and unvented stoves in a rural region of China with a high incidence of lung cancer. Environ. Sci. Technol., 48, 8456–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alemany L., et al. (2014) Time trends of human papillomavirus types in invasive cervical cancer, from 1940 to 2007. Int. J. Cancer, 135, 88–95. [DOI] [PubMed] [Google Scholar]
- 44. Crowe E., et al. (2014) Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ, 348, g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trepo C. (2014) A brief history of hepatitis milestones. Liver Int., 34(suppl. 1), 29–37. [DOI] [PubMed] [Google Scholar]
- 46. Peto T.J., et al. (2014) Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986–90) and in the nationwide immunisation program. BMC Infect. Dis., 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schinazi R., et al. (2014) HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int., 34(suppl. 1), 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. IARC Helicobacter pylori Working Group (2014) Helicobacter pylori eradication as a strategy for preventing gastric cancer. IARC Working Group Reports, No. 8. Available from http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php, Lyon (27 November 2015, date last accessed) [Google Scholar]
- 49. Wright T.C., et al. (2015) Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol., 136, 189–197. [DOI] [PubMed] [Google Scholar]
- 50. Esserman L.J., et al. (2014) Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol., 15, e234–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lauby-Secretan B., et al. (2015) Breast-cancer screening—viewpoint of the IARC Working Group. N. Engl. J. Med., 372, 2353–2358. [DOI] [PubMed] [Google Scholar]
- 52. Thompson I.M., et al. (2014) Prostate cancer screening comes of age. Lancet, 384, 2004–2006. [DOI] [PubMed] [Google Scholar]
- 53. Gupta S., et al. (2014) Challenges and possible solutions to colorectal cancer screening for the underserved. J. Natl Cancer Inst., 106, dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siegel R., et al. (2014) Colorectal cancer statistics, 2014. CA Cancer J. Clin., 64, 104–117. [DOI] [PubMed] [Google Scholar]
- 55. Sankaranarayanan R., et al. (2013) Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol., 49, 314–321. [DOI] [PubMed] [Google Scholar]
- 56. Song P., et al. (2013) Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and china. Liver Cancer, 2, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brown P. (2014) Prevention: targeted therapy-anastrozole prevents breast cancer. Nat. Rev. Clin. Oncol., 11, 127–128. [DOI] [PubMed] [Google Scholar]
- 58. Nan H., et al. (2015) Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA, 313, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Potter J.D. (2014) The failure of cancer chemoprevention. Carcinogenesis, 35, 974–982. [DOI] [PubMed] [Google Scholar]
- 60. Kristensen V.N., et al. (2014) Principles and methods of integrative genomic analyses in cancer. Nat. Rev. Cancer, 14, 299–313. [DOI] [PubMed] [Google Scholar]
- 61. Wang E., et al. (2015) Predictive genomics: a cancer hallmark network framework for predicting tumor clinical phenotypes using genome sequencing data. Semin. Cancer Biol., 30, 4–12. [DOI] [PubMed] [Google Scholar]
- 62. Michailidou K., et al. (2013) Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet., 45, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pashayan N., et al. (2013) Public health genomics and personalized prevention: lessons from the COGS project. J. Intern. Med., 274, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hamilton J.G., et al. (2014) Cancer screening and genetics: a tale of two paradigms. Cancer Epidemiol. Biomarkers Prev., 23, 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stafford P., et al. (2014) Immunosignature system for diagnosis of cancer. Proc. Natl. Acad. Sci. USA, 111, E3072–E3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alix-Panabières C., et al. (2014) Challenges in circulating tumour cell research. Nat. Rev. Cancer, 14, 623–631. [DOI] [PubMed] [Google Scholar]
- 67. Bettegowda C., et al. (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med., 6, 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Madhavan D., et al. (2014) Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res. Treat., 146, 163–174. [DOI] [PubMed] [Google Scholar]
- 69. Alexandrov L.B., et al. (2014) Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr. Opin. Genet. Dev., 24, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scelo G., et al. (2014) Variation in genomic landscape of clear cell renal cell carcinoma across Europe. Nat. Commun., 5, 5135. [DOI] [PubMed] [Google Scholar]
- 71. Zhang B., et al. (2014) Proteogenomic characterization of human colon and rectal cancer. Nature, 513, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Garcia-Closas M., et al. (2014) Combined associations of genetic and environmental risk factors: implications for prevention of breast cancer. J. Natl Cancer Inst., 106, dju305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mabert K., et al. (2014) Cancer biomarker discovery: current status and future perspectives. Int. J. Radiat. Biol., 90, 659–677. [DOI] [PubMed] [Google Scholar]
- 74. Porru S., et al. (2014) Complex relationships between occupation, environment, DNA adducts, genetic polymorphisms and bladder cancer in a case-control study using a structural equation modeling. PLoS One, 9, e94566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ooi A.T., et al. (2014) Molecular profiling of premalignant lesions in lung squamous cell carcinomas identifies mechanisms involved in stepwise carcinogenesis. Cancer Prev. Res. (Phila)., 7, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Westcott P.M., et al. (2014) The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature (published online 2 Nov 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Straif K., et al. (2014) Future priorities for the IARC Monographs. Lancet Oncol., 15, 683–684. [DOI] [PubMed] [Google Scholar]
- 78. Kleinstreuer N.C., et al. (2013) In vitro perturbations of targets in cancer hallmark processes predict rodent chemical carcinogenesis. Toxicol. Sci., 131, 40–55. [DOI] [PubMed] [Google Scholar]
- 79. Grando S.A. (2014) Connections of nicotine to cancer. Nat. Rev. Cancer, 14, 419–429. [DOI] [PubMed] [Google Scholar]
- 80. Bouvard V., et al. (2015) Carcinogenicity of consumption of red and processed meat. Lancet Oncol. [DOI] [PubMed] [Google Scholar]
- 81. Benbrahim-Tallaa L., et al. (2014) Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol., 15, 924–925. [DOI] [PubMed] [Google Scholar]
- 82. Wild C.P., et al. (2013) Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environ. Mol. Mutagen., 54, 480–499. [DOI] [PubMed] [Google Scholar]
- 83. Hampton T. (2008) Cancer prevention efforts stalled. JAMA, 299, 2264. [DOI] [PubMed] [Google Scholar]
- 84. Holford T.R., et al. (2014) Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA, 311, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. La Vecchia C., et al. (2014) Trends in alcohol consumption in Europe and their impact on major alcohol-related cancers. Eur. J. Cancer Prev., 23, 319–322. [DOI] [PubMed] [Google Scholar]
- 86. Altobelli E., et al. (2014) Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev. Med., 62, 132–141. [DOI] [PubMed] [Google Scholar]
- 87. Chen J.G., et al. (2014) Changing rates for liver and lung cancers in Qidong, China. Chem. Res. Toxicol., 27, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khuhaprema T., et al. (2014) Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ Open, 4, e003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Husten C.G., et al. (2013) Understanding the Tobacco Control Act: efforts by the US Food and Drug Administration to make tobacco-related morbidity and mortality part of the USA’s past, not its future. Lancet, 381, 1570–1580. [DOI] [PubMed] [Google Scholar]
- 90. Maurice J. (2014) European Union adds teeth to its anti-tobacco legislation. Lancet, 383, 857–858. [DOI] [PubMed] [Google Scholar]
- 91. Howe M. (2015) Commercial solariums banned in Australia. Lancet. Oncol., 16, e58. [DOI] [PubMed] [Google Scholar]
- 92. Chen Z., et al. (2013) China tackles the health effects of air pollution. Lancet, 382, 1959–1960. [DOI] [PubMed] [Google Scholar]
- 93. Metta E., et al. (2014) Public policy, health system, and community actions against illness as platforms for response to NCDs in Tanzania: a narrative review. Glob. Health Action, 7, 23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Martinez C., et al. (2014) Assessment of the smoke-free outdoor regulation in the WHO European Region. Prev. Med., 64, 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Breslau E.S., et al. (2015) The implementation road: engaging community partnerships in evidence-based cancer control interventions. Health Promot. Pract., 16, 46–54. [DOI] [PubMed] [Google Scholar]
- 96. Chalkidou K., et al. (2014) Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet. Oncol., 15, e119–e131. [DOI] [PubMed] [Google Scholar]
- 97. Venhorst K., et al. (2014) Multi-criteria decision analysis of breast cancer control in low- and middle- income countries: development of a rating tool for policy makers. Cost Eff. Resour. Alloc., 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burger E.A., et al. (2014) Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PLoS One, 9, e89974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Niëns L.M., et al. (2014) Cost-effectiveness of breast cancer control strategies in Central America: the cases of Costa Rica and Mexico. PLoS One, 9, e95836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Goss P.E., et al. (2014) Challenges to effective cancer control in China, India, and Russia. Lancet Oncol., 15, 489–538. [DOI] [PubMed] [Google Scholar]
- 101. Rebbeck T.R. (2014) Precision prevention of cancer. Cancer Epidemiol. Biomarkers Prev., 23, 2713–2715. [DOI] [PubMed] [Google Scholar]
- 102. Cancer Australia (2014) Cancer research in Australia. An overview of funding to cancer research projects and research programs in Australia 2006 to 2011. Cancer Australia, Surry Hills, NSW. [Google Scholar]
- 103.Kuong, K.J. et al. (2013) APOBEC3B mutagenesis in cancer. Nat Genetics, 45, 964–965. [DOI] [PMC free article] [PubMed] [Google Scholar]