Abstract
Yersinia enterocolitica is a major cause of human yersiniosis, with enterocolitis being a typical manifestation. These bacteria can cross the intestinal mucosa, and invade eukaryotic cells by binding to host β1 integrins, a process mediated by the bacterial effector protein invasin. This study examines the role of hypoxia on the internalization of Y. enterocolitica into intestinal epithelial cells, since the gastrointestinal tract has been shown to be physiologically deficient in oxygen levels (hypoxic), especially in cases of infection and inflammation. We show that hypoxic pre-incubation of Caco-2 cells resulted in significantly decreased bacterial internalization compared to cells grown under normoxia. This phenotype was absent after functionally blocking host β1 integrins as well as upon infection with an invasin-deficient Y. enterocolitica strain. Furthermore, downstream phosphorylation of the focal adhesion kinase was also reduced under hypoxia after infection. In good correlation to these data, cells grown under hypoxia showed decreased protein levels of β1 integrins at the apical cell surface whereas the total protein level of the hypoxia inducible factor (HIF-1) alpha was elevated. Furthermore, treatment of cells with the HIF-1 α stabilizer dimethyloxalylglycine (DMOG) also reduced invasion and decreased β1 integrin protein levels compared to control cells, indicating a potential role for HIF-1α in this process. These results suggest that hypoxia decreases invasin-integrin-mediated internalization of Y. enterocolitica into intestinal epithelial cells by reducing cell surface localization of host β1 integrins.
Introduction
The human gastrointestinal (GI) tract is home to an array of bacteria, some commensals that are vital to human digestion and others that can cause acute or chronic infections. GI pathogens have been the subject of extensive studies, and many host-pathogen interactions in this tissue have been fully characterized. Thus, it is important to address the environmental setting in which these interactions occur and the factors that are involved. The GI tract represents its own microenvironment within the body: a vascularized, oxygenated, subepithelial mucosa bordered by the severely anoxic luminal region [1]. The intestinal epithelial layer has been shown to be in a physiological state of oxygen deprivation, also known as hypoxia, characterized by daily fluctuations in oxygen tensions with oxygen levels ranging from 1 to 7% [1–3]. This environment can be challenged even more upon onset of acute infections or chronic inflammation. In fact, infection sites often result in severe hypoxia, with oxygen levels dropping below 1% [4] because of decreased oxygen permeation, increased consumption by invading pathogens and infiltration of recruited immune cells [5,6]. Hypoxia has been shown to lead to numerous changes within host cells, including cytoskeletal rearrangements [7] and alteration of membrane composition [8]. However, it is still not entirely clear whether a hypoxic environment affects internalization of invasive bacteria such as Yersinia enterocolitica into epithelial cells.
Y. enterocolitica is a gram-negative, facultative intracellular zoonotic pathogen that infects the gastrointestinal tract, causing a variety of diseases like gastroenteritis, acute enteritis and enterocolitis especially in children [9]. The most common source of human infections with Y. enterocolitica is ingestion of contaminated food [10]. After ingestion, Y. enterocolitica transverses the intestinal lumen and overlying mucosal layer, across the intestinal epithelial barrier and colonizes the underlying lymphoid tissues [9,11]. The preferential entry of Y. enterocolitica into ileal Peyer’s patches seems to be facilitated by attachment to and penetration of epithelial microfold (M) cells [12–14]. The uptake by epithelial cells is predominantly mediated by invasin of Y. pseudotuberculosis [15,16] and Y. enterocolitica [17,18], but other adhesins like Ail and YadA can contribute to this process [19]. Invasin-promoted internalization is characterized by a “zipper” mechanism [20]. Invasin interacts with high affinity with several members of the β1 integrin family through its extracellular C-terminal region [21]. Interaction of invasin of Y. pseudotuberculosis was shown to bind with a 100 fold higher affinity than the integrin’s natural ligand, fibronectin [22]. Integrins are a family of large transmembrane glycoproteins that function as receptors on the surface of cells, existing as heterodimers of one α and one β subunit, which are non-covalently linked [23]. Among the 18 α and 8 β subunits, β1 integrins are the most widespread [24]. They can be activated by internal as well as external cues, and thus are able to promote inside-out and outside-in signal transduction cascades [25]. Several β1 chain integrins, mainly α5β1 along with α3β1, α4β1, α6β1 and αvβ1, were shown to be receptors for invasin [21]. Invasin binding to integrins triggers receptor clustering, a step that is required for Y. pseudotuberculosis uptake into host cells [26]. Consequently, a series of signaling cues is initiated, promoting the recruitment of tyrosine kinases like the focal adhesion kinase (FAK) and the involvement of the GTPase Rac1 that induces bacterial entry into non-phagocytic cells [27,28].
The goal of this study is to investigate the effect of hypoxia on the β1 integrin-mediated internalization of Y. enterocolitica using Caco-2 cells as a polarized intestinal epithelial cell model. We suggest that cellular changes induced by hypoxia lead to a reduction in cell surface localization of host β1 integrins thus decreasing invasin-integrin-mediated internalization of Y. enterocolitica into intestinal epithelial cells.
Materials and Methods
Cell culture, bacterial strains and growth conditions
Ethics approval was not required since a commercially available human epithelial colorectal adenocarcinoma, Caco-2, cell line (ATCC® HTB-37™) [29] was used in the project. Cells were maintained in high glucose (4.5 g/L) Dulbecco’s modified Eagle medium (DMEM, Sigma), supplemented with 10% heat-inactivated fetal calf serum (FCS, Gibco BRL), and 50 U/ml Penicillin and 50 μg/ml Streptomycin (Sigma, Germany). Caco-2 cells were grown on polystyrene 24 well plates (Sardstedt, Nümbrecht, Germany) for 6 days post confluency.
The bacterial strains used in this study are Y. enterocolitica 8081v bioserotype 1A/O:8, patient isolate, wild-type [30] Y1/07 bioserotype 4/O:3, patient isolate, wild-type and YE21 (Y1, ΔinvA, kanamycin resistant KnR) [31]. Overnight cultures of Y. enterocolitica 8081v were grown at 27°C, and Y. enterocolitica Y1/07 and YE21 were grown at 37°C in Luria-Bertani (LB) broth. The antibiotics used for YE21 selection were carbenicillin 100 mg/ml and kanamycin 50 mg/ml.
Normoxic incubations were performed in a tissue culture incubator at 37°C, 5% CO2 in water saturated air, while hypoxic incubations were performed in an oxygen control hypoxia glove box (Coy Laboratory Products, Grass Lake MI, USA) at 37°C, 1% O2 and 5% CO2 in a humidified (100%) incubation chamber within the glove box. Alternatively, the prolyl-4-hydroxylase inhibitor dimethyloxalylglycine (DMOG; Sigma, Germany) was used to chemically stabilize the HIF-1α subunit under normoxia. DMOG, dissolved in water, was added to the media at 450 μM for 7 hrs.
Oxygen measurements
Immobilized PSt3 oxygen sensor spots (PreSens, Regensburg, Germany) were attached to the inside of 24 well plates and a polymer optical fiber (POF) was connected to a fiber optic oxygen transmitter that relayed the emitted light to a Fibox4 microprocessor (PreSens, Regensburg, Germany). In this manner, oxygen was measured non-invasively and was not consumed during the process of measurement. Caco-2 cells were grown for 6 days under normoxia and then either moved to 1% O2 for 24 hr or kept at normoxia. On day 7 post confluency the cells were infected with Y. enterocolitica O:8 8081v (see below) and dissolved oxygen was measured at time of infection (24 hr), 1.5, 2.5, 4 and 6 hrs post infection.
Infection and internalization
Caco-2 cells were seeded at 0.82 x 104 cells/cm2 in a 24-well plate with growth area of 1.82 cm2 and grown for 6 days post confluence in a normal tissue culture incubator. On day 6, media was changed and two plates were placed at 1% O2 for 24 hr while one plate was left under normoxia for 24 hr. One well of Caco-2 cells was counted and cells were used at 1.65–2.75 x 106 cells/cm2. Cells were washed three times with PBS and incubated in DMEM without FCS or antibiotics. Y. enterocolitica O:8 8081v or O:3 Y1/07 strains were grown till OD600 = 0.5 and used to infect Caco-2 cells at MOI 10. Plates were centrifuged at 142 g for 5 minutes (min) at 20°C and then incubated for 90 min at 37°C at normoxia or hypoxia, accordingly. After 90 min, media was removed and cells were washed with PBS to remove non-associated bacteria. FCS and antibiotic-free media with gentamicin 100 μg/ml (Sigma, Germany) was added to half of the wells for 60 min and the other half was kept with media only. The supernatant of cells incubated with bacteria and gentamicin was plated to ensure bacterial killing, and also taken for cytotoxicity assays. Cells were washed with PBS to remove antibiotics, trypsinized for 2 min with Trypsin-EDTA (Sigma, Germany), and then lysed with 0.1% Triton X-100 in media. Cell lysates were serially diluted and plated on LB agar. The total number of associated bacteria was determined by counting the colony-forming units (CFU) from wells without gentamicin and the number of internalized bacteria was determined by counting the CFU from wells with gentamicin. Internalization was calculated as percentage of gentamicin surviving bacteria relative to the total number of associated bacteria.
Blocking of β1 integrin function
Caco-2 cells were grown for 6 days post confluence in a normal tissue culture incubator. On day 6, media was changed and plates were placed at 1% O2 for 24 hr while one plate was left under normoxia for 24 hr. One hr before infection, media was removed and replaced with media containing 45 μg/ml of either 6S6 anti-β1 integrin (Merck-Millipore, Germany) or the mouse IgG1 (Merck-Millipore, Germany). Cells were incubated for 1 hr at 37°C at normoxia or hypoxia. Cells were then washed and infection proceeded as described above using Y. enterocolitica O8 8081v at MOI 10.
Infected cell lysis and FAK Western blots
Cells were infected as mentioned in the previous section (infection and internalization) and after 1.5 hrs of infection the media was removed and each well was washed twice with phosphate-buffered saline. Cells were lysed with 1% Triton X-100, 2 mM sodium fluoride, 1 mM ethylenediaminetetraacetic acid in phosphate-buffered saline with protease inhibitor mix (Antipain dihydrochloride 1.48 μM, Pepstatin A 1.46 μM, Leupeptin 10.51 μM, Aprotinin 0.768 μM, Trypsin inhibitors 50 μg/ml and phenylmethanesulfonyl fluoride (PMSF) 1 mM; Sigma, Germany). Subsequently, cells were centrifuged at 17, 000 g at 4°C for 10 min and supernatants including cellular proteins were collected and frozen at -20°C until usage. Equal protein amounts (50 μg) of total cell lysates from each sample were denatured in boiling Laemmli buffer plus 50 mM dithiothreitol for 5 min. Samples were then subjected to 8% sodiumdodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Roth, Germany). Total amount of FAK was detected using FAK (D2R2E) rabbit monoclonal antibody and phosphorylated FAK at Tyr397 was detected using P-FAK Y397 (D20B1) rabbit monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA). β-Actin (Santa Cruz Biotechnology, CA, USA) served as loading control. Quantification of band intensities was performed using Image J 1.48v (National Institutes of Health, USA).
Whole cell lysis and HIF-1α Western blots
Whole-cell extracts were obtained from Caco-2 cells grown for 6 days post confluency under normoxia. At day 6, they were either left under normoxia or placed under hypoxia for 24 hr after which they were lysed. Supernatants were removed and cells were washed in cold PBS over ice and scraped into 1 ml of lysis buffer (0.1% Nonidet P40, 300 mM NaCl, 10 mM Tris pH 7.9, 1 mM ethylenediaminetetraacetic acid in phosphate-buffered saline), with protease inhibitor mix (Antipain dihydrochloride 1.48 μM, Pepstatin A 1.46 μM, Leupeptin 10.51 μM, Aprotinin 0.768 μM, Trypsin inhibitors 50 μg/ml and phenylmethanesulfonyl fluoride (PMSF) 1 mM; Sigma, Germany). Subsequently, cells were centrifuged at 17, 000 g at 4°C for 10 min and supernatants including cellular proteins were collected and frozen at -20°C until use. Equal protein amounts (50 μg) of total cell lysates from each sample were denatured in boiling Laemmli buffer plus 50 mM dithiothreitol for 5 min. Samples were then subjected to 8% sodiumdodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Roth, Germany). β1 integrin was detected with a purified mouse anti-Integrin β1 antibody (BD Transduction Laboratories, USA). HIF-1 α was detected with a purified rabbit anti-human HIF-1α antibody (Merck-Millipore, Temecula, CA, USA). β-Actin (Santa Cruz Biotechnology, CA, USA) served as loading control. Quantification of band intensities was performed using Image J 1.48v (National Institutes of Health, USA).
Brush border membrane isolation and sucrase activity
Caco-2 cells grown for 6 days post-confluency under normoxia. At day 6, they were either left under normoxia or placed under hypoxia for 24 hr. Brush border membranes of Caco-2 cells were isolated by the divalent cation precipitation method [32,33]. Cells were homogenized using a Potter–Elvehjem homogenizer in the hypertonic homogenization buffer (300 mM Mannitol, 12 mM Tris-HCl pH 7.1) supplemented with protease inhibitor mix (Antipain dihydrochloride 1.48 μM, Pepstatin A 1.46 μM, Leupeptin 10.51 μM, Aprotinin 0.768 μM, Trypsin inhibitors 50 μg/ml and phenylmethanesulfonyl fluoride (PMSF) 1 mM; Sigma, Germany). The homogenates were passed through a Luer-21 Gage needle and CaCl2 was added to a final concentration of 10 mM and then centrifuged at 5,000 x g for 15 min to obtain the homogenate fraction (H). Homogenates were then incubated at 4°C for 30 min with gentle agitation and centrifuged again at 5,000 x g for 15 min. The pellet was then resuspended in 10 mM Tris-HCl + 150 mM NaCl pH 7.4 to obtain the basolateral and microsomal membrane vesicle fraction (P1). The supernatant was centrifuged at 25,000 x g for 30 min and the pellet was resuspended in 10 mM Tris-HCl + 150 mM NaCl pH 7.4 to yield the apical membrane/brush border membrane fraction (P2) while the supernatant contained all other soluble and small vesicular membrane-bound fraction (S).
Subsequently, 50 μg of total cell lysates from each sample were denatured in boiling Laemmli buffer plus 50 mM dithiothreitol for 5 min. Samples were then subjected to 8% sodiumdodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Roth, Germany). β1 integrins were detected with a purified mouse anti-Integrin β1 antibody (BD Transduction Laboratories, USA) and sucrase isomaltase was detected using mAb anti-SI antibody HBB 3/705 [33] obtained from Drs. Hans-Peter Hauri and Erwin Sterchi (University of Basel and University of Bern, Switzerland).
Sucrase activity in the homogenates, basolateral membranes (P1 fraction), supernatant (S fraction) and brush border membranes (P2 fraction) was measured using 150 mM sucrose added to 25 μl of sample and end glucose was detected using GOD PAP fluid (Axiom Diagnostics, Worms, Germany) at 492 nm. Sucrase specific activity was calculated as μM.hour-1.mg-1 of protein.
Immunofluorescence
Caco-2 cells were grown on glass on cover slips in a 24-well plate for 6 days post confluence in a normal tissue culture incubator. On day 6, media was changed and plates were placed at 1% O2 or left under normoxia for 24 hr. Cells were fixed with ice-cold methanol for 15 min and washed with Tris buffered saline with 0.01% Tween 20 (TBS-T). Coverslips were then incubated in blocking solution of 3% BSA with 0.01% TBS-T for 30 min at room temperature followed by permeabilization using 0.3% Triton X-100 for 15 min at room temperature. After washing, coverslips were incubated with 0.01 mg/ml mouse anti-β1 integrin (Merck-Millipore, Germany) or the mouse IgG1 isotype control (Merck-Millipore, Germany) diluted in 3% BSA with 0.01% TBS-T at room temperature for 2 hr. Coverslips were washed with 0.01% TBS-T and incubated with secondary goat anti-mouse Alexa Fluor® 488-labeled antibody (Invitrogen, Germany) for 45 min at room temperature, protected from light. After washing, coverslips were embedded in ProlongGold + DAPI™ (Invitrogen, Germany). Microscopy was performed using a Leica TCS SP5 confocal fluorescence microscope with a HCX PL APO 40X 0.75–1.25 oil immersion objective. Gain settings were kept the same when acquiring images of cells grown under the two conditions.
Statistical analysis
All experiments were performed in duplicate three independent times. Data were analyzed using Excel 2010 (Microsoft) and GraphPad Prism 6.0 (GraphPad Software). Differences between two or more groups were analyzed by using a One-way ANOVA with Tukey's multiple comparisons test. For Western blots and DMOG internalization statistics, unpaired, two-tailed Student’s t-tests were performed. The significance is indicated as follows: ns = non-significant, * p ≤ 0.05; ** p ≤ 0.01, *** p ≤ 0.001 and **** p < 0.0001.
Results
Characterization of oxygen conditions during Y. enterocolitica invasion into Caco-2 cells
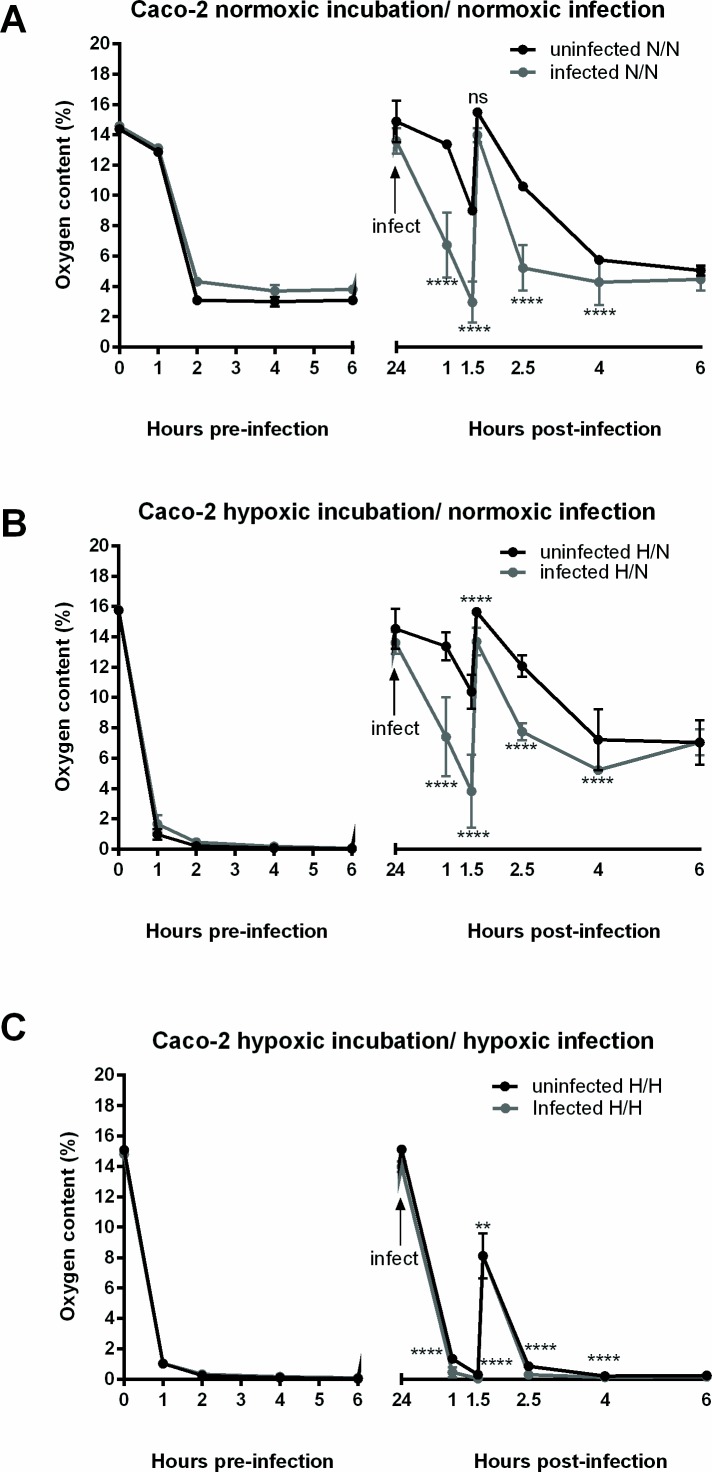
In order to study the host pathogen interactions under hypoxia, the experimental settings of the culture conditions needed to be established. For our purposes, we used Caco-2 cells. This human cell line was grown to a monolayer with differentiated polarized intestinal epithelial cells [34]. Differentiated Caco-2 cells develop brush-border microvilli typical of intestinal enterocytes and express a multitude of intestinal enzymes like sucrase-isomaltase [34,35]. Interestingly, it has been recently shown that in Caco-2 polarized epithelial cell lines, β1 integrins can be found apically at the tight junctions, colocalizing with the zonula occludens proteins [36]. Furthermore, dissolved oxygen levels in the cell culture media were measured using optical sensors, based on the oxygen-dependent quenching of phosphorescent probes that is proportional to the oxygen level in the immediate surroundings [2,37]. Infection incubations were performed under normoxia or hypoxia, thus resulting in three distinct conditions: normoxic pre-incubation / normoxic infection, hypoxic pre-incubation / normoxic infection and hypoxic pre-incubation / hypoxic infection. Oxygen measurements were performed over the course of 6 hours (hr) before infection and 6 hr following infection with Y. enterocolitica 8081v with an MOI of 10 (see experimental procedures for details). Normoxic pre-incubation of uninfected cells resulted in oxygen levels lower than 4% after 6 hr (Fig 1A, left panel). After normoxic infection at time point 24 hr, cells show oxygen levels that decreased much faster than uninfected cells before similar levels (5% O2) are reached after 6 hr (post infection) (Fig 1A, right panel). Hypoxic pre-incubated cells reach levels of approximately 0.04% O2 after 6 hr (Fig 1B and 1C, left panels). Hypoxic pre-incubated cells that were infected under normoxia show a faster decrease in oxygen levels as compared to uninfected cells and finally reach 7% O2 after 6 hr post infection (Fig 1B, right panel). Hypoxic pre-incubated cells that were infected under hypoxia also show a slight yet significant difference in oxygen levels as compared to uninfected cells and finally reach 0.2% O2 after 6 hr of infection (Fig 1C, right panel). It is important to note that after 1.5 hrs of infection in all culture conditions, fresh media with or without gentamicin was added to the cells and corresponds to the peak in oxygen levels that immediately follow.
Fig 1. Oxygen levels in Caco-2 cultures.
Caco-2 cells were grown for 6 days post confluence and then placed under hypoxia or kept under normoxia. (A) Measurements in normoxic pre-incubated and normoxic infected (or uninfected) cells, (B) measurements in hypoxic pre-incubated and normoxic infected (or uninfected) cells and (C) measurements in hypoxic pre-incubated and hypoxic infected (or uninfected) cells. Oxygen peaks represent the addition of fresh media: at time point 24 hr fresh media with bacteria, and at time point 1.5 hr post infection fresh media with gentamicin. Plotted values represent mean ±SEM and are displayed as % oxygen. ** p ≤ 0.01, **** p < 0.0001and ns = non-significant using two-tailed Student’s t-test.
Hypoxic pre-incubation reduces Y. enterocolitica internalization
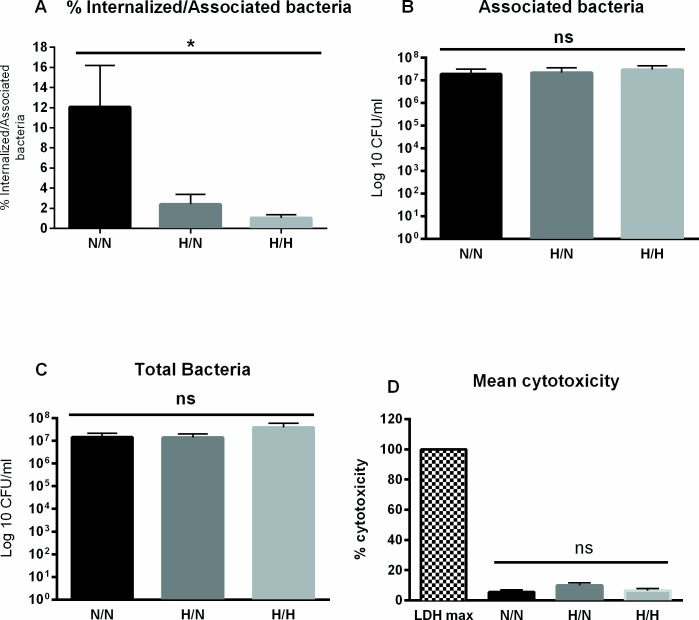
Caco-2 cells were grown for 6 days under normoxia and then either moved to 1% O2 for 24 hr or kept at normoxia. After addition of Y. enterocolitica O:8 8081v at a multiplicity of infection (MOI) 10, plates were centrifuged in order to obtain uniform bacterial attachment to host cells and numbers of intracellular bacteria were identified by gentamicin survival assay [38]. Fig 2A shows that cells pre-incubated under hypoxia had a significantly decreased number of internalized bacteria, after normoxic and hypoxic infection, compared to the normoxic control. Normoxic Caco-2 showed 12% internalized bacteria while hypoxic pre-incubated cells showed 2.4 and 1% internalized bacteria during normoxic and hypoxic infections respectively. Fig 2 shows that there was no significant difference in either the number of associated bacteria (2 B) or in the total bacterial number (2 C) respectively, in the different oxygen incubations. Finally, a lactate dehydrogenase assay (LDH) confirmed no significant cytotoxic effect of hypoxic incubation of Caco-2 cells (Fig 2D).
Fig 2. Y. enterocolitica internalization is reduced in hypoxic incubated cells.
Y. enterocolitica serotype O:8 8081v was used to infect Caco-2 cells (MOI 10) pre-incubated at normoxia or hypoxia for 24 hr. The infection was also performed at normoxia or hypoxia. (A) The percentage of internalized bacteria was significantly reduced in hypoxia pre-incubated cells. There was no significant difference in the number of associated bacteria (B) or in bacterial growth (C) in the cells grown under either condition. (D) Twenty-four hr incubation under hypoxia did not result in significant differences in cytotoxicity as compared to 24 hr under normoxia. * p ≤ 0.05 using one-way ANOVA. Plotted values represent mean ±SEM.
Beta one (β1) integrin-mediated internalization
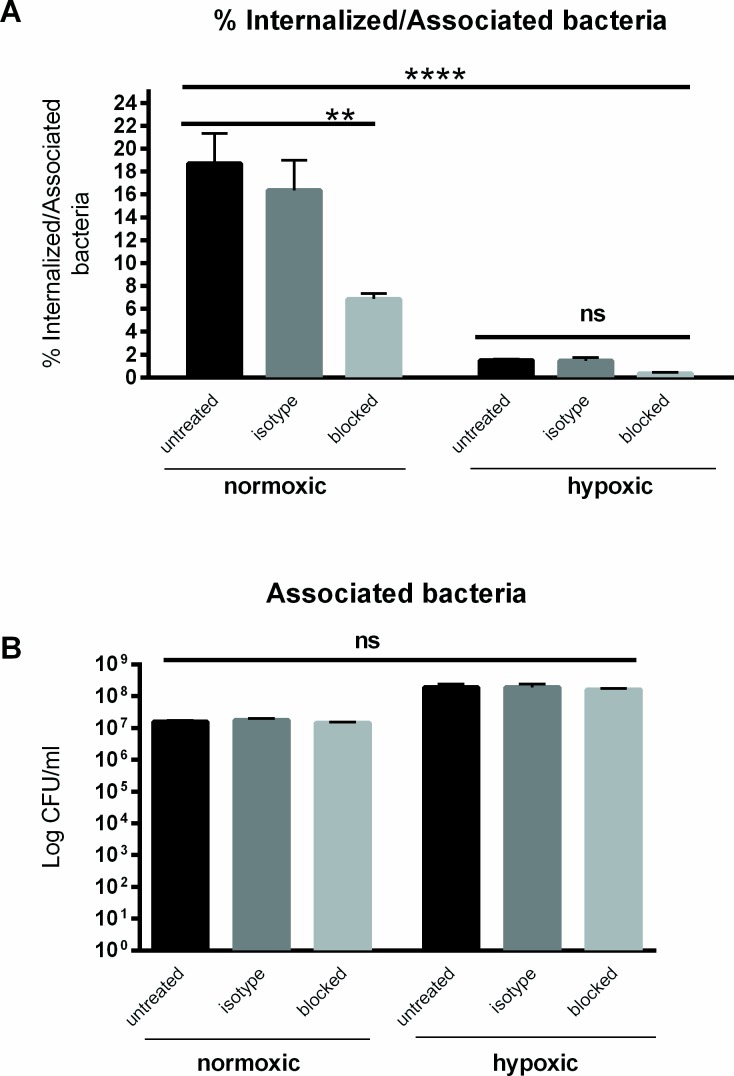
In order to confirm the role of host β1 integrins in Yersinia enterocolitica entry into intestinal epithelial cells, β1 integrins were functionally blocked by using a 6S6 anti-β1 integrin antibody that binds to the extracellular fragment of the receptor. The normoxic or hypoxic incubated Caco-2 cells were treated for 1 hour and were then infected with Y. enterocolitica O:8 8081v at MOI 10. The results in Fig 3A show a significant decrease in bacterial internalization in β1-integrin-blocked cells as compared to the controls under normoxia. Percent internalization was 6.8% for blocked as compared to 18.7% in untreated cells and 16% in IgG1 isotype-treated cells, in line with previous blocking studies [21]. Blocking of β1 integrins under hypoxia resulted in a slight but not significant decrease, 0.3% for blocked compared to 1.5 and 1.4% in untreated and isotype control cells, respectively (Fig 3A). In summary, blocking under hypoxia revealed a strong decrease in internalization when compared to blocking under normoxia, whereas the number of associated bacteria was comparable between the blocked and untreated controls under either oxygen condition (Fig 3B).
Fig 3. Integrin blocking decreases internalization.
Cells were treated with 45 μg/ml of 6S6 integrin blocking antibody, 45 μg/ml of IgG1 isotype control or left untreated for one hour before infection. (A) The percentage of internalized bacteria in cells blocked with anti-integrin antibody was significantly decreased. There was no significant difference between untreated or antibody blocked cells under hypoxia. (B) There was no significant difference in the number of associated bacteria under any condition. **** p<0.0001 using One way ANOVA test, and ** p ≤ 0.01 and ns = non-significant using Tukey's multiple comparisons test.
Invasin-mediated internalization
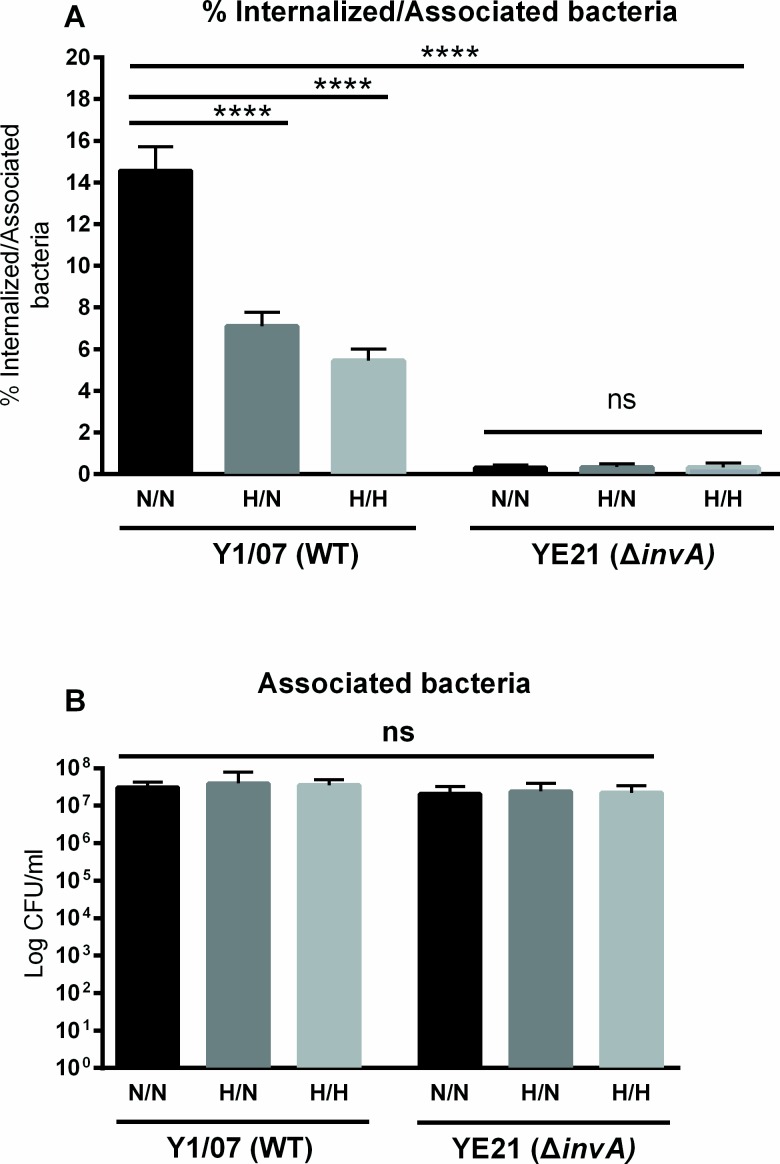
In order to investigate the invasin-β1 integrin mediated internalization, two strains of Y. enterocolitica serotype O:3 were used to infect normoxic and hypoxic incubated Caco-2 cells. The Y. enterocolitica strain Y1/07 is a wild type invasin-expressing strain while YE21 is the respective invasin-deficient mutant strain (ΔinvA) [31]. Similarly to the infection with 8081v, 6-day post confluent Caco-2 cells were pre-incubated either at normoxia or hypoxia for 24 hr. Infection incubations were also performed under normoxia or hypoxia. The results in Fig 4A show that the wild type strain was internalized significantly less in cells pre-incubated under hypoxia, similar to the O:8 serotype. Infection with the invasin mutant showed a highly significant decrease in internalization as compared to the infection with the wild type strain (Fig 4A). Similar to the Y. enterocolitica O:8 8081v wildtype strain, the number of associated bacteria showed no significant difference between the different oxygen conditions for either bacterial strain (Fig 4B).
Fig 4. Yersinia invasin is required for internalization.
Y. enterocolitica serotype O:3 Y1/07 (MOI 10) and the isogenic inv deletion mutant YE21 were used to infect Caco-2 cells pre-incubated at normoxia or hypoxia for 24 hrs. The infection was also performed at normoxia or hypoxia. (A) The percentage of internalized wild type bacteria was significantly reduced in hypoxia pre-incubated cells. Infection with the invasin-deficient strain YE21 showed no significant difference between the different oxygen conditions. (B) There was no significant difference in the number of associated bacteria in the cells grown under either condition. **** p < 0.0001 using One way ANOVA test, and **** p < 0.0001 and ns = non-significant using Tukey's multiple comparisons test.
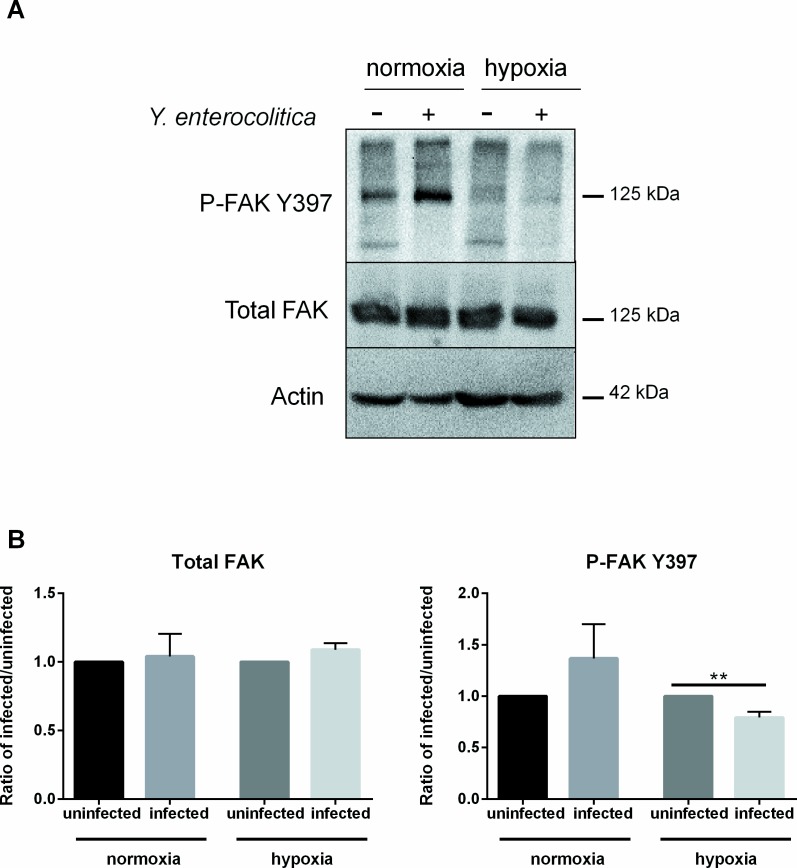
Influence of oxygen levels on FAK activation
The efficient uptake of Yersinia spp. into non-phagocytic cells via the invasin-integrin pathway requires the FAK that plays a central role in downstream signaling events [39]. Binding of integrin leads to an increase in tyrosine phosphorylation levels in the cell, specifically at tyrosine 397, identified as the major site of autophosphorylation in cell adhesion [40,41]. Therefore, in order to determine whether the hypoxia-induced decrease in internalization is also correlating with altered levels of phosphorylated FAK (p-FAK), total and p-FAK level were analyzed in cells that were pre-incubated under normoxia or hypoxia and then infected with Y. enterocolitica. Western blots show an increase in p-FAK at the site Y397 after infection as compared to uninfected cells under normoxia (Fig 5A). Under hypoxia, the levels of p-FAK are low in uninfected cells, and were further reduced upon infection (Fig 5A). Quantification of the Western blots shows that when compared to uninfected cells, infected cells show a distinct but not significant increase in p-FAK under normoxia and a significant decrease in p-FAK under hypoxia (Fig 5B). Total FAK levels showed no overall difference between infected and uninfected cells under either oxygen condition (Fig 5B).
Fig 5. Analysis of total and phosphorylated FAK.
Levels of total FAK and p-FAK Y397 from Caco-2 cells pre-incubated under hypoxia or normoxia for 24 hr and then infected with Y. enterocolitica. (A) Western blots and (B) their quantification. Values are presented as a ratio of infected over uninfected in case of normoxia or hypoxia, respectively. ** p ≤ 0.01 using two-tailed Student’s t-test.
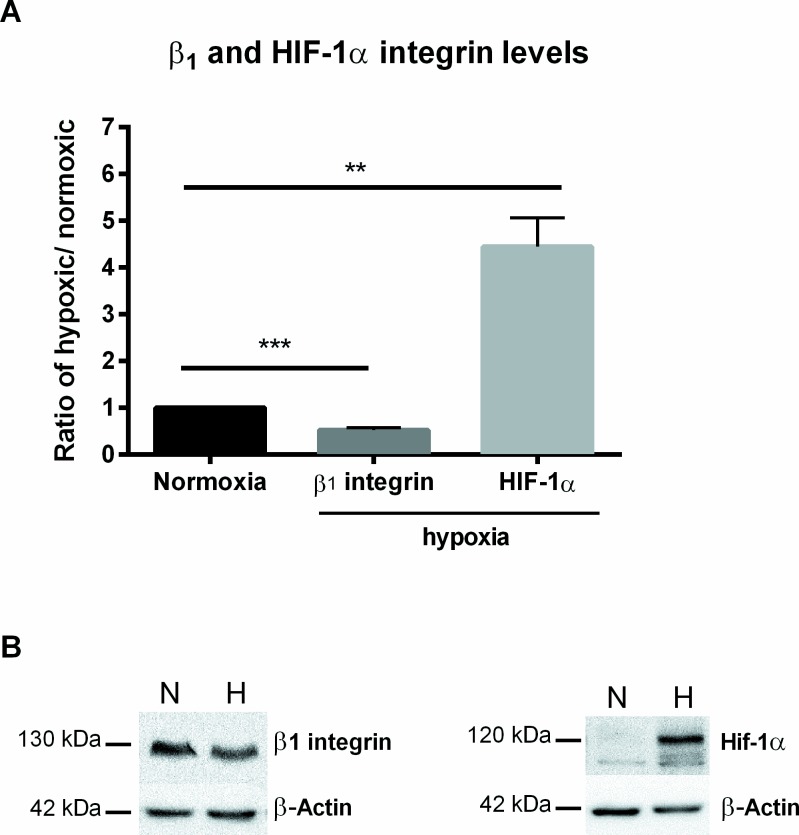
Beta one (β1) integrin and HIF-1 alpha (α) protein levels
The uptake of Y. enterocolitica into Caco-2 cells requires binding to host β1 integrins, and since decreased bacterial entry was seen under hypoxia, it was important to investigate whether reduced oxygen conditions induce changes in β1 integrin protein levels. Thus, Western blots were performed on whole cell lysates from 7-day post confluent Caco-2 cells incubated under normoxia or hypoxia for 24 hr. Interestingly, β1 integrin protein levels were significantly decreased (0.5-fold) under hypoxia. Lower β1 integrin protein levels (Fig 6) may explain the hypoxia-mediated decrease of the Y. enterocolitica internalization rate. At the same time, protein level of the transcription factor hypoxia inducible factor HIF-1α, a global regulator of cellular response to hypoxia [42] was significantly increased (4-fold) in hypoxic incubated cells (Fig 6).
Fig 6. Quantification of Western blots of β1 integrin and HIF-1α.
(A) Caco-2 cells pre-incubated under hypoxia as compared to the normoxic controls for 24 hr. Quantification of Western blot values are presented as a ratio over the respective normoxic value. (B) Representative Western blots showing β1 integrin at 130 kDa and HIF-1α at 120kDa. ** p ≤ 0.01, *** p ≤ 0.001 using two-tailed Student’s t-test.
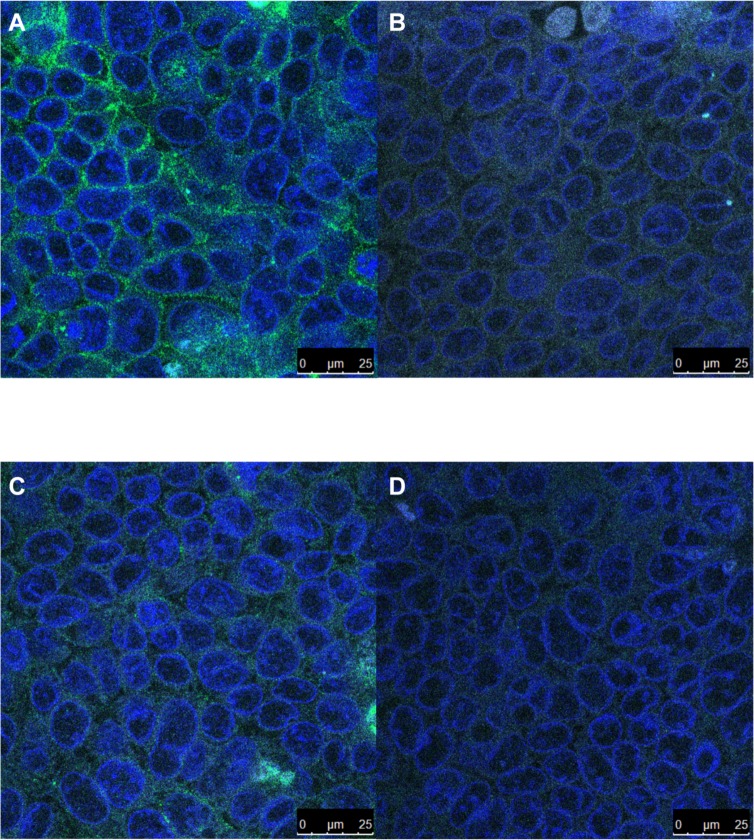
In order to confirm the decrease in β1 integrin protein levels seen in the Western blots, immunofluorescent visualization of β1 integrins in 7-day post confluent Caco-2 cells incubated under normoxia or hypoxia for 24 hr was performed. Representative images shown in Fig 7 confirm a distinct decrease in β1 integrin intensity, visualized in green, and distribution on the cells in hypoxic samples (Fig 7C) compared to the normoxic controls (Fig 7A). The isotype controls for normoxic and hypoxic staining are shown in Fig 7B and 7D respectively.
Fig 7. Decreased β1 integrin under hypoxia.
Representative fluorescent micrographs of β1 integrin abundance under normoxia (A) or hypoxia (C) with the mouse IgG1 isotype control (B and D). Green: β1 integrin, Blue: DAPI.
Apical localization of β1 integrin
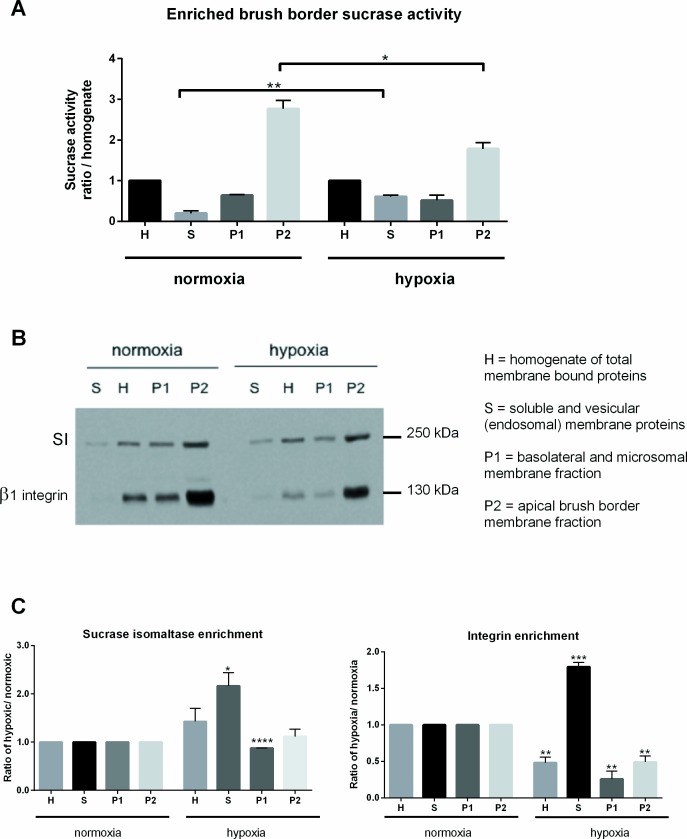
Differentiated Caco-2 cells display well-developed, brush-border membranes and express the active, apically enriched, intestinal disaccharidase sucrase-isomaltase (SI) [34,35]. To monitor any changes in cell polarization as a consequence of hypoxic exposure, the enrichment levels and activity of brush border SI was assessed in hypoxia pre-incubated cells. Furthermore, apical cell surface enrichment of β1 integrin was determined in hypoxic pre-incubated cells and compared to normoxic pre-incubated cells. For this, brush border membranes were separated from intracellular and basolateral membranes by the use of divalent ions (Ca2+) and subsequent separation by centrifugation (see material and methods section).
First, sucrase activity was assessed in the brush border (P2) fraction versus the total cellular homogenates (H). As expected for well-differentiated cells, under normoxia, the activity of sucrase in P2 was approximately 3-fold higher than in the homogenate [33]. Interestingly, sucrase activity was significantly lower under hypoxia, with activity of sucrase in P2 less than 2-fold higher than in the homogenate fraction (Fig 8A). Next, the patterns of SI protein enrichment in the different membrane fractions was analyzed by Western blots (Fig 8B). As expected, SI protein bands were mostly enriched in the P2 fraction under normoxia, in good correlation with the specific activity (Fig 8A). Under hypoxia, SI bands were significantly increased in the soluble and homogenate fractions with a slight decrease in P1 (Fig 8C).
Fig 8. Analysis of brush border membrane protein enrichment.
(A) Analysis of sucrase activity in the different membrane fractions under normoxia and hypoxia. (B) Western blots showing the localization of SI and β1 integrin in the different membrane fractions under normoxia and hypoxia. (C) Quantification of SI and β1 integrin enrichment in the different membrane fractions under normoxia and hypoxia. Values are presented as a ratio of each fraction over homogenate. * p ≤ 0.05; ** p ≤ 0.01, *** p ≤ 0.001 and **** p < 0.0001 using two-tailed Student’s t-test.
Localization of β1 integrin under normoxia was highly enriched in the P2 fraction under normoxia, confirming its apical cell surface localization. Under hypoxia, β1 integrin levels were reduced in the H, P1 and P2 fractions accompanied by a significant increase in soluble vesicular membrane localization compared to normoxia (Fig 8C).
In summary, these results indicate that under normoxia, in 7-day postconfluent Caco-2 cells, β1 integrin receptors are found on the apical surface. In contrast, after 24 hrs of incubation under hypoxia, apical cell surface localization of β1 integrins was significantly decreased, in agreement with the observed decreased bacterial internalization shown above.
Treatment of Caco-2 cells with DMOG reduces Y. enterocolitica internalization
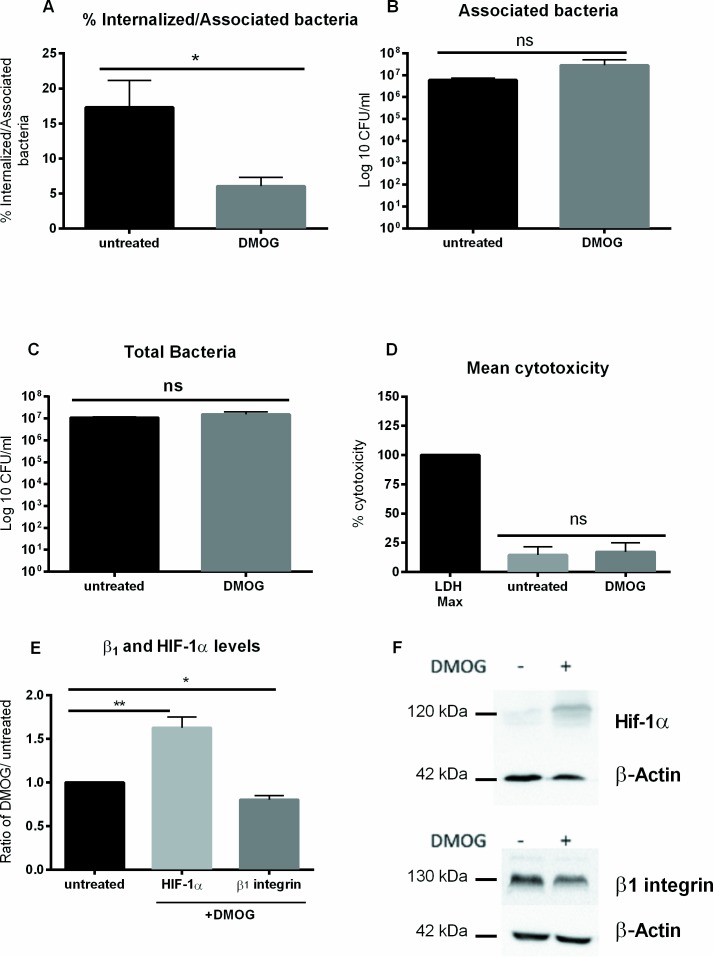
In order to determine whether HIF-1α plays a role in Y. enterocolitica uptake, a pharmacological agent was used to stabilize HIF-1α under normoxia. Dimethyloxalylglycine (DMOG) is a competitive pan inhibitor of prolyl-4-hydroxylases that degrade HIF-1α and it has been effectively used to stabilize HIF-1α in cells under normoxia [43]. Therefore, 7 day post confluent Caco-2 cells were treated with DMOG or with media alone under normoxic conditions. The cells were then infected with Y. enterocolitica O:8 8081v with a MOI 10 (under normoxia), DMOG was kept in the media of treated cells throughout the infection process. Fig 9A shows a significant decrease in bacterial internalization in cells treated with DMOG (6%) as compared to the untreated control (17.3%). Neither the number of associated bacteria or bacterial growth control showed a significant difference between DMOG treated cells and untreated controls (Fig 9B and 9C, respectively). Cytotoxicity of DMOG on Caco-2 cells was determined by performing an LDH assay, and no significant cytotoxic effect of DMOG treatment was found (Fig 9D). Furthermore, we found that DMOG treatment resulted in a slight, but significant decrease (0.8-fold) in β1 integrin and a significant increase (1.6-fold) in HIF-1α protein levels as compared to the untreated control (Fig 9E and 9F). These data imply a possible involvement of HIF-1α in the decreased β1 integrin levels that in turn lead to a reduced internalization of Y. enterocolitica.
Fig 9. Treatment with DMOG mimics hypoxic results.
Y. enterocolitica serotype O:8 8081v was used to infect Caco-2 cells (MOI 10) incubated at normoxia and treated with DMOG for 7 hr. The infection was performed at normoxia. (A) The percentage of internalized bacteria was significantly reduced in DMOG treated cells. There was no significant difference in the number of associated bacteria (B) or in bacterial growth (C) in the cells between treated and untreated. (D) Treatment with DMOG did not result in significant differences in cytotoxicity. (E) Western blots of β1 integrin and HIF-1α and their (F) quantification in Caco-2 cells treated with DMOG for 7 hr as compared to untreated controls. * p ≤ 0.05; ** p ≤ 0.01 using two-tailed Student’s t-test.
Discussion
The zoonotic bacterium Yersinia enterocolitica colonizes the human intestinal epithelium and its uptake is mediated by bacterial invasins that bind to host cell surface β1 integrins. In human intestinal enterocytes, β1 integrins are mostly localized on the basal and basolateral surfaces, however, on M cells, they are found mostly apically [44,45]. Studies have shown that M cells are the primary site of in vivo intestinal epithelial invasion by Yersinia species [12–14]. However, since oral in vivo infection with Y. enterocolitica was found to be lethal to mice, a direct correlation to human infections cannot be made [46]. Furthermore, since M cells represent less than 1% of the total human intestinal surface [47,48], it is much more relevant and efficient to study bacterial invasion in polarized epithelial cells models such as CHO, HEp-2, MDCK and Caco-2 [36,49,50]. Caco-2 cells are a well-established model for the intestinal epithelium, due to their ability to polarize and differentiate into intestinal epithelial cells [34]. It has been described that Caco-2 cells exist in three different states in culture: homogeneously undifferentiated at subconfluence, heterogeneously polarized and differentiated between 0 and 20 days after confluence, and homogeneously polarized and differentiated after 30 days [51]. Furthermore, in the intermediate state, all cells exhibit apical cell-cell junctions and polarization but they display a high degree of heterogeneity in the organization of the apical surface and the development of the brush border membrane [52]. In parallel to this differentiation process, a differential pattern of expression of extra cellular matrix (ECM) proteins and integrins along the crypt-to-villus is seen in epithelial cells [53]. While undifferentiated cells express integrins along the entire cell surface, as cells differentiate, the integrins begin to exhibit a shift from a lateral to a more basal distribution [50,54]. Interestingly, a comparative study of monocultures of Caco-2 on plastic plates and co-cultures with human intestinal mesenchymal (HIM) cells revealed a better basal distribution of β1 integrins in the co-culture system [55]. Conversely, β1 integrins in polarized MDCK and Caco-2 cells displayed an apicolateral distribution, where they colocalized with tight junction proteins and enabled Y. pseudotuberculosis internalization [36]. This disparity may be due to the varying differentiation stages at which the cells were used. Indeed, studies have shown that at advanced stages of cellular differentiation, bacterial internalization frequencies are substantially reduced [50,56,57]. We have shown that in our Caco-2 culture system, 7-day postconfluent cells display an apical cell surface localization of β1 integrins (Fig 8).
This may explain that Y. enterocolitica uptake can still be detected in differentiated Caco-2 cells in a cell culture model. In this study we show that hypoxic pre-incubated cells show less internalization of Yersinia enterocolitica compared to cells kept under normoxia. This phenomenon was in line with decreased protein levels of host β1 integrin in hypoxic cells. The results of hypoxic pre-incubation of Caco-2 cells infected with Y. enterocolitica under normoxia largely excludes any effects of hypoxia on the bacterial expression of invasin, however, we cannot discount the possibility that such an effect may exist and contribute to the decrease in internalization. Furthermore, two other loci have been identified in Y. enterocolitica, Ail and YadA that contribute to cell surface attachment to host cells [17,58–60]. Y. pseudotuberculosis strains lacking invasin were still able to associate with Caco-2 cells although internalization was abolished [50].
Our results show that bacterial internalization is decreased under hypoxia and abolished in the absence of bacterial invasin or active β1 integrins. Furthermore, reduced levels of phosphorylated FAK (p-FAK) were confirmed in hypoxia pre-incubated cells infected with Y. enterocolitica. Since invasin-mediated binding of β1 integrins promotes recruitment of tyrosine kinases like FAK [39], these data support our hypothesis that the invasin-integrin-mediated internalization of Y. enterocolitica is altered under hypoxia.
The GI tract has been described to be in a state of constant, low grade inflammation associated with hypoxia, with intestinal epithelial cells playing a pivotal role in mucosal immunity and response to this inflammation [61]. Furthermore, chronic inflammation can be found in cases of inflammatory bowel disease (IBD), which has also been shown to result in hypoxic conditions [62]. In fact, intestinal epithelial cells have revealed a strong resilience to low oxygen conditions and have efficiently adapted to this physiological state [63]. Among these adaptation mechanisms is the accumulation of HIF-1, a transcription factor consisting of two subunits: the oxygen regulated α- and a constitutively expressed β-subunit [42,64]. HIF-1α has now been shown to be present ubiquitously in human tissues and plays an important role in the cellular adaptation to hypoxia [65]. Under normoxic conditions, the HIF-1α subunit is rapidly degraded by ubiquitination and subsequent proteosomal degradation mediated by oxygen- and iron- dependent prolyl hydroxylases (PHDs) [66,67]. The HIF-prolyl hydroxylases are dioxygenase enzymes that require oxygen and 2-oxoglutarate, rendering them key oxygen sensors [68,69]. Hypoxic conditions allow for HIF-1α accumulation due to the interruption of its degradation pathway [70,71]. Following HIF-1α stabilization, it dimerizes with the HIF-1β subunit and subsequently binds to specific hypoxia response elements (HREs) on target genes [71–73]. HIF-1α binding regulates the transcription of several target genes that encode, among others, angiogenic factors, proliferation and survival factors, glucose transporters, glycolytic enzymes, and antimicrobial factors [74,75].
Here, we have shown that the decreased internalization of Y. enterocolitica in hypoxic-treated Caco-2 cells goes along with increased protein levels of HIF-1α. Furthermore, the pan-hydroxylase inhibitor DMOG that is commonly used to stabilize HIF-1α [43] shows a similar phenotype. These data lead to the hypothesis that HIF-1α may contribute to this process. However, an isoform of HIF-1α, HIF-2α, may also be involved in this mechanism since it shares several regulatory functions with HIF-1α and is subject to a similar oxygen-dependent degradation by prolyl hydroxylases [76]. Therefore, studies with genetically modified cells are needed to verify this hypothesis. Interestingly, several studies described an effect of hypoxia on integrin expression, and in fact, a binding site for the transcription factor HIF-1α has been found on the β1 integrin (ITGB1) gene promoter in colonic fibroblasts that results in a significant increase in ITGB1 induction under hypoxia [77,78]. Besides transcriptional regulation of ITGB1, however, many hypoxia-induced post-translational modifications have also been reported [78–80]. In renal epithelial cells, hypoxia results in over-activation of the calcium-dependent cysteine protease, calpain, which then leads to unrestrained cleavage of integrins [81,82]. In several cell types, hypoxia increased β1 integrin mRNA levels but hindered maturation and localization, thus resulting in decreased protein levels or activation [78,83,84]. Additionally, oxygen-dependent modifications of the host cytoskeleton significantly affect the paracellular permeability, intracellular transport and the general endocytic uptake of particles [7]. Finally, an exposure to hypoxia induces significant remodeling of the host cell membrane microdomains (lipid rafts) in alveolar epithelial cells [8]. Lipid rafts have been shown to function in cell signaling, intracellular membrane transport, cell adhesion and host-pathogen interactions [8,85,86]. Whether the hypoxia-induced changes in the host cytoskeleton and membrane microdomains play a role in decreased bacterial entry into epithelial cells remains unclear.
The intricate relationship between hypoxia, infection and inflammation has been thoroughly investigated and besides HIF-1α various transcription factors are involved in this cellular stress response pathways, including Nuclear Factor Kappa Beta (NFκB) and cAMP Responsive Element Binding protein (CREB), among many others [87,88]. Furthermore, many of these investigations have been performed in vivo, where all these factors contribute to the immune response during hypoxia and infection [1,3,89]. Future experiments with genetically modified cells will be directed to characterize the cellular pathways involved in the hypoxia-modulated internalization of Y. enterocolitica into Caco-2 cells.
Interestingly, a decrease of bacterial internalization under hypoxia was shown for Shigella flexneri into host epithelial cells in a predicted HIF-1α -dependent manner [90]. Moreover, Pseudomonas aeruginosa entry into alveolar cells was decreased under hypoxia and after DMOG treatment, confirming the role of HIF-1α in an in vivo pneumonia model [91]. However, there has been no mention of the involvement of the β1 integrins in the hypoxia-induced decrease in internalization. Furthermore, evaluation of HIF-1α stabilization by pharmacological inhibition of prolyl hydroxylases, namely the HIF-1-specific PHD inhibitor AKB-4924, have revealed an important role for HIF-1α in boosting the innate immune response of keratinocytes against skin infections [92] and of the intestinal epithelium in murine colitis [93]. In light of these results, understanding the effects of hypoxia on epithelial cells during infections offers a new potential for pharmacological interference and the use of HIF-1α as a therapeutic target [5,6,94].
Acknowledgments
This work was partially supported by DFG grant KO 3552/4-1 (MvK-B); N.Z. was funded by the German Academic Exchange Service (DAAD).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was partially supported by DFG grant KO 3552/4-1 (MvK-B); N.Z. was funded by the German Academic Exchange Service (DAAD). Both funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Taylor CT, Colgan SP: Hypoxia and gastrointestinal disease. J Mol Med 2007;85:1295–1300. [DOI] [PubMed] [Google Scholar]
- 2.Carreau A, Hafny‐Rahbi B, Matejuk A, Grillon C, Kieda C: Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 2011;15:1239–53. 10.1111/j.1582-4934.2011.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glover L, Colgan S: Hypoxia and Metabolic Factors That influence inflammatory bowel disease pathogenesis. Gastroenterology 2011;140:1748–1755. 10.1053/j.gastro.2011.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melican K, Boekel J, Månsson L, Sandoval R, Tanner G, Källskog Ö, et al. Bacterial infection‐mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cellular Microbiol 2008; 10.1111/j.1462-5822.2008.01182.x [DOI] [PubMed] [Google Scholar]
- 5.Bhandari T, Nizet V: Hypoxia-inducible factor (HIF) as a pharmacological target for prevention and treatment of infectious diseases. Infect Dis Ther 2014;3:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinkernagel A, Johnson R, Nizet V: Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med 2007;85:1339–1346. [DOI] [PubMed] [Google Scholar]
- 7.Bouvry D, Planès C, Malbert-Colas L, Escabasse V, Clerici C: Hypoxia-induced cytoskeleton disruption in alveolar epithelial cells. Am J Respir Cell Mol Biol 2006;35:519–527. [DOI] [PubMed] [Google Scholar]
- 8.Botto L, Beretta E, Bulbarelli A, Rivolta I, Lettiero B, Leone BE, et al. Hypoxia induced modifications in plasma membranes and lipid microdomains in A549 cells and primary human alveolar cells. J Cell Biochem 2008;105:503–513. 10.1002/jcb.21850 [DOI] [PubMed] [Google Scholar]
- 9.Bottone E: Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev 1997;10:257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottone EJ, Chester B, Malowany MS, Allerhand J: Unusual Yersinia enterocolitica isolates not associated with mesenteric lymphadenitis. Appl Microbiol 1974. May 3;27:858–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis R, Horn F: Enteropathogenic Escherichia coli, Samonella, Shigella and Yersinia: cellular aspects of host-bacteria interactions in enteric diseases. Gut Pathog 2010;2:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanski Naumann, Hahn Riecken: Determinants of invasion and survival of Yersinia enterocolitica in intestinal tissue. Med Microbiol Immunol 1988; 10.1007/BF00191063 [DOI] [PubMed] [Google Scholar]
- 13.Grützkau A, Hanski C, Hahn H, Riecken EO: Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 1990;31:1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark AM, Hirst BH, Jepson MA: M-cell surface β 1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun 1998;66:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isberg RR, Voorhis DL, Falkow S: Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 1987. 5;50:769–78. [DOI] [PubMed] [Google Scholar]
- 16.Isberg RR, Falkow S: A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 1985. 2;317:262–4. [DOI] [PubMed] [Google Scholar]
- 17.Miller VL, Finlay BB, Falkow S: Factors essential for the penetration of mammalian cells by Yersinia. Curr Top Microbiol Immunol 1988. 5;138:15–39. [PubMed] [Google Scholar]
- 18.Young Miller, Falkow Schoolnik: Sequence, localization and function of the invasin protein of Yersinia enterocoiitica. Molecul Microbiol 1990;4:1119–1128. [DOI] [PubMed] [Google Scholar]
- 19.Eitel J, Dersch P: The YadA protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect Immun 2002. 1;70:4880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller VL, Falkow S: Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun 1988. 2;56:1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isberg RR, Leong JM: Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 1990;60:861–871. [DOI] [PubMed] [Google Scholar]
- 22.Nhieu GT Van, Isberg RR: The Yersinia pseudotuberculosis invasin protein and human fibronectin bind to mutually exclusive sites on the alpha 5 beta 1 integrin receptor. J Biol Chem 1991. 3;266:24367–75. [PubMed] [Google Scholar]
- 23.Srichai MB, Pozzi A: Integrin Structure and Function In Zent R. and Pozzi A. ed. Cell-extracellular matrix interactions in cancer. Springer Science; p 19–41. 2010, pp 19–41. [Google Scholar]
- 24.Hynes RO: Integrins: Bidirectional, allosteric signaling machines. Cell 2002;110:673–687. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MA, Schaller MD, Ginsberg MH: Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995;11:549–99. [DOI] [PubMed] [Google Scholar]
- 26.Dersch P, Isberg R: A region of the Yersinia pseudotuberculosis invasin protein enhances integrin‐mediated uptake into mammalian cells and promotes self‐association. EMBO J 1999;18:1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alrutz MA, Isberg RR: Involvement of focal adhesion kinase in invasin-mediated uptake. PNAS 1998; 10.1073/pnas.95.23.13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong KW, Isberg RR: Yersinia pseudotuberculosis spatially controls activation and misregulation of host cell Rac1. Plos Pathog 2005; 10.1371/journal.ppat.0010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogh J, Fogh JM, Orfeo T: One hundred and twenty-seven cultured human tumor cel lines producing tumors in nude mice. J Natl Cancer Inst 1977;59:221–226. [DOI] [PubMed] [Google Scholar]
- 30.Pepe J, Badger J, Miller V: Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol 1994;11:123–135. [DOI] [PubMed] [Google Scholar]
- 31.Uliczka F, Pisano F, Schaake J, Stolz T, Rohde M, Fruth A, et al. Unique cell adhesion and invasion properties of yersinia enterocolitica O:3, the most frequent cause of human yersiniosis. PLoS Pathog. 2011;7 10.1371/journal.ppat.1002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz J, Preiser H, Maestracci D, Ghosh BK, Cerda JJ, Crane RK. Purification of the human intestinal brush border membrane. Biochim Biophys Acta- Biomembranes. 1973;323(1):98–112 [DOI] [PubMed] [Google Scholar]
- 33.Hein Z, Schmidt S, Zimmer K-P, Naim H (2011) The dual role of annexin II in targeting of brush border proteins and in intestinal cell polarity. Differentiation 81: 243252 10.1016/j.diff.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 34.Pinto M, Robine-Leon S, Appay M-DC, Kedinger M, Triadou N, Dussaulx E, et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 1983;47:323–330. [Google Scholar]
- 35.Zweibaum A, Triadou N, Kedinger M, Augeron C, Robine‐Léon S, Pinto M, et al. Sucrase‐isomaltase: A marker of foetal and malignant epithelial cells of the human colon. Int J Cancer 1983;32:407–412. [DOI] [PubMed] [Google Scholar]
- 36.Tafazoli F, Holmström A, Forsberg Å, Magnusson K-E: Apically exposed, tight junction-associated β1-integrins allow binding and YopE-mediated perturbation of epithelial barriers by wild-type Yersinia Bacteria. Infect Immun 2000;68:5335–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderkooi JM, Maniara G, Green TJ, Wilson DF: An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem 1987;262:5476–82. [PubMed] [Google Scholar]
- 38.Mandell GL: Interaction of Intraleukocytic Bacteria and Antibiotics. J Clin Invest 1973;52:1673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alrutz M a, Isberg RR. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc Natl Acad Sci U S A. 1998;95: 13658–63. 10.1073/pnas.95.23.13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994. 2;14(3):1680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan J-L. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol 1997. 29: 1085–1096. 10.1016/S1357-2725(97)00051-4 [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL, Wang GL: A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992;12:5447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472. [DOI] [PubMed] [Google Scholar]
- 44.Beaulieu JF: Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci 1992;102 (3):427–36. [DOI] [PubMed] [Google Scholar]
- 45.Hamzaoui N, Kernéis S, Caliot E, Pringault E: Expression and distribution of beta1 integrins in in vitro-induced M cells: implications for Yersinia adhesion to Peyer’s patch epithelium. Cell Microbiol 2004. 3;6:817–28. [DOI] [PubMed] [Google Scholar]
- 46.Curfs J, Meis J, Fransen J, Lee H, Hoogkamp-Korstanje J: Interactions of Yersinia enterocolitica with polarized human intestinal Caco-2 cells. Med Microbiol Immunol 1995;184 10.1007/BF00224348 [DOI] [PubMed] [Google Scholar]
- 47.Trier JS, Madara JL: Morphology of mucosa of small intestine New York, Raven Press, 1988, p 953–955. [Google Scholar]
- 48.Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR: Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun 1999;67:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finlay B, Falkow S: Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie 1988;70:1089–1099. [DOI] [PubMed] [Google Scholar]
- 50.Coconnier MH, Bernet-Camard MF, Servin AL: How intestinal epithelial cell differentiation inhibits the cell-entry of Yersinia pseudotuberculosis in colon carcinoma Caco-2 cell line in culture. Differentiation 1994. 2;58:87–94. [DOI] [PubMed] [Google Scholar]
- 51.Vachon PH, Beaulieu JF: Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 1992. August 6;103:414–23. [DOI] [PubMed] [Google Scholar]
- 52.Vachon P, Perreault N, Magny P, Beaulieu J: Uncoordinated, transient mosaic patterns of intestinal hydrolase expression in differentiating human enterocytes. J Cell Physiol 1996;166:198–207. [DOI] [PubMed] [Google Scholar]
- 53.Schreider C, Peignon G, Thenet S, Chambaz J, Pinçon-Raymond M: Integrin-mediated functional polarization of Caco-2 cells through E-cadherin-actin complexes. J Cell Sci 2002;115:543–52. [DOI] [PubMed] [Google Scholar]
- 54.Kaiserlian D, Rigal D, Abello J, Revillard JP: Expression, function and regulation of the intercellular adhesion molecule-1 (ICAM-1) on human intestinal epithelial cell lines. Eur J Immunol 1991;21:2415–21. [DOI] [PubMed] [Google Scholar]
- 55.Vachon PH, Durand J, Beaulieu JF: Basement membrane formation and re-distribution of the beta 1 integrins in a human intestinal co-culture system. Anat Rec 1993;235:567–76. [DOI] [PubMed] [Google Scholar]
- 56.Gaillard JL, Finlay BB: Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun 1996; Available from: http://iai.asm.org/content/64/4/1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira SH, Cervante MP, Bentzmann S, Plotkowski MC: Pseudomonas aeruginosa entry into Caco-2 cells is enhanced in repairing wounded monolayers. Microb Pathog 1997;23:249–55. [DOI] [PubMed] [Google Scholar]
- 58.Miller VL, Bliska JB, Falkow S: Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol 1990;172:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bliska JB, Copass MC, Falkow S: The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun 1993;61:3914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pepe JC, Wachtel MR, Wagar E: Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun 1995; Available from: http://iai.asm.org/content/63/12/4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colgan SP, Curtis VF, Campbell EL: The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis 2013;19:2238–2244. 10.1097/MIB.0b013e31828dcaaf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatoum OA, Binion DG, Gutterman DD: Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease. Eur J Clin Invest 2005;35:599–609. [DOI] [PubMed] [Google Scholar]
- 63.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, et al. Hypoxia-inducible factor 1–dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Medic 2001;193:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS 1995;92:5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semenza GL: Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999;15:551–78. [DOI] [PubMed] [Google Scholar]
- 66.Bruick RK, McKnight SL: A Conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001;294:1337–1340. [DOI] [PubMed] [Google Scholar]
- 67.Lee J-W, Bae S-H, Jeong J-W, Kim S-H, Kim K-W: Hypoxia-inducible factor (HIF-1)α: its protein stability and biological functions. Exp Mol Med 2004;36:112. [DOI] [PubMed] [Google Scholar]
- 68.Fraisl P, Aragonés J, Carmeliet P: Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 2009;8:139–52. 10.1038/nrd2761 [DOI] [PubMed] [Google Scholar]
- 69.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke JJ, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001;107:43–54. [DOI] [PubMed] [Google Scholar]
- 70.Kallio P, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1α: Posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. PNAS 1997;94:5667–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maxwell P, Wiesener M, Chang G-W, Clifford S, Vaux E, Cockman M, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors foroxygen-dependent proteolysis. Nature 1999;399:271–275. [DOI] [PubMed] [Google Scholar]
- 72.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE: Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. PNAS 1991;88:5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fandrey J: Hypoxia-inducible gene expression. Respir Physiol 1995;101:1–10. [DOI] [PubMed] [Google Scholar]
- 74.Wang GL, Semenza GL: General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. PNAS 1993;90:4304–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Semenza GL: Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–32. [DOI] [PubMed] [Google Scholar]
- 76.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt K-U, Talks KL, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1. Blood 1998;92:2260–2268. [PubMed] [Google Scholar]
- 77.Keely S, Glover L, MacManus C, Campbell E, Scully M, Furuta G, et al. Selective induction of integrin β1 by hypoxia-inducible factor: implications for wound healing. FASEB J 2009;23:1338–1346. 10.1096/fj.08-125344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rana M, Srivastava J, Yang M, Chen C, Barber D: Hypoxia increases the abundance but not the assembly of extracellular fibronectin during epithelial cell transdifferentiation. J Cell Sci 2015;128:1083–9. 10.1242/jcs.155036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren Y, Hao P, Law SK, Sze SK: Hypoxia-induced changes to integrin α 3 glycosylation facilitate invasion in epidermoid carcinoma cell line A431. Mol Cell Proteomics : MCP 2014;13:3126–37. 10.1074/mcp.M114.038505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu B, Li S, Yao Y, Lin Z: Changes in beta(1) integrin in renal tubular epithelial cells after intrauterine asphyxia of rabbit pups. Journal of perinatal medicine 2009;37:59–65. 10.1515/JPM.2009.009 [DOI] [PubMed] [Google Scholar]
- 81.Saido TC, Sorimachi H, Suzuki K: Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J 1994;8:814–22. [PubMed] [Google Scholar]
- 82.Wilson PD, Hartz PA: Mechanisms of cyclosporine A toxicity in defined cultures of renal tubule epithelia: a role for cysteine proteases. Cell Biol Int Rep 1991;15:1243–58. [DOI] [PubMed] [Google Scholar]
- 83.Zuk A, Bonventre JV, Brown D, Matlin KS: Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol 1998;275:C711–31 [DOI] [PubMed] [Google Scholar]
- 84.Aoyama K, Ozaki Y, Nakanishi T, Ogasawara M, Ikuta K, Aoki K, et al. Cleavage of integrin by mu-calpain during hypoxia in human endometrial cells. Am J Rep Immunol 2004;52:362–9 [DOI] [PubMed] [Google Scholar]
- 85.Brown DA, London E: Structure and Function of Sphingolipid- and Cholesterol-rich Membrane Rafts. J Biol Chem 2000;275:17221–4. [DOI] [PubMed] [Google Scholar]
- 86.Lindner R, Naim H: Domains in biological membranes. Exp Cell Res 2009;315:2871–8. 10.1016/j.yexcr.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 87.Cummins EP, Taylor CT: Hypoxia-responsive transcription factors. Pflügers Archiv : Eur J Physiol 2005;450:363–71. [DOI] [PubMed] [Google Scholar]
- 88.Haase VH: Pathophysiological Consequences of HIF Activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci 2009;1177:57–65. 10.1111/j.1749-6632.2009.05030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schaffer K, Taylor C: The impact of hypoxia on bacterial infection. FEBS J 2015;282:2260–2266. 10.1111/febs.13270 [DOI] [PubMed] [Google Scholar]
- 90.Lima C, Santos S dos, Junior D de, Lima C, Santos S dos, Junior D de: Hypoxic stress, hepatocytes and Caco-2 viability and susceptibility to Shigella flexneri invasion. Rev Inst Med Trop Sao Paulo 2013;55 10.1590/S0036-46652013000500008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schaible B, McClean S, Selfridge A, Broquet A, Asehnoune K, Taylor C, et al. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE 2013;8: e56491 10.1371/journal.pone.0056491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Köckritz-Blickwede M, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med 2012;90:1079–89. 10.1007/s00109-012-0882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol 2014;7:114–123. 10.1038/mi.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zinkernagel A, Peyssonnaux C, Johnson R, Nizet V. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis 2008;197:214–7. 10.1086/524843 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.