Abstract
Sickle cell disease (SCD) substantially alters renal structure and function, and causes various renal syndromes and diseases. Such diverse renal outcomes reflect the uniquely complex vascular pathobiology of SCD and the propensity of red blood cells to sickle in the renal medulla because of its hypoxic, acidotic, and hyperosmolar conditions. Renal complications and involvement in sickle cell nephropathy (SCN) include altered haemodynamics, hypertrophy, assorted glomerulopathies, chronic kidney disease, acute kidney injury, impaired urinary concentrating ability, distal nephron dysfunction, haematuria, and increased risks of urinary tract infections and renal medullary carcinoma. SCN largely reflects an underlying vasculopathy characterized by cortical hyperperfusion, medullary hypoperfusion, and an increased, stress-induced vasoconstrictive response. Renal involvement is usually more severe in homozygous disease (sickle cell anaemia, HbSS) than in compound heterozygous types of SCD (for example HbSC and HbSβ+-thalassaemia), and is typically mild, albeit prevalent, in the heterozygous state (sickle cell trait, HbAS). Renal involvement contributes substantially to the diminished life expectancy of patients with SCD, accounting for 16–18% of mortality. As improved clinical care promotes survival into adulthood, SCN imposes a growing burden on both individual health and health system costs. This Review addresses the renal manifestations of SCD and focuses on their underlying mechanisms.
Introduction
Few diseases give rise to such diverse renal manifestations as does sickle cell disease (SCD). Such involvement adversely affects virtually all major physiological processes in the kidney, and leads to complications that are common and chronic on the one hand (such as impaired urinary concentrating ability), and those that are rare and uniformly fatal on the other (such as renal medullary carcinoma).1–15 Box 1 summarizes the spectrum of manifestations and processes that characterize sickle cell nephropathy (SCN).
Renal involvement can occur throughout the life of a patient with SCD. Manifestations such as hyperfiltration, hypertrophy, and impaired urinary concentrating ability are described as early as in infancy. Microalbuminuria is observed in some patients in childhood, whereas haematuria and acute kidney injury (AKI) can occur at any age. Macroalbuminuria tends to occur in early to middle adulthood, and can be accompanied by regression of the glomerular filtration rate (GFR) to the normal range. In the later decades, the risk of chronic kidney disease (CKD), progressive reduction of GFR, and end-stage renal disease (ESRD) increases.
This steady, age-dependent accrual of adverse renal sequelae shortens the average lifespan of patients with SCD. Proteinuria and a reduced GFR are risk factors associated with increased mortality among patients with SCD;16–18 approximately 16–18% of overall mortality in this patient group is ascribed to kidney disease.19,20 Once ESRD is reached, the mortality of patients who are on haemodialysis and have SCD is increased severalfold relative to the mortality of patients who are on haemodialysis but do not have SCD.21 Thus, although the average lifespan of patients with SCD has increased during recent decades owing to improved management of complications outside the kidney, the age-dependent accrual of kidney disease contributes substantially to the still increased mortality in SCD.
Prefaced by an overview of SCD, this Review addresses renal manifestations in SCD and their underlying pathogenetic mechanisms.
Overview of SCD
SCD is one of the most frequent hereditary haematologic diseases in the world. Its most severe and common form, sickle cell anaemia (SCA), results from homozygosity for the mutant form of the gene that encodes β-globin. SCA is also the most severe form of SCD in terms of its renal manifestations. These renal manifestations are generally less severe in the compound heterozygous forms of SCD (such as HbSC and HbSβ+-thalassaemia), and are comparatively mild in the heterozygous state, sickle cell trait (SCT). The current global population of patients with SCD is substantial: in 2010, SCT afflicted approximately 5.5 million neonates, and 312,000 neonates were born with SCA.22 In the coming decades, this worldwide burden is expected to markedly increase,23 thereby underscoring the importance of gaining a full understanding of the renal complications of SCD.
Mutant sickle β-globin results from substitution of valine for glutamic acid at its sixth amino acid. The resulting sickle haemoglobin (HbS) polymerizes when the concentration of its deoxygenated form (deoxyHbS) exceeds a critical threshold. Thus, polymerization is governed by local oxygen tension, and promoted by both acidosis (which decreases the affinity of HbS for oxygen) and hyperosmolarity (which increases red blood cell [RBC] haemoglobin [Hb] concentration). Additionally, sickle RBCs exhibit abnormally high adhesion to the endothelium, owing to acquired membrane changes and to retained adhesion receptors on reticulocytes, especially the stress reticulocytes. This adhesion event slows microvascular transit, thereby enabling the sickling process.24,25
The ambient conditions of the renal medulla promote polymerization of HbS. The medullary milieu is hypoxic and acidotic, and its hyperosmolality dehydrates the RBC, thereby increasing the intracellular concentration of HbS. Moreover, the relatively slow blood flow in the vasa recta prolongs RBC transit through the medulla, thereby increasing the propensity for polymerization of HbS. Finally, slow vasa recta blood flow would promote RBC adhesion to the endothelium, as such adhesion can be enhanced by low shear stress.
Vascular stasis induces ischaemia that can progress to infarction. Alternatively, vascular stasis can resolve, leading to ischaemia–reperfusion and accompanying processes such as oxidative stress and inflammation. Ischaemia–reperfusion pathobiology in SCD is unique, in that occlusions are generally microvascular, multifocal, and recurrent.26 All these processes are relevant to the renal complications of SCD, as are other processes such as endothelial activation and dysfunction, aberrant vascular reactivity, nitric oxide (NO) deficiency, haemolysis, and procoagulant processes (Figure 1).8,13
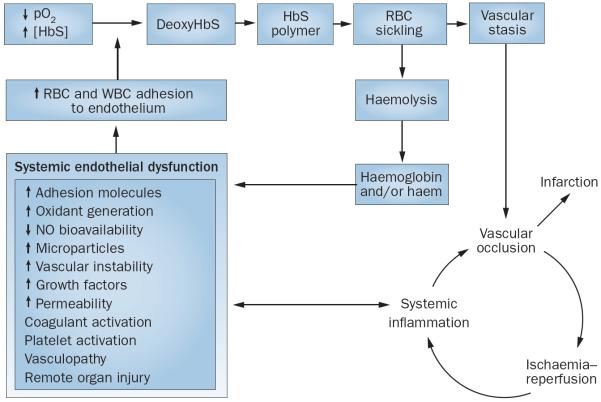
Figure 1.
The pathobiology of sickle cell disease. The formation of deoxyHbS leads to RBC sickling and vascular stasis. Stasis induces vascular occlusion, which either leads to infarction or resolves and causes ischaemia–reperfusion and its accompanying processes. Recurrent cycles of ischaemia–reperfusion in microcirculatory beds amplify organ injury (the `big bang' effect) because they induce inflammation and endothelial dysfunction, both regionally and systemically. Endothelial dysfunction promotes adhesion of RBCs and WBCs to the endothelium. This adhesion is critical because it impedes the transit of RBCs through the microcirculation, thereby promoting RBC sickling and vascular stasis. This vascular stasis explains why RBC sickling occurs in the microcirculation, despite the fact that the time required for sickling usually exceeds RBC transit through the microcirculation. Additional pathobiological pathways include haemolysis and increased free plasma haemoglobin. Plasma haemoglobin scavenges NO from the endothelium. Autoxidation and degradation of HbS lead to the release of free haem, which is toxic to the endothelium via its pro-oxidant and proinflammatory properties. Abbreviations: deoxyHbS, deoxygenated HbS; HbS, sickle haemoglobin; NO, nitric oxide; pO2, partial pressure of oxygen; RBC, red blood cell; WBC, white blood cell.
Renal vasculopathy in SCD
Vasculopathies can arise from either functional or structural changes in the vasculature. The presence of a renal vasculopathy in SCD is indicated by alterations in renal haemodynamics. These alterations accompany changes in the systemic circulation, and underlie the pathogenesis of most manifestations of SCN. As noted in a previous review, SCD exhibits a “perfusion paradox” that is characterized by “a curious coexistence of contrasting perfusion profiles in the circulatory system: hypoperfusion is endemic in microcirculatory beds occluded by haemoglobin S-containing erythrocytes while hyper perfusion characterizes the systemic (macro) circulation and a number of regional vascular circuits.”8
In young patients (generally those in the late teens to early thirties) who have SCD without major organ damage, plasma volume and cardiac output are higher than in healthy individuals; as systemic blood pressure in these patients is usually lower than in healthy individuals, systemic vascular resistance is diminished. Hyperperfusion, which occurs systemically, also occurs regionally; blood flow is increased in the forearm, pedal cutaneous area, brain, and kidney.8,27 The combination of the hyperperfused whole kidney and the microcirculatory regional defects in the renal medulla thus exemplifies, in a single organ, the perfusion paradox in SCD.8
Increased renal plasma flow in SCD is accompanied by increased renal blood flow and GFR.28,29 This supranormal GFR subsides to a normal GFR with either ageing or development of CKD, and to a subnormal GFR as CKD progresses. Hyperfiltration can occur as early as in infancy in patients with SCD,30 and is also exhibited in transgenic murine models of SCD.31 Filtration fraction is reduced in patients with SCD, and this observation might reflect the selective loss of juxtamedullary nephrons, which exhibit a higher filtration fraction than do cortical nephrons.
Whole-kidney GFR is the sum of GFRs in all functional nephrons. If one assumes that the number of nephrons in the kidney of a patient with SCD is unchanged from that of healthy individuals, then the increased whole-kidney GFR observed in SCD reflects increased single-nephron GFR (SNGFR). SNGFR is determined by glomerular plasma flow rate, the transcapillary hydraulic pressure gradient, the glomerular ultrafiltration coefficient (Kf), and plasma oncotic pressure.32 In young adults who have SCD and exhibit renal hyperperfusion and hyperfiltration, functional measurements and mathematical modelling demonstrate that hyperfiltration is principally driven by increased glomerular plasma flow rate and Kf; elevations in transcapillary hydraulic pressure gradient values are marginal.33 Haemodynamic alterations in SCD are assigned considerable importance in the pathogenesis of glomerular disease in SCN, as discussed below.
Kf is of interest in SCD for at least two additional reasons. First, Kf is the product of the total filtering surface area and the glomerular hydraulic permeability; glomerular hypertrophy has long been recognized as a feature of SCD, and an increased Kf value might reflect an increased filtering surface area. Second, the decline in GFR to a normal level as albuminuria occurs and to a subnormal level with the development of CKD partly reflects a progressive decrease in Kf.34
The basis for renal hyperperfusion remains unresolved. Such hyperperfusion cannot be readily ascribed to anaemia or increased plasma volume because correction of anaemia by HbA-containing RBC transfusions does not reverse hyperperfusion,35 and chronic anaemia from causes other than SCD is not usually associated with renal hyperperfusion.36 Hyperperfusion likely reflects the activity of vasorelaxant species, especially because renal vascular resistance (RVR) is reduced.8 Such vasorelaxant species include prostaglandins, the production of which increases in the kidney of patients with SCD, possibly because of medullary ischaemia.3 Although NO is also implicated in hyperperfusion, no evidence to date demonstrates an increase in RVR when NO synthase (NOS) isoforms are inhibited. Indeed, SCD is commonly viewed as a state characterized by a deficiency of and/or resistance to NO. Increased production of kallikrein might also underlie renal hyperperfusion.37
A possible basis for renal hyperperfusion involves the haem oxygenase–carbon monoxide (HO–CO) system. In SCD, haem oxygenase (HO-1 protein) is induced in the kidney and other organs,38 in the vasculature,38 in circulating leucocytes,39 and in circulating endothelial cells.38 Such induction is likely driven by haem, oxidative stress, and inflammation. Markedly increased systemic levels of CO (a vasorelaxant product of HO) reflect the induction of HO-1 in the kidney, vasculature, other tissues, and circulating cells.40,41 Induction of HO–CO might contribute to systemic hyperperfusion and to regional hyperperfusion of the kidney and other vascular beds. Moreover, as previously suggested,13 CO-driven vasodilatation could explain the puzzling association of hyperfiltration (which is usually associated with renal vasodilatation) with haemolysis (which is usually associated with vasoconstriction because of NO binding by haemoglobin) described in SCD.42
In contrast to whole-kidney blood flow, medullary blood flow is regarded as being markedly reduced in SCD. This consensus is based on prominent vasa recta vasoocclusion observed on histological examination and in classical microangiographic studies in humans.43 These studies demonstrate marked loss of vasa recta that supply the inner medulla, and abrupt termination and distortion of the few remaining vasa recta.43 Older studies that were based on renal extraction of para-aminohippurate and which suggest that medullary blood flow is increased29 are uninterpretable because such extraction does not reliably reflect medullary blood flow when whole-kidney blood flow is increased.
Pathogenesis of SCN
A functional renal vasculopathy sets the stage for SCN; hyperperfusion underlies many of the observed cortical changes, and hypoperfusion underlies changes in the medulla (Figure 2). Aberrant renal vascular responses to stress occur in SCD, leading to exaggerated renal vasoconstriction. Cyclical episodes of renal ischaemia and ischaemia-reperfusion predispose to recurrent subclinical and clinical AKI (Figure 2). The key elements in this synthesis of the pathogenesis of SCN are discussed below.
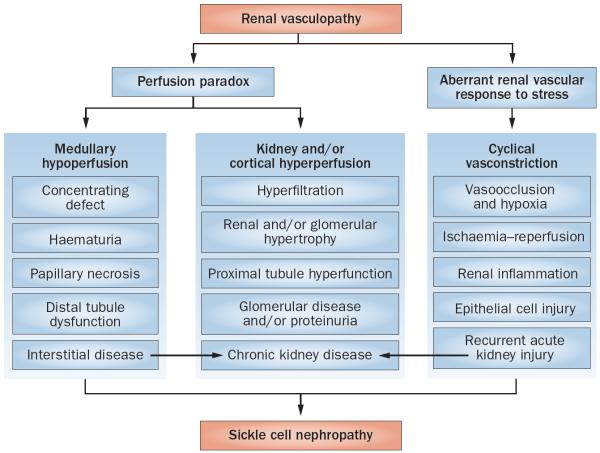
Figure 2.
Salient pathogenetic processes in the development of sickle cell nephropathy. Sickle cell nephropathy largely reflects an underlying functional vasculopathy. This vasculopathy leads to a perfusion paradox, wherein medullary hypoperfusion occurs in conjunction with kidney and/or cortical hyperperfusion. The renal vasculopathy also leads to aberrant renal vascular responses to stress that occur systemically or in distant organs and tissues. This response is characterized by enhanced renal vasoconstriction and resultant vasoocclusion. Recurrent cycles of ischaemia and ischaemia–reperfusion injury thus occur, thereby leading to subclinical and clinical acute kidney injury. These processes summate in the initiation and progression of sickle cell nephropathy.
Increased renal growth
Increased renal growth occurs in SCD, but the reasons are unclear.1,44 This increased growth can be observed as early as in infancy, when renal enlargement correlates with hyperfiltration.30,45 Interestingly, SCD is one of several conditions in which increased renal growth accompanies hyperperfusion; the other conditions include the remnant kidney, diabetes mellitus, pregnancy, and increased dietary protein intake.32,46–48 The `renal work hypothesis' provides an explanation for the association of increased renal blood flow with kidney size in these conditions, and might also apply to SCD. This hypothesis proposes that alteration of renal haemodynamics leads to increased renal growth:46 hyperperfusion - driven hyperfiltration delivers increased amounts of salt and water to the proximal tubule, which increases tubular reabsorption of sodium and water to restore glomerulotubular balance. Proximal tubular reabsorption of sodium is the main determinant of renal oxygen consumption, which increases to sustain increased tubular transport of sodium. This demand for increased renal metabolic work stimulates the requisite mitochondrial processes and adaptive cellular responses, culminating in proximal tubular and overall renal enlargement. Systemic levels of assorted growth factors are also elevated in SCD,49 and these growth factors might exert nephrotrophic effects.
Glomerular enlargement in disease models commonly accompanies increased glomerular plasma flow rates and intraglomerular pressure,32,47 in a process similar to the hypertrophy of blood vessels that are subjected to increased blood flow and pressure. This complex phenomenon might be relevant to the glomerular hypertrophy that occurs in SCD.
Glomerular and other histologic lesions
Renal histologic findings in SCD include congestion of the cortical and glomerular capillaries, mesangial proliferation, thickening and reduplication of the glomerular basement membrane, glomerulosclerosis, tubular deposition of iron, congestion of the vasa recta, interstitial oedema, tubulointerstitial inflammation, and fibrosis.1,50–54 Focal segmental glomerulosclerosis (FSGS) and its variants are the major glomerular lesions in SCD.55–57 Histologic patterns such as membranoproliferative glomerulonephritis and thrombotic microangiopathy occur less frequently than FSGS, and immune reactants, when detected, are usually considered nonspecific.
Proteinuria and chronic kidney disease
Proteinuria is age-dependent in SCD. When assessed either as microalbuminuria (30–300 mg/g creatinine) or macroalbuminuria (>300 mg/g creatinine), and as comprehensively reviewed in 2014,15 proteinuria occurs in up to 27% of patients in the first three decades,15,58 and in up to 68% of older patients.15,59 The natural history of CKD in SCD remains poorly defined, particularly the extent to which microalbuminuria progresses to macroalbuminuria, and macroalbuminuria progresses to nephrotic-range proteinuria. Albuminuria levels >500 mg/g creatinine are associated with progressive CKD, as shown in a retrospective study that involved 98 patients over 5 years.60 In this study, the prevalence of CKD rose from 29% to 42% over this period, and the prevalence of patients with CKD in stages 1, 2, 4, and 5 increased.60 Nephrotic-range proteinuria occurs in 4% of patients with SCD and presages progressive CKD.61 Prior studies demonstrate that irreversible kidney damage (defined by a serum creatinine level >132.6 μmol/l) occurs in approximately 12% of patients with SCD.62
Proteinuria and/or CKD are associated with a number of factors in addition to older age, and variably so depending upon the study. These factors are prehypertensive blood pressure profiles,63 increased blood pressure,64–66 low haemoglobin levels,67,68 haemolysis,69–71 leucocytosis,72 haematuria,73 prior vasoocclusive crisis,62 the βS-gene haplotype,73 pulmonary hypertension,74 stroke,72 acute chest syndrome,75 and infection with parvovirus B19.76 Development of SCN is thus complex, as it can be influenced by blood pressure, haematologic profiles, inflammation, assorted extrarenal processes, and vasoocclusive disease in distant organs.
Albuminuria is usually more severe in SCA than in other types of SCD, and is more likely to occur in patients who express specific single nucleotide polymorphisms in the MYH9 and APOL1 genes, which are associated with an increased risk of CKD in African Americans.77 Conversely, microdeletions in the gene that encodes α-globin (reflecting a form of α-thalassaemia trait) leads to a lower prevalence of macroalbuminuria among patients with SCA.78 Genetic polymorphisms of bone morphogenetic protein receptor 1B also influence GFR in SCA.79
Defects in glomerular permselectivity exist in patients who have SCD with proteinuria, as shown by fractional clearance of IgG and neutral dextran sieving.33,34 In patients with proteinuria and in whom GFR is either increased or within the normal range, the radius of restrictive pores in the glomerular filtration barrier is increased;33,34 in patients with proteinuria and reduced GFR, macromolecular flux through the shunt pathway is substantially increased.34 Interestingly, patients with albuminuria and SCD exhibit increased urinary excretion of markers of tubular injury (KIM-1 and NAG).80 Whether such findings reflect tubular injury induced by glomerular proteinuria or tubular injury as a contributor to impaired tubular albumin reabsorption is largely unaddressed.
Angiotensin-converting-enzyme inhibitors are employed to reduce proteinuria and delay the progression of CKD in patients with SCD.55 Although this approach has not been vigorously tested in patients with SCD,81 it is recommended on the basis of the general efficacy of these agents in CKD. Recommendations made by an expert panel in 2014 include the initiation of treatment with these agents in patients with microalbuminuria and macroalbuminuria, even when blood pressure is normal.82 Interest also surrounds the use of hydroxyurea, as such treatment diminishes renal enlargement and improves urinary concentrating ability in infants,83 decreases glomerular hyperfiltration in children,84 and is associated with less albuminuria in adults.75
Acute kidney injury
AKI occurs in 4–10% of hospitalized patients with SCD, and is more frequent in patients with acute chest syndrome (13.6%) than in patients with painful crisis (2.3%).85,86 AKI is prognostically important in SCD, as it predicts a less favourable outcome among patients who are transferred to the intensive care unit.87 AKI develops in some 75% of episodes of painful crisis that are complicated by acute multiorgan failure; haemodialysis is needed during 18% of these episodes.88 In prospectively studied patients, a reversible 15% reduction in creatinine clearance occurs during painful crisis.89 Even in patients with painful crisis but a normal serum creatinine level (an imperfect marker of GFR in SCD because of the relatively high tubular secretion of creatinine), acute subclinical tubular injury occurs, as evidenced by more than a twofold increase in urinary excretion of NGAL.90 Factors that predispose to AKI include volume depletion, rhabdomyolysis, infections, and the use of nonsteroidal analgaesics.1–6
Pathogenesis of progressive CKD
CKD progresses because of interactions among multiple processes in the vascular, glomerular, tubular, and interstitial compartments of the kidney.91,92 Information regarding such processes in SCN is limited; the following sections broadly outline possible pathogenetic pathways relevant to progressive SCN.
Haemodynamic glomerular injury
SNGFR and glomerular plasma flow rate are predicted to increase in SCD, along with marginal increases in glomerular capillary pressure. As shown by substantial experimental data, these haemodynamic alterations can damage the glomerular endothelium, the podocyte, and the glomerular filtration barrier, thereby causing proteinuria; proteinuria extends injury into the tubulointerstitial compartment.7,32,47,91 Damage to podocytes leads to focal adhesions to the parietal epithelium.93 These adhesions cause FSGS, the misdirection of glomerular filtration, and the development of interstitial inflammation.
Increased glomerular growth
Glomerular enlargement is considered a precursor of glomerular injury.32,47 Glomerular enlargement increases wall tension in the capillary loops, even when intraglomerular pressure is unchanged, in accordance with Laplace's Law (which states that wall tension in spherical objects is the product of intraluminal pressure and radius of the sphere).47 Increased wall tension might damage the glomerular endothelium, the podocyte, and the filtration barrier in SCD. Additionally, if hypertrophy of the podocyte does not proceed at the same pace as the glomerular enlargement, areas of the glomerular basement membrane will be bereft of podocyte foot processes; these denuded areas adhere to the parietal epithelium, thereby predisposing to FSGS.
Endothelium–podocyte/pericyte crosstalk
Podocyte integrity—the main determinant of glomerular permselectivity—is critically dependent upon a healthy glomerular endothelium.94 Endothelial integrity is, in turn, dependent upon podocyte-derived signals. For example, vascular endothelial growth factor (VEGF), which is produced by podocytes, maintains the health of the neighbouring endothelium through endothelial production of NO and other mechanisms. Abundant evidence for endothelial dysfunction in SCD exists;8,13 indeed, such evidence is fundamental to the concept of SCD as a vasculopathic state.95,96 The same dysfunction in the glomerular endothelium would promote an activated, proinflammatory, and procoagulant endothelial phenotype; inflammatory mediators (such as endothelin-1 [ET-1] and tumour necrosis factor) from the glomerular endothelium would, in turn, engage receptors on podocytes, thereby causing podocyte injury and proteinuria. Additionally, the procoagulant endothelial phenotype would promote formation of microthrombi in the capillary loops. Such pathogenetic processes originating in the dysfunctional glomerular endothelium could thus contribute to histologic lesions such as FSGS, membranoproliferative glomerulonephritis, and thrombotic microangiopathy.
Studies of soluble fms-like tyrosine kinase 1 (sFLT-1) in SCD provide new insights into endothelial dysfunction in SCD and its relevance to glomerular injury.74 sFLT–1 binds VEGF and disrupts the trophic effects of VEGF on the endothelium, thereby leading to endothelial dysfunction. Plasma levels of sFLT–1 are increased in SCD, and might thereby predispose to albuminuria.74 Endothelial dysfunction in SCD could also result from the pro-oxidant effects of ET-1 and the binding of NO by HbS, as plasma levels of both ET-1 and HbS are elevated in SCD.
A healthy endothelium is necessary for the normal behaviour of pericytes. Pericytes regulate medullary blood flow in the healthy kidney, and transform into myofibroblasts in the diseased kidney; chronic tubulointerstitial disease is largely driven by myofibroblast proliferation and migration.92,97 Endothelial dysfunction in SCD might perturb the behaviour of pericytes, leading to impaired medullary blood flow and transformation of pericytes into myofibroblasts, thus setting the stage for CKD.
Haemolysis-induced renal injury
Haemolysis prominently occurs in SCD. Free plasma HbS, which is not haptoglobin-bound, exists as dimers that readily traverse the glomerular filtration barrier. Haemoglobinuria leads to tubular incorporation of HbS followed by intracellular break-down of HbS and haem. Iron thus accumulates in renal tubules and accounts for the clinical observation that renal iron content detected by MRI correlates with the severity of haemolysis.98,99
Several studies have shown an association of haemolysis with proteinuria and CKD,69–71 and further observations indicate that CKD and its progression are associated with haemoglobinuria.100 In one patient cohort, CKD and haemoglobinuria were present in 58% and 36% of patients, respectively; in another, they were present in 54% and 20% of patients, respectively. In both cohorts, haemoglobinuria independently associated with CKD.100 Moreover, in a longitudinal analysis over 32 months, haemoglobinuria was associated with the progression of CKD.100
Repetitive exposure of the kidney to haem proteins induces CKD;101 the condition is exaggerated when haem-degrading capacity is impaired.102 Renal sensitivity to haemoglobin increases with age,103 and age is a major risk factor for CKD among patients with SCD. Haemolysis can lead to renal injury in SCD through the following mechanisms. HbS is an unstable protein that undergoes autoxidation and denaturation to produce oxidants and free haem. Trafficking of HbS across podocytes and tubular epithelial cells can impose oxidant-mediated cellular injury. Additionally, haem is a ligand for the Toll-like receptor 4,104 which is present on endothelial, mesangial, and tubular epithelial cells, and on podocytes; proinflammatory responses in these cells can thus be triggered by haem.105 Interestingly, haem promotes proliferation of smooth muscle cells,106 which are phenotypically similar to mesangial cells. Mesangial trafficking of HbS and haem, along with mesangial phagocytosis of fragmented sickle RBCs, might underlie mesangial proliferation, which is often observed in SCD. Finally, the pro-oxidant effects of HbS and haem might upregulate proinflammatory and fibrogenic genes, leading to infiltrative and fibrosing processes in the glomerular and tubulointerstitial compartments.
Repetitive vascular and other acute insults
Repetitive but mild acute insults or a single severe acute insult to the kidney can cause CKD.101,107 This concept is especially applicable to SCN: SCD is characterized by intermittent episodes of vasoocclusion, and the kidney in SCD shows an increased susceptibility to a single acute insult.108,109
In a transgenic murine model of SCD, a relatively brief episode of acute renal ischaemia instigates a striking reduction in GFR, a marked increase in RVR, rapid extension of vascular congestion from the inner medulla into the cortex, tubular necrosis, and upregulation of proinflammatory mediators such as tumour necrosis factor and inducible NOS;108,109 induction of inducible NOS causes nitrosative stress and tubular damage, as shown in prior studies.110 ATP is promptly depleted following ischaemia, thereby compromising homeostatic processes, including those that phosphorylate and activate proteins that are critical for cell survival.109 Interestingly, unilateral ischaemia exaggerates vasoocclusion in the contralateral, nonischaemic kidney, and bilateral renal ischaemia triggers vasoocclusion in the lungs. These findings indicate the propensity for localized renal injury to instigate vasoocclusion and resultant ischaemia in distant, previously nonischaemic microcirculatory beds in the kidney and elsewhere.108
Vascular congestion and vasoocclusion, especially when repetitive, can promote CKD in at least four ways. First, vasoocclusion induces endothelial injury, which triggers the release of proinflammatory and profibrogenic cytokines. Second, vasoocclusion causes hypoxia in the cortex and medulla that, in turn, elicits hypoxia-inducible factor-1α (HIF1α)-dependent and HIF1α-independent pathways of CKD.111,112 In CKD that develops independently of SCD, interstitial capillary rare fraction in the diseased kidney causes renal hypoxia, which constitutes one of the final common pathways for progressive CKD.113,114 Thus, the origins of hypoxia and hypoxia-dependent pathways of renal injury in SCN are at least twofold: vasoocclusion in SCD, and capillary rare fraction that occurs irrespective of cause. Third, sufficiently severe acute insults to the tubular epithelium can cause capillary rarefraction, interstitial disease, and glomerulosclerosis.115 Fourth, the resolution of vasoocclusion ushers in ischaemia–reperfusion and accompanying pathobiological processes, including proinflammatory and profibrogenic pathways.26,108,109
Contributors to renal vascular congestion and dysfunction during vasoocclusive disease include oxidative stress,38 ET-1,116 thrombospondin,117 and adenosine.118 Even in steady-state SCD, when infection, pain and other disease are absent, plasma ET-1 levels are increased, as is renal expression of prepro-ET-1 mRNA; ET-1 receptor blockers attenuate renal vascular congestion, inflammation, and vasoconstriction induced by hypoxia.116 During vasoocclusive episodes, thrombospondin levels increase in the plasma, where it promotes the shedding of micro particles from RBCs; these microparticles induce oxidant-mediated endothelial injury, increase RBC adhesion to the endothelium, and promote renal vasoocclusive disease.117 Plasma levels of adenosine (an ATP break-down product) are increased in steady-state SCD; adenosine promotes RBC sickling by increasing levels of 2,3-diphosphoglycerate in RBCs.118 Strategies that chronically reduce plasma adenosine levels also reduce renal vascular congestion, microinfarcts, histological injury and proteinuria, and increase urinary concentrating ability.118 Interrupting these mediators of renal vasoocclusion thus provides a strategy for decreasing CKD in SCD.
Tubular hyperfunction
Increased GFR in SCD would, predictably, increase proximal tubular sodium reabsorption because of glomerulotubular balance. Hyperfunctioning of the proximal tubule exists in SCD, as evidenced by increased tubular reabsorption of β2-microglobulin protein and phosphate (the latter being linked to sodium reabsorption); increased proximal tubular secretion of uric acid and creatinine; and increased transport maximum for para-aminohippurate. Increased sodium reabsorption increases oxygen consumption, and such hypermetabolism of renal tubules predisposes to tubulointerstitial injury through oxidative stress and other mechanisms.119 Additionally, greater oxygen consumption during hypermetabolism might exacerbate renal hypoxia in SCD.
Other pathogenetic pathways
Clinical studies suggest that renal immune injury might result from tubular injury and resultant tubular antigen exposure,120 and that glomerular injury might arise from iron-mediated processes.121 Experimental studies suggest that opiates also contribute to SCN.122
Urinary concentrating defect
Nephrogenic in origin, impaired urinary concentrating ability frequently occurs in SCD, and can appear as early as in infancy.1,3–5,12 This impairment can lead to enuresis and polyuria, and increases the risk of dehydration. Before the age of 15 years, but not thereafter, this impairment can be corrected by repeated transfusions of HbA-containing RBCs.123 Adults with SCD can rarely achieve a urine osmolality greater than 450 mOsm/kg following water restriction, and this impairment is vasopressin-resistant.3 Impaired free water reabsorption accompanies this loss of concentrating ability, whereas the diluting ability is intact.3
RBC sickling and congestion in the vasa recta, and resultant ischaemia, impair both solute reabsorption by the ascending limb of the long loops of Henle and the capacity of the vasa recta to serve as countercurrent exchangers.1,3 These impairments lead to a decrease in solute accrual and retention in the medullary interstitium, thereby reducing interstitial osmolality. Reabsorption of water across antidiuretic-hormone-stimulated collecting ducts is critically dependent upon interstitial osmolality; as the latter is reduced in SCD, concentrating ability is impaired. In time, recurrent cycles of ischaemia and infarction cause vasa recta rarefraction, the haphazard formation of collaterals, and loss of the long loops of Henle that descend into the inner medulla and papilla.43 Such structural abnormalities underlie the failure to correct this impaired concentrating ability with HbA-containing RBC transfusions later in life.
The preservation of diluting ability despite an impairment of free water reabsorption in SCD draws upon the fact that the long loops of Henle that descend into the inner medulla and papilla derive from the juxtamedullary nephrons, the efferent arterioles of which supply the accompanying vasa recta;124 juxtamedullary nephrons represent approximately 15% of the nephron population. 85% of nephrons have short loops and peritubular capillaries that descend no deeper than the outer medulla. Unlike the population of juxtamedullary nephrons, this population of superficial nephrons escapes the damaging effects of vasoocclusion in the vasa recta (and are relatively intact prior to the development of CKD). The relative preservation of this nephron population (85% of the total population) explains why diluting ability is intact, as this function is essentially carried out by the thick ascending limb. The preservation of this nephron population also explains why the upper limit of urinary concentration in adults with SCD is approximately 450 mOsm/kg, as this value is the osmolality achieved in the outer medulla.3
Haematuria
Haematuria is among the most common renal manifestations of SCD, and can present as microscopic or macroscopic haematuria; the latter can be life-threatening.1,4–12 Haematuria reflects capillary congestion, especially in the medullary vessels, with extravasation of RBCs into the tubular lumen. Congestion and rupture of the sub mucosal capillaries in the renal pelvis can also be responsible for haematuria; these vessels branch from the efferent arterioles of the juxtamedullary nephrons, from which the vasa recta also arise.1,3 Blockage of the vasa recta can divert blood flow to capillaries that supply the mucosa of the renal pelvis and to the peritubular capillaries of the juxtamedullary nephrons. Haematuria emanates more frequently from the left kidney than the right because of the so-called nutcracker phenomenon imposed upon the left renal vein as it passes between the aorta and the superior mesenteric artery; this phenomenon leads to compression of the vein.
Haematuria might also reflect the presence of renal papillary necrosis caused by vasoocclusion in the vasa recta.9 Besides painless haematuria, renal papillary necrosis can also present with asymptomatic radio-graphic findings, urinary tract obstruction and infection, and flank pain and fever.
In rare cases, haematuria is caused by renal medullary carcinoma, a locally invasive, fast-growing malignancy that usually presents within the first few decades, and causes death within 2 years of diagnosis.9 Reported cases predominantly involve SCT rather than SCA. This malignancy can present as haematuria, pain, a renal mass, or constitutional symptoms; its pathogenesis is essentially unknown.125–127
Distal nephron dysfunction
Impaired medullary perfusion in SCD can cause distal nephron dysfunction, which manifests as an acidification defect and/or an impaired ability to secrete potassium.
Earlier studies demonstrate the presence of an incomplete distal renal tubular acidosis (RTA) in steady-state SCD, with little apparent acid-base abnormality apart from a mild respiratory alkalosis in some patients.1,3,4 Generation and maintenance of an adequate proton gradient for acid excretion are energy-dependent processes that might be impaired by medullary ischaemia. Subsequent studies in 2014 emphasize the importance of RTA in SCD: retrospective analyses of over 400 patients demonstrate that more than 40% of patients with SCD exhibit a metabolic acidosis ascribed to an under lying normokalaemic RTA that arises from diminished availability of ammonium.128
Impaired potassium excretion in SCD likely reflects resistance of the distal nephron to aldosterone.129 Hyperkalaemic hyperchloraemic metabolic acidosis is also recognized in patients with SCD, and reflects either a type IV RTA defect or a selective aldosterone deficiency.130 Hyporeninaemic hypoaldosteronism can occur in SCD in rare cases.130
Sickle cell trait
In SCT, only 40% of RBC haemoglobin content is HbS: the rest is normal HbA. Nevertheless, this amount of HbS is sufficient to cause common complications such as haematuria and impaired concentrating ability.131,132 Renal microangiographic studies show distortion and destruction of the vasa recta in SCT, but considerably less than in SCD.43 Volume contraction due to the urinary concentrating defect likely contributes to the increased risk of AKI, renal infarction, and rhabdomyolysis seen in these patients, especially under physical stress in hot and humid conditions.131,132
Considerable interest exists in whether the presence of SCT predisposes an individual to CKD.132,133 Evidence has been mixed: divergent conclusions were reached as to whether the prevalence of SCT is greater in African American patients with ESRD than in individuals without ESRD,134,135 and whether SCT increases the risk of proteinuria in individuals with diabetes mellitus.136,137 However, based on data from five population-based prospective studies that involved a total of approximately 16,000 African Americans, a 2014 analysis demonstrated that SCT is clearly associated with an increased risk of CKD and a reduction in GFR.138 Prior studies indicated that the presence of SCT in individuals with polycystic kidney disease predisposes them to ESRD.139
SCT confers at least one benefit: a reduction in the severity of malaria and its complications.140 Experimental studies demonstrate that such protection is conferred by the induction of HO-1.141
Conclusions
A current paradigm for the renal complications of SCD is that medullary ischaemia induces synthesis of vasodilatory prostaglandins, which elicit renal hyperperfusion.3 Medullary ischaemia also causes distal nephron dysfunction, which then instigates proximal tubular hyperfunction as a compensatory response.3
The present Review takes into account the following considerations. First, the majority of renal complications of SCD result from an underlying functional vasculopathy, in which renal hyperperfusion and medullary hypoperfusion (which together represent the renal perfusion paradox) occur concomitantly, rather than medullary hypoperfusion serving as the forerunner of renal hyperperfusion.8 Second, renal hyperperfusion is an organ-specific manifestation of an enhanced vasodilatory response that is exhibited systemically and in specific regional beds in SCD, and one that conceivably underlies the low blood pressure and reduced prevalence of systemic hypertension in SCD. Third, renal hyper filtration increases proximal tubular transport to maintain glomerulotubular balance; hyperfunction of the proximal nephron is caused by hyperfiltration of the upstream glomerulus, rather than by hypofunctioning of the downstream distal nephron.8 Fourth, increased glomerular size in SCD results from the sustained increase in glomerular perfusion, whereas increased renal metabolic work might drive proximal tubular growth and consequent kidney growth in SCD. Fifth, vascular instability exists in the kidney in SCD, as demonstrated by an exaggerated renal vasoconstrictive response to stress despite augmented vasorelaxation of the kidney in steady-state SCD.8 Vasorelaxant systems, if maximally upregulated in steady-state SCD, might thus lack the functional reserve needed to counter vasoconstrictive stress imposed upon the kidney.8
From a therapeutic standpoint, the kidney as an organ affected by SCD presents unique challenges. First, therapeutic approaches need to be cognizant of the marked heterogeneity of renal involvement on the one hand, but tailored to individual variability in specific renal manifestations on the other. Second, the natural history and pathobiology of some prominent renal manifestations of SCD, such as proteinuria, are still poorly understood. Current therapeutic approaches are largely adopted from other kidney diseases82 without being specifically geared to either the milieu or nuances of SCD. Third, interruption of a given pathophysiologic system could exert divergent effects that mitigate the severity of one manifestation but exacerbate the severity of another. For example, the renin–angiotensin system contributes to FSGS in a murine model of SCD but reduces the severity of the concentrating defect; interruption of the renin–ngiotensin system—a frequently employed therapeutic approach in SCD—might therefore protect against one renal complication but exacerbate the other.142 Fourth, the sickle milieu causes a multitude of pathobiologic processes, and the relative contributions of these processes to the renal complications of SCD are still being defined. Although no all-encompassing solution exists for these challenges, one general approach worth consideration is a multi-modal therapeutic strategy that seeks to interrupt the critical steps in salient pathogenetic pathways.143
Key points.
-
■
Sickle cell nephropathy (SCN) largely reflects an underlying renal vasculopathy characterized by the coexistence of cortical hyperperfusion, medullary hypoperfusion, and an increased renal vasoconstrictive response to systemic and regional stress
-
■
Renal involvement is usually more severe in sickle cell anaemia (HbSS) than in other types of sickle cell disease (HbSC and HbSβ+-thalassaemia), and is typically mildest in sickle cell trait
-
■
Proteinuria and decreased glomerular filtration rate are independent risk factors for increased mortality in sickle cell disease; 16–18% of overall mortality is estimated to arise from kidney disease
-
■
The most frequent glomerulopathy in SCD is focal segmental glomerulosclerosis, a lesion considered to be mediated by alterations in glomerular haemodynamics
-
■
Haematuria and loss of concentrating ability are among the most frequent renal syndromes caused by sickle cell disease, both of which are also common in sickle cell trait
-
■
Angiotensin-converting-enzyme inhibitor therapy is recommended for patients with SCN and microalbuminuria and proteinuria, even in the presence of normal blood pressure
Box 1 | Renal manifestations of sickle cell disease.
Alterations in renal haemodynamics
-
■
Increased renal blood flow rate
-
■
Increased renal plasma flow rate
-
■
Increased glomerular filtration rate
-
■
Decreased renal vascular resistance
-
■
Decreased filtration fraction
-
■
Decreased medullary perfusion
Renal and glomerular enlargement
Hyperfunction of the proximal tubule
-
■
Increased reabsorption of phosphate and β2-microglobulin
-
■
Increased secretion of creatinine and uric acid
-
■
Increased transport maximum of para-aminohippurate
Glomerulopathies
-
■
Focal segmental glomerulosclerosis
-
■
Membranoproliferative glomerulonephritis
-
■
Thrombotic microangiopathy
Tubular deposits of iron
Chronic tubulointerstitial disease
Impaired function of the distal nephron
-
■
Decreased urinary concentrating ability
-
■
Partial distal renal tubular acidosis
-
■
Impaired renal potassium excretion
Increased susceptibility to acute kidney injury
Chronic kidney disease and its progression to end-stage renal disease
Haematuria
Renal papillary necrosis
Increased susceptibility to urinary tract infections
Renal medullary carcinoma
Acknowledgements
K.A.N. is supported by NIH grants DK47060 and DK70124. We gratefully acknowledge the secretarial expertise of Mrs Kara Sloan in the preparation of this manuscript.
Footnotes
Competing interests The authors declare no competing interests.
Author contributions Both authors contributed equally to researching the data for the article, discussion of its content, writing the article and reviewing and/or editing of the manuscript before submission.
References
- 1.Buckalew VM, Jr, Someren A. Renal manifestations of sickle cell disease. Arch. Intern. Med. 1974;133:660–669. [PubMed] [Google Scholar]
- 2.Alleyne GA. The kidney in sickle cell anemia. Kidney Int. 1975;7:371–379. doi: 10.1038/ki.1975.54. [DOI] [PubMed] [Google Scholar]
- 3.de Jong PE, Statius van Eps LW. Sickle cell nephropathy: new insights into its pathophysiology. Kidney Int. 1985;27:711–717. doi: 10.1038/ki.1985.70. [DOI] [PubMed] [Google Scholar]
- 4.Allon M. Renal abnormalities in sickle cell disease. Arch. Intern. Med. 1990;150:501–504. [PubMed] [Google Scholar]
- 5.Pham PT, Pham PC, Wilkinson AH, Lew SQ. Renal abnormalities in sickle cell disease. Kidney Int. 2000;57:1–8. doi: 10.1046/j.1523-1755.2000.00806.x. [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am. J. Hematol. 2000;63:205–211. doi: 10.1002/(sici)1096-8652(200004)63:4<205::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Wesson DE. The initiation and progression of sickle cell nephropathy. Kidney Int. 2002;61:2277–2286. doi: 10.1046/j.1523-1755.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- 8.Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation. 2004;11:179–193. doi: 10.1080/10739680490278592. [DOI] [PubMed] [Google Scholar]
- 9.Scheinman JI. Sickle cell disease and the kidney. Nat. Clin. Pract. Nephrol. 2009;5:78–88. doi: 10.1038/ncpneph1008. [DOI] [PubMed] [Google Scholar]
- 10.da Silva GB, Jr, Libério AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann. Hematol. 2011;90:1371–1379. doi: 10.1007/s00277-011-1327-8. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br. J. Haematol. 2011;155:287–297. doi: 10.1111/j.1365-2141.2011.08853.x. [DOI] [PubMed] [Google Scholar]
- 12.Becker AM. Sickle cell nephropathy: challenging the conventional wisdom. Pediatr. Nephrol. 2011;26:2099–2109. doi: 10.1007/s00467-010-1736-2. [DOI] [PubMed] [Google Scholar]
- 13.Nath KA, Katusic ZS. Vasculature and kidney complications in sickle cell disease. J. Am. Soc. Nephrol. 2012;23:781–784. doi: 10.1681/ASN.2011101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe CC, Thein SL. How I treat renal complications in sickle cell disease. Blood. 2014;123:3720–3726. doi: 10.1182/blood-2014-02-557439. [DOI] [PubMed] [Google Scholar]
- 15.Ataga KI, Derebail VK, Archer DR. The glomerulopathy of sickle cell disease. Am. J. Hematol. 2014;89:907–914. doi: 10.1002/ajh.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darbari DS, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS ONE. 2013;8:e79923. doi: 10.1371/journal.pone.0079923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladwin MT, et al. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS ONE. 2014;9:e99489. doi: 10.1371/journal.pone.0099489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmariah H, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am. J. Hematol. 2014;89:530–535. doi: 10.1002/ajh.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt OS, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 20.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr. Blood Cancer. 2013;60:1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 21.McClellan AC, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br. J. Haematol. 2012;159:360–367. doi: 10.1111/bjh.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piel FB, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. [PubMed] [Google Scholar]
- 25.Hebbel RP. Perspectives series: cell adhesion in vascular biology. Adhesive interactions of sickle erythrocytes with endothelium. J. Clin. Invest. 1997;99:2561–2564. doi: 10.1172/JCI119442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebbel RP. Ischemia–reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol. Oncol. Clin. North Am. 2014;28:181–198. doi: 10.1016/j.hoc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Prohovnik I, et al. Cerebral hyperemia, stroke, and transfusion in sickle cell disease. Neurology. 1989;39:344–348. doi: 10.1212/wnl.39.3.344. [DOI] [PubMed] [Google Scholar]
- 28.Etteldorf JN, Smith JD, Tuttle AH, Diggs LW. Renal hemodynamic studies in adults with sickle cell anemia. Am. J. Med. 1955;18:243–248. doi: 10.1016/0002-9343(55)90239-4. [DOI] [PubMed] [Google Scholar]
- 29.Hatch FE, Jr, Azar SH, Ainsworth TE, Nardo JM, Culbertson JW. Renal circulatory studies in young adults with sickle cell anemia. J. Lab. Clin. Med. 1970;76:632–640. [PubMed] [Google Scholar]
- 30.Ware RE, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J. Pediatr. 2010;156:66–70. e1. doi: 10.1016/j.jpeds.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bank N, et al. Renal nitric oxide synthases in transgenic sickle cell mice. Kidney Int. 1996;50:184–189. doi: 10.1038/ki.1996.301. [DOI] [PubMed] [Google Scholar]
- 32.Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin. Nephrol. 2003;23:194–199. doi: 10.1053/anep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt F, et al. Early glomerular dysfunction in patients with sickle cell anemia. Am. J. Kidney Dis. 1998;32:208–214. doi: 10.1053/ajkd.1998.v32.pm9708603. [DOI] [PubMed] [Google Scholar]
- 34.Guasch A, Cua M, Mitch WE. Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int. 1996;49: 786–791. doi: 10.1038/ki.1996.109. [DOI] [PubMed] [Google Scholar]
- 35.Statius van Eps LW, Schouten H, La Porte-Wijsman LW, Struyker Boudier AM. The influence of red blood cell transfusions on the hyposthenuria and renal hemodynamics of sickle cell anemia. Clin. Chim. Acta. 1967;17:449–461. doi: 10.1016/0009-8981(67)90222-7. [DOI] [PubMed] [Google Scholar]
- 36.Bradley SE, Bradley GP. Renal function during chronic anemia in man. Blood. 1947;2:192–202. [PubMed] [Google Scholar]
- 37.Bergmann S, Zheng D, Barredo J, Abboud MR, Jaffa AA. Renal kallikrein: a risk marker for nephropathy in children with sickle cell disease. J. Pediatr. Hematol. Oncol. 2006;28:147–153. doi: 10.1097/01.mph.0000203722.91189.9d. [DOI] [PubMed] [Google Scholar]
- 38.Nath KA, et al. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am. J. Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jison ML, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sears DA, Udden MM, Thomas LJ. Carboxyhemoglobin levels in patients with sickle-cell anemia: relationship to hemolytic and vasoocclusive severity. Am. J. Med. Sci. 2001;322:345–348. doi: 10.1097/00000441-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Sylvester KP, et al. Exhaled carbon monoxide levels in children with sickle cell disease. Eur. J. Pediatr. 2005;164:162–165. doi: 10.1007/s00431-004-1605-8. [DOI] [PubMed] [Google Scholar]
- 42.Haymann JP, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin. J. Am. Soc. Nephrol. 2010;5:756–761. doi: 10.2215/CJN.08511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J. Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet. 1970;1:450–452. doi: 10.1016/s0140-6736(70)90836-6. [DOI] [PubMed] [Google Scholar]
- 44.Walker TM, Beardsall K, Thomas PW, Serjeant GR. Renal length in sickle cell disease: observations from a cohort study. Clin. Nephrol. 1996;46:384–388. [PubMed] [Google Scholar]
- 45.McCarville MB, et al. Abdominal ultrasound with scintigraphic and clinical correlates in infants with sickle cell anemia: baseline data from the BABY HUG trial. AJR Am. J. Roentgenol. 2011;196:1399–1404. doi: 10.2214/AJR.10.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fine L. The biology of renal hypertrophy. Kidney Int. 1986;29:619–634. doi: 10.1038/ki.1986.45. [DOI] [PubMed] [Google Scholar]
- 47.Hostetter TH. Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 1995;57:263–278. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- 48.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nature Rev. Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 49.Brittain JE, Parise LV. Cytokines and plasma factors in sickle cell disease. Curr. Opin. Hematol. 2007;14:438–443. doi: 10.1097/MOH.0b013e3282a4a673. [DOI] [PubMed] [Google Scholar]
- 50.Pitcock JA, Muirhead EE, Hatch FE, Johnson JG, Kelly BJ. Early renal changes in sickle cell anemia. Arch. Pathol. 1970;90:403–410. [PubMed] [Google Scholar]
- 51.Walker BR, Alexander F, Birdsall TR, Warren RL. Glomerular lesions in sickle cell nephropathy. JAMA. 1971;215:437–440. [PubMed] [Google Scholar]
- 52.Elfenbein IB, Patchefsky A, Schwartz W, Weinstein AG. Pathology of the glomerulus in sickle cell anemia with and without nephrotic syndrome. Am. J. Pathol. 1974;77:357–374. [PMC free article] [PubMed] [Google Scholar]
- 53.Tejani A, et al. Renal lesions in sickle cell nephropathy in children. Nephron. 1985;39:352–355. doi: 10.1159/000183404. [DOI] [PubMed] [Google Scholar]
- 54.Bhathena DB, Sondheimer JH. The glomerulopathy of homozygous sickle hemoglobin (SS) disease: morphology and pathogenesis. J. Am. Soc. Nephrol. 1991;1:1241–1252. doi: 10.1681/ASN.V1111241. [DOI] [PubMed] [Google Scholar]
- 55.Falk RJ, et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N. Engl. J. Med. 1992;326:910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- 56.Nasr SH, Markowitz GS, Sentman RL, D'Agati VD. Sickle cell disease, nephrotic syndrome, and renal failure. Kidney Int. 2006;69:1276–1280. doi: 10.1038/sj.ki.5000234. [DOI] [PubMed] [Google Scholar]
- 57.Maigne G, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010;89:18–27. doi: 10.1097/MD.0b013e3181ca59b6. [DOI] [PubMed] [Google Scholar]
- 58.Dharnidharka VR, Dabbagh S, Atiyeh B, Simpson P, Sarnaik S. Prevalence of microalbuminuria in children with sickle cell disease. Pediatr. Nephrol. 1998;12:475–478. doi: 10.1007/s004670050491. [DOI] [PubMed] [Google Scholar]
- 59.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: prevalence and clinical correlates of progressive renal failure. J. Am. Soc. Nephrol. 2006;17:2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 60.Gosmanova EO, Zaidi S, Wan JY, Adams-Graves PE. Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J. Investig. Med. 2014;62:804–807. doi: 10.1097/01.JIM.0000446836.75352.72. [DOI] [PubMed] [Google Scholar]
- 61.Bakir AA, et al. Prognosis of the nephrotic syndrome in sickle glomerulopathy. A retrospective study. Am. J. Nephrol. 1987;7:110–115. doi: 10.1159/000167444. [DOI] [PubMed] [Google Scholar]
- 62.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 63.Becker AM, et al. Blood pressure abnormalities in children with sickle cell anemia. Pediatr. Blood Cancer. 2014;61:518–522. doi: 10.1002/pbc.24843. [DOI] [PubMed] [Google Scholar]
- 64.Thompson J, Reid M, Hambleton I, Serjeant GR. Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch. Intern. Med. 2007;167:701–708. doi: 10.1001/archinte.167.7.701. [DOI] [PubMed] [Google Scholar]
- 65.Gordeuk VR, et al. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am. J. Hematol. 2008;83:15–18. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE. Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr. Nephrol. 2011;26:1285–1290. doi: 10.1007/s00467-011-1857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faulkner M, et al. Severe anemia: a risk factor for glomerular injury in sickle cell disease. J. Natl Med. Assoc. 1995;87:209–213. [PMC free article] [PubMed] [Google Scholar]
- 68.Lebensburger J, et al. Protective role of hemoglobin and fetal hemoglobin in early kidney disease for children with sickle cell anemia. Am. J. Hematol. 2011;86:430–432. doi: 10.1002/ajh.21994. [DOI] [PubMed] [Google Scholar]
- 69.Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr. Nephrol. 2010;25:2123–2127. doi: 10.1007/s00467-010-1560-8. [DOI] [PubMed] [Google Scholar]
- 70.Day TG, Drasar ER, Fulford T, Sharpe CC, Thein SL. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica. 2012;97:201–205. doi: 10.3324/haematol.2011.050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamideh D, et al. Albuminuria correlates with hemolysis and NAG and KIM-1 in patients with sickle cell anemia. Pediatr. Nephrol. 2014;29:1997–2003. doi: 10.1007/s00467-014-2821-8. [DOI] [PubMed] [Google Scholar]
- 72.Wigfall DR, Ware RE, Burchinal MR, Kinney TR, Foreman JW. Prevalence and clinical correlates of glomerulopathy in children with sickle cell disease. J. Pediatr. 2000;136:749–753. [PubMed] [Google Scholar]
- 73.Powars DR, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann. Intern. Med. 1991;115:614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- 74.Ataga KI, et al. Urinary albumin excretion is associated with pulmonary hypertension in sickle cell disease: potential role of soluble fms-like tyrosine kinase-1. Eur. J. Haematol. 2010;85:257–263. doi: 10.1111/j.1600-0609.2010.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol. Dial. Transplant. 2014;29:1211–1218. doi: 10.1093/ndt/gft295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wierenga KJ, et al. Glomerulonephritis after human parvovirus infection in homozygous sickle-cell disease. Lancet. 1995;346:475–476. doi: 10.1016/s0140-6736(95)91324-6. [DOI] [PubMed] [Google Scholar]
- 77.Ashley-Koch AE, et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br. J. Haematol. 2011;155:386–394. doi: 10.1111/j.1365-2141.2011.08832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guasch A, et al. Evidence that microdeletions in the alpha globin gene protect against the development of sickle cell glomerulopathy in humans. J. Am. Soc. Nephrol. 1999;10:1014–1019. doi: 10.1681/ASN.V1051014. [DOI] [PubMed] [Google Scholar]
- 79.Nolan VG, et al. Estimated glomerular filtration rate in sickle cell anemia is associated with polymorphisms of bone morphogenetic protein receptor 1B. Am. J. Hematol. 2007;82:179–184. doi: 10.1002/ajh.20800. [DOI] [PubMed] [Google Scholar]
- 80.Sundaram N, et al. Biomarkers for early detection of sickle nephropathy. Am. J. Hematol. 2011;86:559–566. doi: 10.1002/ajh.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasongko TH, Nagalla S, Ballas SK. Angiotensin-converting enzyme (ACE) inhibitors for proteinuria and microalbuminuria in people with sickle cell disease. Cochrane Database Syst. Rev. 2013;3:CD009191. doi: 10.1002/14651858.CD009191.pub2. [DOI] [PubMed] [Google Scholar]
- 82.Yawn BP, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 83.Alvarez O, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr. Blood Cancer. 2012;59:668–674. doi: 10.1002/pbc.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aygun B, et al. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am. J. Hematol. 2013;88:116–119. doi: 10.1002/ajh.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int. J. Artif. Organs. 1990;13:347–351. [PubMed] [Google Scholar]
- 86.Audard V, et al. Acute kidney injury in sickle patients with painful crisis or acute chest syndrome and its relation to pulmonary hypertension. Nephrol. Dial. Transplant. 2010;25:2524–2529. doi: 10.1093/ndt/gfq083. [DOI] [PubMed] [Google Scholar]
- 87.Cecchini J, et al. Outcomes of adult patients with sickle cell disease admitted to the ICU: a case series. Crit. Care Med. 2014;42:1629–1639. doi: 10.1097/CCM.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 88.Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am. J. Med. 1994;96:155–162. doi: 10.1016/0002-9343(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 89.Aderibigbe A, Arije A, Akinkugbe OO. Glomerular function in sickle cell disease patients during crisis. Afr. J. Med. Med. Sci. 1994;23:153–160. [PubMed] [Google Scholar]
- 90.Audard V, et al. First evidence of subclinical renal tubular injury during sickle-cell crisis. Orphanet J. Rare Dis. 2014;9:67. doi: 10.1186/1750-1172-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 92.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases–insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 94.Garg P, Rabelink T. Glomerular proteinuria: a complex interplay between unique players. Adv. Chronic Kidney Dis. 2011;18:233–242. doi: 10.1053/j.ackd.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- 96.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am. J. Hematol. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peppiatt-Wildman CM. The evolving role of renal pericytes. Curr. Opin. Nephrol. Hypertens. 2013;22:10–16. doi: 10.1097/MNH.0b013e32835b4e6e. [DOI] [PubMed] [Google Scholar]
- 98.Schein A, Enriquez C, Coates TD, Wood JC. Magnetic resonance detection of kidney iron deposition in sickle cell disease: a marker of chronic hemolysis. J. Magn. Reson. Imaging. 2008;28:698–704. doi: 10.1002/jmri.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vasavda N, et al. Renal iron load in sickle cell disease is influenced by severity of haemolysis. Br. J. Haematol. 2012;157:599–605. doi: 10.1111/j.1365-2141.2012.09093.x. [DOI] [PubMed] [Google Scholar]
- 100.Saraf SL, et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br. J. Haematol. 2014;164:729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int. 2000;57:2423–2433. doi: 10.1046/j.1523-1755.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 102.Nath KA, et al. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 103.Nath KA, et al. Age sensitizes the kidney to heme protein-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 2013;304:F317–F325. doi: 10.1152/ajprenal.00606.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Belcher JD, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J. Am. Soc. Nephrol. 2007;18:414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 106.Moraes JA, et al. Heme modulates smooth muscle cell proliferation and migration via NADPH oxidase: a counter-regulatory role for heme oxygenase system. Atherosclerosis. 2012;224:394–400. doi: 10.1016/j.atherosclerosis.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 107.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nath KA, et al. Transgenic sickle mice are markedly sensitive to renal ischemia–reperfusion injury. Am. J. Pathol. 2005;166:963–972. doi: 10.1016/S0002-9440(10)62318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Juncos JP, et al. Early and prominent alterations in hemodynamics, signaling, and gene expression following renal ischemia in sickle cell disease. Am. J. Physiol. Renal Physiol. 2010;298:F892–F899. doi: 10.1152/ajprenal.00631.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bank N, et al. Inhibition of nitric oxide synthase ameliorates cellular injury in sickle cell mouse kidneys. Kidney Int. 2000;58:82–89. doi: 10.1046/j.1523-1755.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- 111.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 112.Sun K, Xia Y. New insights into sickle cell disease: a disease of hypoxia. Curr. Opin. Hematol. 2013;20:215–221. doi: 10.1097/MOH.0b013e32835f55f9. [DOI] [PubMed] [Google Scholar]
- 113.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 114.Long DA, Norman JT, Fine LG. Restoring the renal microvasculature to treat chronic kidney disease. Nat. Rev. Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- 115.Grgic I, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabaa N, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J. Clin. Invest. 2008;118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Camus SM, et al. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood. 2012;120:5050–5058. doi: 10.1182/blood-2012-02-413138. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat. Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nath KA, Salahudeen AK, Clark EC, Hostetter K, Hostetter TH. Role of cellular metabolites in progressive renal injury. Kidney Int. Suppl. 1992;38:S109–S113. [PubMed] [Google Scholar]
- 120.Strauss J, Pardo V, Koss MN, Griswold W, McIntosh RM. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. I. Studies on nature of glomerular-bound antibody and antigen identification in a patient with sickle cell disease and immune deposit glomerulonephritis. Am. J. Med. 1975;58:382–387. doi: 10.1016/0002-9343(75)90604-x. [DOI] [PubMed] [Google Scholar]
- 121.McCoy RC. Ultrastructural alterations in the kidney of patients with sickle cell disease and the nephrotic syndrome. Lab. Invest. 1969;21:85–95. [PubMed] [Google Scholar]
- 122.Weber ML, et al. Morphine promotes renal pathology in sickle mice. Int. J. Nephrol. Renovasc. Dis. 2012;5:109–118. doi: 10.2147/IJNRD.S33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Itano HA, Keitel HG, Thompson D. Hyposthenuria in sickle cell anemia: a reversible renal defect. J. Clin. Invest. 1956;35:998–1007. doi: 10.1172/JCI103360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am. J. Physiol. Renal Physiol. 2003;284:F253–F266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 125.Hakimi AA, et al. Renal medullary carcinoma: the Bronx experience. Urology. 2007;70:878–882. doi: 10.1016/j.urology.2007.06.1124. [DOI] [PubMed] [Google Scholar]
- 126.Gatalica Z, et al. Renal medullary carcinomas: histopathologic phenotype associated with diverse genotypes. Hum. Pathol. 2011;42:1979–1988. doi: 10.1016/j.humpath.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 127.Shetty A, Matrana MR. Renal medullary carcinoma: a case report and brief review of the literature. Ochsner J. 2014;14:270–275. [PMC free article] [PubMed] [Google Scholar]
- 128.Maurel S, et al. Prevalence and correlates of metabolic acidosis among patients with homozygous sickle cell disease. Clin. J. Am. Soc. Nephrol. 2014;9:648–653. doi: 10.2215/CJN.09790913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DeFronzo RA, Taufield PA, Black H, McPhedran P, Cooke CR. Impaired renal tubular potassium secretion in sickle cell disease. Ann. Intern. Med. 1979;90:310–316. doi: 10.7326/0003-4819-90-3-310. [DOI] [PubMed] [Google Scholar]
- 130.Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am. J. Med. 1982;72:188–192. doi: 10.1016/0002-9343(82)90809-9. [DOI] [PubMed] [Google Scholar]
- 131.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am. J. Med. 2009;122:507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 132.Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am. Soc. Hematol. Educ. Program. 2010;2010:418–422. doi: 10.1182/asheducation-2010.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shaw C, Sharpe CC. Could sickle cell trait be a predisposing risk factor for CKD? Nephrol. Dial. Transplant. 2010;25:2403–2405. doi: 10.1093/ndt/gfq186. [DOI] [PubMed] [Google Scholar]
- 134.Derebail VK, et al. High prevalence of sickle cell trait in African Americans with ESRD. J. Am. Soc. Nephrol. 2010;21:413–417. doi: 10.1681/ASN.2009070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hicks PJ, et al. Sickle cell trait is not independently associated with susceptibility to end-stage renal disease in African Americans. Kidney Int. 2011;80:1339–1343. doi: 10.1038/ki.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ajayi AA, Kolawole BA. Sickle cell trait and gender influence type 2 diabetic complications in African patients. Eur. J. Intern. Med. 2004;15:312–315. doi: 10.1016/j.ejim.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Bleyer AJ, et al. Sickle cell trait and development of microvascular complications in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2010;5:1015–1020. doi: 10.2215/CJN.08841209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Naik RP, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312:2115–2125. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yium J, Gabow P, Johnson A, Kimberling W, Martinez-Maldonado M. Autosomal dominant polycystic kidney disease in blacks: clinical course and effects of sickle-cell hemoglobin. J. Am. Soc. Nephrol. 1994;4:1670–1674. doi: 10.1681/ASN.V491670. [DOI] [PubMed] [Google Scholar]
- 140.Rosenthal PJ. Lessons from sickle cell disease in the treatment and control of malaria. N. Engl. J. Med. 2011;364:2549–2551. doi: 10.1056/NEJMcibr1105118. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira A, et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell. 2011;145:398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 142.Roy S, et al. Increased oxidative stress in sickle cell disease activates the renin-angiotensin-TGF-β pathway to mediate sickle nephropathy. 55th ASH Annual Meeting and Exposition. 2013 https://ash.confex.com/ash/2013/webprogram/Paper64925.html.
- 143.Hebbel RP, Vercellotti G, Nath KA. A systems biology consideration of the vasculopathy of sickle cell anemia: the need for multi-modality chemo-prophylaxsis. Cardiovasc. Hematol. Disord. Drug Targets. 2009;9:271–292. doi: 10.2174/1871529x10909040271. [DOI] [PMC free article] [PubMed] [Google Scholar]