Abstract
Objective
Short sleep duration is a significant risk factor for weight gain, particularly in African Americans and men. Increased caloric intake underlies this relationship but it remains unclear whether decreased energy expenditure is a contributory factor. The current study assessed the impact of sleep restriction and recovery sleep on energy expenditure in African American and Caucasian men and women.
Methods
Healthy adults participated in a controlled laboratory study. After two baseline sleep nights, subjects were randomized to an experimental (n=36; 4h sleep/night for 5 nights followed by 1 night 12h recovery sleep) or control condition (n=11; 10h sleep/night). Resting metabolic rate and respiratory quotient were measured using indirect calorimetry in the morning after overnight fasting.
Results
Resting metabolic rate—the largest component of energy expenditure—decreased after sleep restriction (−2.6%, p=0.032) and returned to baseline levels after recovery sleep. No changes in resting metabolic rate were observed in control subjects. Relative to Caucasians (n=14), African Americans (n=22) exhibited comparable daily caloric intake but a lower resting metabolic rate (p=0.043) and higher respiratory quotient (p=0.013) regardless of sleep duration.
Conclusions
Sleep restriction decreased morning resting metabolic rate in healthy adults, suggesting that sleep loss leads to metabolic changes aimed at conserving energy.
Keywords: Sleep restriction, energy balance, race differences
Epidemiological and laboratory studies have consistently found that short sleep duration is a significant risk factor for weight gain and obesity1–4, particularly in African Americans and men5–8. Increased caloric intake underlies the relationship between short sleep duration and weight gain3,4,9,10, but it remains unclear whether decreased energy expenditure—an important contributor to energy balance and weight management11,12—also plays a role.
Sleep loss can change energy expenditure by affecting each of its components: resting metabolic rate (RMR), diet-induced thermogenesis (DIT) and physical activity13. Because energy expended during sleep is less than energy expended during wake14, the increased energy requirement associated with sleep restriction produces negative energy balance and weight loss over time if diet remains constant. To protect against this state, it is hypothesized that the body employs compensatory responses to increase energy intake and conserve energy14. If more energy is consumed and conserved than needed (overcompensation), positive energy balance and weight gain occurs.
Several studies support this compensatory hypothesis. Additional energy required for extended wakefulness from acute total sleep deprivation and sleep restriction is 135 kcal/day15 and ~100 kcal/day4,16, respectively. Under ad libitum feeding conditions, subjects consume ~500 additional daily calories when sleep restricted3; thus, during extended wakefulness adults overcompensate for increases in energy cost with marked increases in energy intake. Some laboratory studies, but not all15–18, have observed decreased RMR19–21, DIT20, and physical activity22,23 during the day following sleep loss, suggesting a metabolic adaptation to conserve energy in response to the previous day’s extended wakefulness.
Substrate utilization, the type of fuel used for metabolism, is assessed using respiratory quotient (RQ; CO2 produced/O2 consumed). RQ ranges from 0.63 (0.7: fat metabolism) to 1.3 (1.0: carbohydrate metabolism) with higher RQ values associated with overfeeding and weight gain12,24,25. Some studies have found that sleep loss increases RQ17,21, others have found it has no effect15,18.
Few studies have assessed the impact of recovery sleep on energy expenditure. Sleeping metabolic rate was lower during recovery sleep from acute total sleep deprivation than during baseline sleep in a small sample of young adults15. RMR decreased following 3 weeks of sleep restriction combined with circadian disruption and returned to baseline levels after 9 nights of recovery sleep in young adults19. Previous studies examining the effect of sleep loss and recovery sleep on energy expenditure have controlled caloric intake which does not mimic what occurs when adults are sleep restricted outside of the laboratory. Therefore, an experiment examining the impact of sleep restriction and recovery sleep on energy expenditure under ad libitum feeding conditions is critically needed to more closely approximate real world conditions.
No studies to date have examined race and gender differences in the energy expenditure response to sleep restriction. We found that men increased caloric intake to a greater degree than women during sleep restriction, whereas African Americans and Caucasians exhibited comparable daily caloric intake levels26. Given that African Americans gained more weight than Caucasians during sleep restriction3 but did not have greater caloric intake, sleep restriction may affect energy expenditure differently in these racial groups.
The aim of the current study was to investigate the impact of sleep restriction and subsequent recovery sleep on energy expenditure in a sample of healthy African American and Caucasian men and women. We hypothesized resting metabolic rate would be reduced and respiratory quotient would be increased following sleep restriction and these measures would show a compensatory return to baseline levels after a night of recovery sleep. Considering that African Americans gain more weight but exhibit a similar increase in caloric intake compared to Caucasians when sleep restricted, we hypothesized resting metabolic rate would be lower and respiratory quotient would be higher following sleep restriction in African Americans.
Methods
Subjects
Healthy adults (21–50y) were recruited in response to study advertisements. They reported habitual nightly sleep durations between 6.5h–8.5h, bedtimes between 2200h-0000h and wake-times between 0600h–0900h; reports were confirmed using actigraphy. They had no evidence of habitual napping, sleep disturbances, or extreme morningness/eveningness (assessed by questionnaire27). Subjects were free of acute/chronic medical/psychological conditions, as established by interviews, clinical history, questionnaires, physical examinations, and blood (including a fasting glucose test) and urine tests. They were nonsmokers and did not participate in shift work, transmeridian travel, or irregular sleep-wake routines in the 60 days prior to the study. Enrolled subjects were monitored at home with actigraphy, sleep-wake diaries, and time-stamped call-ins to assess bedtime and waketime during the week before and after the in-laboratory phase. They were not permitted to use caffeine, alcohol, tobacco, and medications (except oral contraceptives) in the week before the laboratory study, as verified by urine screenings. Sleep disorders were excluded by a night of laboratory polysomnography and oximetry measurements.
The studies were approved by the Institutional Review Board of the University of Pennsylvania and carried out in accordance with approved guidelines. All subjects provided written informed consent before enrollment and were compensated for participation.
Experimental design
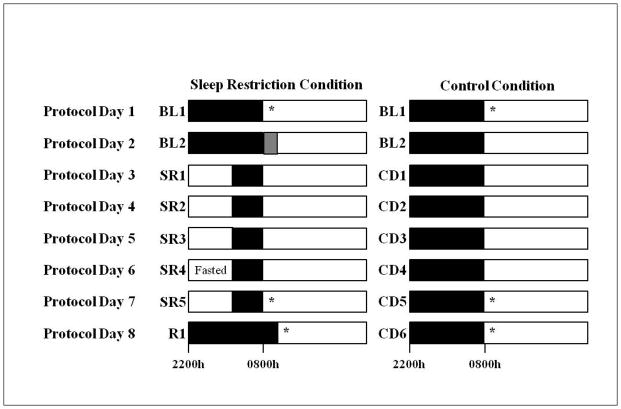
Subjects participated in one of two protocols in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania and were studied for 14 or 18 consecutive days continuously, in a laboratory protocol with daily clinical checks of vital signs and symptoms by nurses (with a physician on call). Only data collected during the first 8 days of both protocols were used in the current study. A subset of subjects in the current cohort were part of larger cohorts in two previously published studies (n=6 control subjects and n=15 sleep-restricted subjects). Subjects were randomized to either the experimental or control condition (Figure 1). In both protocols, the experimental condition consisted of two baseline nights of 10h/12h time-in-bed (TIB) per night (BL1-2; 2200h-0800h/1000h) followed by five nights of sleep restricted to 4h TIB per night (SR1-5; 0400h–0800h) and one night of 12h TIB recovery sleep (R1; 2200h-1000h). This dose of sleep restriction produces neurobehavioral deficits in healthy adults28 and is within the range of sleep loss occurring due to lifestyle factors29. The control condition consisted of 10h TIB per night (2200h–0800h) each night.
Figure 1.
Protocol schedule
The sleep restriction condition consisted of two baseline nights of 10h or 12h TIB per night (2200h–0800h/1000h) followed by five nights of sleep restricted to 4h TIB per night (0400h–0800h) and one night of 12h TIB recovery sleep (2200h-1000h). The control condition consisted of 8 consecutive nights of 10h TIB per night (2200h-0800h). Food/drink intake was ad libitum; however, prior to the measurement on SR5, sleep-restricted subjects were awake for 6h of the 10h overnight fast (2200h-0359h) and were only allowed to consume water during this time. *Body composition and metabolic measurements were collected during the morning after the first night of baseline sleep, after the fifth night of sleep restriction and after the night of recovery sleep, or after corresponding nights in the control condition. To control for morning-evening effects, measurements occurred during the two hours following waketime (between 0800h–1000h on BL1, SR5/CD5 and CD6 and between 1000h–1200h on R1). Upon awakening, subjects used the restroom. Each subject’s body composition was assessed and then subjects remained in bed in a supine position for the remainder of the testing period. After the body composition measurement, each subject’s metabolic rate was assessed. Black bars=sleep periods; white bars=wake periods; gray bar=additional 2h sleep period for subjects participating in one of the protocols.
Subjects were not permitted to leave the laboratory during the protocol. Subjects were ambulatory and were permitted to watch television, read, play video/board games, and perform other sedentary activities between test bouts but they were not allowed to exercise. Subjects wore a wrist actigraph throughout the in-laboratory protocol. On certain protocol days, subjects wore ambulatory electroencephalography and electrocardiography recording equipment for 24h intervals. The light levels were held constant at <50 lux during scheduled wakefulness and <1 lux during sleep periods. Ambient temperature was maintained 22°–24°C. Subjects were monitored by trained staff continuously to ensure adherence. Food/drink was ad libitum throughout the protocol (caffeine was prohibited).
Body composition and metabolic measurements were collected after an overnight fast in the morning following BL1, the fifth night of sleep restriction or control sleep and the night of recovery sleep or sixth night of control sleep (Figure 1). To control for morning-evening effects, measurements occurred during the two hours following waketime (BL1, SR5/CD5, CD6: 0800h–1000h, R1: 1000h–1200h). Prior to the SR5 measurement, subjects were awake for 6h of the 10h fast (2200h-0359h) and could only consume water during this time. Upon awakening, subjects were instructed to use the restroom. Each subject’s body composition was assessed and then he/she remained in bed in a supine position for the remainder of the testing period.
Measures
Each subject’s body composition was measured using bioelectrical impedance analysis (Omron HBF-510W, 4-Limb device), following voiding. Body weight, fat percentage, and fat free mass (FFM) were collected.
Subjects selected meals/snacks by choosing from menu options, selecting food/drink available in the kitchen within the laboratory suite and making requests to the study staff. Subjects were allowed to consume food/drink at any time during the protocol other than when they were completing neurobehavioral tests or sleeping. All food was weighed and recorded prior to being provided to the subjects. Each day, a detailed description of the items, the amount consumed and intake time were recorded by trained monitors. Additionally, food/drink that was leftover after each meal was weighed and recorded. Intake data were entered into The Food Processor SQL program (ESHA Research, Salem, OR), a validated30 professional nutrition analysis software that provides components of food/drink intake including calories and macronutrients.
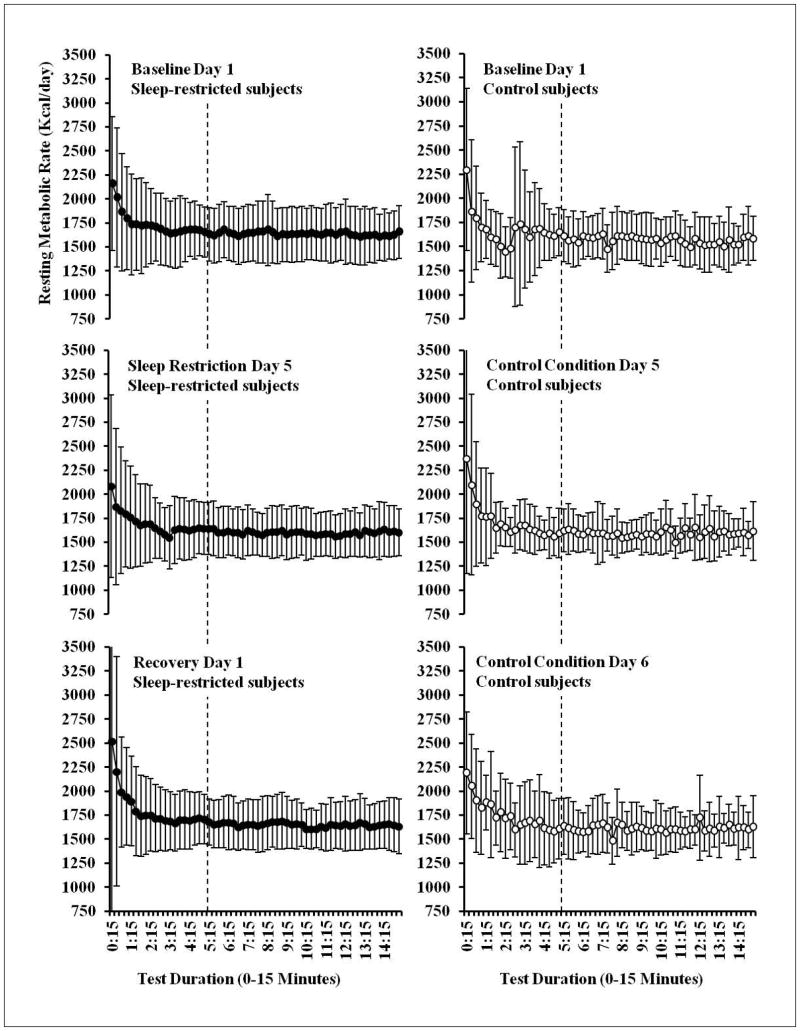
Metabolic rate (kcal/day) was measured using indirect calorimetry with a validated31,32 ventilated hood system (TrueOne 2400 metabolic cart, Parvo Medics, Sandy, UT, USA). Before each measurement day, a flow meter calibration was conducted and before each use, the metabolic cart was calibrated with reference gas. After achieving steady state (5 minutes, Figure 2), expired gases were collected for 10 minutes and used to calculate metabolic rate. To test the reliability of this measurement, metabolic rate was assessed in a subset of subjects (n=13, 4 women) on two occasions separated by at least 3 months. During both assessments metabolic rate was measured after a baseline night of sleep in the laboratory and after an overnight fast. The measurement of resting metabolic rate was stable (Assessment 1: 1561.2 kcal/day, Assessment 2: 1571.0 kcal/day; Cronbach’s alpha=0.79, Intraclass Correlation Coefficient=0.80) between measurements. Trained technicians monitored subjects and instructed them to keep their eyes open to ensure they remained awake during the tests.
Figure 2.
Metabolic measurements during each protocol day
Metabolic rate (kcal/day) was measured using indirect calorimetry with a validated ventilated hood system (TrueOne 2400 metabolic cart, Parvo Medics, Sandy, UT, USA). Before each measurement day, a flow meter calibration was conducted and before each use, the metabolic cart was calibrated with reference gas. After achieving steady state (5 minutes, denoted with the dotted line), expired gases were collected for 10 minutes and used to calculate metabolic rate. Trained technicians monitored subjects and instructed them to keep their eyes open to ensure they remained awake during the tests. Data shown as mean ± SD.
Statistical analysis
Independent sample t-tests compared demographic and sleep variables between sleep-restricted and control subjects and between sleep-restricted gender and race groups. Wilcoxon signed rank tests compared the percent of African Americans, percent of women, as well as pre-study sleep timing between sleep-restricted and control subjects and between sleep-restricted gender and race groups. Mixed model ANOVAs compared changes across protocol days for RMR and RQ between sleep-restricted and control subjects and between sleep-restricted gender and race groups. Planned comparisons were conducted using repeated measures ANOVA and Wilcoxon signed rank tests. All subjects were included in all analyses except for in-laboratory sleep duration; polysomnography data was unavailable for n=10 sleep-restricted subjects. Statistical analyses were completed using IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY). Effect sizes were calculated using Cohen’s d33.
Results
Subject characteristics
Sleep-restricted subjects (n=36) did not differ from control subjects (n=11) in age, BMI, FFM, or in the percentage of African Americans or women (ps>0.66; Table 1). Sleep duration and timing during the week prior to the in-laboratory study were assessed using wrist actigraphy; there were no differences in pre-study sleep duration, onset, offset or midpoint between sleep-restricted and control subjects (ps>0.13). During the in-laboratory study, sleep duration (assessed using polysomnography) did not differ between sleep-restricted subjects and control subjects during baseline sleep (sleep-restricted subjects: 529.9±55.6 min, control subjects: 494.4±64.3 min, p=0.09). Compared to control subjects, sleep-restricted subjects exhibited a shorter sleep duration during sleep restriction (SR1/CD1: sleep-restricted subjects: 223.5±14.5 min, control subjects: 482.8±84.6 min, p<0.001 and SR5/CD5: sleep-restricted subjects: 229.0±10.9 min, control subjects: 450.4±93.3 min, p<0.001) and a longer sleep duration during recovery sleep (sleep-restricted subjects: 661.5±40.2 min, control subjects: 481.6±71.8 min, p<0.001).
Table 1.
Mean (SD) Subject Demographic Characteristics
| Age y | Weight kg | BMI kg/m2 | Fat-Free Mass kg | % Body Fat | Pre-Study Sleep Duration | Pre-Study Sleep Midpoint | |
|---|---|---|---|---|---|---|---|
| Control Subjects (n=11) | 34.0 (9.4) | 73.4(11.5) | 24.3 (3.4) | 52.6 (9.8) | 28.3(10.5) | 7h 57m(30.6 m) | 03:53(33.0 m) |
| Sleep-Restricted Subjects (n=36) | 33.8 (9.2) | 74.0(12.3) | 24.8 (3.1) | 52.2 (11.5) | 30.1(9.4) | 7h 55m(26.4 m) | 03:41(43.2 m) |
| Men (n=20) | 37.4(9.5) | 79.4(12.4) | 24.7(3.1) | 60.8(7.8) | 23.7(6.1) | 7h 53m(21.6 m) | 03:40(42.6 m) |
| Women (n=16) | 29.4 (6.7) | 67.2(8.4) | 25.0(3.3) | 41.6(3.6) | 38.1(6.2) | 7h 57m(32.4 m) | 03:44(45.0 m) |
| Caucasians (n=14) | 34.1(7.1) | 75.8(12.4) | 25.0(3.1) | 55.0(12.0) | 28.1(8.8) | 7h 53m(24.0 m) | 03:50(37.8 m) |
| African Americans (n=22) | 33.7(10.5) | 72.9(12.4) | 24.7(3.2) | 50.5(11.1) | 31.4(9.8) | 7h 56m(28.8 m) | 03:35(46.8 m) |
Body composition was assessed using bioelectrical impedance analysis.
Pre-study sleep duration and timing were assessed using wrist actigraphy.
Sleep-restricted African Americans and Caucasians did not differ in age, BMI, FFM or the percentage of women (ps>0.26, Table 1). Sleep-restricted men and women did not differ in BMI or the percentage of African Americans (ps>0.49, Table 1). However, compared to women, men were older (p=0.008) and had greater FFM (p<0.001). In sleep-restricted subjects, there were no gender or race differences in pre-study sleep duration, onset, offset or midpoint (all p>0.15, Table 1) or in sleep duration during the in-laboratory study (ps>0.12).
Weight Gain and Caloric Intake
Sleep-restricted subjects gained 1.31±1.22 kg from baseline day 1 (BL1) to sleep restriction day 5 (SR5; t35=6.42, p<0.001), whereas the change in weight in control subjects (0.62±1.33 kg) from BL1 to control day 5 (CD5) was not significant (p=0.15). The change in weight from BL1 to SR5/CD5 was not significantly different between sleep-restricted and control subjects (covariates: age, BMI, gender and race, p=0.097).
Sleep-restricted subjects and control subjects did not differ in caloric or macronutrient intake during BL1, SR5/CD5 or recovery day 1/control day 6 (R1/CD6; ps>0.05). However, sleep-restricted subjects consumed more calories per day (F1,42=5.30, p=0.026), a higher percentage of calories from fat (F1,42=5.25, p=0.027) and a lower percentage of calories from protein (F1,42=7.71, p=0.008), on average, from baseline day 2 (BL2, when kept awake until 0400h) to sleep restriction day 4 (SR4, the day prior to the metabolic measurement that occurred upon waking on SR5) than control subjects on corresponding days (BL2 to CD4) (covariates: age, gender and race).
Although the difference was not significant, sleep-restricted African Americans gained more weight from BL1 to SR5 than Caucasians (African Americans: 1.57±1.08 kg, Caucasians: 0.89±1.34 kg, covariates: age, BMI, gender, p=0.084). African Americans and Caucasians showed comparable daily caloric intake during the study (BL1-R1; covariates: age and gender, ps>0.24). Sleep-restricted men and women did not significantly differ in weight gain from BL1 to SR5 (men: 1.47±1.13 kg, women: 1.10±1.33 kg, p=0.58; covariates: age, BMI, race); however, men consumed more calories throughout the study than women (BL1: p=0.091, BL2-SR4: p=0.021, SR5: p=0.002, and R1: p=0.002).
Resting metabolic rate
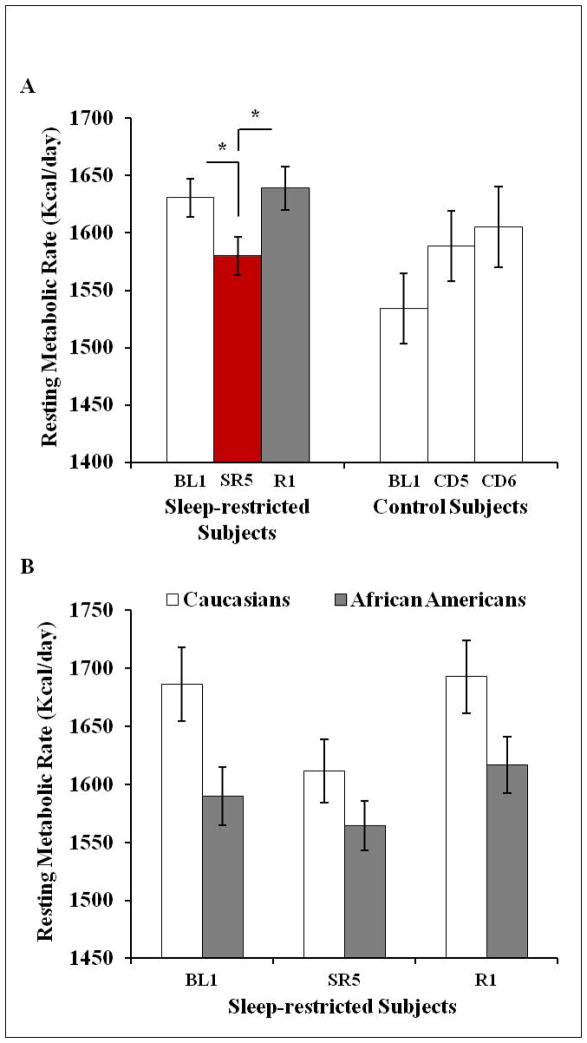
A mixed-model ANOVA, with RMR measurement day as the repeated-measures variable (BL1, SR5/CD5 and R1/CD6), condition (control versus sleep-restriction) as the independent variable, and age, gender, race, FFM, and caloric intake during sleep restriction/corresponding control condition days (BL2-SR4/CD4) as covariates, was conducted. There was a significant day X condition interaction (F2,80=6.10, p=0.003); RMR varied across the three measurements in sleep-restricted subjects (F2,70=7.79, p=0.001) but not in control subjects (p=0.42). In sleep-restricted subjects, RMR was lower on SR5 compared to BL1 (p=0.032, dz=0.37, raw change: −41.72 kcal/day, −2.6%), and higher on R1 compared to SR5 (p<0.001, dz=0.84, raw change: 60.02 kcal/day, +3.8%) but did not differ between BL1 and R1 (p=0.24) (Figure 3A). Comparisons were also significant when using non-parametric tests (BL1 to SR5: p=0.021, SR5 to R1: p<0.001).
Figure 3.
Resting metabolic rate across protocol days
(A) A mixed-model ANOVA, with RMR measurement day as the repeated-measures variable (BL1, SR5/CD5 and R1/CD6), condition (control versus sleep restriction) as the independent variable and age, gender, race, FFM, and caloric intake during sleep restriction/corresponding control condition days (BL2-SR4/CD4) as a covariates, revealed that there was a significant day X condition interaction (p=0.003). RMR varied across the three measurements in sleep-restricted subjects (p=0.001) but not in control subjects. In sleep-restricted subjects, RMR was lower on SR5 compared to BL1 (p=0.032, raw change: −41.72 kcal/day, −2.6%), and higher on R1 compared to SR5 (p<0.001, raw change: 60.02 kcal/day, +3.8%) but did not differ between BL1 and R1. (B) In sleep-restricted subjects, a mixed-model ANOVA, with RMR measurement day as the repeated-measures variable (BL1, SR5 and R1), gender and race as independent variables and age, FFM, and BL2-SR4 caloric intake as covariates, revealed no significant day X race or day X gender interactions. There was a significant main effect of race (p=0.043) such that African Americans exhibited a lower RMR than Caucasians during the study; however, there was no main effect of gender (p=0.38). Data shown as mean ± SEM, *p<0.05.
In sleep-restricted subjects, a mixed-model ANOVA, with RMR measurement day as the repeated-measures variable (BL1, SR5 and R1), gender and race as independent variables and age, FFM, and BL2-SR4 caloric intake as covariates was conducted. There were no significant day X race (p=0.25) or day X gender interactions (p=0.85). There was a significant main effect of race (F1,29=4.50, p=0.043, d=0.60) such that African Americans exhibited a lower RMR than Caucasians during the study (Figure 3B) and no main effect of gender (p=0.38).
Respiratory quotient
A mixed-model ANOVA, with RQ measurement day as the repeated-measures variable (BL1, SR5/CD5 and R1/CD6), condition (control versus sleep-restriction) as the independent variable, and age, gender, race, FFM, and caloric intake during sleep restriction/control condition (BL2-SR4/CD4) as covariates, was conducted. The day X condition interaction was not significant (p=0.22). For both groups, RQ was higher on SR5/CD5 (sleep-restricted subjects: p<0.001; control subjects: p=0.019) and R1/CD6 (sleep-restricted subjects: p<0.001; control subjects: p=0.012) compared to BL1, but did not differ between SR5/CD5 and R1/CD6 (sleep-restricted subjects: p=0.57; control subjects: p=0.22; Table 2).
Table 2.
Mean (SD) RQ across Protocol Days
| BL1 | SR5/CD5 | R1 | Change in RQ (SR5 – BL1) | |
|---|---|---|---|---|
| Control Subjects (n=11) | 0.86 (0.04) | 0.91 (0.07)* | 0.93 (0.09)* | 0.05 (0.06) |
| Sleep-Restricted Subjects (n=36) | 0.85 (0.05) | 0.89 (0.06)* | 0.90 (0.07)* | 0.05 (0.06) |
| Men (n=20) | 0.84 (0.05) | 0.91 (0.06)* | 0.92 (0.07)* | 0.06 (0.05) |
| Women (n=16) | 0.85 (0.04) | 0.88 (0.05) | 0.87 (0.06) | 0.03 (0.06) |
| Caucasians (n=14) | 0.83 (0.04) | 0.87 (0.06)* | 0.88 (0.06)* | 0.04 (0.06) |
| African Americans (n=22) | 0.86 (0.05) | 0.91 (0.05)* | 0.91 (0.07)* | 0.05 (0.06) |
p<0.05, compared to BL1
In sleep-restricted subjects, a mixed-model ANOVA, with RQ measurement day as the repeated-measures variable (BL1, SR5 and R1), gender and race as independent variables and age, FFM, and BL2-SR4 caloric intake as covariates was conducted. There was not a significant day X race interaction (p=0.89) but there was a significant day X gender interaction (p=0.034). RQ varied across the three measurements in men (F2,38=14.93, p<0.001) but not in women (p=0.097). Although both gender groups exhibited an increase in RQ from BL1 to SR5 and R1, the increase was more marked in men (BL1 to SR5: p<0.001, BL1 to R1: p<0.001) and not significant in women (BL1 to SR5: p=0.07, BL1 to R1: p=0.15; Table 2).There was a significant main effect of race (F1,29=7.00, p=0.013, d=0.70) such that African Americans exhibited a higher RQ than Caucasians during the study (Table 2) but no main effect of gender (p=0.88).
Discussion
Resting metabolic rate (RMR)—the largest (60–70%) component of energy expenditure—was significantly reduced after sleep restriction and rebounded to baseline levels after one night of recovery sleep. Our finding is consistent with a study reporting reductions in RMR following three weeks of sleep restriction (5.6h sleep/24h) and forced desynchrony (28h “days”), and a return in RMR to baseline levels following 9 nights of recovery sleep with circadian re-entrainment (10h sleep/24h) under controlled caloric intake conditions19. Our results demonstrate that sleep restriction, without significant circadian disruption and under ad libitum feeding conditions, decreased RMR. Furthermore, RMR returned to baseline levels after only one night of recovery sleep, consistent with the time course of the caloric intake response3.
Other studies have found no effect of sleep restriction on RMR9,10 when assessed the day following sleep loss. Discrepant findings may be due to differences in study design. For example, studies allowed subjects to leave the laboratory to walk outside, consume caffeine daily during the study, or use a gym at their leisure, thereby potentially confounding the assessment of RMR9,10.
The compensatory hypothesis posits that sleep restriction produces weight gain due to the overcompensation of neuroendocrine, metabolic and behavioral responses aimed to increase energy intake and conserve energy in order to counteract the additional energy requirement associated with extended wakefulness14. Although the observed change in RMR was small (~50 kcal/day), our study provides further support for this hypothesis. Previous research has found that under non-sleep restriction conditions, short-term (2–7 days) overfeeding leads to weight gain (+0.5–1.8 kg) and increased RMR (+1.1–11.7%)34,35. In the current study, food/drink was ad libitum. We estimated the amount of daily calories each subject would need to consume in order to maintain weight during the study (each subject’s measured RMR multiplied by 1.4, to account for energy needed for sedentary activity levels in the laboratory environment)38. Using this estimation, subjects consumed 35% more calories than needed during sleep restriction (BL2-SR4). For example, if it was estimated that a subject needed to consume 2000 calories to maintain weight, he/she consumed 700 additional calories during sleep restriction. Despite this recent history of overconsumption, subjects exhibited a decrease in RMR measured in the morning after the fifth night of sleep restriction. Thus, sleep restriction produces metabolic responses to substantially increase caloric intake and to conserve energy which is conducive to weight gain over time. By contrast, recovery sleep reverses these responses.
It remains unknown which mechanisms may underlie the effect of sleep loss on changes in resting metabolic rate. In contrast to humans, rodents exhibit marked increases in energy expenditure in response to sleep deprivation which prevents weight gain despite increased caloric intake37. More research in humans is needed to better understand what mechanisms may underlie the decrease in resting metabolic rate that we observed the morning following sleep restriction; decreases in energy used for immune, endocrine and sympathetic nervous system functions are areas that should be examined.
Consistent with previous studies17,21, sleep restriction increased RQ, which has been associated with weight gain12,24. However, this increase was also observed in control subjects, suggesting that factors other than sleep restriction, such as overeating25 and living in a sedentary laboratory environment, contributed to the RQ effect.
Relative to Caucasians, African Americans exhibited a lower RMR and a higher RQ regardless of sleep duration but had comparable caloric intake during the study. Although there is debate whether or not race differences in RMR or RQ are associated with weight gain/obesity38, it is possible that these differences led to greater weight gain during our in-laboratory study. African Americans—who consistently report shorter sleep durations39 and have a higher prevalence of overweight/obesity (particularly women) in population studies40—may need to compensate for a lower RMR by reducing caloric intake to a greater degree than Caucasians or by increasing physical activity in order to prevent weight gain.
The current study had several limitations. Because subjects needed to remain in the laboratory for the duration of the protocol, body composition was assessed using bioelectrical impedance analysis which has limited sensitivity; therefore, we were unable to examine changes in body composition after sleep loss. Due to time restrictions related to our protocol, we used a metabolic cart with 15-minute test duration to assess metabolic rate. Whole-room indirect calorimetry systems with continuous recordings for multiple hours provide a more complete measure of changes in energy expenditure13. Physical activity was not directly assessed in the current study and was kept sedentary within the confines of our laboratory; future studies are needed to assess if there are race or gender differences in activity levels. Finally, in our subjects we did not systematically monitor the menstrual cycle and allowed the use of oral contraceptives. Only n=2 women were taking oral contraceptives at the time of the study and all women except for two reported experiencing regular menstrual cycles (one woman was not actively menstruating due to oral contraceptive use and one woman had a partial hysterectomy).
Conclusion
Sleep restriction decreased morning resting metabolic rate in healthy adults, suggesting that sleep loss leads to metabolic changes aimed at conserving energy. A lower resting metabolic rate—combined with the increased caloric intake that occurs during sleep restriction—places adults who are habitual short sleepers at heightened risk for weight gain and obesity.
What is already known about this subject?
Habitual short sleep duration is consistently associated with weight gain and increased risk for obesity
Sleep restriction leads to weight gain and increased caloric intake
What does your study add?
Resting metabolic rate is decreased after sleep restriction under ad libitum feeding conditions in healthy diverse men and women
Acknowledgments
FUNDING: NIH grants R01 NR004281 (DFD) and F31 AG044102 (AMS); the Department of the Navy, Office of Naval Research Award No. N00014-11-1-0361 (NG); and Clinical and Translational Research Center (CTRC) grant UL1TR000003.
The authors thank the participants in the experiments and the faculty and staff who helped acquire the data.
Footnotes
DISCLOSURE: Dr. Spaeth and Dr. Goel have no conflicts of interest. Dr. Dinges is compensated for serving on a scientific advisory council for Mars, Inc.
AUTHOR CONTRIBUTIONS: The authors’ responsibilities were as follows: AMS, DFD and NG designed research, AMS conducted research, AMS analyzed data, AMS, DFD and NG wrote the manuscript, and AMS had primary responsibility for final content. All authors read and approved the final manuscript.
References
- 1.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among U.S. adults. Obesity. 2014;22:598–607. doi: 10.1002/oby.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 3.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36:981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer KA, Wall MM, Larson NI, Laska MN, Neumark-Sztainer D. Sleep duration and BMI in a sample of young adults. Obesity. 2012;20:1279–1287. doi: 10.1038/oby.2011.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang TC, Matthews SA, Chen VY. Stochastic variability in stress, sleep duration, and sleep quality across the distribution of body mass index: insights from quantile regression. Int J Behav Med. 2014;21:282–291. doi: 10.1007/s12529-013-9293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donat M, Brown C, Williams N, Pandey A, Racine C, McFarlane SI, et al. Linking sleep duration and obesity among black and white US adults. Clin Pract. 2013;10 doi: 10.2217/cpr.13.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98:E703–707. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filozof C, Gonzalez C. Predictors of weight gain: the biological-behavioural debate. Obes Rev. 2000;1:21–26. doi: 10.1046/j.1467-789x.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 13.Leonard WR. Laboratory and field methods for measuring human energy expenditure. Am J Hum Biol. 2012;24:372–384. doi: 10.1002/ajhb.22260. [DOI] [PubMed] [Google Scholar]
- 14.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–1801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013;98:1433–1439. doi: 10.3945/ajcn.113.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr. 2011;94:804–808. doi: 10.3945/ajcn.111.017632. [DOI] [PubMed] [Google Scholar]
- 18.Shechter A, Rising R, Wolfe S, Albu JB, St-Onge MP. Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int J Obes (Lond) 2014;38:1153–1158. doi: 10.1038/ijo.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–1236. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 21.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 23.Bromley LE, Booth JN, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35:977–984. doi: 10.5665/sleep.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra M, Scalfi L, Contaldo F, Pasanisi F. Fasting respiratory quotient as a predictor of long-term weight changes in non-obese women. Ann Nutr Metab. 2004;48:189–192. doi: 10.1159/000079556. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt SL, Kealey EH, Horton TJ, VonKaenel S, Bessesen DH. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int J Obes (Lond) 2013;37:1192–1197. doi: 10.1038/ijo.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaeth AM, Dinges DF, Goel N. Gender and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100:559–566. doi: 10.3945/ajcn.114.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 29.Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hise ME, Sullivan DK, Jacobsen DJ, Johnson SL, Donnelly JE. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr. 2002;75:263–267. doi: 10.1093/ajcn/75.2.263. [DOI] [PubMed] [Google Scholar]
- 31.Bassett DR, Howley ET, Thompson DL, King GA, Strath SJ, McLaughlin JE, et al. Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J Appl Physiol. 2001;91:218–224. doi: 10.1152/jappl.2001.91.1.218. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JA, Watras AC, O’Brien MJ, Luke A, Dobratz JR, Earthman CP, et al. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009;109:128–132. doi: 10.1016/j.jada.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863–875. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joosen AM, Bakker AH, Westerterp KR. Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol Behav. 2005;85:593–597. doi: 10.1016/j.physbeh.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Harris AM, Jensen MD, Levine JA. Weekly changes in basal metabolic rate with eight weeks of overfeeding. Obesity. 2006;14:690–695. doi: 10.1038/oby.2006.78. [DOI] [PubMed] [Google Scholar]
- 36.Black AE. Physical activity levels from a meta-analysis of doubly labeled water studies for validating energy intake as measured by dietary assessment. Nutr Rev. 1996;54:170–174. doi: 10.1111/j.1753-4887.1996.tb03924.x. [DOI] [PubMed] [Google Scholar]
- 37.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol. 1993;264:R376–387. doi: 10.1152/ajpregu.1993.264.2.R376. [DOI] [PubMed] [Google Scholar]
- 38.Gannon B, DiPietro L, Poehlman ET. Do African Americans have lower energy expenditure than Caucasians? Int J Obes Relat Metab Disord. 2000;24:4–13. doi: 10.1038/sj.ijo.0801115. [DOI] [PubMed] [Google Scholar]
- 39.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]