Abstract
The conjugated estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) is designed to minimize the undesirable effects of estrogen in the uterus and breast tissues and to allow the beneficial effects of estrogen in other estrogen-target tissues, such as the bone and brain. However, the molecular mechanism underlying endometrial and breast safety during TSEC use is not fully understood. Estrogen receptor α (ERα)–estrogen response element (ERE)–DNA pull-down assays using HeLa nuclear extracts followed by mass spectrometry–immunoblotting analyses revealed that, upon TSEC treatment, ERα interacted with transcriptional repressors rather than coactivators. Therefore, the TSEC-mediated recruitment of transcriptional repressors suppresses ERα-mediated transcription in the breast and uterus. In addition, TSEC treatment also degraded ERα protein in uterine tissue and breast cancer cells, but not in bone cells. Interestingly, ERα-ERE-DNA pull-down assays also revealed that, upon TSEC treatment, ERα interacted with the F-box protein 45 (FBXO45) E3 ubiquitin ligase. The loss-of- and gain-of-FBXO45 function analyses indicated that FBXO45 is involved in TSEC-mediated degradation of the ERα protein in endometrial and breast cells. In preclinical studies, these synergistic effects of TSEC on ERα inhibition also suppressed the estrogen-dependent progression of endometriosis. Therefore, the endometrial and breast safety effects of TSEC are associated with synergy between the selective recruitment of transcriptional repressors to ERα and FBXO45-mediated degradation of the ERα protein.
Introduction
Estrogen receptors (ERs) are members of the nuclear receptor superfamily of ligand-inducible transcription factors that regulate essential cellular processes, such as development, metabolism, reproduction, and behavior, by regulating the transcription of ER target genes (Burns and Korach, 2012). The working model for ERα action indicates that ligand-bound receptor binds to the enhancers/promoters of target genes that contain the estrogen-response element and sequentially recruits multiple coregulator complexes to these regulatory regions; this results in chromatin remodeling and assembly of the transcriptional preinitiation complex as well as the downstream processing events involved in mRNA synthesis and maturation (McKenna and O'Malley, 2002; Green and Carroll, 2007; Foulds et al., 2013).
Because of its essential role in estrogen target tissues, aberrant regulation of the ERα level and activity is associated with estrogen-related progression of human disease (Deroo and Korach, 2006; Burns and Korach, 2012). Therefore, the development of tissue-specific modulators of ERα activity is a critical step to effectively treat the estrogen-related disease progression in specific tissues while minimizing side effects in certain other tissues. This goal led to the development of selective estrogen-receptor modulators (SERMs) (Levenson and Jordan, 1999). The SERMs act as tissue-specific estrogen-receptor agonists in the bone, brain, cardiovascular system, vagina, and urogenital system and as estrogen-receptor antagonists in the breast, endometrium, pelvic floor, and in venous thrombosis (Morello et al., 2002; Maximov et al., 2013). The first generation of SERMs included the drug tamoxifen (Ward, 1973; Jordan and Koerner, 1975). Clinical studies revealed that treatment with tamoxifen significantly reduced the incidence of breast cancer in high-risk patients (Jordan, 1988; Morello et al., 2003). However, chronic tamoxifen treatment has stimulatory effects on endometrial cells, increasing the risk of development and progression of endometrial cancer (Rutqvist, 1993; Iqbal et al., 2012). To overcome the undesirable side effects of tamoxifen, raloxifene (second-generation SERM) was developed (Black et al., 1994). Because raloxifene reduced the incidence of invasive breast cancer in postmenopausal women with osteoporosis, raloxifene is the current preferred drug for women at risk for vertebral fracture who have an elevated risk of breast cancer (Ko and Jordan, 2011; Gizzo et al., 2013). Unlike tamoxifen, raloxifene does not increase the risk of uterine cancers (Vogel et al., 2006; DeMichele et al., 2008; Pinkerton and Goldstein, 2010), even when combined with vaginal estrogen (Parsons et al., 2003; Pinkerton et al., 2003). To improve upon the beneficial effects of raloxifene, third-generation SERMs were developed, including bazedoxifene (BAZ), which prevented and treated postmenopausal osteoporosis without adverse stimulation of the breast and endometrium (Komm and Chines, 2012).
To maximize the tissue-specific effects of SERMs and minimize their side effects, a tissue-selective estrogen complex (TSEC) has been developed (Archer, 2010; Pickar and Mirkin, 2010). The final goal of TSEC therapy is to combine the desired tissue-selective properties of SERMs with the beneficial effects of estrogen. The TSEC would ideally have estrogen-receptor antagonist activity in the breast and endometrium, reduced vasomotor symptoms, and improved lipid profile and bone mineral density compared with a SERM- or estrogen-alone treatment (Mirkin et al., 2014). The first-generation TSEC was a combination of BAZ and conjugated estrogen (CE), which had unique molecular properties compared with the individual components (Mirkin et al., 2014). For example, the BAZ/CE TSEC combination was associated with a less than 1% rate of endometrial hyperplasia. This rate was not significantly different from a placebo-treated group during a 2-year study (Pickar et al., 2009). When the incidence of uterine bleeding in postmenopausal women was studied, the mean number of bleeding or spotting days in women treated with TSEC was not significantly different from a placebo-treated group during 2 years of therapy (Archer et al., 2009). Thus, TSEC therapy can be used to treat menopausal symptoms without stimulation of the endometrium. Treatment with TSEC for 24 months also did not affect the mammographic density of postmenopausal women with breast cancer compared with a placebo-treated group (Harvey et al., 2013). Therefore, the endometrial and breast-cell stimulatory effects of the accompanying CE were completely abrogated by BAZ in TSEC treatment (Song et al., 2013). Collectively, the TSEC has an antiestrogenic effect on the growth of breast cancer cells and prevents the development of endometrial hyperplasia in postmenopausal women. However, the molecular mechanisms underlying endometrial and breast safety during TSEC administration are not understood yet; elucidation of these mechanisms is important for the use of TSEC in future clinical therapy. Here, we analyze the molecular functions of TSEC and suggest dual mechanisms by which TSEC inhibits ERα function in endometrial and breast cells.
Materials and Methods
Experimental Animals.
Wild-type (C57BL/6J) mice were used. All experiments involving animals were conducted in accordance with the National Institutes of Health standards for the use and care of animals, with protocols approved by Baylor College of Medicine. C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA).
Cell Culture and Reagents.
The HeLa human cervical carcinoma, MCF7 human breast cancer, and HTB-85 human osteosarcoma cell lines were obtained from the American Type Culture Collection (Manassas, VA). HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). MCF7 cells were maintained in minimum essential medium supplemented with 10% FBS. HTB-85 cells were cultured in DMEM containing 10% FBS. 4-Hydroxytamoxifen, Carbobenzoxy-Leu-Leu-leucinal (MG132), and ethanol-soluble estrogen (E2) were purchased from Sigma-Aldrich (St. Louis, MO). Bazedoxifene and CE (Premarin) were obtained from Pfizer Inc. (New York, NY). For cell culture, a mixture of the unconjugated forms of the 10 most abundant CE components of CE (Premarin; Pfizer Inc.) was prepared using the same relative proportions as the previously described CE formulation (Chang et al., 2010), and a 1 mM CE stock solution was prepared using 1 mM solutions of each of the 10 components mixed according to their respective proportions.
Western Blot Analysis.
Primary antibodies against the following proteins were used: SRC-1 (Abcam, Cambridge, MA), SRC-2 (Bethyl Laboratories, Montgomery, TX), SRC-3 (BD Biosciences, San Jose, CA), tubulin (Santa Cruz Biotechnologies, Dallas, TX), F-box protein 45 (FBXO45; Novus Biologicals, Littleton, CO), ERα (SC-542; Santa Cruz Biotechnologies), and BCL-6 corepressor (BCOR; Bethyl Laboratories). Membranes containing proteins were incubated with secondary horseradish peroxidase–tagged antibodies (Sigma-Aldrich), and the signals were visualized using ECL plus (Amersham, Pittsburgh, PA).
Immunohistochemistry.
Immunostaining was performed with 10% neutral-buffered, formalin-fixed, and paraffin-embedded sections of mouse tissue. Antibodies against FBXO45 (Novus Biologicals), ERα (Santa Cruz Biotechnologies), and Ki-67 (Abcam) were used. The specific antigens were visualized with the DAB substrate kit (Vector Laboratories, Burlingame, CA). The immunostaining intensity was quantified using the ImageJ program, which was developed by the National Institutes of Health. Scale bar is 20 µm.
Uterotrophic Assay.
Uterotrophic assays were performed to screen for potential estrogenic activity. CE, BAZ, and TSEC were administered daily via subcutaneous injection in 6-week-old ovariectomized female mice. Ovariectomy was performed as previously described (Han et al., 2005). The animals were dosed for 3 consecutive days and necropsied approximately 24 hours after the final dose to determine wet and blotted uterine weights.
Estrogen Response Element–DNA Pull-Down Assays.
The estrogen response element (ERE)–DNA pull-down reaction conditions and generation of biotinylated EREs were described previously (Foulds et al., 2013). In brief, 4 µg of ERE DNA, 1 mg of HeLa nuclear extract, 0.5 µg of recombinant human ERα protein (Abcam), and 60 μl of Dynabeads M-280 Streptavidin were mixed with NETN (0.5% Nonyl Phenoxypolyethoxylethanol (NP)-40, 1 mM EDTA, 50 mM Tris-HCl (PH7.5) and 150 mM NaCl) buffer and then incubated with vehicle, 10 nM CE, 100 nM BAZ, and 10 nM CE + 100 nM BAZ (TSEC) at 4°C for 1.5 hours with constant shaking. The pellet was washed twice with NETN (0.5% NP40, 1 mM EDTA, 50 mM Tris-HCl (PH7.5) and 150 mM NaCl) buffer, 80% of the pellet was used for liquid chromatography mass spectroscopy (MS) analyses, and 20% was used for Western blotting analyses.
Protein Identification by MS.
For MS analyses, nearly all of the ERE pull-down reaction (80%) was separated using one-dimensional SDS-PAGE. All ERα-interacting proteins were isolated from the gel, and their identities were determined by MS as described previously (Foulds et al., 2013).
TSEC-Mediated ERα Degradation in Breast Cells.
MCF7 breast cancer cells were cultured in six-well plates in duplicate and maintained in regular medium supplemented with 10% FBS. Prior to the experiment, the cells were deprived of estrogen for 2 days by incubating in stripped serum medium followed by treatment with vehicle, 10 nM CE, 100 nM BAZ, 100 nM 4-hydroxytamoxifen (4-HT), 10 nM CE plus 100 nM BAZ (TSEC), and 10 nM CE plus 100 nM 4HT for 24 hours. The cells were then lysed, and the RNA and protein were extracted. For Western blotting analyses, the proteins were separated on a 10% SDS-PAGE gel, transferred into a nitrocellulose membrane, and incubated with anti-ERα and antitubulin antibodies overnight at 4°C followed by incubation with horseradish peroxidase–conjugated anti-rabbit antibody (Sigma-Aldrich) for 1 hour. Signals were detected using the ECL Plus system (Pierce, Waltham, Massachusetts). Transcript levels were analyzed by quantitative polymerase chain reaction using Applied Biosystems (Waltham, Massachusetts). cDNAs were synthesized, and TaqMan probes for ERα, progesterone receptor (PR), and 18S ribosomal RNA (rRNA; Applied Biosystems) were used to measure ERα, PR, and 18S rRNA levels. The quantitative polymerase chain reaction results were analyzed to determine the ratio of our target gene in our treated sample relative to our untreated sample using delta-delta cT method, and the fold enrichment was calculated relative to the 18S rRNA level.
Effect of FBXO45 Gain-of-Function and Loss-of-Function on ERα Stability.
Knockdown experiments were performed in HeLa cells transfected with ERα by treating them with 100 nM nontargeting small interfering RNA (siRNA) or FBX045 (SMARTpool; Dharmacon, Lafayette, CO) siRNA for 48 hours. The cells were then incubated with vehicle, 10 nM CE, 100 nM BAZ, and 10 nM CE plus 100 nM BAZ (TSEC) for 24 hours. Cell lysates were prepared and used for Western blotting analyses with specific antibodies against FBX045 (Abcam), ERα, and tubulin. To examine the effect of FBXO45 gain-of-function, HeLa cells were transiently transfected with 100 ng each of ERα and FBXO45 expression vectors (gift from Hirobumi Tada, Keio University School of Medicine, Shinjuku, Tokyo, Japan) followed by treatment with vehicle, 10 nM CE, 100 nM BAZ, and 10 nM CE plus 100 nM BAZ (TSEC). Samples were collected at 0, 1, 3, 6, and 9 hours after treatment, and levels of ERα and FBXO45 were analyzed by Western blotting, as described earlier.
Coimmunoprecipitation and Western Blotting.
For coimmunoprecipitation assays, HeLa cells were transiently transfected with 100 ng each of ERα and FBX045 expression vectors or empty vectors. After 48 hours, cells were treated with vehicle, 10 nM CE, 100 nM BAZ, and 10 nM CE plus 100 nM BAZ (TSEC) in the presence or absence of 10 µM MG132 for 6 hours. The lysates were incubated with ERα, Flag, or IgG antibodies under constant rotation. Protein G beads were added 2 hours later and incubated for an additional 2 hours, followed by extensive washing [20 mM HEPES (pH 7.6), 150 mM KCl, 1 mM Dithiothreitol, 0.1% NP40, and 8% glycerol supplemented with protease and phosphatase inhibitors]. The protein G beads were boiled in 1× Laemmli buffer, and the coprecipitated proteins were separated by SDS-PAGE. The proteins were transferred onto nitrocellulose membranes, and Western blotting was performed and analyzed with specific antibodies.
Administration of CE, BAZ, and TSEC to Mice with Surgically Induced Endometriosis.
The surgical treatments of mice were performed under aseptic conditions with anesthesia. Endometriosis was surgically induced in mice with a modification of a previously described method (Cummings and Metcalf, 1995). In brief, 6-week-old C57BL/6 mice were ovariectomized. After 2 weeks, we implanted sterile 60-day-release pellets containing 0.36 mg of 17-β estradiol (Innovative Research of America, Sarasota, FL) into ovariectomized mice. Two days later, we isolated one uterine horn of each mouse under anesthesia. In a Petri dish containing warmed DMEM/F-12 (Invitrogen, Carlsbad , CA) supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin, we longitudinally cut the uterine horns with a pair of scissors. We then isolated a tissue sample using a 2-mm dermal biopsy punch (Miltex, York, PA) and subsequently sutured one endometrial fragment to the mesenteric membrane attached to the intestine in the same mouse through a midline incision (7-0 braided silk suture; Ethicon). We closed the abdominal incision with a 5-0 braided silk suture (Ethicon, Blue Ash, OH) in a continuous fashion. Ectopic lesions were developed and established for 21 days after endometriosis induction. Subsequently, the mice were injected with vehicle, CE (2.5 mg/kg), BAZ (2.5 mg/kg), and TSEC (2.5 mg/kg CE plus 2.5 mg/kg BAZ) on alternate days for 21 days, and then the ectopic lesions were harvested for analyses. All experiments were repeated at least three times.
Statistical Analysis.
Statistical analysis was performed using a paired Student t test in GraphPad Prism 5.00 (GraphPad, La Jolla, CA).
Results
Corepressor Components Recruitment to ERα by TSEC.
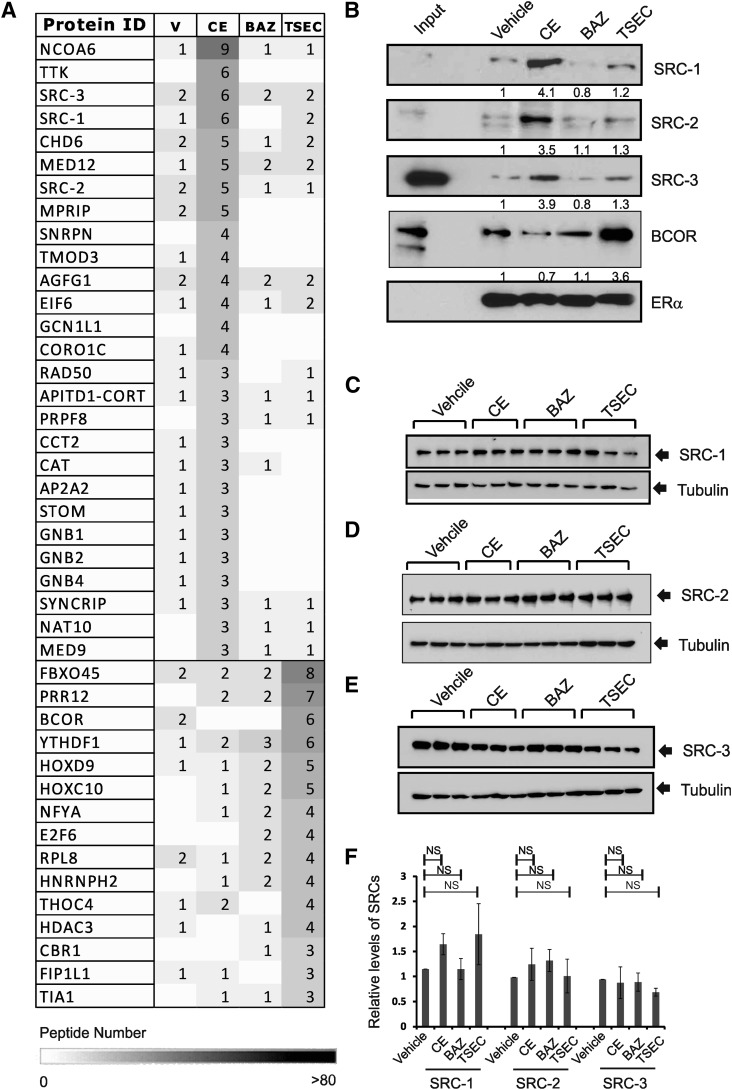
The physical and functional interactions between ERα and its coregulators are essential for the regulation of ERα-mediated transcription upon estrogenic stimulation (Foulds et al., 2013). In this regard, ERα recruits different coregulators to regulate target gene expression upon various external stimuli (Foulds et al., 2013). Therefore, we hypothesized that the TSEC should generate unique ERα-coregulator combinations to confer endometrial and breast safety. ERα activity determined by PR expression in HeLa cells transfected with human ERα expression vector revealed that 10 nM CE stimulated ERα, activity and 100 nM, but not 10 nM, BAZ inhibited ERα activity that was stimulated by CE (Supplemental Fig. 1). To examine the coregulators bound to ERα upon CE, BAZ, and TSEC treatment, ERα-ERE pull-down assays were performed using a biotinylated DNA fragment containing the ERE in HeLa cell nuclear extracts (Foulds et al., 2013). Compared with vehicle, 10 nM CE also actively enhanced interaction of ERα with steroid receptor coactivators (SRCs), and this CE-mediated interaction was strongly inhibited by 100 nM, but not 10 nM, BAZ (Supplemental Fig. 2, A and B). Interestingly, the amount of SRCs recruited to ERα by 10 nM CE was less than by 10 nM E2. Therefore, the reduced amounts of coactivator recruitments to ERα might provide the lesser estrogenic activity of CE compared with estradiol. Based on these observations, 10 nM CE plus 100 nM BAZ combinations have been applied as a TSEC in this study. The proteins that coprecipitated with ERα in response to each hormone were identified by mass spectrometric analyses (Fig. 1A). Compared with other hormone treatments, CE treatment enhanced the recruitment of transcriptional coactivators (such as SRCs, NCoA6, RAD50, and Mediator complex components) to ERα bound to the ERE DNA fragment (Fig. 1A). Consistent with the mass spectrometry analyses, the ERα-ERE pull-down assays followed by Western blot analyses indicated that SRC-1, SRC-2, and SRC-3 were significantly recruited to ERα upon CE treatment compared with treatment with other agents (Fig. 1B). However, less SRC-1, -2, and -3 was recruited to BAZ- and TSEC-bound ERα compared with CE treatment (Fig. 1, A and B). Notably, the SRC expression levels in HeLa cells were not altered by CE, BAZ, or TSEC compared with vehicle treatment (Fig. 1, C–F).
Fig. 1.
Differential recruitment of coregulators to ERα upon CE, BAZ, and TSEC treatment. (A) ERα/ERE-DNA pull-down analyses were performed using HeLa nuclear extracts in the presence of vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE plus 100 nM BAZ) as described in Materials and Methods. Proteins associated with ERα after different hormone treatments were identified by MS analyses, and the peptide numbers were listed. (B) After ERα/ERE-DNA pull-down analyses, the levels of SRC-1, SRC-2, SRC-3, BCOR, and ERα in the precipitates were determined by Western blotting analyses. SRC-1 (C), SRC-2 (D), and SRC-3 (E) protein levels in HeLa cells treated with hormone for 24 hours were determined by Western blotting analyses (n = 3/group). The tubulin level in each group was used as a protein loading control. (F) The ratios of SRCs to tubulin levels in (C)–(E) are shown in a graph. NS, nonspecific, Student’s t test. Values represent the average ± S.E.M. of three independent experiments.
In contrast, the ERα-ERE pull-down assays coupled with mass spectrometric analyses revealed that TSEC treatment led to increased recruitment of different coregulators, usually transcriptional repressor components, such as BCOR and histone deacetylase3 (Fig. 1A). The TSEC-induced recruitment of BCOR to ERα was confirmed using ERα-ERE pull-down assays followed by Western blotting analyses (Fig. 1B). Collectively, the TSEC-induced recruitment of transcriptional repressors relative to transcriptional activators correlates with inhibition of ERα target gene transcription in endometrial cells upon TSEC treatment.
Degradation of the Uterine ERα Protein by TSEC to Prevent Endometrial Stimulation.
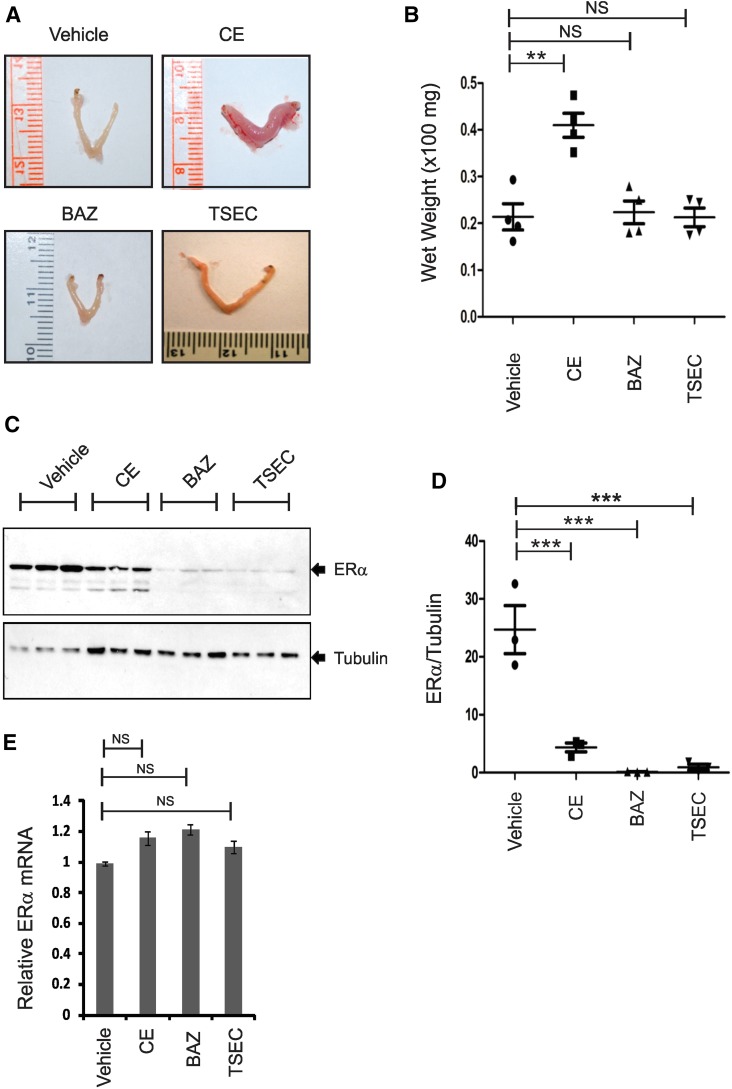
To analyze the endometrial effects of TSEC in more detail, uterotrophic assays were performed using ovariectomized female mice: on the 14th day after ovariectomy, the mice were randomly divided into four groups and then administered vehicle, CE (2.5 mg/kg), BAZ (2.5 mg/kg), or TSEC containing both CE (2.5 mg/kg) and BAZ (2.5 mg/kg) daily for 3 days. Six hours after the final hormone injection, uteri were isolated from each group of mice and weighed. Compared with vehicle treatment, CE treatment significantly increased uterine weight, but both BAZ and TSEC did not increase uterine weight (Fig. 2, A and B). Unlike CE, therefore, BAZ and TSEC had no endometrial stimulatory effect (Fig. 2, A and B). Furthermore, the presence of BAZ in the TSEC suppressed CE-mediated endometrial stimulation.
Fig. 2.
TSEC causes ERα protein degradation in murine uteri to prevent ERα-mediated endometrial stimulation. (A) Uteri were isolated from ovariectomized mice treated daily with vehicle, CE (2.5 mg/kg), BAZ (2.5 mg/kg), and TSEC (2.5 mg/kg CE plus 2.5 mg/kg BAZ) for 3 days, and then uterine morphology was examined. (B) The uterine wet weights in each group described in (A) are shown in a graph (n = 4/group). (C) ERα and tubulin (loading control) protein levels in the uteri in (A) were determined by Western blotting analyses (n = 3/group). (D) The ratios of ERα to tubulin levels in (C) are shown in a graph (n = 3/group). (E) ERα mRNA and 18S rRNA levels in the uteri in (A) were measured. The relative ERα mRNA levels in each hormone-treated uterus were determined as compared with the RNA levels in the vehicle-treated group after normalization with 18S rRNA levels. **P < 0.01; ***P < 0.001. NS, nonspecific versus control, Student’s t test. Values represent the average ± S.E.M. of three independent experiments.
To elucidate the endometrial safety of TSEC, uterine ERα levels were measured in each hormone-treated group. Compared with vehicle treatment, CE, BAZ, and TSEC treatments reduced uterine ERα protein levels (Fig. 2, C and D). To determine whether the reduction in ERα protein level was correlated with downregulation of the ERα mRNA level, uterine ERα mRNA levels were measured in each hormone-treated group. Notably, uterine ERα mRNA levels were not altered by hormone treatment compared with vehicle treatment (Fig. 2E). Therefore, CE-mediated ERα degradation is associated with endometrial stimulation, an effect observed previously (Nawaz et al., 1999; Callige and Richard-Foy, 2006). However, TSEC-mediated ERα degradation occurred in the absence of endometrial stimulation in mice.
TSEC-Mediated ERα Degradation Prevents ERα Activity in Human Breast Cancer Cells.
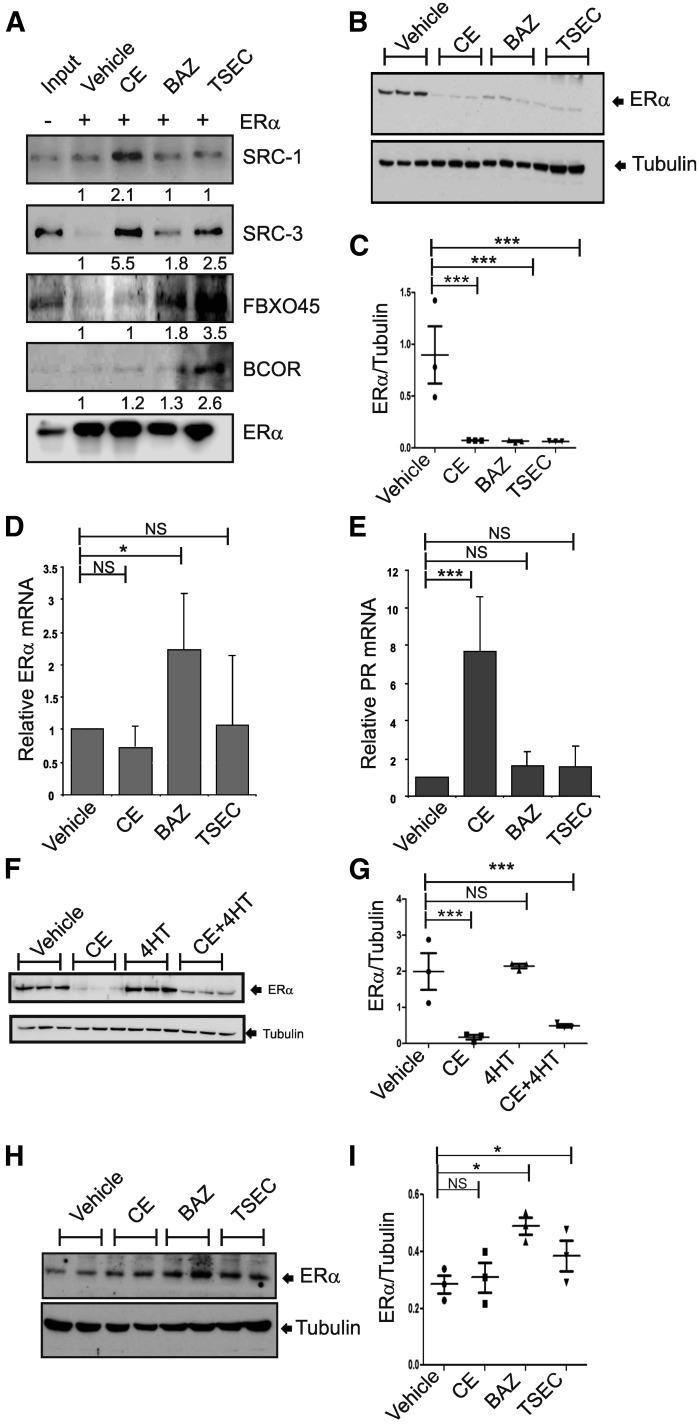
To examine the effect of TSEC on breast ERα, MCF7 human breast cancer cells were selected. Consistent with HeLa nuclear extracts, the ERα-ERE pull-down assays followed by Western blot analyses with MCF7 nuclear extracts indicated that coactivators (SRC-1 and SRC-3) were significantly recruited to ERα upon CE treatment compared with treatment with other agents (Fig. 3A). In contrast to coactivators, BCOR (transcriptional repressor) and FBXO45 effectively interact with ERα upon TSEC treatment compared with other hormones in the MCF7 nuclear extracts (Fig. 3A). Therefore, breast ERα has similar responses upon CE and TSEC treatment compared with endometrial ERα.
Fig. 3.
TSEC causes ERα protein degradation in human breast cancer cells to inhibit ERα activity. (A) ERα/ERE-DNA pull-down analyses were performed using MCF7 nuclear extracts in the presence of vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE plus 100 nM BAZ). After ERα/ERE-DNA pull-down analyses, the levels of SRC-1, SRC-3, FBXO45, BCOR, and ERα in the precipitates were determined by Western blotting analyses. (B) MCF7 cells were treated with vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE and 100 nM BAZ) for 24 hours. Subsequently, the ERα and tubulin protein levels in MCF7 cells were determined by Western blotting analyses (n = 3/group). (C) The ratios of ERα to tubulin in the MCF7 cells in (B) are shown in a graph (n = 3/group). (D) The total RNA was isolated from the MCF7 cells in (B), and the levels of ERα mRNA and 18S rRNA were determined by real-time reverse-transcription polymerase chain reaction analyses. The ERα mRNA levels in each group were normalized to the 18S rRNA levels. The normalized ERα mRNA levels in hormone-treated MCF7 cells relative to vehicle-treated MCF7 cells are shown in a graph (n = 3/group). (E) PR mRNA levels were determined in the MCF7 cells described in (B) and normalized to the 18S rRNA levels. The normalized PR mRNA levels in hormone-treated MCF7 cells relative to vehicle-treated MCF7 cells are shown in a graph (n = 3/group). (F) MCF7 cells were treated with vehicle, CE (10 nM), 4-hydroxytamoxifen (4-HT; 100 nM), and CE (10 nM) plus 4-HT (100 nM) for 24 hours. Subsequently, the ERα and tubulin protein levels were determined by Western blotting analyses (n = 3/group). (G) The ratio of ERα to tubulin in (F) is shown in a graph. (H) HTB-85 cells were treated with vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE plus 100 nM BAZ) for 24 hours. Subsequently, the ERα and tubulin protein levels in HTB-85 cells were determined by Western blotting analyses (n = 2/group). (I) The ratios of ERα to tubulin in the HTB-85 cells are shown in a graph (n = 3/group). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significantly different versus vehicle, Student’s t test. Values represent the average ± S.E.M. of three independent experiments.
To investigate the effect of TSEC on breast ERα protein levels, MCF7 human breast cancer cells were treated with CE (10 mM), BAZ (100 mM), or TSEC (containing CE and BAZ) for 1 day. Subsequently, the ERα protein level in each group of cells was determined by Western blot analysis. Consistent with endometrial ERα expression, the breast ERα levels in MCF7 cells were significantly reduced by CE, BAZ, and TSEC treatment compared with vehicle treatment (Fig. 3, B and C). Breast ERα RNA levels in MCF7 cells were not altered by CE, BAZ, or TSEC treatment compared with vehicle treatment (Fig. 3D). These data indicate that CE and TSEC also cause degradation of the ERα protein in breast cancer cells.
To explain the functional correlation between ERα degradation and ERα activity in breast cancer cells, we measured levels of PR because PR is the direct ERα target gene (Nagai and Brentani, 2008). Compared with vehicle treatment, CE treatment increased PR mRNA levels in MCF7 cells, whereas BAZ and TSEC treatment did not (Fig. 3E). Therefore, similar to the endometrial data, CE-mediated ERα degradation was associated with induction of ERα activity, whereas BAZ- and TSEC-mediated ERα degradation correlated with suppression of ERα activity in MCF7 cells.
The SERMs, BAZ, and tamoxifen inhibit ERα activity in breast cancer cells (Lewis-Wambi et al., 2011; Cirillo et al., 2013). However, these drugs inhibit ERα activity through different mechanisms. For example, the 4-hydroxytamoxifen–mediated inhibition of ERα activity was not associated with a reduction in ERα protein levels in MCF-7 cells compared with vehicle treatment (Fig. 3, F and G). Therefore, these data indicate that a unique ERα destabilization activity of BAZ and TSEC effectively downregulates ERα activity in both endometrial and breast cells.
To determine whether this unique molecular property of TSEC correlates with tissue specificity, ERα levels in bone cells (HTB-85, osteosarcoma) were determined upon each treatment. In contrast to endometrial and breast cells, CE, BAZ, and TSEC did not reduce ERα levels in HTB-85 cells compered to vehicle treatment (Fig. 3, H and I). Interestingly, BAZ and TSEC led to a small increase in ERα levels in bone cells compared with vehicle and CE treatment. We conclude that TSEC treatment differentially modulates the ERα protein level in a tissue-specific manner.
Interaction of ERα with FBXO45 upon TSEC Treatment.
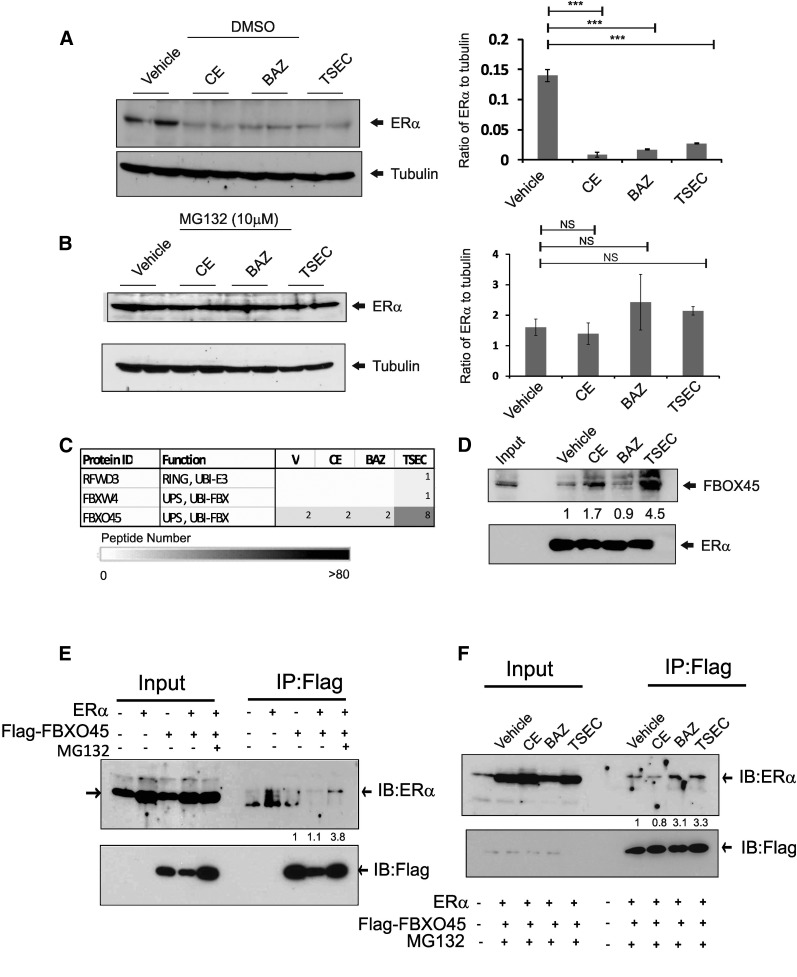
Our data revealed that CE- and TSEC-mediated ERα degradation was associated with differential cellular regulation of ERα activity (Figs. 2 and 3). This observation suggests that distinct proteasome complexes might be involved in CE- versus TSEC-mediated ERα degradation. To test this hypothesis, we analyzed whether the ubiquitination-dependent proteasome might be differentially recruited during CE- versus TSEC-mediated ERα degradation. In the presence of DMSO, CE, BAZ, and TSEC treatments, but not vehicle treatment, ERα protein degradation occurred in HeLa cells (Fig. 4A). However, all of these ERα degradations were prevented by the addition of the proteasome inhibitor MG132 (Fig. 4B). Therefore, both CE- and TSEC-induced ERα protein degradations require a ubiquitin-dependent proteasome system.
Fig. 4.
ERα interacts with FBXO45 upon TSEC treatment. (A and B) HeLa cells in a six-well plate were transfected with 100 ng of ERα expression vector. After 48 hours, cells were treated with vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE plus 100 nM BAZ) for 24 hours in the absence (A) or presence (B) of MG132 (10 µM). Subsequently, the ERα and tubulin protein levels in HeLa cells were determined by Western blotting analyses (n = 2/group). The ratios of ERα to tubulin in HeLa cells are shown graphically (n = 4/group). (C) Peptide numbers of the ubiquitin ligases that were specifically associated with TSEC-bound ERα after ERα/ERE-DNA pull-down analyses. (D) ERα/ERE-DNA pull-down analysis was performed in the presence of hormone, and the FBXO45 levels in the precipitant were determined by Western blotting analyses. (E) HeLa cells were transiently transfected with expression vectors for ERα and/or Flag-tagged FBXO45. After 48 hours, anti-Flag antibodies were used to immunoprecipitate Flag-tagged FBXO45 from each cell lysate in the absence or presence of MG132 (10 µM). The levels of ERα and FBXO45 in the immunoprecipitates were determined by Western blotting analyses. (F) HeLa cells were transiently transfected with expression vectors for ERα and Flag-FBXO45. After 48 hours, cells were treated with vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE plus 100 nM BAZ) for 6 hours. Using anti-Flag antibodies, Flag-FBXO45 was immunoprecipitated from each group, and the ERα levels in the immunoprecipitates were determined by Western blotting analyses. ***P < 0.001, Student’s t test. Values represent the average ± S.E.M. of three independent experiments. FBX, F-Box; IB, Immuno Blot assay; IP, immunoprecipitation; NS, not significantly different versus vehicle; RING, Really Interesting New Gene; UBI, Ubiquitin; UPS, Ubiquitin Proteasome System.
The ubiquitin-dependent proteasome systems consist of several components that are involved in specific cellular processes (Devoy et al., 2005; Konstantinova et al., 2008). Therefore, we analyzed whether different components of the ubiquitin proteasome complex might be involved in CE- versus TSEC-mediated ERα degradation. ERE-DNA pull-down assays coupled with mass spectrometry analyses revealed that FBXO45, a component of an E3 ubiquitin ligase complex (Saiga et al., 2009; Tada et al., 2010), was significantly recruited to the ERα/ERE complex in the presence of TSEC compared with vehicle (Fig. 4, C and D). However, less FBXO45 protein was recruited to the ERα/ERE complex upon E2 or CE treatment compared with TSEC treatment (Fig. 4D; Supplemental Fig. 2C). BAZ did not stimulate FBXO45 recruitment to the ERα/ERE complex compared with vehicle (Fig. 4D). Immunoprecipitation with HeLa cells transfected with ERα and/or Flag-tagged FBOX45 cDNA using Flag antibody also revealed the interaction of FBXO45 with ERα in the presence of MG132 (Fig. 4E). To test whether this interaction was enhanced by TSEC treatment, HeLa cells were transiently transfected with expression vectors for ERα and Flag-tagged FBXO45, and then treated with vehicle, CE, BAZ, or TSEC. Flag-tagged FBXO45 was then immunoprecipitated using the Flag antibody, and ERα levels in the precipitated pellets were determined using Western blot analyses. Consistent with the results of the ERE-DNA pull-down assay (Fig. 4C), the interaction of ERα with FBXO45 was enhanced by TSEC treatment compared with vehicle and CE treatment (Fig. 4F). In addition, BAZ treatment also enhanced the interaction of ERα with FBXO45 compared with vehicle and CE in HeLa cells (Fig. 4F). This BAZ-mediated interaction of ERα with FBXO45 was not detected using the ERE-DNA pull-down assay (Fig. 4D). Therefore, these data indicate that the FBXO45/ubiquitin proteasome complex interacts with ERα only upon TSEC treatment in ERE-DNA.
Degradation of the ERα Protein by the FBOX45/Ubiquitination by TSEC.
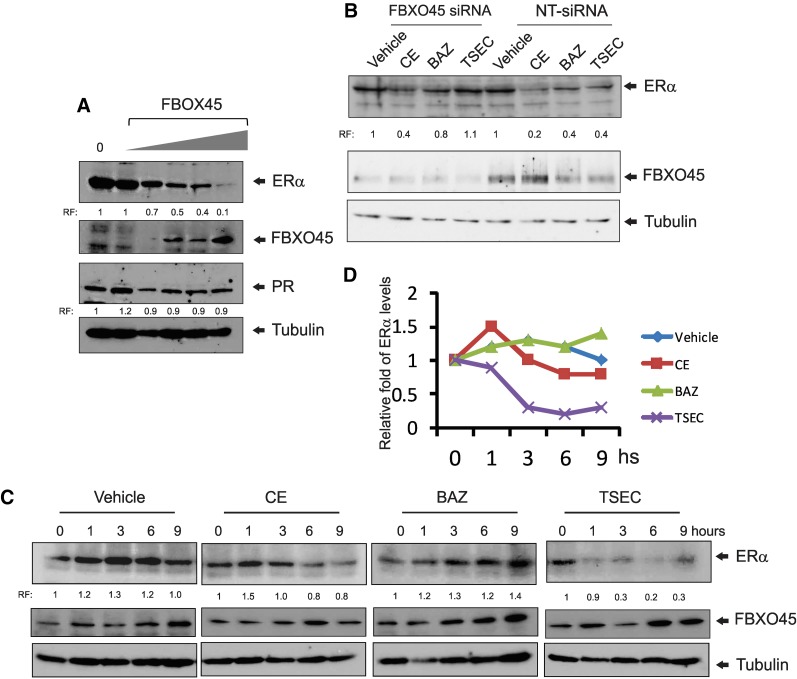
TSEC induced an interaction of ERα with FBXO45. Next, we analyzed whether this interaction is directly correlated with TSEC-induced ERα degradation. HeLa cells were transiently transfected with ERα and different amounts of FBXO45 expression vectors, and the ERα protein levels were determined by Western blot analyses. The ERα protein levels gradually decreased in the presence of increased FBXO45 levels compared with cells transfected with the empty expression vector (Fig. 5A). However, PR protein levels were not reduced in the presence of higher FBXO45 levels (Fig. 5A). Therefore, elevation of the FBXO45 level caused the specific degradation of ERα, but not PR, in HeLa cells.
Fig. 5.
FBXO45 involves in TSEC-mediated ERα degradation. (A) HeLa cells were transfected with an ERα expression vector and different amounts of expression vectors for Flag-tagged FBXO45. The ERα, FBXO45, PR, and tubulin protein levels in each group were determined by Western blotting analyses. (B) HeLa cells transfected with ERα were treated with 100 nM nontargeting siRNA or FBXO45 siRNA for 3 days followed by vehicle, CE (10 nM), BAZ (100 nM), and TSEC (10 nM CE and 100 nM BAZ) for 24 hours. Subsequently, the ERα, FBXO45, and tubulin protein levels in each group were determined by Western blotting analyses. (C) HeLa cells were transfected with expression vectors for ERα and FBXO45. The cells were then treated with vehicle, 10 nM CE, 100 nM BAZ, or TSEC (10 nM CE plus 100 nM BAZ) for 0, 1, 3, 6, and 9 hours. Subsequently, the ERα, FBXO45, and tubulin protein levels in each group were determined by Western blotting analyses. (D) Quantification of ERα levels in panel C is shown in the graph. hs, hours; RF, relative fold compared with control.
The effects of FBXO45 gain-of-function and loss-of-function in TSEC-induced ERα degradation were examined with HeLa cells transfected with ERα. To reduce FBXO45 protein levels in HeLa cells, the cells were treated with siRNA targeting FBXO45. As a control, HeLa cells were treated with nontargeting siRNA (NT-siRNA). Subsequently, both groups of cells were treated with vehicle, CE, BAZ, or TSEC to test their effects on ERα degradation. After hormone treatment, the ERα and FBXO45 protein levels in both groups of HeLa cells were determined by Western blotting analyses. FBXO45 levels were significantly reduced by FBXO45 siRNA compared with NT-siRNA in HeLa cells (Fig. 5B). In the NT-siRNA–treated HeLa cells, ERα protein levels were reduced by CE, BAZ, and TSEC treatments compared with the vehicle (Fig. 5B). However, BAZ and TSEC treatment did not reduce ERα protein levels in FBXO45 siRNA-treated cells compared with the vehicle (Fig. 5B). Notably, CE treatment reduced the ERα protein level in FBXO45 siRNA-treated HeLa cells compared with vehicle treatment (Fig. 5B). Therefore, FBXO45 is required for TSEC-mediated degradation, whereas it is not involved in CE-mediated ERα degradation in HeLa cells.
To examine whether increased FBXO45 expression degrades ERα protein in a TSEC-dependent manner, HeLa cells were transiently transfected with expression vectors for ERα and FBXO45. Subsequently, the cells were stimulated with vehicle, CE, BAZ, or TSEC, and then the ERα protein levels in these cells were determined by Western blot analysis. The ERα protein levels were not significantly reduced 9 hours after CE treatment compared with vehicle treatment (Fig. 5, C and D). BAZ treatment does not reduce ERα protein levels compared with vehicle treatment at 9 hours after treatment (Fig. 5, C and D). However, ERα protein levels were rapidly and significantly reduced by TSEC treatment compared with vehicle treatment (Fig. 5, C and D). Therefore, overexpressing FBXO45 effectively caused ERα protein degradation upon treatment with TSEC, but not with other agents.
Suppression of Ectopic Lesion Growth in Mice with Endometriosis by TSEC.
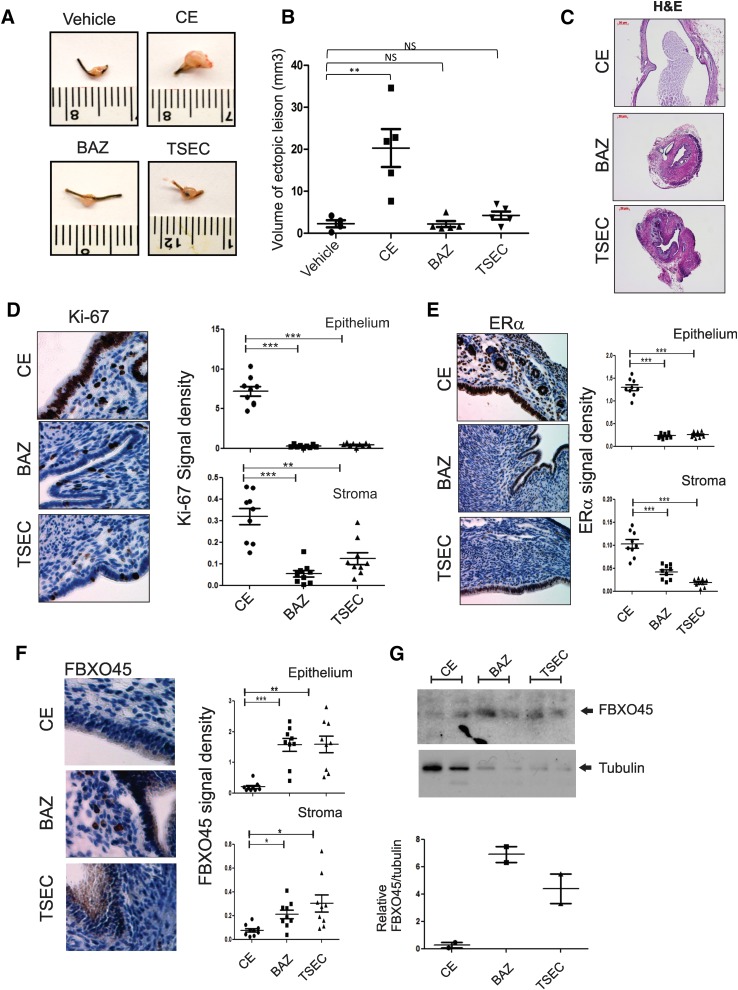
Endometriosis is an estrogen-dependent disease, because estrogen signaling plays an essential role in the pathogenesis of endometriosis (Bulun, 2009). Studies using ERα knockout mice with surgically induced endometriosis revealed that ERα has an essential role in the growth of ectopic lesions (Burns et al., 2012). Since the TSEC/FBXO45 axis is involved in ERα degradation in endometrial tissues (Fig. 5), TSEC might be applied for an alternative endometriosis treatment, because TSEC minimizes the side effects of systemic estrogen-deficiency therapy in other estrogen target tissues, such as brain and bone, during treatment. To test this hypothesis, mice with surgically induced endometriosis were administered CE, BAZ, and TSEC. CE treatment increased the volume of the ectopic lesion in mice with endometriosis compared with vehicle treatment, most likely due to its endometrial stimulatory effect (Fig. 6, A and B). However, compared with vehicle treatment, BAZ and TSEC did not stimulate ectopic lesion growth in mice with endometriosis (Fig. 6, A and B). The presence of BAZ in TSEC suppressed the CE-mediated ectopic lesion growth during endometriosis. In addition, histologic analyses revealed that CE-treated ectopic lesions had well developed cyst formation (Fig. 6C). This endometriotic cyst formation was not well developed in ectopic lesions treated with BAZ and TSEC as compared with CE-treated ectopic lesions (Fig. 6C). In addition to the cyst formation, immunohistochemistry (IHC) analyses with Ki-67 antibody revealed that proliferative activities in epithelial and stromal compartments of ectopic lesions treated with BAZ and TSEC were significantly reduced compared with ectopic lesions treated with CE (Fig. 6D). Collectively, TSEC treatment inhibited proliferative activity of ectopic lesions to prevent their progression, whereas CE promoted proliferative activity of ectopic lesions for the establishment of ectopic lesions.
Fig. 6.
TSEC treatment inhibited the progression of ectopic lesions in mice with surgically induced endometriosis. (A and B) Ectopic lesions were isolated from each group of mice treated with different hormones (A), and then the lesion volumes were calculated and presented graphically (n = 5/group) (B). (C) Hematoxylin and eosin analyses of ectopic lesions treated with each ligand in (A). (D–F) Expression levels of Ki-67 (D), ERα (E), and FBXO45 (F) in each type of ectopic lesion described in (A) were determined using immunohistochemistry and are shown in the graph (n = 9/group). (G) Expression levels of FBXO45 in eutopic endometrium treated with each hormone were determined using Western blot analyses and are shown in the graph. *P < 0.05; **P < 0.01; ***P < 0.001. NS, nonspecific versus control, Student’s t test.
Since TSEC degrades ERα protein to suppress ERα-mediated signaling in endometrial tissues (Fig. 2), we analyzed whether TSEC caused the degradation of ERα in ectopic lesions to inhibit the estrogen-dependent growth of these lesions. ERα levels in ectopic lesions were determined by IHC analyses using an ERα antibody. TSEC-treated ectopic lesions had lower ERα protein levels in both epithelial and stromal compartments of ectopic lesions compared with CE-treated ectopic lesions (Fig. 6E). Notably, IHC and Western blot analyses using an FBXO45 antibody revealed that FBXO45 levels were elevated in BAZ- and TSEC-treated ectopic lesions compared with CE-treated lesions (Fig. 6, F and G), and TSEC-mediated elevation of FBXO45 levels appears to enhance TSEC-induced ERα degradation to suppress the growth of ectopic lesions. Together, our data suggest that TSEC-induced ERα degradation in the endometrium might be used as an alternative endometriosis treatment to minimize the side effects of estrogen deprivation in other estrogen target tissues that are associated with certain current endometriosis treatments.
Discussion
Hormone replacement therapy has been used for the treatment of postmenopausal symptoms. To maximize the benefits of hormone replacement therapy, TSEC was developed, which is a new menopausal therapy that combines a SERM with one or more estrogens. The goal of TSEC therapy is to reduce menopausal symptoms (e.g., hot flashes and vulvar/vaginal atrophy), prevent osteoporosis, and improve lipid parameters while inhibiting estrogenic stimulation of the breast and endometrium.
Although TSEC is known to inhibit estrogenic stimulation in the breast and endometrium, the molecular mechanisms underlying this function of TSEC were not understood. Here, we observed two new molecular mechanisms by which TSEC confers endometrial and breast safety effects. First, TSEC recruited corepressors, rather than coactivators, to ERα. In contrast, however, CE actively recruited coactivators to ERα compared with vehicle treatment, leading to enhanced ERα activity. The differential recruitment of ERα coregulators by CE versus TSEC is likely associated with distinct ERα conformational changes induced by these agents. For example, in the presence of a single hormone (such as CE and BAZ), each hormone-bound ERα generates homodimer binding to EREs to regulate ERα target genes. In the presence of TSEC, however, one agonist (CE)-bound ERα monomer binds to an antagonist (BAZ)-bound ERα to generate a distinct heterodimeric ERα conformation, which then binds to ERα target genes (Liu et al., 2013). This unique heterodimeric ERα conformation generated by TSEC appears to induce an interaction of ERα with certain corepressors, leading to inhibition of ERα activity.
In addition, TSEC induced ERα degradation via the FBXO45/ubiquitin proteasome, leading to suppression of ERα activity in endometrial and breast cells. CE also induced the degradation of ERα in endometrial and breast cancer cells by the ubiquitin-dependent proteasome. However, each hormone-induced ERα degradation was accompanied by an opposite effect on ERα activity: TSEC-induced ERα degradation was associated with inhibition of ERα activity, whereas CE-mediated ERα destabilization was correlated with enhancement of ERα activity. Previous studies also revealed that enhanced estradiol-induced ERα activation is associated with ERα degradation (Wijayaratne and McDonnell, 2001; Callige and Richard-Foy, 2006), and a specific E3 ubiquitin ligase, such as E6-AP, might be involved in this process (Li et al., 2006; Sun et al., 2012). However, the molecular mechanism underlying the functional correlation between estrogen-induced ERα degradation and ERα activation has not been fully elucidated. BAZ also causes ERα protein degradation similar to CE. However, BAZ-mediated ERα degradation is linked to the prevention of expression of ERα target genes, such as cyclin D1, in MCF-7:5C cells (Lewis-Wambi et al., 2011). The mechanism underlying BAZ-induced ERα degradation has not been investigated.
TSEC significantly reduced CE-mediated enhancement of ERα activity in endometrial and breast cancer cells by degrading ERα protein, in contrast with the E2-induced interaction of ERα with E6-AP for the ERα activation (Sun et al., 2012). However, TSEC caused a differential interaction of ERα with FBXO45 for the suppression of ERα activity. FBXO45 modulates the stability of key factors that are involved in the regulation of cell-cycle arrest and apoptosis in response to DNA damage–induced cellular stress by its ubiquitin ligase activity (Melino, 2003). For example, FBXO45 promotes the proteasome-dependent degradation of p73 to promote cell survival (Peschiaroli et al., 2009; Tada et al., 2010). In the case of TSEC treatment, FBXO45/ubiquitin proteasome promoted ERα degradation to suppress ERα activity leading to suppression of endometrial stimulation effects. The unique heterodimeric ERα conformation induced by TSEC appears to generate a specific interaction with FBXO45 (Liu et al., 2013). Collectively, TSEC treatment effectively prevents ERα activity by dual synergistic effects: recruitment of corepressors to ERα at target gene promoters and degradation of the ERα protein by a FBXO45/ubiquitin proteasome system in endometrial and breast cells.
Current endometriosis treatments induce systemic estrogen deficiency caused by the gonadotropin-releasing hormone agonists depot medroxyprogesterone acetate and danazol; aromatase inhibitors also are used (Telimaa et al., 1987; Haney and Weinberg, 1988; Telimaa, 1988; Fedele et al., 2000; Vigano et al., 2003; Selak et al., 2007). However, these estrogen-deficiency therapies can have harmful side effects in other estrogen target tissues, such as the brain and bone in young reproductive-age women (Compston et al., 1995; Vanderschueren et al., 1997; Hughes et al., 2007). Therefore, alternative endometriosis therapies that have fewer side effects in estrogen target tissues are needed. In this context, TSEC is an appropriate candidate for the next generation of endometriosis therapy because it effectively degrades ERα in endometriotic tissues but not in other estrogen target tissues, such as bone. Consistent with our study, other murine endometriosis models also revealed that TSEC effectively suppresses mouse endometriotic lesion growth compared with controls (Naqvi et al., 2014). Collectively, TSEC treatment should have selective therapeutic effects in endometrial tissues by virtue of its synergistic dual ERα inhibitory effects.
Supplementary Material
Acknowledgments
The authors thank the Cancer Prevention and Research Institute of Texas (RP120092) Facilities Support Award and NCI P30 CA123125 Cancer Center Support Grant for the Proteomics Core.
Abbreviations
- BAZ
bazedoxifene
- BCOR
BCL-6 corepressor
- CE
conjugated estrogen
- DMEM
Dulbecco’s modified Eagle’s medium
- E2
ethanol-soluble estrogen
- ER
estrogen receptor
- ERE
estrogen response element
- FBS
fetal bovine serum
- FBXO45
F-box protein 45
- IHC
immunohistochemistry
- MS
mass spectroscopy
- NT-siRNA
nontargeting siRNA
- PR
progesterone receptor
- rRNA
ribosomal RNA
- SERM
selective estrogen-receptor modulator
- siRNA
small interfering RNA
- SRC
steroid receptor coactivator
- TSEC
tissue-selective estrogen complex
Authorship Contributions
Participated in research design: Han, Begum, O’Malley.
Conducted experiments: Han, Begum.
Contributed new reagents or analytic tools: Foulds, Hamilton, Bailey, Malovannaya, Chan, Qin.
Performed data analysis: Han, Begum, O’Malley.
Wrote or contributed to the writing of the manuscript: Han, Begum, O’Malley.
Footnotes
This work was supported in part by the U.S. National Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD082786], the Komen Foundation, and Pfizer Pharmaceuticals.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Archer DF. (2010) Tissue-selective estrogen complexes: a promising option for the comprehensive management of menopausal symptoms. Drugs Aging 27:533–544. [DOI] [PubMed] [Google Scholar]
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. (2009) Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 92:1039–1044. [DOI] [PubMed] [Google Scholar]
- Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR, et al. (1994) Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 93:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. (2009) Endometriosis. N Engl J Med 360:268–279. [DOI] [PubMed] [Google Scholar]
- Burns KA, Korach KS. (2012) Estrogen receptors and human disease: an update. Arch Toxicol 86:1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. (2012) Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology 153:3960–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calligé M, Richard-Foy H. (2006) Ligand-induced estrogen receptor alpha degradation by the proteasome: new actors? Nucl Recept Signal 4:e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Wang Y, Bodine PV, Nagpal S, Komm BS. (2010) Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol 118:117–124. [DOI] [PubMed] [Google Scholar]
- Cirillo F, Nassa G, Tarallo R, Stellato C, De Filippo MR, Ambrosino C, Baumann M, Nyman TA, Weisz A. (2013) Molecular mechanisms of selective estrogen receptor modulator activity in human breast cancer cells: identification of novel nuclear cofactors of antiestrogen-ERα complexes by interaction proteomics. J Proteome Res 12:421–431. [DOI] [PubMed] [Google Scholar]
- Compston JE, Yamaguchi K, Croucher PI, Garrahan NJ, Lindsay PC, Shaw RW. (1995) The effects of gonadotrophin-releasing hormone agonists on iliac crest cancellous bone structure in women with endometriosis. Bone 16:261–267. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL. (1995) Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol 9:233–238. [DOI] [PubMed] [Google Scholar]
- DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, Schinnar R, Burgh D, Berlin M, Rubin SC, et al. (2008) Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol 26:4151–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. (2006) Estrogen receptors and human disease. J Clin Invest 116:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoy A, Soane T, Welchman R, Mayer RJ. (2005) The ubiquitin-proteasome system and cancer. Essays Biochem 41:187–203. [DOI] [PubMed] [Google Scholar]
- Fedele L, Bianchi S, Zanconato G, Tozzi L, Raffaelli R. (2000) Gonadotropin-releasing hormone agonist treatment for endometriosis of the rectovaginal septum. Am J Obstet Gynecol 183:1462–1467. [DOI] [PubMed] [Google Scholar]
- Foulds CE, Feng Q, Ding C, Bailey S, Hunsaker TL, Malovannaya A, Hamilton RA, Gates LA, Zhang Z, Li C, et al. (2013) Proteomic analysis of coregulators bound to ERα on DNA and nucleosomes reveals coregulator dynamics. Mol Cell 51:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizzo S, Saccardi C, Patrelli TS, Berretta R, Capobianco G, Di Gangi S, Vacilotto A, Bertocco A, Noventa M, Ancona E, et al. (2013) Update on raloxifene: mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet Gynecol Surv 68:467–481. [DOI] [PubMed] [Google Scholar]
- Green KA, Carroll JS. (2007) Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer 7:713–722. [DOI] [PubMed] [Google Scholar]
- Han SJ, Jeong J, Demayo FJ, Xu J, Tsai SY, Tsai MJ, O’Malley BW. (2005) Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol 25:8150–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney AF, Weinberg JB. (1988) Reduction of the intraperitoneal inflammation associated with endometriosis by treatment with medroxyprogesterone acetate. Am J Obstet Gynecol 159:450–454. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. (2013) Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause 20:138–145. [DOI] [PubMed] [Google Scholar]
- Hughes E, Brown J, Tiffin G, Vandekerckhove P. (2007) Danazol for unexplained subfertility. Cochrane Database Syst Rev (1):CD000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Ginsburg OM, Wijeratne TD, Howell A, Evans G, Sestak I, Narod SA. (2012) Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev 38:318–328. [DOI] [PubMed] [Google Scholar]
- Jordan VC. (1988) Long-term tamoxifen therapy to control or to prevent breast cancer: laboratory concept to clinical trials. Prog Clin Biol Res 262:105–123. [PubMed] [Google Scholar]
- Jordan VC, Koerner S. (1975) Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer 11:205–206. [DOI] [PubMed] [Google Scholar]
- Ko SS, Jordan VC. (2011) Treatment of osteoporosis and reduction in risk of invasive breast cancer in postmenopausal women with raloxifene. Expert Opin Pharmacother 12:657–674. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Komm BS, Chines AA. (2012) Bazedoxifene: the evolving role of third-generation selective estrogen-receptor modulators in the management of postmenopausal osteoporosis. Ther Adv Musculoskelet Dis 4:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova IM, Tsimokha AS, Mittenberg AG. (2008) Role of proteasomes in cellular regulation. Int Rev Cell Mol Biol 267:59–124. [DOI] [PubMed] [Google Scholar]
- Levenson AS, Jordan VC. (1999) Selective oestrogen receptor modulation: molecular pharmacology for the millennium. Eur J Cancer 35:1628–1639. [DOI] [PubMed] [Google Scholar]
- Lewis-Wambi JS, Kim H, Curpan R, Grigg R, Sarker MA, Jordan VC. (2011) The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor α and cyclin D1. Mol Pharmacol 80:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li Z, Howley PM, Sacks DB. (2006) E6AP and calmodulin reciprocally regulate estrogen receptor stability. J Biol Chem 281:1978–1985. [DOI] [PubMed] [Google Scholar]
- Liu S, Han SJ, Smith CL. (2013) Cooperative activation of gene expression by agonists and antagonists mediated by estrogen receptor heteroligand dimer complexes. Mol Pharmacol 83:1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov PY, Lee TM, Jordan VC. (2013) The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8:135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474. [DOI] [PubMed] [Google Scholar]
- Melino G. (2003) p73, the “assistant” guardian of the genome? Ann N Y Acad Sci 1010:9–15. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Ryan KA, Chandran AB, Komm BS. (2014) Bazedoxifene/conjugated estrogens for managing the burden of estrogen deficiency symptoms. Maturitas 77:24–31. [DOI] [PubMed] [Google Scholar]
- Morello KC, Wurz GT, DeGregorio MW. (2002) SERMs: current status and future trends. Crit Rev Oncol Hematol 43:63–76. [DOI] [PubMed] [Google Scholar]
- Morello KC, Wurz GT, DeGregorio MW. (2003) Pharmacokinetics of selective estrogen receptor modulators. Clin Pharmacokinet 42:361–372. [DOI] [PubMed] [Google Scholar]
- Nagai MA, Brentani MM. (2008) Gene expression profiles in breast cancer to identify estrogen receptor target genes. Mini Rev Med Chem 8:448–454. [DOI] [PubMed] [Google Scholar]
- Naqvi H, Sakr S, Presti T, Krikun G, Komm B, Taylor HS. (2014) Treatment with bazedoxifene and conjugated estrogens results in regression of endometriosis in a murine model. Biol Reprod 90:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. (1999) Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A, Merritt D, Rosen A, Heath H, 3rd, Siddhanti S, Plouffe L, Jr, Study Groups on the Effects of Raloxifene HCI With Low-Dose Premarin Vaginal Cream (2003) Effect of raloxifene on the response to conjugated estrogen vaginal cream or nonhormonal moisturizers in postmenopausal vaginal atrophy. Obstet Gynecol 101:346–352. [DOI] [PubMed] [Google Scholar]
- Peschiaroli A, Scialpi F, Bernassola F, Pagano M, Melino G. (2009) The F-box protein FBXO45 promotes the proteasome-dependent degradation of p73. Oncogene 28:3157–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar JH, Mirkin S. (2010) Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 16:121–128. [DOI] [PubMed] [Google Scholar]
- Pickar JH, Yeh IT, Bachmann G, Speroff L. (2009) Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 92:1018–1024. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Goldstein SR. (2010) Endometrial safety: a key hurdle for selective estrogen receptor modulators in development. Menopause 17:642–653. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Shifren JL, La Valleur J, Rosen A, Roesinger M, Siddhanti S. (2003) Influence of raloxifene on the efficacy of an estradiol-releasing ring for treating vaginal atrophy in postmenopausal women. Menopause 10:45–52. [DOI] [PubMed] [Google Scholar]
- Rutqvist LE. (1993) Long-term toxicity of tamoxifen. Recent Results Cancer Res 127:257–266. [DOI] [PubMed] [Google Scholar]
- Saiga T, Fukuda T, Matsumoto M, Tada H, Okano HJ, Okano H, Nakayama KI. (2009) Fbxo45 forms a novel ubiquitin ligase complex and is required for neuronal development. Mol Cell Biol 29:3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak V, Farquhar C, Prentice A, Singla A. (2007) Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev (4):CD000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Santen RJ, Wang JP, Yue W. (2013) Inhibitory effects of a bazedoxifene/conjugated equine estrogen combination on human breast cancer cells in vitro. Endocrinology 154:656–665. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhou W, Kaliappan K, Nawaz Z, Slingerland JM. (2012) ERα phosphorylation at Y537 by Src triggers E6-AP-ERα binding, ERα ubiquitylation, promoter occupancy, and target gene expression. Mol Endocrinol 26:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Okano HJ, Takagi H, Shibata S, Yao I, Matsumoto M, Saiga T, Nakayama KI, Kashima H, Takahashi T, et al. (2010) Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J Biol Chem 285:3840–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telimaa S. (1988) Danazol and medroxyprogesterone acetate inefficacious in the treatment of infertility in endometriosis. Fertil Steril 50:872–875. [DOI] [PubMed] [Google Scholar]
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. (1987) Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol 1:13–23. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, van Herck E, Nijs J, Ederveen AG, De Coster R, Bouillon R. (1997) Aromatase inhibition impairs skeletal modeling and decreases bone mineral density in growing male rats. Endocrinology 138:2301–2307. [DOI] [PubMed] [Google Scholar]
- Viganò P, Mangioni S, Odorizzi MP, Chiodini A, Rocca S, Chiodo I. (2003) Use of estrogen antagonists and aromatase inhibitors in endometriosis. Curr Opin Investig Drugs 4:1209–1212. [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741. [DOI] [PubMed] [Google Scholar]
- Ward HW. (1973) Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. BMJ 1:13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayaratne AL, McDonnell DP. (2001) The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem 276:35684–35692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.