Abstract
Afferent connections to the sensory inner and outer hair cells in the cochlea refine and functionally mature during the thyroid hormone (TH)- critical period of inner ear development that occurs perinatally in rodents. In this study, we investigated the effects of hypothyroidism on afferent type II innervation to outer hair cells (OHCs) using the Snell dwarf mouse (Pit1dw). Using a transgenic approach to specifically label type II spiral ganglion neurons, we found that a lack of TH causes persistence of excess type II SGN connections to the OHCs, as well as continued expression of the hair cell functional marker, otoferlin, in the OHCs beyond the maturation period. We also observed a concurrent delay in efferent attachment to the OHCs. Supplementing with TH during the early postnatal period from postnatal day (P) 3 to P4 reversed the defect in type II SGN pruning but did not alter otoferlin expression. Our results show that hypothyroidism causes a defect in the large-scale pruning of afferent type II spiral ganglion neurons in the cochlea, and a delay in efferent attachment and the maturation of otoferlin expression. Our data suggest that the state of maturation of hair cells, as determined by otoferlin expression, may not regulate the pruning of their afferent innervation.
1.
The inner (IHCs) and outer hair cells (OHCs) in the mature organ of Corti are innervated by the spiral ganglion neurons (SGNs). Mature IHCs are innervated with 90% to 95% type I SGN fibers (Liberman, 1980). The remaining 5 to 10% of SGNs are type II unmyelinated neurons that project toward OHC and spiral toward the base to contact multiple OHCs (Liberman, 1980; Brown, 1987). At birth, a mouse cochlea contains an overabundance of type II SGNs. These type II afferent neurons and their fibers make multiple contacts with OHCs, resulting in a surplus number of synapses by the first postnatal week. By the age of onset of hearing, the excess type II SGNs die, and consequently, more than 96% of the OHC afferent synapses are pruned back (Rueda et al., 1987; Echteler, 1992; Barclay et al., 2011). This maturation process takes place during thyroid hormone (TH) critical period on cochlear development that is in the perinatal period in mice (Deol, 1973; Uziel, 1986; Knipper et al., 1998). An early study by Uziel et al (Uziel et al., 1983a) describes defective synaptic pruning in the OHCs of hypothyroid rats, including the persistence of afferent connections that fail to regress normally in the postnatal period. Rueda and colleagues in another study on rats showed that hypothyroid animals had 22% more total SGNs compared to age-matched controls (Rueda et al., 2003). These studies underscore the importance of TH in the OHC afferent pruning process. However, several important questions remain, such as: 1) What is the effect of hypothyroidism on the synapses of type II SGNs with OHCs? 2) Does supplementing with TH during the early postnatal period restore normal afferent innervation in the hypothyroid OHCs? 3) What is the impact of defective afferent pruning on expression of functionally relevant afferent proteins in the OHC such as otoferlin (OTOF)? 4) Does defective afferent pruning correlate with the delay in OHC efferent innervation? 5) What are the long-term effects of hypothyroidism on OHC synapses? Answers to these questions will provide important insights into the fundamental mechanisms involved in OHC synaptic refinement and maintenance as well as the role of TH in these events.
In an earlier study, we found striking structural defects and functional delays in the pruning of IHC afferent synapses in the Snell dwarf (Pit1dw, officially Pou1f1dw) hypothyroid mouse model (Sundaresan et al., 2015). In the current study, we focused on the OHCs and examined the role of TH in pruning type II SGNs. We used a transgenic approach to specifically label type II SGNs and count them after the pruning period. We also quantified the number of synaptic ribbons under hypothyroid conditions at different time points during the early postnatal period as well as in the adult mouse. In addition, we examined the ultrastructural pattern of efferent and afferent connectivity to the OHCs and the expression of OTOF, a synaptic vesicle protein involved in neurotransmitter release, under normal and hypothyroid conditions. Furthermore, we established the critical window for TH action on OHC afferent refinement by supplementing Pit1dw mice with TH for finite time windows in the early postnatal period. We report that a defect in large-scale pruning of type II SGN is responsible for the abnormal retention of afferent innervation in hypothyroid OHCs.
2. Experimental Procedures
2.1 Mice
The following strains of mice were used in our experiments: 1) Pit1dw: DW/J Mlphln Pit1dw/J mice were obtained from the Jackson Laboratory in 1990 and maintained at Stanford University. 2) Peripherin-GFP (PGFP): This transgenic mouse was obtained from Joseph Sarsero, MCRI, Australia. These mice were crossed with the Pit1dw strain to generate Pit1dw-PGFP mice. 3) Mafb-GFP (MGFP): This strain was obtained from Satoru Takahashi, University of Tsukuba, Tsukuba, Japan. These mice were crossed with the Pit1dw strain to generate Pit1dw - MGFP mice.
To induce hypothyroidism, pregnant dams were fed with an iodine-deficient diet containing 0.15% propylthiouracil (PTU) (Harlan Labs, Teklad Animal Diet #95125) throughout the gestation period. Pups were maintained on this diet until the completion of the experiment at which point they were sacrificed. Established procedures for animal care and genotyping were used, including feeding mice a higher fat chow designed for breeding (PMI5020), delaying weaning of mutants until 35 days old, and housing mutants with normal littermates to provide warmth (Karolyi et al., 2007). Control mice were fed normal chow. In all experiments, at least 4 animals of each genotype were analyzed for each age group studied unless stated otherwise. Postnatal day zero (P0) was designated as the day of birth. All experiments were approved by the University Committee on the Use and Care of Animals and conducted in accord with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals.
2.2 TH injection
Pit1dw mice and control wild type (WT) mice were subcutaneously injected for specific durations with either 20 ng T3/g body weight or PBS as a negative control. T3 solution (20ng/ul) was prepared by dissolving 1 mg of T3 (3,3,5-triiodo-L-thyronine sodium salt, Sigma Aldrich, St. Louis, MO) in 1 mL 1N NaOH and 49mL PBS. Radioimmunoassay was used to confirm that this dose of T3 was sufficient to induce normal T3 levels in Pit1dw mice (Sundaresan et al., 2015). Similar studies by other groups have shown that replacement with T3 rather than T4 is more effective since it bypasses the necessary conversion of T4 to the more active T3 in vivo (Weiss et al., 1998; Sui et al., 2006). Mice were sacrificed at P14. n≥4 for all treatment groups and controls.
2.3 Immunofluorescence Staining
Preparation of whole mount cochlear tissues and the procedures for immunostaining on these tissues have been previously described (Mustapha et al., 2009). Inner ears were dissected into cold phosphate-buffered saline (PBS) and after opening the oval and round windows and the bone at the cochlear apex, cochleae were perfused with 4% paraformaldehyde in PBS and were left in fixative solution for additional 10 minutes. Samples were washed in PBS for 10 minutes and then blocked in PBS containing 0.5% Triton X-100 plus 5% bovine serum albumin for 30 minutes at room temperature. The same blocking buffer was used for diluting antibodies. Primary antibodies were incubated at 4 °C for 36 hours followed by three washes in PBS with 0.1% Tween 20. Secondary antibodies were added for 1 hour at room temperature, followed by three washes in PBS with 0.1% Tween 20. The following primary antibodies were used: goat anti-CtBP2 polyclonal for RIBEYE (1:200, Santa Cruz Biotechnology), rabbit anti-SHANK1 polyclonal (1:200, Neuromics), rabbit anti-GLUTR2/3 polyclonal for glutamate receptor (1:200, Millipore), rabbit anti-synaptophysin polyclonal (1:300, Pierce Biotechnology), and anti-OTOF (1:500, a gift from Drs. Saaid Safieddine and Christine Petit, Pasteur Institute, France). Secondary antibodies used were Alexa Fluor 488-conjugated anti-goat and Alexa Fluor 546-conjugated anti-rabbit (1:500 dilution, Invitrogen). After immunostaining, the cochleae were decalcified in 10% EDTA at room temperature and further fixed by immersion in 4% paraformaldehyde in PBS for 15 minutes. Cochleae were washed in PBS and mounted on slides in ProLong (Invitrogen) anti-fading media.
2.4 Transmission Electron Microscopy (TEM)
TEM analyses were done as previously described (Mustapha et al., 2009). Briefly, animals were anesthetized and fixed with an intracardiac perfusion of 2.5% glutaraldehyde in 0.15M cacodylate buffer, pH 7.2 with 0.1% tannic acid. Inner ears were removed and further fixed in the same solution for 2 hours. The ears were then decalcified with 3% EDTA with 0.25% glutaraldehyde for one week at 4°C. They were then postfixed in 1% osmium tetraoxide, dehydrated with increasing ethanol concentrations, and embedded in Embed 812 epoxy resin. Embedded ears were sectioned, stained with uranyl acetate and lead citrate, and examined on a Phillips CM-100 TEM. A minimum of four animals per group was examined.
2.5 Cochlear RNA extraction and quantitative RT-qPCR
Cochleae were dissected, and RNA extraction and cDNA preparation were performed as previously described (Mendus et al., 2014). To quantify mRNA expression levels, the cDNA was amplified using Taqman PCR assays (Applied Biosystems, Foster City, CA). We used their proprietary probes and primers for OTOF (assay ID Mm00453299_m1) and GAPDH (assay ID Mm99999915_g1). All Taqman qPCR assays were performed on a BIO-RAD CFX96™ Real-Time System (Bio-Rad, Hercules, CA) with accompanying software for data analysis. Expression of the gene of interest was normalized with respect to the internal standard, GAPDH. Statistical analyses were performed using Student’s t test on Microsoft Excel (Microsoft Corp, Redmond, WA). p<0.05 was considered statistically significant.
2.6 Quantification and Statistical Analysis
Quantification of RIBEYE puncta was performed as previously described (Mendus et al., 2014). A minimum of 30 outer hair cells per ear and an “n” of four to seven animals per genotype or treatment group were counted for each marker. Marker counts are shown as box and whisker plots that represent every data point in the set. The whiskers represent the minimum and maximum values in the specific group examined. Outliers in the data set are also shown. Statistical analyses were performed using an analysis of variance (ANOVA) (SPSS Statistics Premium Grad Pack, IBM Corp.). Two-way ANOVA of the immunohistochemistry data was performed with RIBEYE count as the dependent variable with genotype (wild type or Pit1dw) and treatment or age as independent factors for these tests. On the basis of significant main effects and interactions from this statistical test, planned comparisons were performed either by use of Student’s t test or a one-way ANOVA with marker counts as dependent variable, followed by Scheffe’s post hoc test to identify differences between genotypes, or across ages or treatment groups (SPSS, and Microsoft Excel). In all statistical tests, a p < 0.05 was considered statistically significant. p values ≥ 0.001 for significant comparisons are mentioned in the appropriate results section.
3. Results
3.1 Abnormal persistence of afferent connections to OHCs of Pit1dw mutants
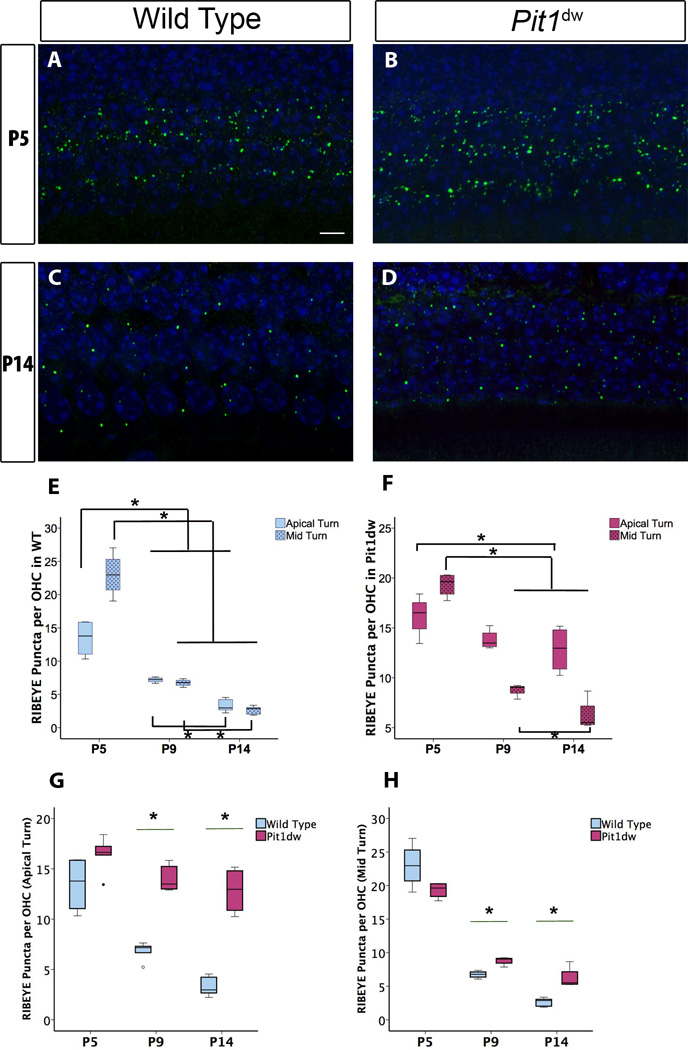
Previous studies have shown a drastic reduction in the number of ribbon synapses during the OHC maturation period (P6 to P12) in mice (Sobkowicz et al., 1982; Huang et al., 2012). To study the role of TH in the elimination of these synapses, we compared presynaptic ribbons in the OHCs of Pit1dw and WT mice by immunohistochemistry with the ribbon marker, RIBEYE, at P5, P9, and P14. We also tried to detect the postsynaptic markers GLUTR2/3 and SHANK1 by immunohistochemistry, but were unable to detect distinct puncta for these markers beyond P5. Also, postsynaptic puncta marked by GLUTR2/3 or SHANK1 immunoreactivity were not juxtaposed with RIBEYE puncta in our experiments. Therefore, we considered RIBEYE puncta only for our analyses. Confocal images from the apical turn of the cochlea are shown in Figure 1A–D. RIBEYE-positive puncta from Pit1dw and WT samples were quantified using 3D–reconstructions of confocal images. For statistical analysis, two-way ANOVAs were performed with RIBEYE puncta count as dependent variable and genotype (WT or Pit1dw) and age (P5, P9, and P14) as independent factors. This analysis showed a significant main effect of genotype by age interaction (F2,25=14.501, p<0.001). Planned comparisons were performed with a one-way ANOVA, with RIBEYE puncta count as dependent variable and either age or genotype as independent factor as indicated below. This analysis was followed by Scheffe’s post hoc test to identify differences between genotypes or across the ages tested. Wild type OHCs showed a normal pattern of presynaptic ribbon loss between the ages of P5, P9, and P14 in both the apical and mid turns of the cochlea (Figure 1A,C,E). In WT mice, RIBEYE counts at P9 and P14 were 49.4% and 75.2% lower than those at P5 in the apical turn indicating pruning occurred throughout this period (p<0.001 for both P9 vs. P5 and P14 vs. P5) (Figure 1E). There was a 51.2% reduction in RIBEYE counts between P9 and P14 as well (p=0.025). In the mid turn of WT cochlea, we saw a similar pattern of pruning with 70.7% and 88.6 % reduction in RIBEYE counts at P9 and P14, respectively, compared to P5 (p<0.001 for both comparisons). Counts at P14 were also 61% lower than those at P9 (p=0.027). The presynaptic ribbons in the OHCs of the apical turn refined to a lesser extent in Pit1dw mutants compared to WT controls during this time period (Figure 1F). While pruning was delayed in the mutant mice and no significant changes in RIBEYE counts were seen at P9 compared to P5, there was a 22% reduction at P14 compared to levels at P5 (p =0.02). In the mid turn, however, Pit1dw mutants pruned to a greater extent than the apical turn with RIBEYE counts 55.4% lower at P9 and 68% lower at P14 compared to P5 (p<0.001 for both P9 vs. P5 and P14 vs. P5). RIBEYE counts at P9 and P14 were not significantly different (p=0.05) indicating that pruning mainly occurred in the P5-P9 window in these mice.
Figure 1. Outer hair cell (OHC) synaptic refinement is disrupted in Pit1dwmutants.
Projection of confocal sections obtained from the apical turn of cochlear whole mounts stained with the afferent presynaptic marker RIBEYE (in green) at P5 in wild type (WT) (A) and Pit1dw mice (B). The same marker at P14 in WT and Pit1dw mice is shown in (C) and (D) respectively. E, F, Quantification of RIBEYE puncta from the cochlea in WT and Pit1dw mice, respectively at P5, P9, and P14. G, H, Comparison of RIBEYE puncta counts in the OHCs of Pit1dw mutants at P5, P9, and P14 as compared to age-matched WT controls. Box plots represent the entire data set. Statistical tests were performed using ANOVA followed by Scheffe’s post hoc test. n≥4 for all groups. *p<0.05 was considered statistically significant. Statistically significant comparisons are indicated (*).
Next, when we compared the average ribbon numbers per OHC between the Pit1dw mutants and WT mice at P5, they were similar in both the apical and mid turns of the cochlea (p=0.1 for both apical and mid turn comparisons) (Figure 1G,H). However, at P9 and P14, WT mice had significantly lower numbers of ribbons per OHC in the apical turn than Pit1dw mutants (p<0.001 for P9 and p=0.002 for P14) (Figure 1G). RIBEYE counts in the OHCs of the mid turn at P9 and P14 were also both lower in WT compared to Pit1dw mice (p=0.003 for P9 and p=0.016 for P14) (Figure 1H), although these differences were smaller than what was seen in the apical turn. These results taken together indicated a disruption in the refinement of OHC presynaptic ribbons in both turns of the cochlea in the Pit1dw mutants.
3.2 Pit1dw mice have more type II SGNs compared to WT mice
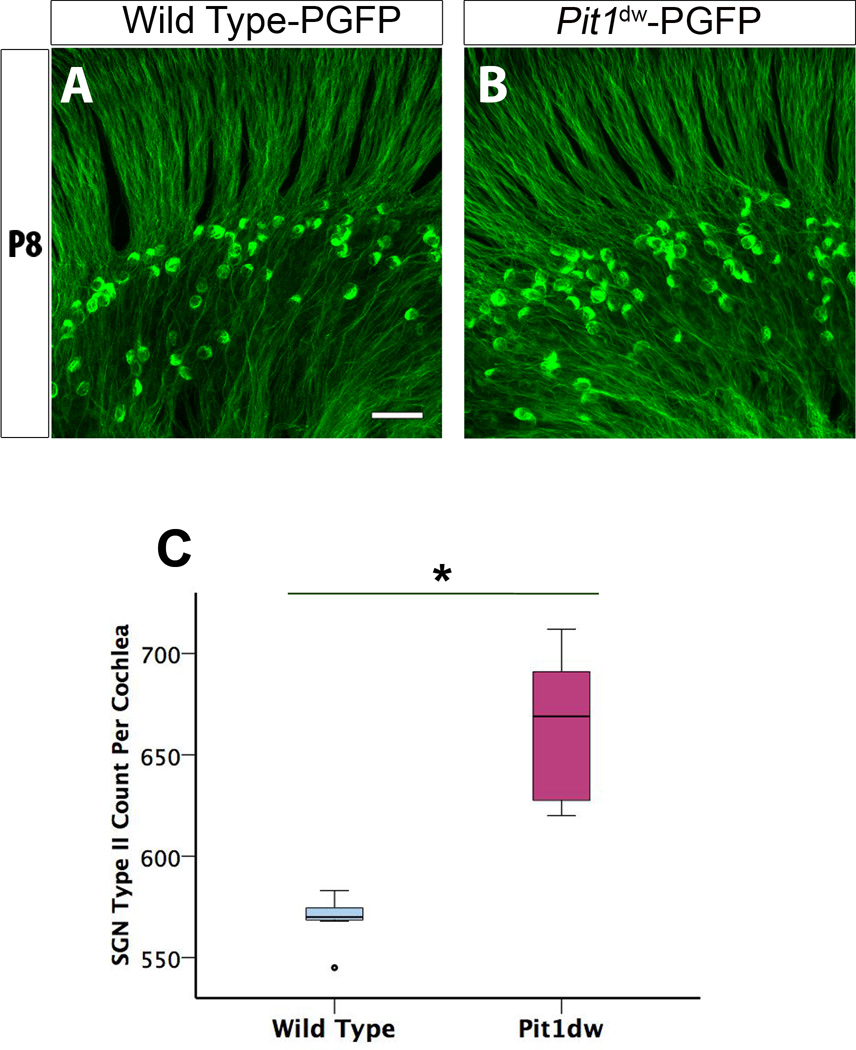
The abnormalities of OHC innervation we observed in Pit1dw mutants may be due to the persistence of afferent fibers and neurons of type II SGNs, which normally regress from the OHCs during the first postnatal week of cochlear development (Rueda et al., 1987; Echteler, 1992). It has been shown that loss of type II SGNs during this period is an important mechanism of pruning of afferent innervation to the OHCs (Huang et al., 2012). We hypothesized that defective pruning of type II SGN fibers could account for the abnormal retention of presynaptic ribbons in Pit1dw mutants. We took advantage of the Peripherin-GFP (PGFP) transgenic mouse model that has the protein peripherin, a commonly used marker for type II SGNs, labeled with GFP (McLenachan et al., 2008). The breeding of PGFP mice with Pit1dw mice allowed a specific detection of type II SGNs in both WT and Pit1dw cochleas from P6 onward (Figure 2A,B). We performed whole mounts of all three regions of the cochlea (apical, mid, and basal), followed by confocal microscopy and 3D–reconstruction using Volocity® software. We then counted the number of peripherin-labeled type II neurons in PGFP- Pit1dw and WT-PFGP mice. We found that PGFP-Pit1dw mice had 19.6% more type II SGN as compared to PGFP controls (Figure 2C). Therefore, defects in pruning of excess afferent synapses to the OHCs of Pit1dw mutants can, at least partially, be attributed to a failure in the retraction of type II SGNs during the normal window of pruning. This result further indicates that TH could facilitate the apoptosis of type II SGNs directly or indirectly in WT mice, a process that is lacking in the Pit1dw mutants. Our data is consistent with an earlier study in hypothyroid rats that also reported an increase in the number of SGNs, but this study did not distinguish between types I and II (Rueda et al., 2003). Our results conclusively demonstrate that the number of SGNII is higher in Pit1dw mutants compared to WT mice. We next examined whether replacement with TH would restore normal pruning in the OHCs of Pit1dw mutants.
Figure 2. Altered pruning of spiral ganglion type II neurons in P8 hypothyroid organ of Corti.
A, B, Projection of confocal sections obtained from whole mount cochlea of PGFP that has type II SGNs labeled with GFP (green) (A) and PGFP-Pit1dw mice (B). C, Quantification of the SG II neurons in the organ of Corti of Pit1dw mutants compared to WT controls. Results are expressed as a box plot showing the entire data set. The scale bar represents 10 µm. n=7 for both PGFP and PGFP- Pit1dw mice. *p<0.001 by Student’s t test.
3.3 Effect of TH replacement on OHC presynaptic ribbon refinement
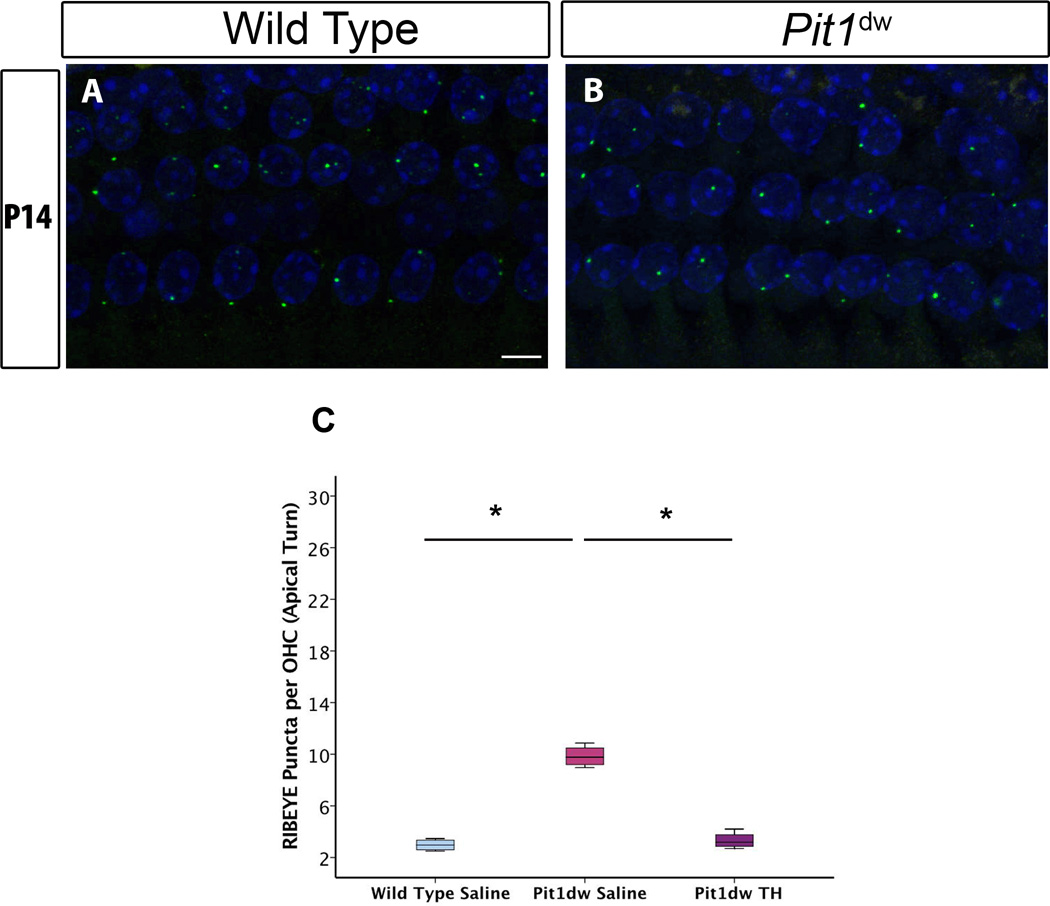
We injected Pit1dw mutants with TH for specific time periods (indicated below) and examined whether TH treatment was able to correct the refinement defect observed with the presynaptic ribbons in the OHCs of these animals. Previous studies by our group have shown that the period from P3 to P8 is critical for TH action on afferent presynaptic pruning in IHCs (Sundaresan et al., 2015). Based on reports in literature that suggest TH levels (and therefore, TH action) in the cochlea are highest in the early postnatal period, we picked a few different treatment windows within this period for TH supplementation (Campos-Barros et al., 2000; Sharlin et al., 2011). We injected Pit1dw mice with TH from: 1) P0 to P5, 2) P3 to P4, 3) P3 to P6, or 4) P3 to P8. We then analyzed cochlear tissue from these mice to determine which of these treatment periods was effective in restoring pruning in the OHCs of Pit1dw mutants. Our analyses showed that treatment from P3 to P4 was sufficient to restore normal ribbon counts in the hypothyroid OHCs. Data from the other treatment periods (P0 to P5, P3 to P6 and P3 to P8) were similar. Only data and images from the apical turn of the P3 to P4 treated mice are shown since effects in the apical turn were more pronounced. Confocal images of cochlear whole mounts stained with antibodies to the presynaptic marker, RIBEYE, are shown from the apical turn for saline-injected WT, and Pit1dw mice TH-treated from P3 to P4, in Figure 3A,B. We quantified the RIBEYE puncta and then performed one-way ANOVA with marker count as dependent variable and treatment group as independent factor (F2,12=28.429, p<0.001) (Figure 3C). At P14, TH-injected Pit1dw mice had significantly lower number of RIBEYE puncta per OHC compared to the same genotype animals that were injected with saline (p<0.001 for both apical and mid turns; only apical turn shown). RIBEYE puncta counts in both the apical and mid turns of TH-treated Pit1dw mice at P14 were not significantly different from those in saline-injected WT controls (p=0.93 for apical turn; p=0.73 for mid turn). We concluded that TH treatment from P3 to P4 was sufficient for restoring normal pruning in the OHCs of the apical and mid turns of the cochlea.
Figure 3. Refinement of presynaptic ribbons in Pit1dw is rescued by thyroid hormone (TH) treatment from P3 to P4.
A, B, Projection of confocal sections obtained from the apical turn of cochlear whole mounts stained with RIBEYE (green) at P14 in saline-treated wild type (WT) (A) and in Pit1dw mice treated with TH from P3 to P4 (B). C, Quantification of RIBEYE puncta from the apical turn of the cochlea in saline-treated WT and TH-treated Pit1dw mice showed no significant differences. n≥4 for each group. Results are expressed as a box plot showing the entire data set. The scale bar represents 10 µm. * p<0.05 from Scheffe’s post hoc tests following an ANOVA.
3.4 The impact of prolonged hypothyroidism on afferent pruning
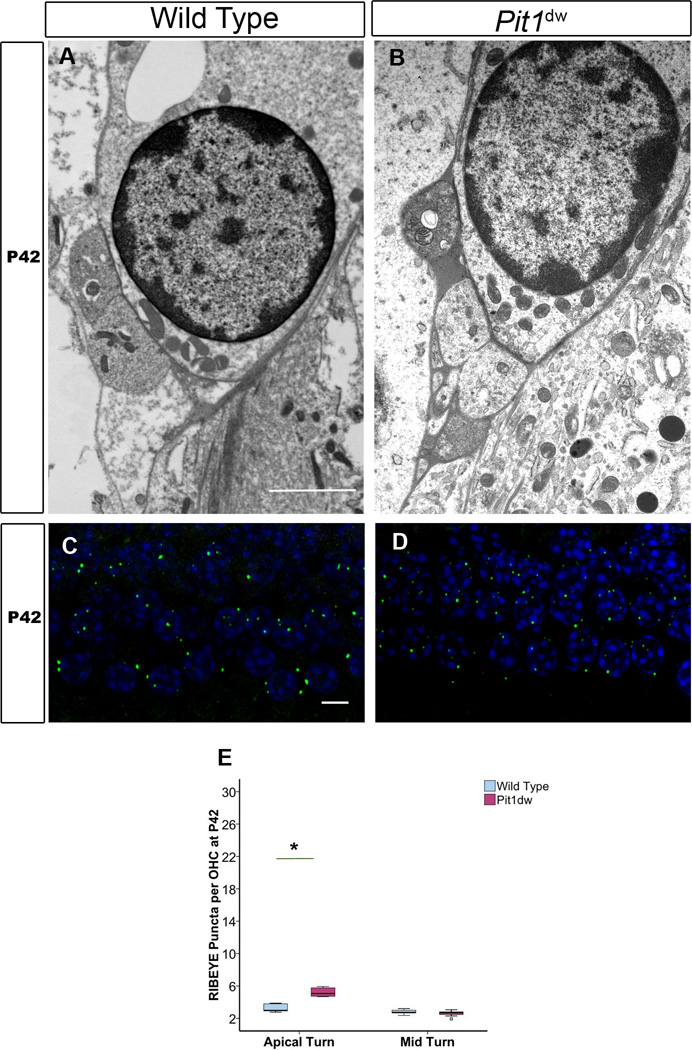
The excess afferent innervation in the apical turn of the Pit1dw mutant mice could either persist with age or prune over period of time if it were due to a simple developmental delay. To test which of these two possibilities, we examined the synapses in Pit1dw mutant OHCs by TEM at P42. A representative image from the mid-basal turn is shown for WT and Pit1dw mutants (Figure 4A,B). Hypothyroid OHCs at P42 retained their immature pattern of innervation, consistent with abnormal persistence of temporary afferent dendrites (Figure 4B). In addition, efferent endings were noted the base of the hypothyroid OHCs where they retained immature axo-dendritic contacts with the abnormally persisting afferents instead of establishing mature axo-somatic connection with OHCs as in WT controls (Figure 4A,B). This observation is consistent with a previous report on hypothyroid rats (Uziel et al., 1983b; Uziel, 1986). Vesicles were noted in the efferent nerve endings indicating these terminals may be functional in the Pit1dw mutants. In order to obtain a more quantitative estimate, we performed immunohistochemistry on a whole mount of the organ of Corti with an anti-RIBEYE antibody to reveal afferent terminals (Figure 4C,D; only apical turn shown). Quantification of RIBEYE counts from apical and mid turns of WT and Pit1dw mice at P42 showed similar numbers in the mid turn but significantly higher numbers in the apical turn for the Pit1dw mice (p<0.001) (Figure 4E). RIBEYE counts in the apical turn of the Pit1dw mutants were not as high at P42 compared to the same genotype at P9 and P14 (Figure 1H), but still indicated that the afferents in this region did not prune completely by P42.
Figure 4. Transmission electron microscopy (TEM) analysis of OHCs at P42 and ribbon quantification.
Representative TEM images from the mid-basal turn of and WT (A) and Pit1dw mutant cochleas at P42 (B). The scale bar is 2 µm. C, D, Projection of confocal sections obtained from the apical turn of cochlear whole mounts stained with RIBEYE from WT (C) and Pit1dw mutants (D). E, Quantification of RIBEYE puncta from whole mounts of WT and Pit1dw mutant cochleas stained with an anti-RIBEYE antibody. Data is represented as a box plot showing the entire data set. The scale bar represents 10 µm. * p<0.05 from Scheffe’s post hoc tests following an ANOVA.
The TEM images represent a smaller number of cells per sample than the RIBEYE immunohistochemistry data and this might account for the discrepancy in the persistent afferent innervation seen with the TEM that is not supported by the quantification of the RIBEYE presynaptic puncta in the mid turn at P42. Another possibility is that presynaptic pruning perhaps preceded postsynaptic removal, as we previously reported for the IHCs (Sundaresan et al., 2015). However, since we did not use a postsynaptic marker (such as GLUT2/3 or SHANK1) in this immunohistochemistry analysis, we cannot rule out the possibility of persistent postsynaptic afferent innervation that might correlate with the TEM data.
3.5 Effect of abnormal afferent fiber persistence on efferent connectivity
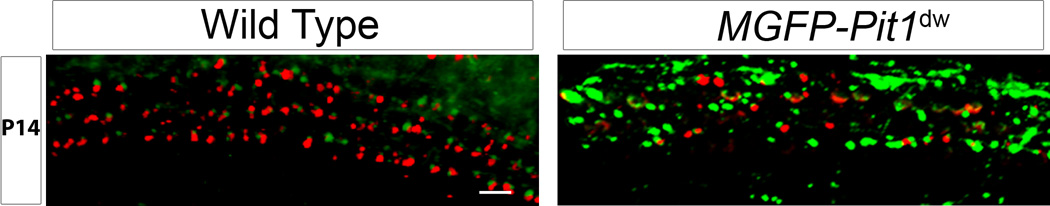
To further examine the immature pattern of efferent connectivity to the Pit1dw mutant OHCs observed using TEM (Figure 4B) and determine whether it correlated with the abnormal persistence of afferent fibers, we performed immunohistochemistry on cochlear whole mounts for the presynaptic efferent marker, synaptophysin. For this study, Mafb-GFP (MGFP) mice crossed with the Pit1dw strain (MGFP-Pit1dw) were examined for the expression pattern of the efferent marker, synaptophysin (Knipper et al., 1995), and compared with the control MGFP strain. Mafb is a transcription factor expressed in both types of neurons and therefore, both type I and type II SGNs and their fibers/terminals are labeled green with GFP (Lu et al., 2011; Yu et al., 2013). At P30, the controls showed a greater number of synaptophysin positive puncta with fewer afferent (GFP-positive) puncta whereas the reverse was observed in age-matched MGFP-Pit1dw cochleae (apical turn, Figure 5A,B). Lower synaptophysin expression correlated with an abnormal retention of afferent innervation in the OHCs of the apical turn in Pit1dw mice (Figure 1B).
Figure 5. Low efferent marker synaptophysin expression in Pit1dw OHCs.
Representative images from apical turn sections showing immunohistochemistry for efferent marker, synaptophysin (red), and type I and type II SGNs (green) from Mafb-GFP (MGFP) mice (A) and hypothyroid MGFP-Pit1dw mice (B).
To get a more quantitative estimate of the ratio of efferent to afferent puncta in these mice, we counted the number of synaptophysin-positive and RIBEYE-positive puncta in the OHCs of Pit1dw and WT mice at P30. As mentioned earlier, we were unable to obtain distinct punctate staining with afferent postsynaptic markers beyond P5, and therefore considered the presynaptic ribbons as representing the afferent synapse. We found that Pit1dw mice had 87% lower numbers of synaptophysin puncta (8.16±1.47 per 30 OHCs; mean±SD) compared to WT mice (62.83±3.65 per 30 OHCs; mean±SD) in the apical turn, indicating greatly reduced efferent innervation to the OHCs in the Pit1dw mice (p<0.001, Student’s t test, n=6 both genotypes). In addition, the ratio of RIBEYE presynaptic puncta to synaptophysin puncta was 25.7 to 1 in Pit1dw mice, and 1.14 to 1 in WT mice, in the apical turn (p<0.001, Student’s t test). These results show that the retention of afferent contacts in the Pit1dw mice correlated with their abnormally low efferent numbers. Knipper and colleagues reported normal efferent innervation to the OHCs in a model of drug-induced perinatal hypothyroidism (from embryonic day 17 to P9) using synaptophysin for an efferent marker (Knipper et al., 2000). Our results suggest that prolonged absence of TH beyond this perinatal period disrupts normal afferent and efferent innervation of the OHCs.
It is possible that the persistence of the afferent connections to the OHCs prevents incoming efferent fibers from making contacts with their target hair cells. On the other hand, the transient efferent to afferent axo-dendritic connection may be involved in afferent elimination during development. In addition to the actions of the efferent input, OHCs may themselves play an important role in establishing mature pattern of innervation. In order to investigate the role of the presynaptic activity of OHCs in the maturation of afferent type II SGN connections, we examined the level and pattern of OTOF expression in the Pit1dw mutants.
3.6 Otoferlin expression persists in the OHCs of hypothyroid mice
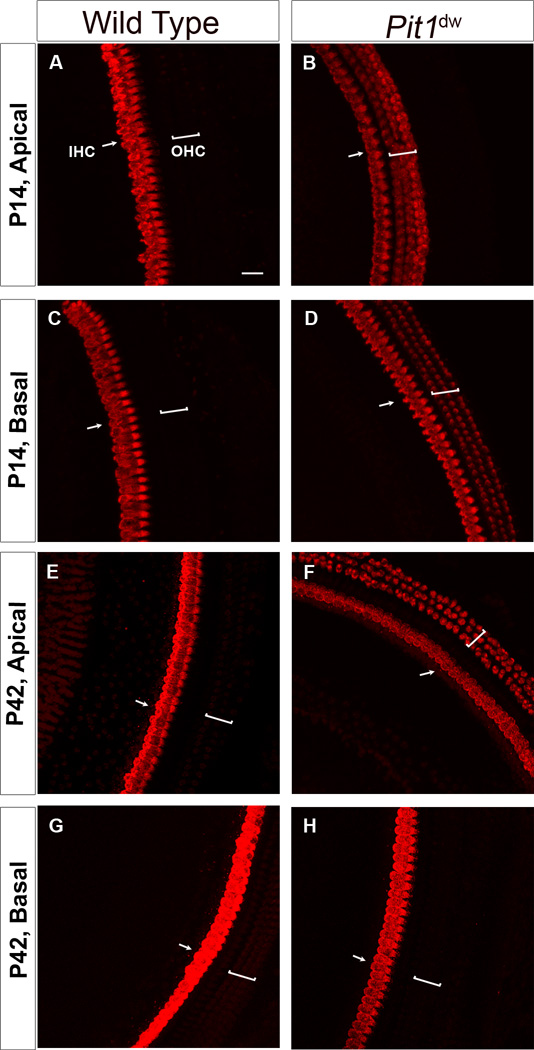
OTOF plays a major role in exocytosis at both the IHC and immature OHC ribbon synapses (Roux et al., 2006; Beurg et al., 2008). In normal mice, OTOF protein level increases in both IHCs and OHCs from E18 to P6. From P6 onward, OTOF immunoreactivity vanishes in most OHCs in parallel with the regression of temporary OHC afferent synaptic contacts (Sobkowicz et al., 1982; Roux et al., 2006; Beurg et al., 2008). We found persistent immature OTOF expression by immunohistochemistry in the OHCs of Pit1dw mutants in all cochlear regions up to P21 (P7, P11, P14, P21, P42 and P90 were analyzed but only P14 and P42 data are shown) (Figure 6A,B,C,D). At P42 and P90, OTOF expression persisted in the apical and upper mid-turn regions of the organ of Corti (Figure 6E,F,G,H). However, OTOF expression was not detectable in the OHCs of the lower mid and basal turns at these adult ages in the Pit1dw mutants. In addition to the protein expression pattern, we looked at OTOF mRNA levels by quantitative RT-PCR using RNA from WT and Pit1dw mutants. Expression of OTOF mRNA was 70% higher in the organ of Corti of mutants as compared to WT (p=0.009 by Student’s t test) (data not shown). This increase in OTOF mRNA levels could account for the higher persistent expression of the protein in the OHCs of Pit1dw mice.
Figure 6. Abnormal persistence of otoferlin expression in adult Pit1dw OHCs.
Projection of confocal sections obtained from immunohistochemistry of cochlear whole mounts with an antibody to the presynaptic vesicle protein, otoferlin, in WT and Pit1dw mutants at P14 and P42. Both apical and basal turns of the cochlea were analyzed. A,B,C,D, apical and basal turns from P14 WT (A,C) and Pit1dw mutants (B,D). E,F,G,H, apical and basal turns from P42 WT (E,G) and Pit1dw mutants (F,H).
4. Discussion
Neuronal death is a common phenomenon in the development of neuronal tissues and is necessary for the formation of a mature neuronal network (Katz and Lasek, 1978; Catsicas et al., 1987; Williams and Herrup, 1988). During cochlear development as well, a significant loss of SGNs occurs between P1 and P7 (Rueda et al., 1987). Studies have shown that 25% of total SGNs lost during this period are of the type II kind (Chiong et al., 1993; Echteler et al., 2005; Barclay et al., 2011). This early postnatal period is also considered a critical phase for TH action on cochlear neurodevelopment (Uziel, 1986; Rusch et al., 2001). Our group has recently shown that TH supplementation in the time window from P3 to P8 is sufficient to restore normal synaptic pruning in the IHCs of the hypothyroid Pit1dw mutant mice (Sundaresan et al., 2015). However, pruning of afferent IHC innervation differs from OHC synaptic pruning since it is caused by retraction of afferent type I neurite branches rather than by the death of type II SGNs. In the current study, we found that TH also regulates type II SGN pruning as demonstrated by persistent attachment of type II SGNs to the OHCs of Pit1dw mutant mice. This OHC afferent synaptic pruning defect was reversible with TH treatment from P3 to P4. In comparison, a longer treatment window (from P3 to P8) was necessary to reverse an afferent pruning defect in the IHCs (Sundaresan et al., 2015).
Our current study shows that hypothyroidism in the Pit1dw mutants somewhat disrupts the programmed death of type II SGNs that occurs normally during cochlear development. This result is consistent with a previous study on propylthiouracil (PTU)-treated rats even though it did not differentiate between the SGN types that are affected by the absence of TH (Rueda et al., 2003). While the focus of this study is on TH regulation of the pruning and maintenance of OHC afferent synapses, it will be interesting to check in future studies whether the retained afferent fibers are functional and whether hypothyroid OHCs can generate an action potential. Previous studies have shown that pre-hearing type II SGNs are excited by ATP, and may be important for sensing painful sound and receiving the medial olivocochlear efferent feedback to the cochlea (Weisz et al., 2009, 2012; Flores et al., 2015; Froud et al., 2015; Liberman and Liberman, 2015). We found that the persistence of type II SGNs was correlated with more afferent and lower efferent innervation to the OHCs in the Pit1dw mutants. It is unclear whether the persistence of the afferent connections to the OHCs impedes incoming efferent fibers from making proper contacts with their target hair cells. Studies, including our current one, have reported that the myelinated medial efferent fibers make a temporary axo-dendritic connection with unmyelinated afferent type II fibers before they make a final axo-somatic connection with the OHCs (Nadol, 1983). It is tempting to speculate that these transient connections to the afferent fibers by the incoming efferent fibers may result in the weakening of afferent synapses and cause subsequent synaptic pruning or axonal retraction. An early study of OHC synapse number during development in a cultured organ of Corti preparation, however, showed that normal afferent pruning, as reflected by the number of the presynaptic ribbons, was achieved even in the absence of any efferent connections, arguing against any efferent role in this process (Sobkowicz et al., 1982).
If the pruning signals are not originating from the incoming efferent fibers, other potential sources for type II SGN pruning signals are either the hair cells, the supporting cells or SGNs themselves. One possibility is that the hair cells need to mature to be able to express the correct cues to attract the incoming efferent fibers and repel the excess afferent connections. The state of maturation of a hair cell can be determined in many ways, including the expression of key functional proteins. Appropriate TH levels at specific developmental time periods were also required for the normal expression of the motor protein, prestin, and ion channels, KCNQ4 and BK in the hair cells (Knipper et al., 2001; Rusch et al., 2001; Winter et al., 2007; Mustapha et al., 2009). Whether the immature hair cell phenotype in hypothyroid animals as observed with disruption in the expression of key functional proteins is the cause or the effect of the lack of afferent refinement is an interesting question. In this study we examined whether afferent pruning correlated with the expression of OTOF under hypothyroid conditions. In our experiments, we found that OTOF expression persisted in the OHCs of all cochlear turns in Pit1dw mutant mice at P14 while it was not detectable in age-matched WT controls. The persistence of OTOF expression in the apical and upper mid turn OHCs at later ages (P42 and P90) in the Pit1dw mutants, therefore, may reflect an immature phenotype. A persistence of OTOF expression was also observed by our group studying a similar hypothyroid mouse model, Prop1df/df (Fang et al., 2012) but not in other hypothyroid reported models (Brandt et al., 2007; Sendin et al., 2007). This current study is a more comprehensive look, however, at the correlation between persistence of OTOF expression and afferent innervation in the OHCs. Afferent synapses in mutant OHCs even in the apical turn did prune somewhat over a longer period of time as indicated by lower ribbon synaptic puncta at P42 in the Pit1dw mice, even though OTOF expression persisted at that age. We also looked at OTOF expression in the Pit1dw mice at P14 after TH supplementation from P3-P4, and found that levels were unchanged in the mid turn compared to untreated littermates (Table 1). Data on presynaptic marker quantification, and OTOF expression detection in the OHC are compiled in Table 1 for a number of developmental stages for both WT and Pit1dw mice for easy comparison. Since afferent pruning to the OHCs was restored by TH replacement in the Pit1dw mice, we propose that the state of maturity of the hair cell, as determined by OTOF expression, may not regulate the process of afferent pruning. While additional markers or measures of OHC functional maturation are needed to confirm this theory, we recently reported similar observations with regard to IHC maturation and afferent synapse pruning (Sundaresan et al., 2015).
Table 1.
Summary of otoferlin expression and quantification of afferent presynaptic marker RIBEYE (mean±SD per OHC) in Wild Type (WT) and Pit1dw mice.
| Age | P5 | P9 | P14 | P42 | P14 (+TH) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Marker/Region | A | M | A | M | A | M | A | M | A | M |
| Otoferlin (WT) | + | + | − | − | − | − | − | − | NA | NA |
| Otoferlin (Pit1dw) | + | + | + | + | + | + | + | − | + | + |
| RIBEYE (WT) | 13.4 ± 2.8 | 23 ± 3.3 | 9.9 ± 1.6 | 3.0 ± 1.4 | 3.3 ± 1.0 | 2.6 ± 0.6 | 3.2 ± 0.4 | 2.8 ± 0.2 | NA | NA |
| RIBEYE (Pit1dw) | 16.4 ± 1.6 | 19.3 ± 1.2 | 14.0 ± 1.0 | 12.6 ± 2.6 | 12.8 ± 2.3 | 6.2 ± 1.6 | 5.2 ± 0.5 | 2.6 ± 0.3 | 3.3 ± 0.6 | 1.9 ± 0.4 |
Presence of otoferlin immunoreactivity, and quantification of presynaptic marker, RIBEYE, is shown for P5, P9, P14, P42, for both WT and Pit1dw mice, and at P14 following TH-treatment from P3-P4 for Pit1dw mice. Data from the apical and mid turns of the cochlea are shown. + : Present; - : Absent; A : Apical Turn; M : Mid Turn; TH : Thyroid Hormone (P3-P4); NA : Not Applicable
It is possible that supporting cells and or/ type II SGNs may express genes, directly or indirectly regulated by TH, that are involved in the pruning of type II SGNs and/or in guiding efferent fibers innervating the OHCs. For example, guidance molecules expressed in the cochlea such as the class 3 Semaphorins and Plexins, their receptors (Katayama et al., 2013; Coate et al., 2015), the Eph/ephrin signaling molecules (Defourny et al., 2013; Cramer and Gabriele, 2014), neurotrophins such as the brain-derived neurotrophic factor, BDNF, and neurotrophin 3 (Ntf3) (Fariñas et al., 2001; Schimmang et al., 2003; Fritzsch et al., 2005), and transcription factors such as Prox1 (Fritzsch et al., 2010), are all implicated in regulation of spiral ganglion outgrowth or attachment to hair cell targets to achieve normal neural patterning in the cochlea. It is unclear whether TH regulates these genes. In our hands, BDNF and Ntf3 mRNA levels were similar in control and hypothyroid cochleae (data not shown). However, TH may post-transcriptionally regulate these genes, as we have observed in the case of glial glutamate transporter (GLAST) levels in Pit1dw mice (Sundaresan et al., 2015). The expression profiling of candidate genes in supporting cells and SGNs under normal and hypothyroid conditions, or transcriptome analysis of cell-specific TH deficiency may aid in identifying target genes in these cells that are regulated by TH (Dettling et al., 2014). Our current data suggest that while TH is required for normal initiation of synaptic refinement, there maybe one or more other unknown components that also act in conjunction with TH in these events. In the absence of TH, perhaps one or more of these other co-factors enables some of the pruning or death of type II SGNs to occur, albeit in a delayed manner. We observed a similar delayed pruning of type I SGNs innervating the IHCs in the same Pit1dw mouse model (Sundaresan et al., 2015). Peeters and coworkers also recently proposed the idea of other components acting in conjunction with TH in the programmed death of the germinal epithelial ridge in the developing inner ear (Peeters et al., 2015). From our studies, there is sufficient evidence to conclude that TH regulates both the small-scale pruning involving the retraction of neurite branches as in the case of the type I SGNs innervating the IHCs (Sundaresan et al., 2015), as well as the death of type II SGNs on a larger scale (this study). These data point to a global role for TH in synaptic pruning in the cochlea, and also raise possibilities to consider with respect to the action of TH on neuronal maturation in other organs.
5. Conclusions
This study provides important insights into the role of thyroid hormone in the development and maturation of type II spiral ganglion neurons that primarily innervate the mature outer hair cells. Hypothyroid mice retained excess afferent connections to the outer hair cells that are normally pruned away by the second postnatal week during the process of synaptic refinement. Tracing type II SGN fibers specifically using transgenic Peripherin-GFP mice, we have shown that type II fibers were retained in the hypothyroid OHCs. Hypothyroid mice also retained OTOF expression up to P42 in the apical and mid turns of their cochleae. TH supplementation from P3 to P4 restored normal afferent connectivity to OHCs in Pit1dw mice. This did not, however, correct the persistent OTOF expression in the OHCs of these mice. Additionally, we also found that efferent connections to the OHCs were disrupted in the Pit1dw mice. The above data together indicate that thyroid hormone is required for the normal innervation of outer hair cells.
Highlights.
Hypothyroid mice retain excess afferent innervation to outer hair cells
Excess innervation is made up of type II spiral ganglion neurons
Otoferlin expression persists in the outer hair cells of hypothyroid mice
Thyroid hormone given from postnatal day 3-4 restores normal afferent innervation
Afferent pruning may not be regulated by the state of hair cell maturation
Acknowledgements
This work was supported by research and core grants from the National Institute in Deafness and other Communicative Disorders: R01 DC09590 (Dr. Mirna Mustapha), P30 DC010363 (Dr. Stefan Heller, Stanford University). The authors sincerely thank Dr. Srikantan Nagarajan for advice on statistics.
Abbreviations
- ANOVA
Analysis of variance
- GFP
Green fluorescent protein
- GLAST
Glial glutamate transporter
- GLUTR
Glutamate receptor
- IHC
Inner hair cell
- OHC
Outer hair cell
- OTOF
Otoferlin
- PBS
Phosphate-buffered saline
- RT-qPCR
Reverse transcriptase-quantitative polymerase chain reaction
- SGN
Spiral ganglion neuron
- TH
Thyroid hormone
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Barclay M, Ryan AF, Housley GD. Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development. [Accessed March 27, 2014];Neural Dev. 2011 6:33. doi: 10.1186/1749-8104-6-33. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3207869&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. [Accessed July 22, 2015];J Neurosci. 2008 28:1798–1803. doi: 10.1523/JNEUROSCI.4653-07.2008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18287496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Münkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. [Accessed March 21, 2014];J Neurosci. 2007 27:3174–3186. doi: 10.1523/JNEUROSCI.3965-06.2007. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17376979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC. Morphology of Labeled Afferent Fibers in the Guinea Pig Cochlea. 1987;604:591–604. doi: 10.1002/cne.902600411. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci U S A. 2000;97:1287–1292. doi: 10.1073/pnas.97.3.1287. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10655523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas S, Thanos S, Clarke PG. Major role for neuronal death during brain development: refinement of topographical connections. Proc Natl Acad Sci U S A. 1987;84:8165–8168. doi: 10.1073/pnas.84.22.8165. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=299499&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong CM, Burgess BJ, Nadol JB. Postnatal maturation of human spiral ganglion cells: light and electron microscopic observations. Hear Res. 1993;67:211–219. doi: 10.1016/0378-5955(93)90249-z. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8340274. [DOI] [PubMed] [Google Scholar]
- Coate TM, Spita NA, Zhang KD, Isgrig KT, Kelley MW. Neuropilin-2/Semaphorin-3F–mediated repulsion promotes inner hair cell innervation by spiral ganglion neurons. [Accessed August 31, 2015];Elife. 2015 :4. doi: 10.7554/eLife.07830. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26302206. [DOI] [PMC free article] [PubMed]
- Cramer KS, Gabriele ML. Axon guidance in the auditory system: multiple functions of Eph receptors. [Accessed August 24, 2015];Neuroscience. 2014 277:152–162. doi: 10.1016/j.neuroscience.2014.06.068. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defourny J, Poirrier A-L, Lallemend F, Mateo Sánchez S, Neef J, Vanderhaeghen P, Soriano E, Peuckert C, Kullander K, Fritzsch B, Nguyen L, Moonen G, Moser T, Malgrange B. Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. [Accessed August 24, 2015];Nat Commun. 2013 4:1438. doi: 10.1038/ncomms2445. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23385583. [DOI] [PubMed] [Google Scholar]
- Deol MS. Congenital deafness and hypothyroidism. Lancet. 1973;2:105–106. doi: 10.1016/s0140-6736(73)93310-2. [DOI] [PubMed] [Google Scholar]
- Dettling J, Franz C, Zimmermann U, Lee SC, Bress A, Brandt N, Feil R, Pfister M, Engel J, Flamant F, Rüttiger L, Knipper M. Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells. [Accessed September 3, 2015];Mol Cell Endocrinol. 2014 382:26–37. doi: 10.1016/j.mce.2013.08.025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24012852. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci U S A. 1992;89:6324–6327. doi: 10.1073/pnas.89.14.6324. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=49493&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echteler SM, Magardino T, Rontal M. Spatiotemporal patterns of neuronal programmed cell death during postnatal development of the gerbil cochlea. [Accessed July 22, 2015];Brain Res Dev Brain Res. 2005 157:192–200. doi: 10.1016/j.devbrainres.2005.04.004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15939482. [DOI] [PubMed] [Google Scholar]
- Fang Q, Giordimaina AM, Dolan DF, Camper Sa, Mustapha M. Genetic background of Prop1(df) mutants provides remarkable protection against hypothyroidism-induced hearing impairment. [Accessed April 2, 2014];J Assoc Res Otolaryngol. 2012 13:173–184. doi: 10.1007/s10162-011-0302-3. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3298611&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. [Accessed August 26, 2015];J Neurosci. 2001 21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2710117&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Márquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, García-Añoveros J. A Non-canonical Pathway from Cochlea to Brain Signals Tissue-Damaging Noise. Curr Biol. 2015:606–612. doi: 10.1016/j.cub.2015.01.009. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0960982215000111. [DOI] [PMC free article] [PubMed]
- Fritzsch B, Dillard M, Lavado A, Harvey NL, Jahan I. Canal cristae growth and fiber extension to the outer hair cells of the mouse ear require Prox1 activity. [Accessed August 26, 2015];PLoS One. 2010 5:e9377. doi: 10.1371/journal.pone.0009377. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2826422&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. [Accessed August 26, 2015];Hear Res. 2005 206:52–63. doi: 10.1016/j.heares.2004.11.025. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3904737&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froud KE, Wong ACY, Cederholm JME, Klugmann M, Sandow SL, Julien J-P, Ryan AF, Housley GD. Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. [Accessed August 10, 2015];Nat Commun. 2015 6:7115. doi: 10.1038/ncomms8115. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4432632&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L-C, Barclay M, Lee K, Peter S, Housley GD, Thorne PR, Montgomery JM. Synaptic profiles during neurite extension, refinement and retraction in the developing cochlea. Neural Dev. 2012;7:38. doi: 10.1186/1749-8104-7-38. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3545844&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolyi IJ, Dootz GA, Halsey K, Beyer L, Probst FJ, Johnson KR, Parlow AF, Raphael Y, Dolan DF, Camper SA. Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm Genome. 2007;18:596–608. doi: 10.1007/s00335-007-9038-0. [DOI] [PubMed] [Google Scholar]
- Katayama K, Imai F, Suto F, Yoshida Y. Deletion of Sema3a or plexinA1/plexinA3 causes defects in sensory afferent projections of statoacoustic ganglion neurons. [Accessed August 24, 2015];PLoS One. 2013 8:e72512. doi: 10.1371/journal.pone.0072512. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3753268&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Lasek RJ. Evolution of the nervous system: Role of ontogenetic mechanisms in the evolution of matching populations. Proc Natl Acad Sci U S A. 1978;75:1349–1352. doi: 10.1073/pnas.75.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, et al. Thyroid Hormone Deficiency Before the Onset of Hearing Causes Irreversible Damage to Peripheral and Central Auditory Systems. J Neurophysiol. 2000;83:3101–3112. doi: 10.1152/jn.2000.83.5.3101. [DOI] [PubMed] [Google Scholar]
- Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- Knipper M, Richardson G, Mack A, Muller M, Goodyear R, Limberger A, Rohbock K, Kopschall I, Zenner HP, Zimmermann U. Thyroid hormone-deficient period prior to the onset of hearing is associated with reduced levels of beta-tectorin protein in the tectorial membrane: implication for hearing loss. J Biol Chem. 2001;276:39046–39052. doi: 10.1074/jbc.M103385200. [DOI] [PubMed] [Google Scholar]
- Knipper M, Zimmermann U, Rohbock K, Köpschall I, Zenner HP. Synaptophysin and GAP-43 proteins in efferent fibers of the inner ear during postnatal development. [Accessed August 24, 2015];Brain Res Dev Brain Res. 1995 89:73–86. doi: 10.1016/0165-3806(95)00113-r. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8575095. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. [Accessed April 24, 2015];J Assoc Res Otolaryngol. 2015 16:205–219. doi: 10.1007/s10162-015-0510-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25676132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers int he cat cochlea: an electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Lu CC, Appler JM, Houseman EA, Goodrich LV. Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. [Accessed March 27, 2014];J Neurosci. 2011 31:10903–10918. doi: 10.1523/JNEUROSCI.2358-11.2011. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3167573&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLenachan S, Goldshmit Y, Fowler KJ, Voullaire L, Holloway TP, Turnley AM, Ioannou PA, Sarsero JP. Transgenic mice expressing the Peripherin-EGFP genomic reporter display intrinsic peripheral nervous system fluorescence. [Accessed September 16, 2015];Transgenic Res. 2008 17:1103–1116. doi: 10.1007/s11248-008-9210-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18709437. [DOI] [PubMed] [Google Scholar]
- Mendus D, Sundaresan S, Grillet N, Wangsawihardja F, Leu R, Müller U, Jones SM, Mustapha M. Thrombospondins 1 and 2 are important for afferent synapse formation and function in the inner ear. [Accessed April 2, 2014];Eur J Neurosci. 2014 :1–12. doi: 10.1111/ejn.12486. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24460873. [DOI] [PMC free article] [PubMed]
- Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci. 2009;29:1212–1223. doi: 10.1523/JNEUROSCI.4957-08.2009. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19176829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB. Serial section reconstruction of the neural poles of hair cells in the human organ of Corti. II. outer hair cells. [Accessed September 10, 2015];Laryngoscope. 1983 93:780–791. doi: 10.1288/00005537-198306000-00015. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6855401. [DOI] [PubMed] [Google Scholar]
- Peeters RP, Ng L, Ma M, Forrest D. The timecourse of apoptotic cell death during postnatal remodeling of the mouse cochlea and its premature onset by triiodothyronine (T3) [Accessed August 27, 2015];Mol Cell Endocrinol. 2015 407:1–8. doi: 10.1016/j.mce.2015.02.025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25737207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler M-C, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. [Accessed March 27, 2014];Cell. 2006 127:277–289. doi: 10.1016/j.cell.2006.08.040. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17055430. [DOI] [PubMed] [Google Scholar]
- Rueda J, de la Sen C, Juiz JM, Merchán Ja. Neuronal loss in the spiral ganglion of young rats. Acta Otolaryngol. 1987;104:417–421. doi: 10.3109/00016488709128269. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3434262. [DOI] [PubMed] [Google Scholar]
- Rueda J, Prieto JJ, Cantos R, Sala ML, Merchan JA. Hypothyroidism prevents developmental neuronal loss during auditory organ development. Neurosci Res. 2003;45:401–408. doi: 10.1016/s0168-0102(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21:9792–9800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11739587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T, Tan J, Müller M, Zimmermann U, Rohbock K, Kôpschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. [Accessed September 10, 2015];Development. 2003 130:4741–4750. doi: 10.1242/dev.00676. Available at: http://dev.biologists.org/content/130/19/4741.long. [DOI] [PubMed] [Google Scholar]
- Sendin G, Bulankina AV, Riedel D, Moser T. Maturation of ribbon synapses in hair cells is driven by thyroid hormone. [Accessed March 27, 2014];J Neurosci. 2007 27:3163–3173. doi: 10.1523/JNEUROSCI.3974-06.2007. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17376978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlin DS, Visser TJ, Forrest D. Developmental and cell-specific expression of thyroid hormone transporters in the mouse cochlea. [Accessed September 12, 2014];Endocrinology. 2011 152:5053–5064. doi: 10.1210/en.2011-1372. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3230046&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci. 1982;2:942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Wang F, Li BM. Adult-onset hypothyroidism impairs paired-pulse facilitation and long-term potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. [Accessed September 12, 2014];Brain Res. 2006 1096:53–60. doi: 10.1016/j.brainres.2006.04.042. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16725120. [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Kong J-H, Fang Q, Salles F, Wangsawihardja F, Ricci AJ, Mustapha M. Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses. [Accessed October 24, 2015];Eur J Neurosci. 2015 doi: 10.1111/ejn.13081. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26386265. [DOI] [PMC free article] [PubMed]
- Uziel A. Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol Suppl. 1986;429:23–27. doi: 10.3109/00016488609122726. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3461670. [DOI] [PubMed] [Google Scholar]
- Uziel A, Legrand C, Ohresser M, Marot M. Maturational and degenerative processes in the organ of Corti after neonatal hypothyroidism. Hear Res. 1983a;11:203–218. doi: 10.1016/0378-5955(83)90079-5. [DOI] [PubMed] [Google Scholar]
- Uziel A, Pujol R, Legrand C, Legrand J. Cochlear synaptogenesis in the hypothyroid rat. Brain Res. 1983b;283:295–301. doi: 10.1016/0165-3806(83)90186-4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6850354. [DOI] [PubMed] [Google Scholar]
- Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. Thyroid hormone action on liver, heart, and energy expenditure in thyroid hormone receptor beta-deficient mice. Endocrinology. 1998;139:4945–4952. doi: 10.1210/endo.139.12.6412. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9832432. [DOI] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. [Accessed June 26, 2014];Nature. 2009 461:1126–1129. doi: 10.1038/nature08487. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2785502&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJC, Lehar M, Hiel H, Glowatzki E, Fuchs PA. Synaptic transfer from outer hair cells to type II afferent fibers in the rat cochlea. [Accessed October 21, 2015];J Neurosci. 2012 32:9528–9536. doi: 10.1523/JNEUROSCI.6194-11.2012. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3433252&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3424722&tool=pmcentrez&rendertype=abstract. [DOI] [PubMed] [Google Scholar]
- Winter H, Braig C, Zimmermann U, Engel J, Rohbock K, Knipper M. Thyroid hormone receptor alpha1 is a critical regulator for the expression of ion channels during final differentiation of outer hair cells. Histochem Cell Biol. 2007;128:65–75. doi: 10.1007/s00418-007-0294-6. [DOI] [PubMed] [Google Scholar]
- Yu W-M, Appler JM, Kim Y-H, Nishitani AM, Holt JR, Goodrich LV. A Gata3-Mafb transcriptional network directs post-synaptic differentiation in synapses specialized for hearing. [Accessed June 26, 2015];Elife. 2013 2:e01341. doi: 10.7554/eLife.01341. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3851837&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]