Abstract
Marijuana is the most commonly used illicit drug in the United States and its use is rising. Nonetheless, scientific efforts to clarify the risk for addiction and other harm associated with marijuana use have been lacking. Maladaptive decision-making is a cardinal feature of addiction that is likely to emerge in heavy users. In particular, distorted subjective reward valuation related to homeostatic or allostatic processes has been implicated for many drugs of abuse. Selective changes in responses to uncertainty have been observed in response to intoxication and deprivation from various drugs of abuse. To assess for these potential neuroadaptive changes in reward valuation associated with marijuana deprivation, we examined the subjective value of uncertain and certain rewards among deprived and non-deprived heavy marijuana users in a behavioral economics decision-making task. Deprived users displayed reduced valuation of uncertain rewards, particularly when these rewards were more objectively valuable. This uncertainty aversion increased with increasing quantity of marijuana use. These results suggest comparable decision-making vulnerability from marijuana use as other drugs of abuse, and highlights targets for intervention.
Keywords: marijuana, decision-making, reward, uncertainty, drug deprivation
Marijuana is the most commonly used illicit drug in the United States and use has been on the rise among young adults in recent years (Substance Abuse and Mental Health Services Administration, 2011). Increasingly relaxed attitudes (e.g., Johnston, O'Malley, Miech, Bachman, & Schulenberg, 2014; Jones & Saad, 2013) and policies regarding marijuana use likely result from the belief that it is less harmful than alcohol and other drugs (Nutt, King, Saulsbury, & Blakemore, 2007). This mostly unsubstantiated belief highlights the need for increased scientific efforts to clarify the risk for addiction and other harmful consequences associated with heavy marijuana use (Volkow, Baler, Compton, & Weiss, 2014).
Maladaptive decision-making is a cardinal feature of drug addiction that results in substantial harm as heavy drug users repeatedly choose to use drugs despite negative consequences (Bechara, 2003; Schultz, 2011), and display suboptimal decision-making with respect to non-drug rewards (Brevers et al., 2014; Coffey, Gudleski, Saladin, & Brady, 2003; Petry, 2001; Whitlow et al., 2004). Theorists suggest these decision-making deficits can result from multiple specific vulnerabilities including both premorbid risk factors and homeostatic and/or allostatic changes that are caused by the repeated use and withdrawal from drugs (Redish, Jensen, & Johnson, 2008; Schultz, 2011). Specifically, decision-making vulnerabilities in the context of reward uncertainty can impair individuals’ ability to accurately predict reward value in advance of its receipt. These vulnerabilities can also produce wide discrepancies between the subjective and objective valuations of drug and other rewards. In particular, changes in reward prediction and subjective valuation of uncertain (e.g., probabilistic or delayed) rewards may impact drug users’ ability to maintain drug abstinence during withdrawal.
Research on decision-making deficits associated with heavy marijuana use continues to lag far behind that of tobacco, alcohol, and other drugs of abuse. In particular, very few studies have examined decision-making during acute marijuana deprivation, a clinically meaningful period in which drug deprived individuals make critical decisions regarding further use and relapse. Moreover, drug deprivation represents a period in which homeostatic and allostatic changes in decision-making resulting from chronic marijuana use may be sensitively identified.
We have recently documented selective, compensatory changes in the response to uncertain stressors associated with drug administration (alcohol: Bradford et al., 2013; Hefner & Curtin, 2012; Hefner et al., 2013; Moberg & Curtin, 2009) and deprivation/abstinence (alcohol: Moberg & Curtin, in preparation; nicotine: Hogle et al., 2010; marijuana: Hefner et al., in preparation). This motivated us to examine decision-making involving uncertain rewards in the present study. Specifically, we report the effects of marijuana deprivation among heavy marijuana users on subjective value of uncertain rewards in a decision-making task (Huettel, Stowe, Gordon, Warner, & Platt, 2006).
Addiction Allostasis: Stressors, Rewards, and Uncertainty
Following drug administration, normal homeostatic processes attempt to stabilize drug-induced stressor and reward system dysregulation. Over time, repeated periods of drug intoxication and subsequent withdrawal contribute to allostasis – a process by which organisms achieve systemic stability through physiological or behavioral change (George, Le Moal, & Koob, 2012; Koob & Le Moal, 2001). Allostatic neuroadaptations alter homeostatic set-points for responding to stressors and rewards and can powerfully influence the heavy drug user's perceived needs (Koob & Le Moal, 2001; Koob & LeMoal, 2008; Redish et al., 2008; Solomon & Corbit, 1974), increasing vulnerability for maladaptive decision-making.
Allostatic stress neuroadaptations have been implicated as a fundamental etiological mechanism in addiction to alcohol, benzodiazepines, opiates, cocaine, nicotine, and marijuana (Breese, Sinha, & Heilig, 2011; Koob & LeMoal, 2008; Shaham & Hope, 2005; Sinha, 2008; Weiss, 2001). These neuroadaptations exaggerate anxiety and other negative affective response to stressors, particularly during brief or extended periods when drug use is stopped and withdrawal symptoms emerge (nicotine: Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Curtin, Mccarthy, Piper, & Baker, 2006). These neuroadaptations also position stressors as potent instigators of relapse for humans (Breese et al., 2011 (alcohol); Sinha, 2007) and animals (Shaham & Hope, 2005; Weiss, 2001). When stressors are unpredictable or otherwise uncertain, their impact on affective response and on decisions regarding appropriate behavioral response increases (Bach & Dolan, 2012; Grupe & Nitschke, 2013; Paulus & Yu, 2012). Accumulating evidence from our laboratory suggests that allostatic stress neuroadaptations resulting from repeated alcohol and nicotine use and withdrawal may specifically target response to uncertain stressors (i.e., threat of uncertain electric shock: alcohol: Bradford, Shapiro, & Curtin, 2013; Hefner & Curtin, 2012; Hefner, Moberg, Hachiya, & Curtin, 2013; Moberg & Curtin, 2009; Moberg & Curtin, in preparation; nicotine: Hogle & Curtin, 2006; Hogle, Kaye, & Curtin, 2010). Preliminary data demonstrate exaggerated response to uncertain stressors among drug-deprived heavy marijuana users (Hefner et al., in preparation). Changes in stressor reactivity following heavy, chronic marijuana use also appear to contribute to subjective craving for drug rewards; these changes also contribute to relapse following treatment for marijuana abuse (Fox, Tuit, & Sinha, 2012).
Allostatic neuroadaptations following repeated drug use and withdrawal also directly target reward mechanisms (George et al., 2012). These reward neuroadaptations can effectively alter the subjective value the drug user assigns to available rewards (Redish et al., 2008; Robinson & Berridge, 2003) and motivational salience of drugs (Kalivas & Volkow, 2005), influencing decisions about which rewards to pursue (Mizumori & Jo, 2013). Both the pharmacologic high and the relief from aversive withdrawal symptoms represent relatively certain rewards following drug administration among the landscape of other potentially available but more distal and/or uncertain non-drug rewards (e.g., education, career, improved relationships) in the heavy drug user's life. If allostatic neuroadaptations following repeated drug use decrease the subjective valuation of uncertain rewards, the drug user may be increasingly biased to pursue the more certain rewards from drug use rather than other possibilities that may be objectively more valuable (Berridge & Aldridge, 2008). Neuroadaptations biasing individuals against uncertain rewards may be most pronounced during acute deprivation due to increased negative affect, relief from t which may make the certain rewards from drug use even more valuable. Thus, we directly examined the impact of drug deprivation following heavy marijuana use on the subjective reward value assigned to certain and uncertain rewards.
Reward Decision-Making under Uncertainty
Individuals make decisions to pursue specific rewards based on the subjective value they assign to the array of rewards available to them. The value people assign to rewards is subjective and reward values display intra- and inter-individual differences based on preferences, biases, emotional states, and vulnerabilities (Knutson, Taylor, Kaufman, Peterson, & Glover, 2005; Redish et al., 2008; Tversky & Kahneman, 1974). Decisions regarding which rewards to pursue are often made without complete information regarding the potential outcomes. Uncertainty exists when reward receipt is delayed (temporal uncertainty) (Bickel, Koffarnus, Moody, & Wilson, 2014; Bickel & Marsch, 2001) or when multiple potential reward outcomes are associated with a specific course of action (probabilistic uncertainty) (Shead, Callan, & Hodgins, 2008; Shead & Hodgins, 2009; Yi, Chase, & Bickel, 2007). With probabilistic uncertainty, uncertainty increases when the probability of receiving potential rewards is unknown (Huettel et al., 2006; Platt & Huettel, 2008) and when the variance (difference) between each of the potential rewards is high (Christopoulos, Tobler, Bossaerts, Dolan, & Schultz, 2009; Tobler, Christopoulos, O'Doherty, Dolan, & Schultz, 2009).
People tend to make disadvantageous decisions when uncertainty exists (Tversky & Kahneman, 1974). Much like uncertain stressors are more aversive than certain stressors (Bradford et al., 2013; Grupe & Nitschke, 2013; Hefner & Curtin, 2012), uncertain rewards are less desirable than certain rewards; uncertainty aversion leads humans and animals to subjectively ‘discount’ or devalue uncertain rewards relative to their objective reward value (for review, see: Green & Myerson, 2004)., When choosing between certain and probabilistic (uncertain) rewards, people prefer certain rewards even when the objective value (i.e., probability weighted sum of reward outcomes) of both rewards is identical. The objective value of the uncertain reward must be substantially higher than the certain reward to overcome our innate uncertainty aversion (Huettel et al., 2006; Platt & Huettel, 2008).
Though all individuals make suboptimal decisions under certain circumstances, impaired decision-making is a cardinal feature of addiction, with drug users pursuing drugs despite negative consequences (American Psychological Association, 2013; Redish, 2008). Redish and colleagues (2008) have developed an influential, integrative perspective on addiction etiology that identifies numerous specific vulnerabilities in the decision-making system that contribute to addiction. Although some of these vulnerabilities are pre-morbid risk factors, short term perturbations in homeostasis and persistent allostatic neuroadaptations in reward valuation are proposed to be key etiological mechanisms with high potential to produce harm. In fact, biased or otherwise impaired reward prediction, estimation, and valuation feature prominently in numerous contemporary perspectives on addiction (Bickel et al., 2014; Robinson & Berridge, 2003; Schultz, 2011; Wiers & Stacy, 2006).
Drug users exhibit biased decision-making such that they prefer certain rewards relative to uncertain rewards at greater rates than the general population, even when pursuing these certain rewards is disadvantageous over the long-term (Bickel & Marsch, 2001; Bickel, Odum, & Madden, 1999; Brevers et al., 2014; Johnson, Bickel, & Baker, 2007; Platt, Watson, Hayden, Shepherd, & Klein, 2010). This uncertainty aversion has been most clearly observed as “delay discounting” associated with temporally uncertain, delayed drug rewards including cigarettes (Bickel, Odum, & Madden, 1999; Johnson, Bickel, & Baker, 2007), opioids (Madden, Petry, Badger, & Bickel, 1997), cocaine (Coffey, Gudleski, Saladin, & Brady, 2003), and alcohol (Goudriaan, Grekin, & Sher, 2007; Petry, 2001). Furthermore, drug deprivation has been shown to increase delay discounting of monetary and drug rewards.
As noted earlier, decision-making deficits among heavy marijuana users have not been as thoroughly investigated as other drugs. That which exists has primarily focused on differences between heavy marijuana users and non-users, making it difficult to determine if observed differences result from pre-morbid risk factors or consequences of repeated marijuana use (Bechara, Damasio, Damasio, & Anderson, 1994; Hermann et al., 2009; Johnson et al., 2010; Wesley, Hanlon, & Porrino, 2011; Whitlow et al., 2004). Experimental manipulations of marijuana deprivation in heavy marijuana users are needed to test for allostatic neuroadaptations in reward valuation and decision-making. Preliminary evidence indicates that neural processing is altered during reward anticipation among one week abstinent marijuana users (van Hell et al., 2010). This may have important implications for relapse among abstinence seekers, ongoing decisions to use marijuana, and/or the pursuit of alternative non-drug rewards among marijuana users. If robust changes in subjective reward valuation and impaired decision-making result from heavy marijuana use, the potential for societal harm may be high and likely to grow as use escalates with changing attitudes and legal landscape.
The Present Study
We examined the effects of acute (3 days) marijuana deprivation on heavy marijuana users’ subjective reward valuation of certain vs. uncertain monetary rewards in a modified version of a decision-making task developed by Huettel and colleagues (2006). We recruited a sample of heavy users given the likelihood of neuroadaptive changes in these users. We chose to examine subjective valuation of monetary rewards for ease of administration, calculation of objective (vs. subjective) value and the correspondingly robust literature on decision-making regarding these rewards (Bickel & Marsch, 2001; Richards, Zhang, Mitchell, & de-Wit, 1999; Tobler, Christopoulos, O'Doherty, Dolan, & Schultz, 2009). Further, similar distortions in subjective reward value of monetary and drug rewards manifest during drug deprivation (Field, Santarcangelo, Sumnall, Goudie, & Cole, 2006; Giordano et al., 2002; Yi & Landes, 2012).
On each trial, participants chose whether they would prefer to receive a single pre-specified monetary value (i.e., the “certain reward”) or one of two different monetary values (i.e., the “uncertain reward”) whose probability of receipt was either known (50%-50%) or unknown. Across trials, we manipulated the values of the monetary rewards to vary the objective utility of the uncertain reward (i.e., the probability weighted sum of the two monetary values that comprised the uncertain reward minus the monetary value of the certain reward). We also manipulated the uncertain reward variance (i.e. discrepancy between the two monetary values of the uncertain reward).
Following Huettel and colleagues (2006), we expected all participants to display uncertainty aversion and prefer the certain reward (except when objective utility of the uncertain reward was substantially higher). We expected uncertainty aversion to be greater on unknown (relative to known) probability and high (relative to low) uncertain reward variance trials because these trials involve more uncertainty. Most importantly, we predicted that deprived marijuana users would display greater uncertainty aversion than non-deprived users, particularly at higher levels of uncertain reward utility when the preference for the uncertain reward would otherwise be increased. We examined interactions between Deprivation Group and Uncertainty Type (known vs. unknown probability) and Uncertain Reward Variance (low vs. high) to determine if the predicted deprivation effect on uncertainty aversion was robust across reward characteristics and contexts or alternatively, observed selectively under specific parameters.
Method
Participants
One hundred four heavy marijuana users (i.e., reporting marijuana use ≥5 days per week, ≥twice per day on days when used, for ≥6 months) were recruited from the greater Madison, WI area via flyer and online advertisements. Participants with current diagnoses of alcohol or other drug dependence (other than marijuana dependence)1 or reporting use of psychotropic medication(s), engagement in psychological treatment (within the last 6 months) or current or past diagnosis of psychotic disorders were excluded from the study. The sample included equal numbers of men and women with a mean age of 22.2 years (SD =1.0, range = 18 – 34). We provide additional detail about the sample characteristics in Table 1.
Table 1.
Participant Characteristics by Deprivation Group
| Deprived | Non-Deprived | p | |
|---|---|---|---|
| Age | 22.3 (3.6) | 22.1 (3.3) | .756 |
| Sex | 1.000 | ||
| Male | 50.0% | 50.0% | |
| Female | 50.0% | 50.0% | |
| Marijuana Use Disorder Diagnosis (current)a | .204 | ||
| No diagnosis | 25.0% | 11.5% | |
| Marijuana abuse | 25.0% | 30.8% | |
| Marijuana dependence | 50.0% | 57.7% | |
| Marijuana Grams Used per Week | 7.2 (7.8) | 7.0 (6.8) | .866 |
| Marijuana Withdrawal Checklist (screening session; α=0.50) | 3.2 (2.4) | 3.0 (2.7) | .704 |
| Marijuana Craving Questionnaire (screening session; α=0.79) | 71.7 (14.5) | 74.8 (13.5) | .260 |
| Creatinine-Normalized THC (screening session) | 427.1 (558.0) | 284.4 (285.0) | .103 |
| Substance Abuse Diagnosis (current; other than marijuana)a,b | 11.5% | 7.7% | .739 |
| Alcohol Abuse Diagnosis (current)a,b | 23.1% | 34.6% | .279 |
| Young Adult Alcohol Problems Screening Test (past year; α=0.88) | 4.4 (3.0) | 5.3 (3.9) | .168 |
| Alcoholic Drinks per Week | 10.1 (10.7) | 9.8 (12.6) | .890 |
| Depression Anxiety Stress Scales (past month) | |||
| Anxiety (α=0.66) | 6.9 (5.2) | 6.8 (5.8) | .943 |
| Depression (α=.82) | 6.2 (5.8) | 6.7 (7.1) | .694 |
| Stress (α=0.79) | 10.4 (7.4) | 9.7 (6.9) | .623 |
NOTES:
N=104; Table entries are mean (standard deviation) unless otherwise indicated, We also report Cronbach's alpha (α) internal consistency reliability for self-report measures with multiple items.
Diagnoses established by interview with Mini-International Neuropsychiatric Interview
Potential participants were excluded for current diagnosis of alcohol or substance (other than marijuana) dependence
General Procedure
Preliminary screening was accomplished when prospective participants called the laboratory to indicate interest in the study. Prospective participants were informed on the phone about the protections of the NIH Certificate of Confidentiality associated with this study, and provided verbal consent to assess their marijuana use and medical history. Those meeting preliminary eligibility criteria during the phone contact were scheduled for a subsequent formal screening session in our laboratory. During this screening session, all participants provided informed consent and were reminded about the protections of the study's NIH Certificate of Confidentiality. A clinician verified inclusion/exclusion criteria by administering a standardized structured interview to assess their medical history, past and current drug use history (using the alcohol and drug use disorders components of the Mini-International Neuropsychiatric Interview adapted to assess past use; Sheehan et al., 1998), and marijuana use patterns. Marijuana use was assessed during a semi-structured interview with a clinician in which participants were queried regarding how often they purchased marijuana, in what amounts, how often they used with others, etc. in order to obtain a personal approximate weekly quantity of use. Participants also provided a urine sample to provide biological verification of their marijuana use.
Marijuana users were randomly assigned to one of two Deprivation Groups (deprived or non-deprived) in this screening session. Participants assigned to the deprived group were instructed to abstain from any use of marijuana (e.g., smoked, ingested, etc.) for three days prior to their experimental session (described below). Those assigned to the non-deprived group were instructed to maintain their typical frequency and quantity of marijuana use. However, they were instructed to refrain from smoking marijuana for at least one hour before the experimental session to avoid acute intoxication effects in that session. All participants were asked to avoid using alcohol or other recreational drugs for 24 hours prior to their experimental session. Those reporting use of nicotine were asked to continue their usual quantity and frequency of use. Experimental sessions were scheduled to occur within 4 – 10 days of the screening session.
On arrival for the experimental session, all participants provided a second urine sample and reported the date/time of their last marijuana use and any recent (i.e., past 24 hour) alcohol or other (one week) drug use. Breath alcohol concentration (BAC) was also assessed in all participants. Deprived participants who were non-compliant with abstinence instructions based on this urine drug test or their self-report were dismissed and given the opportunity to reschedule once. Any participant indicating non-compliance with the alcohol and/or other drug use requirements was dismissed.2
Participants next completed the Reward Uncertainty Decision-Making Task (described below). Following task completion, participants were debriefed, compensated for their participation, and dismissed. Participants were paid $30/hour for total time spent in the laboratory (typically between 2-3 hours). They also received money based their choice associated with one randomly selected trial from the decision making task (range: $4-56). Deprived users were mailed an additional $200 for compliance with the deprivation procedure following confirmation of abstinence via quantitative urinalysis (described below).
Reward Uncertainty Decision-Making Task
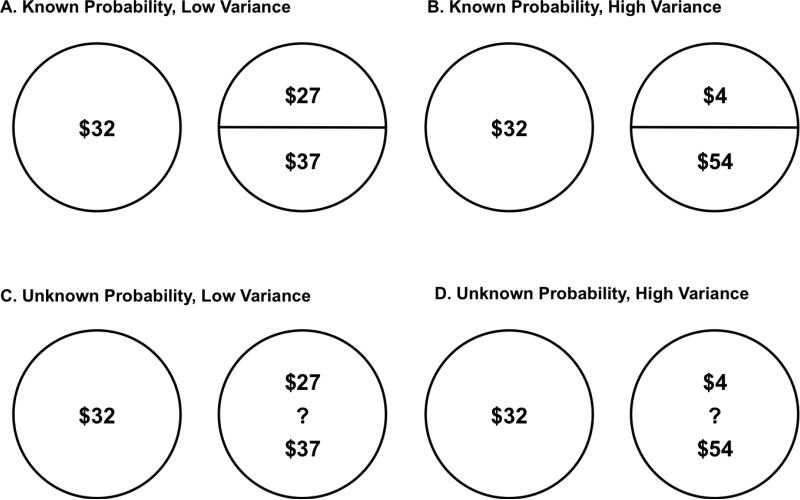
The Reward Uncertainty Decision-Making Task was a modified version of a task developed by Huettel and colleagues (2006). The task included 144 trials where participants were presented simultaneously with “certain” and “uncertain” monetary rewards, indicated by two adjacent circles on a computer monitor. Certain rewards contained a single monetary value presented in the center of a circle. Uncertain rewards contained two monetary values presented vertically within the circle. Exemplar trials are displayed in Figure 1. Participants pressed one of two buttons to indicate whether they preferred to receive the certain or uncertain reward on each trial. If participants chose the certain reward, they were instructed that they could receive the monetary value depicted within that circle at the end of the experiment. If participants chose the uncertain reward, they were instructed that they could receive one of the two monetary values shown in that circle at the end of the experiment. They were instructed that the task was not a math test, there were no right or wrong answers, and that they should decide between the certain and uncertain reward based simply on their personal preference. To motivate task engagement, participants were informed that at the end of the task the computer would randomly select one trial and they would receive the money associated with their decision on that trial as a task performance bonus. Participants were required to respond within 4 s on each trial. Computer feedback was provided on any trial where response time was slower than this cut-off and participants were told that if the computer randomly selected such a trial at the end of the task, they would receive no bonus money. Following instructions and questions, participants completed a series of practice trials to confirm that they understood the task. The task required approximately 20 minutes to complete.
Figure 1.
Participants were presented with trials displaying certain and uncertain rewards. Uncertain rewards varied by Uncertain Reward Variance (low vs. high) Uncertainty Type (known vs. unknown probability). (A) displays trial with low uncertain reward variance ($10 difference between two values displayed in the uncertain reward). (B) displays a known probability trial with high uncertain reward variance ($50). (C) and (D) display unknown probability trials with low ($10) and high ($50) uncertain reward variance, respectively.
The objective utility of the uncertain reward (relative to the certain reward) varied across trials. This Uncertain Reward Utility is defined as the probability weighted sum of the two monetary values that comprised the uncertain reward minus the monetary value of the certain reward. Uncertain Reward Utility can be expressed mathematically as ((0.5 × low uncertain reward value) + (0.5 × high uncertain reward value)) – certain reward value, given that the probability of receiving each of the two values that comprised the uncertain reward was 0.5. We varied the utility of the uncertain reward across 9 ordinal levels (−4 to 4) that represented a range of uncertain and certain reward values where it was objectively advantageous (positive utilities) or disadvantageous (negative utilities) to choose the uncertain reward. An uncertain reward utility of 0 represents a balance point where the expected monetary reward for the uncertain and certain rewards was equal. As described earlier, individuals exhibit a general bias against uncertainty (uncertainty aversion), which was expected to display as reduced subjective preference for the uncertain reward until its objective utility became markedly greater than 0.
Across trials, we manipulated two independent variables that were expected to affect the probability of preferring the uncertain reward. The Uncertain Reward Variance, defined as the difference between the value of the high and low monetary values that comprised the uncertain reward, was either low ($10 difference; e.g., $25 and $35) or high ($50 difference; e.g., $5 and $55). Uncertainty aversion was expected to be greater when Uncertain Reward Variance was high. The Uncertainty Type varied across known and unknown probability trials. On known probability trials (indicated by a line dividing the uncertain reward circle in half), participants were instructed that the probability of receiving each of the two monetary values that comprised the uncertain reward was 0.5 if they selected the uncertain reward. On unknown probability trials (indicated by a question mark in the center of the uncertain reward circle), the two uncertain reward monetary values were displayed but no information was provided about the relative probability of receiving each of those two values. Uncertainty aversion was expected to be greater for unknown probability trials, which included greater uncertainty due to the absence of reward probability information3. Manipulation of uncertain reward utility, uncertainty type and uncertain reward variance yielded 36 unique trial combinations of values across certain and uncertain rewards (9 × 2 × 2) that were presented twice each
Urine Analysis for Marijuana Use
Urine samples from marijuana users were obtained at both screening and experimental sessions for quantitative analysis (United States Drug Testing Laboratories; Des Plaines, IL) to verify compliance with the abstinence instructions for the deprived smokers. To reduce the possible impact of urine dilution on accurate measurement of the THC metabolite, 11-nor-9-carboxy-Δ9-tetrahydocannabinol (THCCOOH), creatinine normalized urine samples were used (Hawks, 1983; Huestis & Cone, 1998). Because of the relatively long half-life of the THC metabolite, a specimen ratio of creatinine-normalized samples at two different time points (Creatinine-Normalized Specimen 2 / Creatinine-Normalized Specimen 1) is necessary to determine detect recent use among chronic marijuana users (Huestis & Cone, 1998). Following previously established procedures and cut scores (Huestis & Cone, 1998; Manno, Ferslew, & Manno, 1984); deprived smokers with specimen ratios greater than 1.5 were considered non-compliant with the abstinence requirement.
Open Science Practices
We support emerging open science guidelines (Nosek et al. 2015). Following these guidelines, we have made the data, analysis scripts, questionnaires, and other study materials associated with this report publicly available via Open Science Framework. These materials can be accessed at https://osf.io/raqm4/.
Results
Participant Characteristics
We report participants’ demographics and history of marijuana use and related experiences by Deprivation Group at screening in Table 1. Consistent with our inclusion criteria, participants were heavy marijuana users with the total sample reporting mean consumption of 7.1 (SD=7.3) grams of marijuana per week. Although not required for inclusion in the study, 52.9% of the sample met criteria for DSM-IV-TR marijuana dependence and 28.9% met criteria for marijuana abuse. As expected given random assignment, there were no significant differences between the deprived and non-deprived users on any of these demographic, trait affect, and alcohol/drug-related measures reported in Table 1.
Deprivation Manipulation Checks
We report results from manipulation checks of our marijuana deprivation procedure in Table 2. As expected, deprived users displayed significantly greater reported time since last use, reported greater withdrawal (Marijuana Withdrawal Checklist; Budney, Novy, & Hughes, 1999) and craving (Marijuana Craving Questionnaire; Heishman, Singleton, & Liguori, 2001) during the experimental session than did non-deprived users. In addition, urinalysis indicated that both overall Creatinine-Normalized THC as well as the specimen ratio of experimental / screening session results were greater in deprived than non-deprived users.
Table 2.
Deprivation Manipulation Checks
| Deprived | Non-Deprived | p | |
|---|---|---|---|
| Days since last marijuana use | 3.7 (0.8) | 0.5 (0.4) | <.001*** |
| Marijuana Withdrawal Checklist (experimental session; α=0.86)a | 7.4 (6.4) | 3.1 (3.3) | <.001*** |
| Marijuana Craving Questionnaire (experimental session; α=0.82)a | 76.2 (11.6) | 71.3 (10.1) | .024* |
| Urinalysis | |||
| Creatinine-Normalized THC (experimental session)a | 123.2 (158.3) | 302.8 (202.1) | <.001*** |
| Specimen ratio (experimental / screening session) | 0.35 (0.25) | 1.26 (1.05) | <.001*** |
NOTES:
N=104; Table entries are mean (standard deviation) unless otherwise indicated. We also report Cronbach's alpha (α) internal consistency reliability for self-report measures with multiple items.
Analyses and descriptive statistics control for scores on same measure from screening session
The Uncertain Reward Decision-Making Task
The odds of choosing the uncertain reward was analyzed in a two-level generalized linear mixed effects model with a binomial error distribution and a logit link function (i.e., multi-level logistic regression) in R (R Development Core Team, 2013) using the lme4 (Bates et al., 2014) and lmSupport (Curtin, 2013) packages. We included fixed effects for the level 2 between-subject variable, Deprivation Group (non-deprived vs. deprived user), and for level 1 within-subject task variables, Uncertain Reward Utility (−4 to 4), Uncertainty Type (known vs. unknown probability), and Uncertain Reward Variance (low vs. high). Fixed effects for the interaction between each of these within-subject task variables and Deprivation Group were also modeled to determine if these task-related variables moderated the Deprivation Group effect. The overall effect of Sex (female vs. male) and its interactions with Deprivation Group and the within-subject task variables were included as covariates to increase power to test for focal effects. Finally, random effects were included for the intercept, Uncertain Reward Utility and each of the within-subject task variables. Two model outliers, based on Bonferroni-corrected (p<.05) standardized residuals, were identified and removed from all analyses of the decision-making task. We report raw parameter estimates (Bs) and odds ratios (OR) to document effect sizes.
Task-related variables and covariates
Consistent with previous research demonstrating uncertainty aversion, participants’ odds of selecting the uncertain (relative to the certain) reward was significantly lower than 1 when the uncertain and certain rewards had equal reward value (i.e., Uncertain Reward Utility=0), B=−1.33, 95% CI(B): [−1.57, −1.10], z=11.01, p<.001, OR=0.26.
We report analyses of the main effects of Uncertain Reward Utility and the three task-related variables as manipulation checks to confirm the validity of the Uncertain Reward Decision Making task functioned. As expected, participants were sensitive to changes in the objective Uncertain Reward Utility, B=0.79, 95% CI(B): [0.72, 0.86], z=22.55, p<.001, OR=2.20. This significant main effect of Uncertain Reward Utility confirms that as the objective monetary reward value of the uncertain reward increased relative to the certain reward, the odds that participants preferred the uncertain reward increased. As expected, there was a main effect of Uncertain Reward Variance, B=−0.52, 95% CI(B): [−0.80, −0.23], z=3.58, p<.001, OR=0.60. This indicates that the odds that participants preferred the uncertain reward was reduced when the difference between the possible values of this uncertain reward was increased (i.e., high vs. low). Similarly, the expected main effect of Uncertainty Type was significant, B=−0.43, 95% CI(B): [−0.57, −0.28], z=5.71, p<.001, OR=0.65. Thus, participants had lower odds of selecting the uncertain reward when no information was provided about the relative probability of receiving each of the two possible values for the uncertain reward (unknown vs. known probability trials), i.e., when uncertainty was highest.
The inclusion of Sex as a covariate to increase power was justified by the significant Sex X Uncertain Reward Utility interaction, B=0.22, 95% CI(B): [0.08, 0.35], z=3.17, p=.002, OR=1.24, indicating that the magnitude of the Utility effect was greater in men than in women.. Of interest, Deprivation Group effects (described next) did not differ by Sex.
Deprivation Group
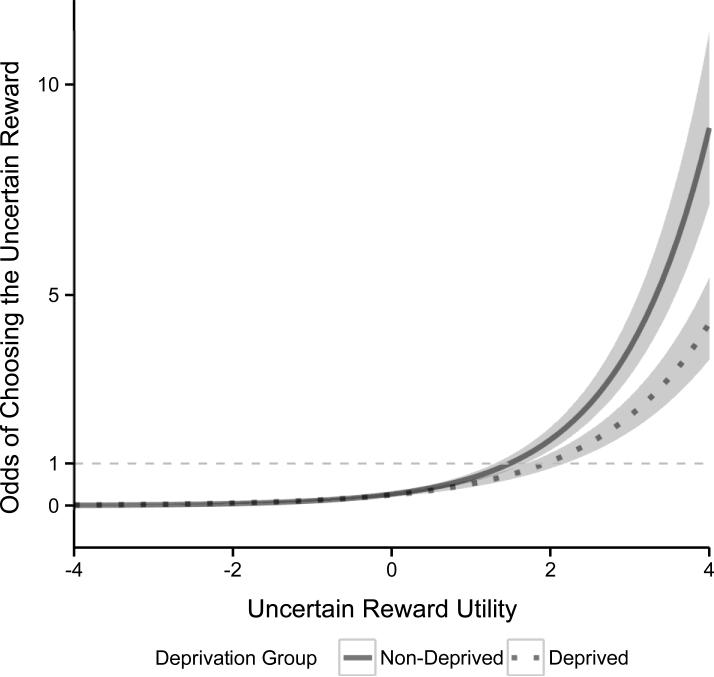
The main effect of Deprivation Group was not significant B=−0.07, 95% CI(B): 0.15, 1.24], z=0.31, p=.761, OR=0.93. However, there was a significant Deprivation Group X Uncertain Reward Utility interaction, B=−0.16, 95% CI(B): [−0.30, −0.03], z=2.37, p=.018, OR=0.85, indicating the effect of Deprivation Group on the odds of preferring the uncertain reward increased as the utility of the uncertain reward increased (see Figure 2). To further describe this interaction, we tested simple effects of Deprivation Group at low (−3) and high (+3) Uncertain Reward Utility. The simple effect of Deprivation Group was not significant when the utility of the uncertain reward was low, B=.42, 95% CI(B): [−0.28, 1.11], z=1.18, p=.238, OR=1.52. This indicates that deprived and non-deprived users preferred the uncertain reward at roughly equivalent rates when the utility of that uncertain reward was low; participants were generally very unlikely to prefer it. In contrast, when the utility of the uncertain reward was high, the simple effect of Deprivation Group was significant, B=−0.56, 95% CI(B): [−1.11, −0.02], z=2.04, p=.041, OR=0.57. This indicates that on trials where it was advantageous to select the uncertain reward because the expected monetary reward was higher for it than the certain reward, deprived users preferred it at significantly lower rates than non-deprived users.
Figure 2. Deprivation Group X Uncertain Reward Utility.
We present the odds of choosing the uncertain reward as a function Deprivation Group and Uncertain Reward Utility. The shaded regions indicate confidence envelopes (±1 standard error) around the point estimates (dark lines) for mean odds from the generalized linear mixed effect model. The Deprivation Group X Uncertain Reward Utility interaction was significant (p=.018). The simple effect of Deprivation Group was not significant (p=.238) at low utility (−3) but was significant (p=.041) at high utility (3).
The other task factors (i.e., Uncertain Reward Variance and Uncertainty Type) did not significantly moderate the Deprivation Group or the Deprivation Group X Uncertain Reward Utility effects. This indicates that the uncertainty aversion characterizing decisions of deprived users when uncertain reward utility was high was robustly displayed across high and low uncertain reward variance and known and unknown probability trials.
Moderation by Marijuana Grams Used per Week
To examine whether quantity of weekly marijuana use moderated the observed effects of Deprivation Group reported above, we added Marijuana Grams Used per Week to the original generalized linear model described earlier, allowing it to interact with the effects of Deprivation and Uncertain Reward Utility.
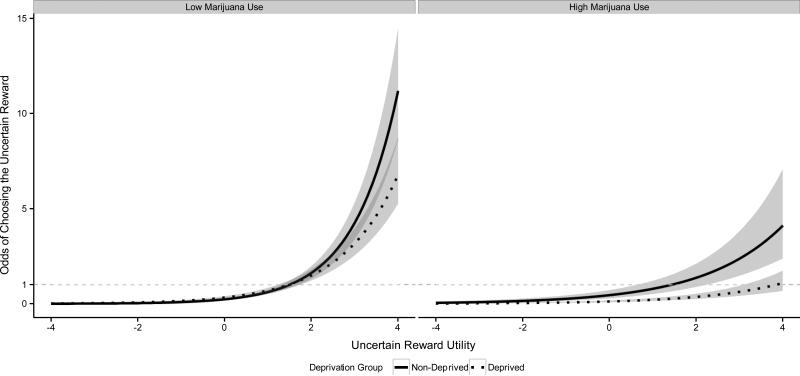
We observed a significant interaction between Marijuana Grams Used per Week and Deprivation Group, B=−0.07, 95% CI(B): [−0.13, −0.01 ], z=2.20, p=.028, OR=0.93, such that the magnitude of the Deprivation Group effect on the odds of preferring the uncertain reward increased as users report higher levels of marijuana use (Figure 3). This moderating effect was observed consistently across the full range of uncertain reward utilities. To further describe this moderating effect, we tested the simple effects of Deprivation Group for low (1.5 grams/week; 5th percentile of sample) and high (25 grams/week; 95th percentile) levels of marijuana use. When level of use was low, the simple effect of Deprivation Group was not significant, B=0.32, 95% CI(B): [−0.26, 0.88], z=1.09 p=.276, OR=1.37. This indicates when weekly marijuana use was low, deprived and non-deprived users selected the uncertain reward at comparable rates across the range of uncertain reward utilities. However, when weekly marijuana use was high, the simple effect of Deprivation Group was significant, B=−1.3, 95% CI(B): [−2.54, −0.09 ], z=2.09 p=.037, OR=0.27. This indicates that when level of weekly use was high, deprived users and preferred the uncertain reward at lower rates than non-deprived users regardless of the level of uncertain reward utility.
Figure 3. Quantity of Marijuana Use Moderates Deprivation Group Effect.
We present the odds of choosing the uncertain reward as a function of Deprivation Group and Uncertain Reward Utility in separate panels for marijuana users with low (1.5 grams/week) and high (25 grams/week) quantity of marijuana use. The shaded regions indicate confidence envelopes (±1 standard error) around the point estimates (dark lines) for mean odds from the generalized linear mixed effect model. The Quantity of Use X Deprivation Group interaction was significant (p=0.028). The simple effect of Deprivation Group was significant for high (p=.037) but not low (p=.276) quantity of use. Note that Quantity of Marijuana Use was modeled quantitatively for analysis. The panels that present point estimates for low and high quantity of use were created only for display.
Discussion
The present study examined the effects of acute (3 days) marijuana deprivation on heavy marijuana users’ subjective valuation of certain vs. uncertain monetary rewards using a modified version of a reward decision-making task. As predicted, participants displayed uncertainty aversion, subjectively over-valuing certain rewards, even when the objective value of these rewards was identical. As expected, preference for uncertain rewards increased with increasing uncertain reward utility. Consistent with the neuroeconomics literature, participants had lower odds of selecting uncertain rewards with unknown probability (putatively more uncertain than known probability trials) (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005; Huettel et al., 2006). Participants also had lower odds of selecting uncertain rewards when reward variance was high vs. low, as expected given the tendency to perceive such circumstances as more uncertain due to greater discrepancy between potential outcomes.
Marijuana Deprivation: Effects, Boundary Conditions, and Moderation by Quantity of Use
Marijuana deprivation further exaggerated reductions in subjective valuation of uncertain rewards. This deprivation effect was observed as a significant Deprivation Group X Uncertain Reward Utility interaction; odds of preferring the uncertain reward were reduced by almost 50% among deprived relative to non-deprived users when uncertain reward utility was moderately high. Given the robust baseline aversion for uncertainty that has been well documented in healthy populations (Tversky & Kahneman, 1974), it is not surprising that deprivation effects were exhibited only at high uncertain reward utility. At lower uncertain reward utility, floor effects likely reduced the sensitivity to detect further reductions in the subjective value of these uncertain rewards by marijuana deprivation (also see Yi, Chase, & Bickel, 2007).
We tested for task moderators of the observed marijuana deprivation effect to establish possible boundary conditions for this decreased subjective valuation of uncertain rewards. Although both Uncertainty Type and Uncertain Reward Variance had significant effects on participants’ subjective valuation of the uncertain reward overall, neither of these factors moderated the deprivation effect. Thus, marijuana deprivation decreased the subjective value of the uncertain rewards even when uncertainty was relatively low (i.e., known probability trials) or when the ‘stakes’ were low (i.e., low Uncertain Reward Variance). Overall, these results suggest marijuana deprivation broadly reduces subjective valuation of uncertain rewards across important characteristics regarding these uncertain rewards.
We also evaluated whether quantity of marijuana use moderated the deprivation effect; indeed weekly marijuana consumption significantly moderated the marijuana deprivation effect. Specifically, the magnitude of the deprivation effect increased with increasing quantity of use. This observation has at least two important implications. First, it increases the construct validity of the deprivation manipulation. Marijuana deprivation would be expected to most robustly affect behavior among individuals for whom deprivation contrasts strongly with their normal pattern of use. Secondly, although we did not directly test for neuroadaptations in the present experiment, this effect is consistent with the homeostatic/allostatic reward neuroadaptation hypothesis, suggesting that the strength of these adaptations may be a function of quantity of use. Conversely, it also begins to define a boundary for harm associated with marijuana use; these compensatory neuroadaptations in uncertain reward valuation emerge only for individuals who display high quantities of use. Furthermore, it should be highlighted that we intentionally recruited individuals who reported relatively high levels of marijuana use for this study, as neuroadaptations in reward processing are expected to manifest in heavier users.
Although our findings are consistent with reward neuroadaptations, it should be noted that alternative theories remain tenable and are not mutually exclusive with this hypothesis. Specifically, working memory and attentional bias may play a role in the observed effects. Reward estimation and prediction under conditions of uncertainty require intact working memory processes within the prefrontal cortex and modulatory inputs from motivation and/or emotion processing areas of the brain, with damage to any of these systems resulting in maladaptive decisions (Mizumori & Jo, 2013). Similarly, increased working memory load leads to impulsive behavior and maladaptive decision-making among vulnerable individuals (Hofmann, Friese, & Strack, 2009; Wiers, Ames, Hofmann, Krank, & Stacy, 2010). Chronic, heavy marijuana use is associated with impairments in memory and attention that worsen with increasing years of regular use (Solowij et al., 2002). Thus, marijuana use may impair decision-making via its impact on the prefrontal cortex (Hermann et al., 2007; Quickfall & Crockford, 2006) and related working memory functions (Fridberg, Gerst, & Finn, 2013). Correspondingly, cognitive enhancement and remediation has been proposed as a promising treatments for marijuana and other drug use disorders (Baskin-Sommers, Curtin, & Newman, 2015; Sofuoglu, Sugarman, & Carroll, 2010). Such cognitive enhancement may influence subjective reward valuation and uncertainty aversion directly or indirectly via effects on attention, working memory, and/or executive function. For example, working memory training has reduced delayed discounting among stimulant addicted individuals (Bickel, Yi, Landes, Hill, & Baxter, 2011).
Clarifications, Caveats, and Future Directions
As described earlier, quantity of use emerged as an important individual difference. Participants also reported diverse patterns of use (e.g., binge vs. steady state use, sharing with others), methods of administration (e.g., joint, water pipe, “spliff”, vaporizer), variable strains, potency, and constituents (e.g., concentration of THC vs. cannabidiol) of marijuana consumed. Research indicates differential subjective, pharmacokinetic, and physiological effects of marijuana when administered via different methods (Cooper & Haney, 2009). Furthermore, drug use patterns involving frequent binge/intoxication and withdrawal vs. steady state dosing may more strongly recruit allostatic changes in brain stress and reward systems (Koob & Kreek, 2007; Koob & Volkow, 2010). Thus, our grams used per week measure is relatively inexact; however despite inherent “noise” in this measure, it remained reliable enough to emerge as a significant moderator of our deprivation effect.
Furthermore, while our behavioral task focused on certain vs. uncertain rewards, participants received a relatively certain and non-trivial payment for time spent in the laboratory. Furthermore, those randomly assigned to abstain received an additional $200 in the mail once their abstinence was confirmed by urine analysis. This compensation could have reduced the subjective value of our task rewards and therefore the sensitivity of the task itself. Furthermore, the relatively large reward for deprivation could have led participants to subvert study protocol (e.g., by substituting another individual's urine for their own). Notwithstanding, the observation of significant and robust task manipulations and a significant deprivation effect on uncertainty aversion suggests that our task remained sensitive to differences in decision making processes.
There are also tradeoffs to using a behavioral task in which multiple responses are made in a short period of time such as the task in this study versus a single decision trial. Of course, heavy marijuana users make both single isolated and repeated related decisions across situations in everyday life such that both single and multiple trial tasks likely possess relevant external validity. Most importantly, trials were presented pseudo-randomly to avoid confounding task manipulations with order effects in this task.
As we provide a cross-sectional snapshot of the effect of marijuana deprivation on subjective valuation of uncertain rewards, it is not possible to determine if the observed effects result from a short-term homeostatic process in response to the most recent drug administration and acute withdrawal or a more persistent allostatic neuroadaptation. If the latter, it is unknown to what degree this allostatic adaptation may be reversible following extended abstinence. These are important questions both with respect to mechanism and the clinical impact of this impaired pattern of decision-making.
We chose to examine probabilistically uncertain monetary rewards in this report in part because these rewards have calculable objective values yet considerable individual differences in the subjective valuation of these rewards are still observed (Huettel et al., 2006; Platt & Huettel, 2008; Richards et al., 1999; Weber & Huettel, 2008). Some research suggests comparable probabilistic discounting across monetary vs. consumable rewards in healthy individuals (Estle, Green, Myerson, & Holt, 2007) and drug users (Field et al., 2006; Giordano et al., 2002; Poltavski & Weatherly, 2013; Yi & Landes, 2012). Nonetheless, future research needs to explicitly examine if our results regarding probabilistic uncertain monetary rewards generalize to probabilistic drug rewards and other consumable and non-consumable probabilistic rewards (e.g., Johnson et al., 2007).
Both temporal delay and probabilistic outcomes can produce uncertainty regarding rewards. Devaluation of delayed and probabilistic rewards may result from shared mechanisms in the processes of these two classes of uncertain rewards (Green & Myerson, 2004; Kalenscher, 2007; Poltavski & Weatherly, 2013; Prelec & Loewenstein, 1991; Rachlin, Raineri, & Cross, 1991). Observed positive correlations between subjective valuation of delayed and probabilistic rewards support this perspective (e.g., Myerson, Green, Hanson, Holt, & Estle, 2003; but see also Shead, Callan, & Hodgins, 2008; Shead & Hodgins, 2009). However, distinct processes may also underlie distortions in subjective valuation of delayed vs. probabilistic rewards in vulnerable populations (Green & Myerson, 2013; Johnson et al., 2010; Myerson et al., 2003). The present task can be easily modified to examine the effect of marijuana deprivation on temporally uncertain rewards, an important next step in our program of research.
Summary and Implications
Increasing marijuana use represents an emerging public health issue that warrants more scientific scrutiny. Public opinion appears to associate marijuana with less harmful consequences than alcohol and other drugs (Johnston et al., 2014; Nutt et al., 2007). Ongoing marijuana use legislation efforts may be premature given the relative dearth of evidence about marijuana's long-term impact on users.
The results described in this report suggest that chronic heavy marijuana use decreases the subjective valuation of probabilistically uncertain rewards across all users during short term drug deprivation. This deprivation effect increases with increasing quantity of use. Nonetheless, this reduction in uncertain reward valuation appears to generalize across important characteristics regarding these uncertain rewards.
To the degree that decision-making vulnerabilities represent a cardinal feature of drug addiction, marijuana appears similar to other drugs of abuse in its potential to recruit addiction mechanisms via homeostatic or allostatic neuroadaptations in subjective reward valuation (Redish et al., 2008). These changes in uncertain reward valuation may have important implications for the individual's subsequent decisions regarding ongoing drug use, relapse following cessation attempts, and perhaps the pursuit of distal or otherwise uncertain non-drug rewards in social, educational, work, and health domains. This bias again uncertain rewards may represent one manifestation of a broader uncertainty aversion that generalizes across rewards and stressors, with implications for ongoing drug use and relapse (Hefner et al., in preparation; Hefner et al., 2013; Hogle et al., 2010; Moberg & Curtin, in preparation). Specifically, our laboratory's program of research suggests that repeated alcohol, marijuana, and other drug use prompts neuroadaptation(s) biasing the user against uncertainty generally, making uncertain rewards less positive and uncertain stressors more negative. It remains unknown if this generic uncertainty aversion results from one common or multiple distinct mechanisms.
The results in this report also have therapeutic implications. The documented therapeutic benefits of contingency management and cognitive-behavioral approaches may be mediated in part through their impact on drug users’ subjective valuation of the landscape of available rewards. For example, contingency management of drug abstinence increases the certainty of non-drug rewards by making them less distal and associated with clearly defined and realistic contingencies (Petry, 2000). This may facilitate the drug users’ decisions to pursue these rewards rather than further drug use. Similarly, skill sets developed via cognitive-behavioral and psychoeducational interventions (stress and anger management, drug refusal skills, problem-solving skills, distress tolerance) may increase perceived efficacy both for resisting short-term drug urges and for obtaining desired but previously less certain rewards in the social, professional, and health domains (e.g., Beck, Wright, Newman, & Liese, 2001). Working memory training and other cognitive remediation approaches hold high promise to impact decision-making deficits in these vulnerable populations of heavy drug users (Sofuoglu et al., 2010). In contrast, psychosocial treatments focusing on long-term negative consequences of drug use and/or distal rewards of drug abstinence may have limited effectiveness for drug users displaying decision making vulnerabilities biasing them towards immediate, certain rewards. Thus, considering the role of subjective reward valuation distortions may enable treatment providers to better prepare drug users for decision making pitfalls that may await them following the initial decision to reduce or abstain from further drug use.
General Scientific Summary.
Following three days of marijuana abstinence, heavy marijuana users showed changes in decision-making such that they avoided uncertain monetary rewards, even when these rewards were objectively more valuable than competing smaller but certain monetary rewards. Heavy marijuana users may prefer certain rewards more generally (e.g., getting high/avoiding unpleasant withdrawal) over alternative, less certain rewards (e.g., pursuing educational and career goals). These findings confirm that heavy marijuana use changes decision-making in similar ways to other additive drugs.
Acknowledgements
This work was supported by National Institute on Drug Abuse grant DA032184 awarded to Dr. Hefner and the University of Wisconsin-Madison Romnes faculty Fellowship awarded to Dr. Curtin. Writing of this manuscript was partially supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. Since Dr. Hefner is an employee of the U.S. Government and contributed to the manuscript as part of her official duties, the work is not subject to U.S. copyright.
Footnotes
We excluded participants with current alcohol or other substance use dependence because the study design required that participants not be under the influence of alcohol or other drugs nor in withdrawal from drugs other than marijuana during the experimental session. However, current alcohol or substance dependence was not observed frequently during study recruitment. These criteria excluded 4 potential participants with current alcohol dependence and 1 potential participant with current cocaine dependence as assessed by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998) during the screening session.
No participants in either group were disqualified for a positive BAC at the experimental session. Similarly, no participants in the Deprived Group were disqualified because of a positive urine drug test indicating recent marijuana use prior to the experimental session. One participant was disqualified from the Deprived Group due to abstaining for seven days. Two participants in the Non-deprived group were disqualified for self-reported use of drugs (other than marijuana) within one week of the experimental session. Finally, 4 participants in the Deprived group and 7 participants in the Non-deprived group were lost to attrition between the screening and experimental session.
Participants completed the task in blocks of threat of electric shock or no threat; they completed 4 blocks (2 of each type) in one of four between subjects counterbalanced orders. A total of 5 shocks were administered. We expected uncertainty aversion to be greater during shock threat trials due to threat-related anxiety. However, this manipulation did not significantly moderate behavior and therefore is not discussed further in this report.
References
- American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Edition (DSM-5) American Psychological Association; 2013. [Google Scholar]
- Bach DR, Dolan RJ. Knowing how much you don't know: a neural organization of uncertainty estimates. Nature Reviews Neuroscience. 2012;13(8):572–586. doi: 10.1038/nrn3289. http://doi.org/10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- Baker T, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Curtin JJ, Newman JP. Altering the Cognitive-Affective Dysfunctions of Psychopathic and Externalizing Offender Subtypes With Cognitive Remediation. Clinical Psychological Science. 2015;3(1):45–57. doi: 10.1177/2167702614560744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Haubo R, Christiansen R, Singmann H. lme4, V1.1-6. 2014 Retrieved from http://cran.r-project.org/web/packages/lme4/index.html.
- Bechara A. Risky business: emotion, decision-making, and addiction. Journal of Gambling Studies / Co-Sponsored by the National Council on Problem Gambling and Institute for the Study of Gambling and Commercial Gaming. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Wright FD, Newman CF, Liese BS. Cognitive Therapy of Substance Abuse (1 edition) The Guilford Press; New York; London: 2001. [Google Scholar]
- Berridge KC, Aldridge JW. Decision utility, the brain, and pursuit of hedonic goals. Social Cognition. 2008;26(5):621–646. doi: 10.1521/soco.2008.26.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–527. doi: 10.1016/j.neuropharm.2013.06.013. http://doi.org/10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. http://doi.org/10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the Future: Working Memory Training Decreases Delay Discounting Among Stimulant Addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. http://doi.org/10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science. 2013;24(12):2541–2549. doi: 10.1177/0956797613499923. http://doi.org/10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & Therapeutics. 2011;129(2):149–171. doi: 10.1016/j.pharmthera.2010.09.007. http://doi.org/10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, Kornreich C, Verbanck P, Noël X. Impaired decision-making under risk in individuals with alcohol dependence. Alcoholism, Clinical and Experimental Research. 2014;38(7):1924–1931. doi: 10.1111/acer.12447. http://doi.org/10.1111/acer.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction (Abingdon, England) 1999;94(9):1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience. 2009;29(40):12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11(1):18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug and Alcohol Dependence. 2009;103(3):107–113. doi: 10.1016/j.drugalcdep.2009.01.023. http://doi.org/10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J. ImSupport. 2014 Retrieved from http://dionysus.psych.wisc.edu/MediaWiki/index.php?title=LmSupport.
- Curtin JJ, Mccarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition And Addiction. Sage Publications Inc.; 2006. pp. 233–250. [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychological Science. 2007;18(1):58–63. doi: 10.1111/j.1467-9280.2007.01849.x. http://doi.org/10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186(2):255–263. doi: 10.1007/s00213-006-0385-4. http://doi.org/10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fox H, Tuit K, Sinha R. Stress system changes associated with marijuana dependence may increase craving for alcohol and cocaine. Human Psychopharmacology: Clinical and Experimental. 2012 doi: 10.1002/hup.2280. n/a. http://doi.org/10.1002/hup.2280. [DOI] [PMC free article] [PubMed]
- Fridberg DJ, Gerst KR, Finn PR. Effects of working memory load, a history of conduct disorder, and sex on decision making in substance dependent individuals. Drug and Alcohol Dependence. 2013;133(2):654–660. doi: 10.1016/j.drugalcdep.2013.08.014. http://doi.org/10.1016/j.drugalcdep.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiology & Behavior. 2012;106(1):58–64. doi: 10.1016/j.physbeh.2011.11.004. http://doi.org/10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163(2):174–182. doi: 10.1007/s00213-002-1159-2. http://doi.org/10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Gloria R, Jaber JN, Baker TB, Curtin JJ. The effect of temporal precision and probability on the response to threat of shock: a fear-potentiated startle study. Psychophysiology. 2009;46(s1):s80. [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcoholism, Clinical and Experimental Research. 2007;31(6):928–938. doi: 10.1111/j.1530-0277.2007.00378.x. http://doi.org/10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. A Discounting Framework for Choice With Delayed and Probabilistic Rewards. Psychological Bulletin. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. http://doi.org/10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. How many impulsivities? A discounting perspective. Journal of the Experimental Analysis of Behavior. 2013;99(1):3–13. doi: 10.1002/jeab.1. http://doi.org/10.1002/jeab.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews. Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. http://doi.org/10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks R. Development in cannabinoid analysis of body fluids: Implications for forensic analysis. In: Agurell S, Dewey W, Willette R, editors. The Cannabinoids: Chemical, Pharmacologic and Therapeutic Aspects. Academic Press; Rockville, MD: 1983. pp. 1–12. Retrieved from http://www.barnesandnoble.com/w/the-cannabinoids-stigagurell/1000716140. [Google Scholar]
- Hefner K, Gloria R, Baker T, Curtin J. Uncovering a potential biological marker for marijuana withdrawal: Startle potentiation to threat. in preparation. [Google Scholar]
- Hefner KR, Curtin JJ. Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. Journal of Psychopharmacology (Oxford, England) 2012;26(2):232–244. doi: 10.1177/0269881111416691. http://doi.org/10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ. Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology. 2013;122(3):756–769. doi: 10.1037/a0033407. http://doi.org/10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction (Abingdon, England) 2001;96(7):1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. http://doi.org/10.1080/09652140120053084. [DOI] [PubMed] [Google Scholar]
- Hermann D, Leménager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision making of heavy cannabis users on the Iowa Gambling Task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. European Addiction Research. 2009;15(2):94–98. doi: 10.1159/000189788. http://doi.org/10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biological Psychiatry. 2007;61(11):1281–1289. doi: 10.1016/j.biopsych.2006.08.027. http://doi.org/10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and Self-Control From a Dual-Systems Perspective. Perspectives on Psychological Science. 2009;4(2):162–176. doi: 10.1111/j.1745-6924.2009.01116.x. http://doi.org/10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43(4):344–356. doi: 10.1111/j.1469-8986.2006.00406.x. http://doi.org/10.1111/j.1469-2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biological Psychiatry. 2010;68(8):687–688. doi: 10.1016/j.biopsych.2010.06.003. http://doi.org/10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology. 1998;22(6):445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Experimental and Clinical Psychopharmacology. 2007;15(2):187–194. doi: 10.1037/1064-1297.15.2.187. http://doi.org/10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18(1):99–107. doi: 10.1037/a0018333. http://doi.org/10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975-2013: Overview, Key Findings on Adolescent Drug Use. Insitute for Social Research, The University of Michigan; Ann Arbor: 2014. [Google Scholar]
- Jones J, Saad L. Gallup Poll Social Series: Crime. Gallup News Service; 2013. [Google Scholar]
- Kalenscher T. Decision making: don't risk a delay. Current Biology: CB. 2007;17(2):R58–61. doi: 10.1016/j.cub.2006.12.016. http://doi.org/10.1016/j.cub.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The Neural Basis of Addiction: A Pathology of Motivation and Choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. http://doi.org/lt;pgt;10.1176/appi.ajp.162.8.1403lt;/pgt; [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. http://doi.org/10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American Journal of Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. http://doi.org/10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal ML. Addiction and the Brain Antireward System. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology Reviews. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. http://doi.org/10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Manno J, Ferslew K, Manno B. Urine excretion patterns of cannabinoids and the clinical appliation of the EMIT-d.a.u. cannabinoid urine assay for substance abuse treatment. In: Agurell S, Dewey W, Willette R, editors. The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. Academic Press, Inc.; New York, NY: 1984. pp. 281–290. [Google Scholar]
- Mizumori SJY, Jo YS. Homeostatic regulation of memory systems and adaptive decisions. Hippocampus. 2013;23(11):1103–1124. doi: 10.1002/hipo.22176. http://doi.org/10.1002/hipo.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. Journal of Abnormal Psychology. 2009;118(2):335–347. doi: 10.1037/a0015636. http://doi.org/10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg C, Curtin J. Stress neuroadaptation to uncertain threat among alcohol dependent individuals in early abstinence. in preparation. [Google Scholar]
- Myerson J, Green L, Hanson S, Holt D, Estle S. Discounting delayed and probabilistic rewards: Processes and traits. Journal of Economic Psychology. 2003;24:619–635. [Google Scholar]
- Nosek BA, et al. Promoting an open research culture. Science. 2015;348:1422–1425. doi: 10.1126/science.aab2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–1053. doi: 10.1016/S0140-6736(07)60464-4. http://doi.org/10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Yu AJ. Emotion and decision-making: affect-driven belief systems in anxiety and depression. Trends in Cognitive Sciences. 2012;16(9):476–483. doi: 10.1016/j.tics.2012.07.009. http://doi.org/10.1016/j.tics.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nature Neuroscience. 2008;11(4):398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Watson KK, Hayden BY, Shepherd SV, Klein JT. Neuroeconomics: Implications for Understanding the Neurobiology of Addiction. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. 2nd ed. CRC Press; Boca Raton (FL): 2010. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK53362/ [PubMed] [Google Scholar]
- Poltavski DV, Weatherly JN. Delay and probability discounting of multiple commodities in smokers and never-smokers using multiple-choice tasks. Behavioural Pharmacology. 2013;24(8):659–667. doi: 10.1097/FBP.0000000000000010. http://doi.org/10.1097/FBP.0000000000000010. [DOI] [PubMed] [Google Scholar]
- Prelec D, Loewenstein G. Decision making over time and under uncertainty: a common approach. Management Science. 1991;37:770–786. [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(3):318–332. doi: 10.1176/jnp.2006.18.3.318. http://doi.org/10.1176/appi.neuropsych.18.3.318. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55(2):233–244. doi: 10.1901/jeab.1991.55-233. http://doi.org/10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. Retrieved from http://www.R-project.org. [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. The Behavioral and Brain Sciences. 2008;31(4):415–437. doi: 10.1017/S0140525X0800472X. discussion 437–487. http://doi.org/10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de-Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71(2):121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential Vulnerabilities of Neuronal Reward, Risk, and Decision Mechanisms to Addictive Drugs. Neuron. 2011;69(4):603–617. doi: 10.1016/j.neuron.2011.02.014. http://doi.org/16/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nature Neuroscience. 2005;8(11):1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Shead NW, Callan MJ, Hodgins DC. Probability discounting among gamblers: Differences across problem gambling severity and affect-regulation expectancies. Personality and Individual Differences. 2008;45(6):536–541. http://doi.org/10.1016/j.paid.2008.06.008. [Google Scholar]
- Shead NW, Hodgins DC. Probability discounting of gains and losses: implications for risk attitudes and impulsivity. Journal of the Experimental Analysis of Behavior. 2009;92(1):1–16. doi: 10.1901/jeab.2009.92-1. http://doi.org/10.1901/jeab.2009.92-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sinha R. The Role of Stress in Addiction Relapse. Current Psychiatry Reports. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. http://doi.org/10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. http://doi.org/10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sugarman DE, Carroll KM. Cognitive function as an emerging treatment target for marijuana addiction. Experimental and Clinical Psychopharmacology. 2010;18(2):109–119. doi: 10.1037/a0019295. http://doi.org/10.1037/a0019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Marijuana Treatment Project Research Group Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA: The Journal of the American Medical Association. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings and Detailed Tables. Department of Health and Human Services; Rockville: 2011. [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Risk-dependent reward value signal in human prefrontal cortex. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0809599106. http://doi.org/10.1073/pnas.0809599106. [DOI] [PMC free article] [PubMed]
- Tversky A, Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science (New York, N.Y.) 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. http://doi.org/10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: an fMRI study. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2010;20(3):153–163. doi: 10.1016/j.euroneuro.2009.11.010. http://doi.org/10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse Health Effects of Marijuana Use. New England Journal of Medicine. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. http://doi.org/10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber BJ, Huettel SA. The neural substrates of probabilistic and intertemporal decision making. Brain Research. 2008;1234:104–115. doi: 10.1016/j.brainres.2008.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Annals New York Academy of Sciences. 2001:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Research. 2011;191(1):51–59. doi: 10.1016/j.pscychresns.2010.10.002. http://doi.org/10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76(1):107–111. doi: 10.1016/j.drugalcdep.2004.04.009. http://doi.org/10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Ames SL, Hofmann W, Krank M, Stacy AW. Impulsivity, impulsive and reflective processes and the development of alcohol use and misuse in adolescents and young adults. Frontiers in Psychology. 2010;1:144. doi: 10.3389/fpsyg.2010.00144. http://doi.org/10.3389/fpsyg.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Handbook of Implicit Cognition and Addiction. SAGE; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Chase WD, Bickel WK. Probability discounting among cigarette smokers and nonsmokers: molecular analysis discerns group differences. Behavioural Pharmacology. 2007;18(7):633–639. doi: 10.1097/FBP.0b013e3282effbd3. http://doi.org/10.1097/FBP.0b013e3282effbd3. [DOI] [PubMed] [Google Scholar]
- Yi R, Landes RD. Temporal and Probability Discounting by Cigarette Smokers Following Acute Smoking Abstinence. Nicotine & Tobacco Research. 2012;14(5):547–558. doi: 10.1093/ntr/ntr252. http://doi.org/10.1093/ntr/ntr252. [DOI] [PMC free article] [PubMed] [Google Scholar]