Abstract
Background
Harmful algal blooms (HABs) caused by the dinoflagellate Cochlodinium polykrikoides lead to severe environmental impacts in oceans worldwide followed by huge economic losses. Algicide agent copper sulfate (CuSO4) is regard as an economical and effective agent for HABs mitigation; its biochemical and physiological effects were revealed in C. polykrikoides. However, molecular mechanisms of CuSO4 effect on the C. polykrikoides, even other HAB species, have not been investigated. The present study investigated the transcriptional response of C. polykrikoides against CuSO4 treatments, with the aim of providing certain molecular mechanism of CuSO4 effect on the C. polykrikoides blooms.
Results
RNA-seq generated 173 million reads, which were further assembled to 191,212 contigs. 43.3 %, 33.9 %, and 15.6 % of contigs were annotated with NCBI NR, GO, and KEGG database, respectively. Transcriptomic analysis revealed 20.6 % differential expressed contigs, which grouped into 8 clusters according to K-means clustering analysis, responding to CuSO4; 848 contigs were up-regulated and 746 contigs were down-regulated more than 2-fold changes from 12 h to 48 h exposure. KEGG pathway analysis of eukaryotic homologous genes revealed the differentially expressed genes (DEGs) were involved in diverse pathway; amongst, the genes involved in the translation, spliceosome, and/or signal transduction genes were highly regulated. Most of photosystem related genes were down-regulated and most of mitochondria related genes were up-regulated. In addition, the genes involved in the copper ion binding or transporting and antioxidant systems were identified. Measurement of chlorophyll fluorescence showed that photosynthesis was significantly inhibited by CuSO4 exposure.
Conclusions
This study reported the first transcriptome of the C. polykrikoides. The widely differential expressed photosystem genes suggested photosynthetic machinery were severely affected, and may further contribute to the cell death. Furthermore, gene translation and transcription processes may be disrupted, inhibiting cell growth and proliferation, and possibly accelerating cell death. However, antioxidant systems resistant to CuSO4 caused stress; mitochondrion may compensate for photosynthesis efficiency decreasing caused energy deficiency. In addition, various signal transduction pathways may be involved in the CuSO4 induced regulation network in the C. polykrikoides. These data provide the potential transcriptomic mechanism to explain the algicide CuSO4 effect on the harmful dinoflagellate C. polykrikoides.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-015-2341-3) contains supplementary material, which is available to authorized users.
Keywords: Cochlodinium polykrikoides, Algicide CuSO4, Trancriptomic response, Differentially expressed genes
Background
Dinoflagellates are a large group of freshwater and marine microalgae; about half of them are photosynthetic, and thus they play a crucial role in aquatic ecosystems. To date, approximately 4500 dinoflagellate species have been described, including more than 2500 extinct species from the fossil record and approximately 2000 living species [1]. Some species are responsible for harmful algal blooms (HABs, sometimes referred to as red-tide), which can severely affect aquaculture and marine environments worldwide. Hence, much effort has been directed at trying to solve problems associated with HABs, including the causes of occurrence, identification of the organisms responsible, bloom dynamics, toxin production and associated genetics, environmental monitoring, management [2–4]. Some dinoflagellates (e.g., Alexandrium tarmarense, Gymnodinium breve, and Prorocentrum minimum) can produce toxins that affect fish, shellfish, mammals, seabirds, and other consumers by persisting in the food chain. In addition, large numbers of dinoflagellate cells clog gills and/or deplete oxygen levels in the water column, sometimes leading to massive morality of marine animals [5, 6]. To reduce these adverse effects, people have employed biological, chemical and physical approaches to control, prevent, and/or mitigate HABs [7, 8]. Amongst these, algicides that affect algae growth such as yellow loess, copper sulfate (CuSO4), hydrogen peroxide (H2O2), and oxidizing chlorine (Cl2) are regarded as effective ways to manage algal blooms, and can be applied in doses considered safe for environmental health [7, 9, 10]. To date, studies on the effects of these algicides on HAB species have mainly focused on the biocidal efficiency, by measuring cell growth, pigment content, and photosynthetic efficiency. Recently cellular and biochemical responses of functional genes, such as those involved in photosynthesis, to algicides have been assessed [9, 11, 12].
As unicellular microeukaryotes, dinoflagellates have distinct genomic characters (e.g., permanently condensed and liquid-crystalline chromosomes, very large nuclear genome sizes, low amounts of histones, ~70 % replacement of thymine with 5-hydroxymethyluracil, etc.). These properties make dinoflagellates an interesting model for genomic research [13]. In addition, some dinoflagellates lack typical eukaryotic transcriptional elements (e.g., TATA boxes) in the upstream regions of coding genes [14]. Hence, they may have specific regulatory mechanisms of gene expression (e.g., spliced leader trans-splicing, post-transcriptional regulation, etc.). Furthermore, dinoflagellate spliced-leader (dinoSL) trans-splicing is known to be a common transcription mechanism in nuclear genomes [15]. However, recent studies have demonstrated that Symbiodinium minutum differs from other dinoflagellates in that not all its nuclear genes are dinoSL trans-splicing [15, 16]. Moreover, Brunelle and Van Dolah [17] found that cell cycle-related genes (e.g., those responsible for producing cell nuclear antigens, ribonucleotide reductase, and replication factor C) were not altered at transcriptional level but at the protein level during the cell cycle in Karenia brevis. Hence, they proposed that expression of these genes was regulated post-transcription in this dinoflagellate. To the best of our knowledge, the transcriptional responses of dinoflagellate genes vary widely in different environmental conditions [18–20]. These findings show that dinoflagellate genes regulation may be affected by environmental changes.
From a molecular perspective, the large genomes of HAB-forming dinoflagellates implies that they should have specific duplication mechanisms (e.g., a permanently present nuclear membrane, even during mitosis) to allow rapid proliferation, especially during bloom initiation. Therefore, dinoflagellate genome and transcriptional studies may help in understanding these molecular mechanisms in HAB-forming species. Recently developed molecular methods (e.g., next generation sequencing and microarrays) have been applied to investigate the genome and transcriptome characteristics of HAB-forming species [16, 18, 20–23]. In addition, the roles of gene expression and regulation in mediating the effects of nutrient availability on HAB-forming dinoflagellate growth have been studied [20, 24]. Despite these advances, few studies have examined the molecular mechanisms leading to HAB termination, particularly the genome-wide gene responses to algicides
The marine dinoflagellate Cochlodinium polykrikoides is widely distributed in tropical and temperate zones throughout the world (see review by Kudela and Gobler [25]). The species causes fish mortality by producing massive amounts of mucous and depleting dissolved oxygen [26]. The HABs formed by C. polykrikoides lead to serious economic losses and environmental impacts. They are highly toxic to organisms that feed upon them, especially fish [26, 27]. Recently, C. polykrikoides has spread to many oceanic regions, including Europe, India, the Middle East, and North America [25]. Many studies on the species have been carried out in the last three decades, including environmental surveys, studies aimed at mitigation of harmful effects, those documenting global expansion, etc. However, studies on the effects of algicides at the cellular and genome level in C. polykrikoides, or other harmful dinoflagellates, are lacking. Such studies are necessary to understand the molecular mechanisms underlying bloom initiation, expansion, and termination.
In recent studies, we examined the physiological responses of C. polykrikoides to a common biocide (hereafter referred to as algicide), CuSO4, and found significant decreases of cell number and the pigment content as well as chlorophyll autofluorescence intensity [10]. These results indicate that the algicide had a considerable effect on C. polykrikoides at the cellular level, even greater than that of other chemicals such as yellow loess (unpublished data). In this study, we tested the effects of the algicide copper sulfate on the transcriptional responses of C. polykrikoides to understand its effects at the molecular level. First, we obtained large-scale cDNA sequences for C. polykrikoides, investigated the transcripts of cells exposed to copper sulfate, and then characterized these with bioinformatics tools, including the NCBI non-redundant protein (NR) database, the gene ontology (GO) database, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. In addition, we investigated the transcriptome response to determine how copper sulfate affects C. polykrikoides at the genomic level and what kinds of gene regulation mechanisms are involved its defensive response.
Methods
Culture and algicide treatment
A strain (CP-01) of C. polykrikoides was obtained from the National Fisheries Research and Development Institute (NFRDI) of Korea, and cultured in f/2 medium at 20 °C under a 12:12-h light–dark cycle with a photon flux density of about 65 μmol photons m−2 s−1.
At the exponential growth phase, cells of C. polykrikoides were exposed to the algicide copper sulfate (CuSO4, Cat. No. C1297, Sigma, MO) with final concentration of 1 mg L−1. The CuSO4 concentration used in this study was selected according to the median effective concentration value (EC50 value) tested by [28], which was 10 times lower than reported EC50 value. The exposed cultures were harvested at 12 h, 24 h, and 48 h, and the untreated cells were used as control.
RNA extraction and cDNA library construction
C. polykrikoides cultures were harvested using centrifugation at 1000 g for 6 min, immediately frozen in liquid nitrogen, and stored at −80 °C until RNA extraction. Preserved cells were physically broken by freeze-thawing in liquid nitrogen, and further homogenized with a mini-beadbeater (BioSpec Products Inc., Bartlesville, OK) with zirconium beads (0.1 mm diameter). Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), and purified using Mini Spin Columns from RNeasy Mini Kits (Qiagen, Valencia, CA). Total RNA integrity and quality were checked using an Agilent 2100 Bioanalyzer (Aglient, Santa Clara, CA). The cDNA library for subsequent cluster generation was prepared using the reagents provided in the Illumina ® TruSeq™ RNA Sample Preparation Kit (RS-122-2001, Illumina Inc., San Diego, CA). Sequencing was finished by a commercial service (Macrogen Inc., Seoul, Korea) using the Illumina HiSeq 2500 (Illumina Inc., San Diego, CA).
Transcriptome assembly and functional annotation
The quality of raw reads was checked with FastQC_v0.10.0 (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/). The raw data were cleaned and trimmed by removing adaptor and low quality reads, and then reads were assembled using Trinity software [29]. The contigs were annotated by BLASTX alignment, with E-value < 0.001, against the NCBI non-redundant protein (NR) database. For pathway enrichment analysis, the contigs were assigned to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [30] using the single-directional best hit (SBH) method contained in the online tool, KEGG Automatic Annotation Server. Functional annotation of contigs by gene ontology (GO) was carried out with Blast2go software [31]. The raw read transcriptome sequences were submitted to the NCBI Sequence Read Archive database (accession number SRR1548539).
Analysis of differentially expressed genes
The gene expression levels of the contigs were calculated as fragments per kilobase of transcript per million mapped reads (FPKM). The degree of differential gene expression in CuSO4 treated samples was calculated by comparison to the control (untreated exponential growth phase samples). The Log2 ratio ≥ 1 (fold change ≥ 2) was used as the threshold to define significantly differentially expressed genes (DEGs). All the DEGs were analyzed by clustering algorithm analysis using K-mean clustering. The identified eukaryote DEGs were mapped to the GO and KEGG databases. In the KEGG analysis, KEGG database assignment showed that some contigs coded for the same proteins, and the KEGG pathway analysis accounted for this when calculating the number of coded proteins. Contigs that coded for the same protein were considered as single genes, and were counted as one. In addition, the DEGs were further manually characterized with GO and NR database annotation by reviewing previous studies. The assembled sequences and raw FPKM values were registered in the GEO database with an accession number GSE75463.
Photosynthesis and oxidative stress measurements
Chlorophyll fluorescence was measured using a Handy PEA (Hansatech Instruments Ltd, Norfolk, UK). The parameters Fo, Fv, and Fm were measured at 0 h, 12 h, 24 h, and 48 h after CuSO4 exposure. The ratios Fv/Fm and Fv/Fo were calculated; Fv/Fm is an indicator of the photosynthetic efficiency, and Fv/Fo is a measure of the activity of the water-splitting complex on the donor side of photosystem II, as well as the size and number of active photosynthetic reaction centers [32, 33]. In addition, the maximum yield of primary photochemistry (ΨEo = TRo/ABS), and efficiency with which a trapped exciton can move an electron into the electron transport chain further than QA- (Ψo = Eto/Tro) were also calculated using a Handy PEA.
To detect reactive oxygen species (ROS), cells were stained with DHR123 (D1054; Sigma) for 1 h. The DHR123 stock solutions were directly added into cell cultures at a final concentration of 5 μM/L. After incubation, cellular ROS content was measured with an LS-55 fluorescence spectrometer (Perkin-Elmer, Waltham, MA). Lipid peroxidation was measured according to the method described in [34]. One-way analysis of variance (ANOVA) with post hoc Dunnett’s multiple comparison test using Graphpad InStat (Graphpad Software, Inc., USA) was used for comparisons between control and treated cultures. Data are represented as mean ± SD, and P < 0.05 was considered statistically significant.
Quantitative real-time PCR for gene validation
Some DEGs of interest were selected and validated using quantitative real-time PCR (qRT-PCR). The primers used in the qRT-PCR are listed in Additional file 1. All qRT-PCR reactions were performed with TOPreal™ qPCR 2X PreMIX (TOP, enzynomics, Korea) in a CFX96 Real-Time PCR Detection System (Bio-Rad; Hercules, CA). The qRT-PCR conditions were as follows: 4 min at 50 °C; 10 min at 95 °C, followed by 40 cycles of 10 s at 95 °C, 15 s at 60 °C, and 15 s at 72 °C. All reactions were performed in triplicate, and the mean value was calculated. The specificity of the amplification was verified through the analysis of a melting curve generated by gradually heating the sample from 65 °C to 95 °C. The α-tubulin (TUA) gene, which has the most stable expression pattern known in the dinoflagellate Prorocentrum minimum [35], was used as an internal control. CT values of qRT-PCR were obtained using CFX96 Real-Time controlling software (Bio-Rad; Hercules, CA). The fold-change relative to control was calculated according to the method of Pfaffl [36]. The Spearman correlation coefficient of the gene expression results from Hiseq2500 sequencing and qRT-PCR were calculated with Origin 8 software (OriginLab Corporation, MA).
Results
Transcriptome and functional gene annotations
To profile the transcriptome of C. polykrikoides, we constructed cDNA libraries, including CuSO4 exposed samples (at 12 h, 24 h, and 48 h), and unexposed control sample. RNA sequencing generated 173 million reads from libraries, containing 26.1 Gb nucleotides. The raw reads were assembled with 90 % similarity and 191,212 contigs with a mean length of 922 bp (Table 1). Contig length ranged from 201 to 36,127 bp (Table 1; Additional file 2). Of these contigs, 102,744 (53.7 %) were 201–600 bp in length, 38,593 (20.2 %) were 601-bp, 23,889 (12.5 %) were 1201–1800 bp, and 25,986 (19.0 %) were longer than 1800 bp.
Table 1.
Summary of the Cochlodinium polykrikoides transcriptome
| Category | Number | N50 (bp) | Total nucleotides (bp) | Maximum length (bp) | Minimum length (bp) |
|---|---|---|---|---|---|
| Read | 172,977,960 | - | 26,119,671,960 | - | - |
| Contigs | 191,212 | 1550 | 176,356,262 | 36,127 | 201 |
In addition, individual contigs were assigned to three different protein databases: NCBI NR, Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) (Table 2). All contigs were aligned on the NCBI NR protein database by BLASTX with an E-value cutoff of 10−4. Of the 191,212 contigs, a total of 82,749 (43.28 %) were annotated in the NCBI NR database. Of these annotated contigs, 82.5 % were assigned to eukaryotes, 16.4 % were assigned to bacteria, and 1.1 % were classified as ‘other’. The superphylum Alveolata accounted for 16.8 % of annotated contigs, including 3.6 % Apicomplexa, 2.1 % Ciliophora, 3.6 % Dinophyta, and 7.6 % Perkinsus. In addition, the same sequences were analyzed to the GO database, and 64,931 contigs (33.96 %) were annotated. Overall, the annotations for these contigs were similar to those obtained from NCBI NR.
Table 2.
Summary of annotation of contigs in each database
| Public protein database | Number of contigs hits | Percentage (%) |
|---|---|---|
| NCBI NR | 82,749 | 43.28 % |
| GO | 64,931 | 33.96 % |
| KEGG | 29,983 | 15.60 % |
In the KEGG analysis, a total of 29,983 contigs (15.6 %) were assigned to 217 KEGG pathways, excluding organismal systems and human diseases. Of these, 2297 genes were assigned to metabolic processing; 1007 to genetic information processing; 717 to environmental information processing; 666 to cellular processes (Fig. 1). The highest number of genes (586) was assigned to signal transduction, followed by carbohydrate metabolism (447 genes) and amino acid metabolism (418 genes). Furthermore, within the third level KEGG pathway (Additional file 3), the highest number of genes (126) was assigned to ribosome, followed by purine metabolism (125 genes), spliceosome (104 genes), and pyrimidine metabolism (99 genes) (Fig. 1).
Fig. 1.

Pathway assignment based on KEGG pathway analysis. The gene numbers were obtained from KEGG pathway online analysis
Clustering of DEGs following algicide exposure
Differential expression of genes in algicide-exposed cells was evaluated using the abundance of transcripts, quantified as FPKM. Contigs with FPKM lower than zero of were excluded from DEG analyses. Based on this cut-off threshold, a total of 100,370 contigs were excluded, and 90,842 contigs were retained in subsequent analyses. Of these, 18,700 contigs (around 20.6 %) showed differentially expressed patterns, as determined by 2-fold changes in expression. Of contigs with 2-fold or greater changes in expression, 3816, 3430, and 5792 were up-regulated and 5304, 2530, 6052 were down-regulated at 12 h, 24 h, and 48 h, respectively (Additional file 4).
Based on K-mean clustering analysis, all contigs were divided into 8 clusters (Fig. 2; Additional file 5). Of these, clusters 1, 3, 5, and 8, which included 5573, 1023, 1933, and 20 contigs, respectively, showed up-regulated patterns of expression within 48 h of algicide exposure. Of these four clusters, cluster 1 was the least up-regulated, with a mean change in expression of around 2-fold and no obvious difference among tested samples. By contrast, expression of contigs in clusters 3 and 8 gradually increased over 48 h, and cluster 8 had greatest increase in expression, which occurred at 48 h. The contigs in cluster 5 were down-regulated at first and then expression gradually increased. Cluster 2 (1077 contigs), cluster 4 (6889 contigs), and cluster 7 (935 contigs) showed no obvious changes or down-regulation. In cluster 6 (1250 contigs), contigs generally displayed up-regulation first and then down-regulation patterns; some contigs showed the highest expression level at 12 h or 24 h (>2 fold changes), and then they showed decreased expression levels i.e., lesser than that of control. Other contigs showed increased expression levels i.e., more than that of control, and then decreased expression levels > 2 fold changes at 48 h.
Fig. 2.
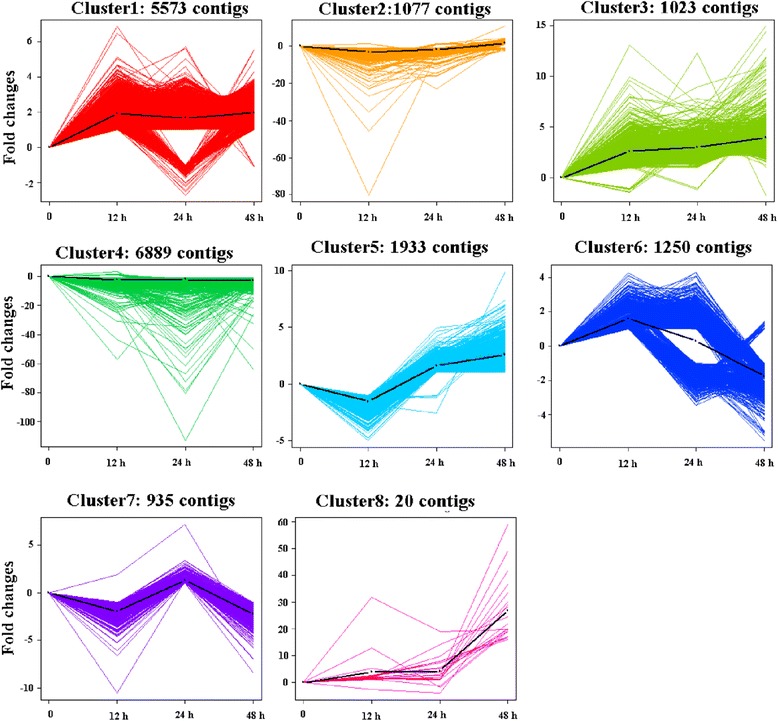
Expression pattern of each cluster of DEGs. A total of 8 clusters were produced; the genes that displayed similar expression pattern were gathered into one cluster
The up- and down-regulated contigs with > 2 fold changes were analyzed separately. Base on this criterion, we detected 848 up-regulated and 726 down-regulated contigs at all time intervals i.e., 12 h, 24 h, and 48 h (Additional file 6). These data were then combined with K-mean clustered contigs with 2-fold changes in expression and overlapping contigs were removed before further analysis. The three up-regulated clusters (clusters 3, 5, and 8) and > 2-fold up-regulated contigs were pooled together into group 1 (3624 contigs in total); and cluster 6 and > 2-fold down-regulated contigs were pooled together into group 2 (1976 contigs in total).
Classification of DEGs by KEGG analysis
The contigs that matched to bacteria and viruses were filtered out, and only eukaryote-matched contigs were subjected to further KEGG pathway analyses. This showed that 1130 eukaryote-matched contigs were assigned to group 1, and these contigs mapped to 157 KEGG pathways (Additional file 3) excluding organismal systems and human disease pathways (Fig. 3). These were assigned to metabolic processing (457 genes), genetic information processing (309 genes), environmental information processing (154 genes), and cellular processes (148 genes). The top three second level pathways were translation (154 genes) (Additional file 7), signal transduction (148 genes) (Additional file 8), and carbohydrate metabolism (122 genes). The top three third level pathways were ribosome (76 genes) (Additional file 7), spliceosome (41 genes) (Additional file 9), and oxidative phosphorylation (36 genes) (Additional file 10). Of interest was that in the transcription pathway analysis 47 genes were identified as spliceosome, but only 2 transcription factors were identified.
Fig. 3.

The pathway assignment of DEGs by KEGG pathway analysis. Each group of DEGs was assigned to KEGG database, and gene numbers were got from KEGG online analysis
In group 2, we detected 374 eukaryote-matched contigs; of these, 133 were assigned to the KEGG database, and mapped to 77 KEGG pathways (Fig. 3, Additional file 3) excluding organismal systems and human disease pathways These were assigned to metabolic processing (64 genes), genetic information processing (45 genes), environmental information processing (23 genes), and cellular processes (20 genes). The top three second level pathways were translation (26 genes) (Additional file 7), signal transduction (23 genes) (Additional file 8), and energy metabolism (16 genes). The top three third level pathways were ribosome (20 genes) (Additional file 7), photosynthesis (Additional file 11) (7 genes), and protein processing in endoplasmic reticulum (6 genes). In the transcription pathway, only one gene was assigned to the spliceosome pathway and no transcription factor was detected.
Photosynthetic and mitochondrial gene responses in C. polykrikoides
Using KEGG pathway analyses, we found that the most affected metabolic pathways were oxidative phosphorylation in group 1 (Additional file 10), and photosynthesis in group 2 (Additional file 11). Since not all the sequences were annotated in the KEGG database, we further characterized the genes, which were involved in photosynthetic light reaction and mitochondria, using NR and GO database annotation. In these analyses, contigs that coded for the same gene were counted as single genes.
Among regulated photosynthesis genes (Additional file 12; Fig. 4), most genes involved in photosystem I (PS I), photosystem II (PS II), and cytochrome b6f complex showed patterns of down-regulation, particularly at 48 h after exposure to copper sulfate. Of a total of nine genes involved in the PS I complex, four genes were down-regulated from 12 h to 48 h after algicide exposure. Other genes were up-regulated and showed highest expression levels at 12 h, and then the expression levels were decreased. Transcriptional expression of the fifteen genes involved in the PS II complex gradually decreased from 12 h or increased at first, and then decreased; nine genes were down-regulated >2-fold at 48 h. As for the chloroplast cytochrome b6f complex, two genes were down-regulated at 48 h, and one gene was up-regulated by >2-fold at 12 h, and expression then decreased. Differing from PS II, PS I, and cytochrome b6f complex, expression of most light-harvesting proteins was up-regulated. Of seven light-harvesting protein genes, three genes were down-regulated > 2-fold at least once (at one time point), and four genes were up-regulated > 2-fold changes at least once. Of eleven photosynthetic electron transport associated genes, expression of four gradually decreased within 48 h; expression of three increased at first and then decreased; two were up-regulated; and two were down-regulated and then increased expression, showing >2-fold changes at 48 h.
Fig. 4.
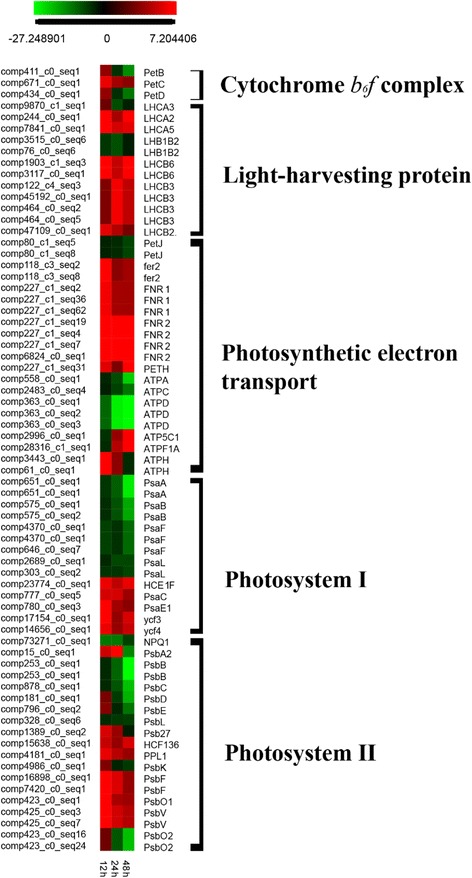
Heat map of DEGs that involved in the photosynthesis light reaction process. Log ratio fold changes were used as relative gene expression level compared to control. The full name of the gene and their characteristic were listed in the Additional file 12
Oxidative phosphorylation is a metabolic pathway that takes place in mitochondria. We examined behavior of certain mitochondrial associated genes which may contribute to mitochondrial composition or have mitochondrion-related functions (Additional file 13; Fig. 5). Of a total of 132 of these genes, 107 were up-regulated by >2-fold. Focusing on mitochondrial complex (MC) I, II, III, IV, and V composition genes, some of MC I and II genes were slightly down-regulated; however, all MC I and II coding genes were up-regulated from 24 h after algicide exposure, attaining >2-fold changes at 48 h. Expression of most of genes that coding for MC III component was increased, and only two genes showed down-regulation patterns. Overall, genes that code for MC IV and V showed up-regulation patterns. These results indicate that the mitochondrial genes examined participate in the cellular response to CuSO4 exposure.
Fig. 5.

Heat map of DEGs that related to the mitochondria. Log ratio fold changes were used as relative gene expression level compared to control. The full name of the gene and their characteristic were listed in the Additional file 13. “*” indicated the controversial genes that present or lost in the dinoflagellate C. polykrikoides
Antioxidant gene responses and copper function genes in C. polykrikoides
Since CuSO4 is a potential reactive oxygen species (ROS) inducer, production of certain antioxidants involved in detoxifying ROS in cells may be affected following exposure. Hence, we examined the behavior of antioxidant genes, including those associated with peroxiredoxin (Prxs), glutathione S-transferase (GST), glutathione reductase (GR), thioredoxin (Trx), etc. (Additional file 14; Fig. 6). Of these, expression of Prxs, GST, GR and Trx associated genes gradually increased following CuSO4 exposure (Fig. 6). In addition, some copper transporting and binding associated genes such as P-type ATPases were found to be expressed differentially (Additional file 15). Furthermore, widely studied chloroplast copper binding protein genes such as Cu/Zn superoxide dismutase (SOD) showed down-regulation patterns at 12 h, and then expression increased a little. Mitochondrial copper binding protein related genes, such as cytochrome C oxidase subunit 17 (COX17), Sco1p as well as some others were up-regulated following copper sulphate exposure (Additional file 13).
Fig. 6.
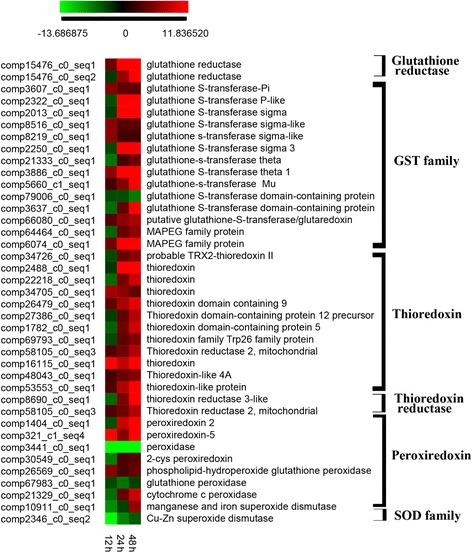
Heat map of DEGs that are coded antioxidant enzymes. Log ratio fold changes were used as relative gene expression level compared to control. The full name of the gene was listed in the Additional file 14
qRT-PCR validation of DEGs identified by transcriptome sequencing
We selected 13 DEGs with >2 fold changes in expression (Additional file 1) and validated our results using qRT-PCR (Fig. 7). Seven of these genes (ATPD - ATP synthase beta, PsbC- PSII CP43 apoprotein, PsaB - PSI P700 apoprotein, rbcL- ribulose 1,5-bisphosphate carboxylase oxygenase form II, PsbB- PSII CP47 apoprotein, COX1 - cytochrome oxidase subunit 1, and CYB - cytochrome b) showed similar results to those obtained via RNA-Seq over the whole test period, with the same expression patterns, but relatively different expression levels. Of the other genes (FNR2 - chloroplast ferredoxin-NADP(+) reductase 2, LHCB3- light-harvesting complex II a/b binding protein 3, CTYC6A - chloroplast cytochrome c6, PsbA2- PSII D1 reaction center protein, PetB - cytochrome b6, and PsbD - photosystem II protein D2), one or two tested samples (different time point) of each gene showed different expression patterns. Overall the results showed similar patterns of expression but with different strengths; the Spearman correlation coefficient (R) of the tested samples was 0.69 (N = 39, P < 0.001).
Fig. 7.
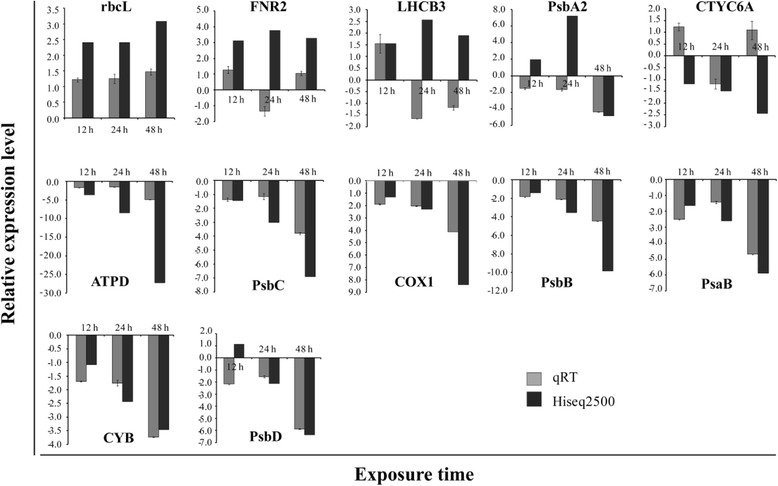
qRT-PCR validation of 13 DEGs identified by RNA-Seq. The relative expression levels of 12 h, 24 h, and 48 h CuSO4 treated sample were presented. The housekeeping gene α-tubulin (TUA) was used as an internal control for qRT-PCR normalization. The relative expression level of control was considered as 1, and the control samples were not shown in the figure
The effect of CuSO4 on C. polykrikoides photosynthesis and lipid peroxidation
Chlorophyll fluorescence parameters were monitored using a pulse amplitude modulation chlorophyll fluorometer. The maximal quantum efficiency of PS II Fv/Fm in C. polykrikoides decreased considerably with increasing CuSO4 exposure time. In addition, Fv/Fo, ΨEo, and Ψo gradually decreased with increasing exposure time to CuSO4 (Fig. 8).
Fig. 8.

Photosynthesis parameters of C. polykrikoides. a Fv/Fm, maximum quantum efficiency of photosystem II; b Fv/Fo, a value that is proportional to the activity of the water-splitting complex on the donor side of the PS II; c TRo/ABS (ΨEo), represents the maximum yield of primary photochemistry; d Eto/Tro (Ψo) represents the efficiency with which a trapped exciton can move an electron into the electron transport chain beyond QA-
The lipid peroxidation was increased in C. polykrikoides after 6 h and 72 h 1 mg L−1 CuSO4 exposure (Additional file 16).
Discussion
HABs caused by the dinoflagellate Cochlodinium polykrikoides are a global concern due to their geographic expansion and harmful environmental impacts. However, molecular understanding of the species has received little attention compared to studies of the species focusing on environmental monitoring, physiology, and toxicology. Those studies of HAB-forming species addressing genetic aspects have focused on discovering genes responsible for toxin production and the effects of nutrient availability on the regulation [21, 24, 37, 38]. In this study, we characterized for the first time genome-scale transcriptomes of C. polykrikoides, and further analyzed DEGs in response to algicide (copper sulfate) exposure to gain insights into the molecular mechanisms underlying the bloom termination process.
The effect of CuSO4 on the C. polykrikoides had been tested in several studies [10, 28]. Although the same strain was employed in these studies, the sensitivity of the cell to CuSO4 was quite varied. Indeed, C. polykrikoides (CP-01) was very tolerant to CuSO4, and other contaminants as well in previous generations [28], the tolerance was decreased in more recent generations [10]. The reason might due to differential composition of multi-clone that happened after several successive transfers. Due to clonal variation, the reduction of Daphnia magna quite varied after stressor exposure like cadmium or cyanobacteria Microcystis aeruginosa [39]. In this study, the CuSO4 concentration we have selected was 10 times lower than that of 72 h-EC50 [28]. Furthermore, the copper concentration, we selected by considering the World Health Organization’s guideline for copper in drinking water, which was 2.0 mg/L [40].
With the functional annotations presently available in three databases used (NR, GO, KEGG), few contigs could be annotated. The highest number of annotations (82,749 contigs, 43.3 %) was obtained from the NR database. One of reason for this was that some contigs were generated by non-coding 5’- or 3’- untranslated regions, another reason might be the presence of many ‘no-hit’ contigs, belonging to undiscovered novel genes and/or non-coding RNAs (e.g., miRNA, siRNA, and rRNA), that are known to be present in dinoflagellates [41, 42]. However, these annotation success rates were higher than those from available transcriptome data for other dinoflagellates, including Alexandrium catenella and Symbiodinium minutum [16, 23]. This suggests that the enough of the functional genes in C. polykrikoides might have been identified in our experiments to sufficiently characterize the whole genome response of the species.
The KEGG pathway analysis provided physiological pathway information for C. polykrikoides. The KEGG pathways of C. polykrikoides were quite different from those of other dinoflagellates. For example, in A. catenella spliceosome, translation factor, and RNA transport were the top pathways [23]. However, in both of C. polykrikoides and A. catenella, many genes that were assigned to genetic metabolism or genetic information processes were identified. In addition, both species have many genes assigned to spliceosome, indicating that spliceosome is likely to be crucial in dinoflagellate genetic processes such as RNA-splicing. These transcriptome data provided basic genetic information on C. polykrikoides, however, further investigations on the characteristics and functions of C. polykrikoides genes are essential.
The mechanism of spliced trans-splicing of mRNA and no typical recognized promoter in the dinoflagellates implied that the dinoflagellate genes expression were regulated by post-transcriptional regulation [15, 43, 44]. Furthermore, investigations on some genes and their protein expressions revealed that they were regulated at protein level. These data were consistent with the post-trancriptional regulation hypothesis [17, 45, 46]. In addition, the low amount of transcriptome were identified in response to stress conditions in some dinoflagellates, for example, Pyrocystis lunula microarray studies showed that around 5.8 % of DEGs (204 in total of 3500 genes) responded to nitrite, and 1.1 % genes to the herbicide paraquat [18]. These data showed that dinoflagellates have no expression preference for transcriptional gene regulation pattern. However, the little higher expression amount transcriptome were also found in some dinoflagellates. For example, transcriptome analyses of Alexandrium fundyense showed that 10 % of signature genes were differentially expressed at two different nutrient conditions [19]. More recently, Johnson et al. [22] reported 29 % of genes were differentially expressed among different life stages in the dinoflagellate Karenia brevis. We detected that expression of 20.6 % of contigs changed following exposure to the algicide CuSO4. In this context, the percentage of response to CuSO4 in this study was not low; this percentage was similar or higher than that shown in other dinoflagellates, suggesting that the algicide might considerably affect molecular genomic processes in C. polykrikoides. These HiSeq results were validated in a separate qRT-PCR assay, with significant correlation (R2 = 0.69, P < 0.05) between results from the two methods.
Environmental stress can cause rapid changes in the production of cellular proteins for survival, and responses are controlled at multiple levels, including transcriptional, post-transcriptional, and translational levels [47]. As a protein involved in genetic processes, ribosome is central to the translation process; ribosomal proteins have functions not only in ribosome composition, but also involved in various regulation processes. Hence, their expression is regulated to balance the protein production in response to environmental changes [48, 49]. In this study, KEGG pathway analyses showed that many ribosomal protein genes were regulated by CuSO4. Similar results have been found in other various organisms, such as fungi, plants, and algae [48, 49]. For example, the ribosomal protein L44 gene was up-regulated by salt, sorbitol, and heavy metal exposure in the fungus Aspergillus glaucus [49]. Differential expression of cytosolic ribosomal protein genes was induced by CuCl2 in marine algae Ulva compressa [50], by various environmental conditions (elevated sugar, nitrogen stress, and UV exposure) in Arabidopsis thaliana [48], and even at different life stages of the dinoflagellate K. brevis [22]. In this study, we found that some differentially expressed ribosomal protein genes, such as those coding for ribosomal proteins L44 (RPL44) and RPL11, were up-regulated by exposure to CuSO4. Taken together with previous findings, our results suggest that ribosomal protein genes (e.g., RPL44, RPL11) may be involved in cellular regulation in response to algicide exposure in dinoflagellates.
Transcription factors commonly regulate gene transcription in various cellular processes [51, 52]. In this study, only a few transcription factors were detected by DEG analysis. This implies that transcription factors were not widely involved in the gene regulation of C. polykrikoides when exposed to CuSO4. This provides further evidence of unusual gene regulation patterns in dinoflagellates, and highlights the need for further investigation of the mechanisms of gene regulation in this group. Nevertheless, these findings suggest that the algicide CuSO4 may disrupt gene transcription and translation in C. polykrikoides, slowing cell growth, accelerating cell death, and thereby inducing HAB termination.
Genes involved in photosynthesis in plants and algae are sensitive to various environmental stimuli, such as excess light, salinity, metals, etc. [53–55]. The transcription of photosystem genes was decreased in the diatom Ceratoneis closterium when exposed to copper [53] and in the green algae Chlorella when treated with Pb2+ [55]. In our DEG analyses, core photosystem coding proteins (e.g., PsaA and PsaB of PS I, PsbB of PS II) were also down-regulated at 12 h after algicide exposure. In contrast, expression of some other photosynthesis-related genes, such as those coding for light harvesting proteins (e.g. LHB3, LHB6), along with PsaC (PS I), and PsbF (PS II) was increased. Similar results have been found in salt stressed rice, the opposite alteration of genes expression pattern of PsaD genes and other tested genes including PsaH, LHCA1, LHCA2 and LHCA4 were found in the salt stressed rice [56]. In addition, in herbicide (atrazine, bentazon) treated soybean expression of PS II type I and type II chlorophyll a/b binding proteins was decreased, while PsbR and PsbS genes, and the oxygen evolving complex were up-regulated [57]. In dichloromethane and dichloroethane treated cells of the algae Chlorella, PsaB genes were up-regulated in the first 12 h, but were down-regulated after 24 h [58]. These results suggest that differential expression of photosystem genes under stress, and that in C. polykrikoides the photosystem apparatus (e.g., PS I and PS II) may be experience ongoing damage with increasing exposure time and doses of CuSO4.
The damage of photosystem by CuSO4 was also supported by our results showing the gradually decreasing photosynthetic efficiency (Fig. 8). This is in accordance with the results of previous research, which showed reduction in both Fv/Fm and Fv/Fo in the black mangle when exposed to copper [59]. Additionally, in the red algae Antithamnion plumula, Fv/Fm decreased and PS II activity gradually decreased with exposure to increasing concentrations of Cu2+ [60]. These findings, taken together with the results of this study, suggest that excess Cu2+ might affect photosystem gene regulation and inhibit photosynthetic efficiency in dinoflagellates. These adverse effects might be due the direct damage to PS II or the inhibition of PS II repair mechanisms [61].
In contrast to photosynthesis-associated genes, we found that most mitochondrial genes were up-regulated in C. polykrikoides when exposed to CuSO4 (see Fig. 5). This is in agreement with the results of a previous study showing that the mitochondrial genes COX11 and COX17 were up-regulated in the algae Ulva compressa when exposed to copper [50]. This increased expression of mitochondrial genes may take place due to damage to mitochondrial proteins [50], or to boost oxidative phosphorylation to increase the probability of cell survival [62, 63]. In addition, many stress responses, such as rebalancing of cellular metabolite concentrations and altered ROS abundance, can be related to mitochondrial processes [63]. Robust mitochondrial function may be crucial to the survival of C. polykrikoides following algicide exposure, and prevention of reduced photosynthetic efficiency depends upon proper mitochondrial function under such stress conditions [63].
Mitochondria and photosystem require copper for their metabolic functions; however, excess copper can induce ROS production and is toxic to most organisms [64, 65]. Indeed, the disturbance of mitochondria and photosynthesis-related genes is an indication of induced ROS production, since these are the organelles that produce intracellular ROS [62, 66]. In this study, we measured ROS production in C. polykrikoides exposed to copper using fluorescence spectrometry. However, we could not quantify the exact amounts of ROS produced, because the naked CuSO4 treated dinoflagellate cells were highly fragile and were easily destroyed during ROS sample preparation. This considerable ROS production was in agreement with the results obtained for another dinoflagellate, Prorocentrum minimum, exposed to the same algicide, CuSO4 [65, 67]. It is likely that that copper induces oxidative stress by facilitating the generation of ROS, and high doses of copper damage C. polykrikoides cells irreversibly [10, 65].
Such cellular oxidative stress can be mitigated by the activation of antioxidant systems [68]. The main antioxidants include glutathione (GSH), thioredoxin (Trxs), peroxiredoxin (Prx), superoxide dismutase (SOD), and catalase [10, 69–71]. In this study, most up-regulated antioxidant genes were related to GSH, Trxs, and Prx production. The up-regulation of antioxidant enzyme (e.g. Prx, Trxs, and GSH) production was increased following copper exposure in the marine algae Ulva compressa [50] and also in the dinoflagellate P. minimum [65]. In particular, Glutathione S-transferase (GST) is a large gene superfamily, the members of which function as antioxidants by conjugating GSH to toxic substances. In addition, differential transcriptional expression of GST members in response to variation in environmental conditions has been found in various organisms [65, 72, 73]. Increased expression of MAPEG (membrane-associated proteins in eicosanoid and glutathione metabolism) family members has been reported in P. minimum in response to exposure to CuSO4 [65]. In addition, reduced GSH production was increased in P. minimum cells treated with CuSO4 [65]. These results suggest that in dinoflagellates GST genes may play an important role in cellular responses to CuSO4 induced stress.
The thioredoxin system includes two antioxidants, Trxs and thioredoxin reductase (TrxR) [74]. Trxs is a protein with a conserved redox site that maintains the intracellular redox state and reduces protein thiols. TrxR is required for the reduction of the active disulfide site in Trx, and plays a role in redox regulation [69]. Prxs family genes also function by via a thiol redox mechanism; they respond to various environmental stresses, such as H2O2, high light, and nutrient deprivation, and they are sensitive to cellular oxidation [75]. Hence, the accumulation of oxidized Prxs may indicate disruption of cellular redox homeostasis [75, 76]. The regulation of Trx and TrxR, as well as Prxs genes may be involved in the response of C. polykrikoides to CuSO4. Similarly, the copper induced expressions of thioredoxin and Prxs genes or their proteins were found in other organisms. For example, the expression of thioredoxin increased by copper in brown algae Ectocarpus siliculosus [77]; expression level of Prx protein increased by copper in macroalgae Scytosiphon gracilis [78]; the Prx enzyme activity was increased in brown alga Dictyota kunthii by excess copper [79]. Furthermore, Prx likely acted as an active antioxidant to control lipid peroxidation generated by copper in the S. gracilis and D. kunthii [78, 79]. Corresponding to increased Prxs gene expression, the lipid peroxidation increased in C. polykrikoides after CuSO4 exposure, which suggests that the Prxs might function in lipid peroxidation by decreasing CuSO4 induced stress in C. polykrikoides. Because glutathione, thioredoxin, Prxs are all elements of the thiol-disulfide redox regulatory network. This suggests that the thiol-disulfide redox system might play a vital role in the CuSO4 induced stress defense network in dinoflagellates.
Conclusions
This study is the first to report transcriptome profiles of the dinoflagellate C. polykrikoides with a focus on the genome-wide molecular responses to the biocide CuSO4. CuSO4 significantly decreased photosynthetic efficiency and induced widespread regulation of gene networks and signal transduction pathways in C. polykrikoides cells. DEG analyses showed that various photosynthetic genes were regulated in C. polykrikoides exposed to CuSO4. Based on these results, we conclude that the photosynthetic machinery might be severely affected when treated with the algicide, leading cell death. In addition, gene translation and transcription processes may be disturbed, and this may further inhibit cell growth and proliferation, possibly accelerating cell death. However, antioxidant systems and mitochondrial genes are likely to be activated in response to the cellular stress caused by CuSO4 exposure, and this might compensate for the decrease in photosynthetic efficiency. These results provide an understanding of the molecular basis of the cellular and genomic responses of HAB forming dinoflagellate cells exposed to algicides, and of the HAB termination process.
Acknowledgement
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2013R1A1A2013596, and 2015M1A5A1041805), and by a grant from the National Fisheries Research and Development (R2015047) funded to J.-S. Ki.
Abbreviations
- ATPD
ATP synthase beta
- COX (11; 17)
Cytochrome c oxidase assembly protein subunit (11; 17)
- COX1
Cytochrome oxidase subunit 1
- CuSO4
Copper sulfate
- CYB
Cytochrome b
- CYTC6A
Chloroplast cytochrome c6
- DEGs
Differentially expressed genes
- FNR2
Chloroplast ferredoxin-NADP(+) reductase
- FPKM
Fragments per kilobase of transcript per million mapped reads
- GO
Gene ontology
- GSH
Glutathione
- GST
Glutathione S-transferase
- HABs
Harmful algal blooms
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LHCA (1, 2, 4)
Light-harvesting complex I chlorophyll a/b binding protein (1, 2, 4)
- LHCB (3, 6)
Light-harvesting complex II chlorophyll a/b binding protein (3, 6)
- MC
Mitochondrial complex
- PetB
cytochrome b6
- NR
Non-redundant protein
- Prx
Peroxiredoxin
- PS I
Photosystem I
- PS II
Photosystem II
- PsaA
PS I P700 chlorophyll a apoprotein A1
- PsaB
PSI P700 apoprotein A2
- PsaC
PS I subunit VII
- PsaD
PS I subunit II
- PsaH
PS I subunit VI
- PsbA2
PSII D1 reaction center protein
- PsbB
PSII CP47 apoprotein
- PsbC
PSII CP43 apoprotein
- PsbD
photosystem II protein D2
- PsbF
PS II cytochrome b559 subunit beta
- PsbR
PS II cytochrome b559 subunit beta
- PsbS
PS II 22 kDa protein
- rbcL
Ribulose 1,5-bisphosphate carboxylase oxygenase form II
- SBH
Single-directional best hit
- SOD
Superoxide dismutase
- TrxR
Thioredoxin reductase
- Trxs
Thioredoxin
Additional files
The primers and full name of genes that used in the qRT-PCR validation. (XLSX 12 kb)
The contigs length distribution of the C. polykrikoides transcriptome. The gray bar indicated the total contigs, and white indicated contigs that have annotation in NR database. (PPTX 174 kb)
KEGG pathways of DEGs. The third level pathways of group 1 and group 2 were list in the excel file. Gene numbers indicated the total gene numbers that assigned to each third level pathway. (XLSX 16 kb)
The numbers of DEGs. The DEGs numbers with 2.0-fold cut-off at 12 h, 24 h, and 48 h. (PPTX 82 kb)
K -means clustering heat map of DEGs. Total 8 clusters were shown; the cluster 8 was not recognized clearly since there were only 20 contigs. (PPTX 187 kb)
Analysis of contigs showing fold change more than 2.0-fold. (A) Up-regulation; (B) Down-regulation. (PPTX 157 kb)
DEGs that involved in translation. The genes were identified by KEGG pathway analysis. (XLSX 37 kb)
DEGs that involved in signal transduction. The genes were identified by KEGG pathway analysis. (XLSX 34 kb)
DEGs that involved in spliceosome. The genes were identified by KEGG pathway analysis. (XLSX 14 kb)
DEGs that involved in oxidative phosphorylation. The genes were identified by KEGG pathway analysis. “*” indicated the controversial genes that present or lost in the dinoflagellate C. polykrikoides. (XLSX 17 kb)
DEGs that involved in photosynthsis. The genes were identified by KEGG pathway analysis. (XLSX 12 kb)
Differentially expressed photosynthesis related genes. The genes were manually summarized by reviewing GO and NR annotation. (XLSX 22 kb)
Differentially expressed mitochondria related genes. The genes were manually summarized by reviewing GO and NR annotation. “*” indicated the controversial genes that present or lost in the dinoflagellate C. polykrikoides. (XLSX 34 kb)
Differentially expressed antioxidant genes. The genes were manually summarized by reviewing GO and NR annotation. (XLSX 14 kb)
Differentially expressed copper binding or transport genes. The genes were manual summarized by reviewing GO and NR annotation. (XLSX 14 kb)
Lipid peroxidation levels. Lipid peroxidation levels expressed as malondialdehyde (MDA) C. polykrikoides after 6 and 72 h exposure. Significant differences between the control and treated groups, as determined using one-way ANOVA, are highlighted **P < 0.01. (PPTX 163 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RYG performed the experiments, analyzed the data, and wrote the manuscript; WH performed the experiments and analyzed the data; YSS contributed to the experimental idea, provided reagents and materials; SJK conceived and designed project, analyzed the data, and wrote the manuscript. All the authors read and approved the final manuscript.
Contributor Information
Ruoyu Guo, Email: ganyuasg@163.com.
Young Sang Suh, Email: yssuhkorea@korea.kr.
Jang-Seu Ki, Email: kijs@smu.ac.kr.
References
- 1.Taylor FJR, Hoppenrath M, Saldarriaga J. Dinoflagellate diversity and distribution. Biodivers Conserv. 2008;17(2):407–18. doi: 10.1007/s10531-007-9258-3. [DOI] [Google Scholar]
- 2.Anderson DM, Cembella AD, Hallegraeff GM. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Ann Rev Mar Sci. 2012;4:143–76. doi: 10.1146/annurev-marine-120308-081121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glibert PM, Icarus Allen J, Artioli Y, Beusen A, Bouwman L, Harle J, et al. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Glob Chang Biol. 2014;20(12):3845–58. doi: 10.1111/gcb.12662. [DOI] [PubMed] [Google Scholar]
- 4.Miao C, Tang Y, Zhang H, Wu Z, Wang X. Harmful algae blooms removal from fresh water with modified vermiculite. Environmental Technolog. 2014;35(1–4):340–6. doi: 10.1080/09593330.2013.828091. [DOI] [PubMed] [Google Scholar]
- 5.Smayda TJ. What is a bloom? A commentary. Limnol Oceanogr. 1997;42(5part2):1132–6. doi: 10.4319/lo.1997.42.5_part_2.1132. [DOI] [Google Scholar]
- 6.Harvey EL, Menden-Deuer S. Predator-induced fleeing behaviors in phytoplankton: a new mechanism for harmful algal bloom formation? PLoS ONE. 2012;7(9):e46438. doi: 10.1371/journal.pone.0046438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HG. Mitigation and controls of HABs. In: Graneli E, Turner J, editors. Ecology of harmful algae, vol. 189. Springer Verlag, Berlin, Heidelberg; 2006. p. 327–38.
- 8.Secher S. Measures to control harmful algal blooms. The Plymouth Student Scientist. 2009;2(1):212–27. [Google Scholar]
- 9.Qian H, Yu S, Sun Z, Xie X, Liu W, Fu Z. Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol. 2010;99(3):405–12. doi: 10.1016/j.aquatox.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Ebenezer V, Lim WA, Ki J-S. Effects of the algicides CuSO4 and NaOCl on various physiological parameters in the harmful dinoflagellate Cochlodinium polykrikoides. J Appl Phycol. 2014;26(6):2357–65. doi: 10.1007/s10811-014-0267-9. [DOI] [Google Scholar]
- 11.Gouvêa SP, Boyer GL, Twiss MR. Influence of ultraviolet radiation, copper, and zinc on microcystin content in Microcystis aeruginosa (Cyanobacteria) Harmful Algae. 2008;7(2):194–205. doi: 10.1016/j.hal.2007.07.003. [DOI] [Google Scholar]
- 12.Jeong HJ, Kim HR, Kim KI, Kim KY, Park KH, Kim ST, et al. NaOCl produced by electrolysis of natural seawater as a potential method to control marine red-tide dinoflagellates. Phycologia. 2002;41(6):643–56. doi: 10.2216/i0031-8884-41-6-643.1. [DOI] [Google Scholar]
- 13.Hackett JD, Bhattacharya D. The genomes of dinoflagellates. In: Katz LA, Bhattacharya D, editors. Genomics and evolution of microbial eukaryotes. New York: Oxford University Press; 2006. pp. 48–63. [Google Scholar]
- 14.Hackett JD, Anderson DM, Erdner DL, Bhattacharya D. Dinoflagellates: a remarkable evolutionary experiment. Am J Bot. 2004;91(10):1523–34. doi: 10.3732/ajb.91.10.1523. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Hou Y, Miranda L, Campbell DA, Sturm NR, Gaasterland T, et al. Spliced leader RNA trans-splicing in dinoflagellates. Proc Natl Acad Sci. 2007;104(11):4618–23. doi: 10.1073/pnas.0700258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoguchi E, Shinzato C, Kawashima T, Gyoja F, Mungpakdee S, Koyanagi R, et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr Biol. 2013;23(15):1399–408. doi: 10.1016/j.cub.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 17.Brunelle SA, Van Dolah FM. Post-transcriptional regulation of s-phase genes in the dinoflagellate, Karenia brevis. J Eukaryot Microbiol. 2011;58(4):373–82. doi: 10.1111/j.1550-7408.2011.00560.x. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto OK, Hastings J. Novel dinoflagellate clock-related genes identifed through microarray analysis. J Phycol. 2003;39(3):519–26. doi: 10.1046/j.1529-8817.2003.02170.x. [DOI] [Google Scholar]
- 19.Erdner DL, Anderson DM. Global transcriptional profiling of the toxic dinoflagellate Alexandrium fundyense using massively parallel signature sequencing. BMC Genomics. 2006;7(1):88. doi: 10.1186/1471-2164-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moustafa A, Evans AN, Kulis DM, Hackett JD, Erdner DL, Anderson DM, et al. Transcriptome profiling of a toxic dinoflagellate reveals a gene-rich protist and a potential impact on gene expression due to bacterial presence. PLoS ONE. 2010;5(3) doi: 10.1371/journal.pone.0009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang I, Beszteri S, Tillmann U, Cembella A, John U. Growth-and nutrient-dependent gene expression in the toxigenic marine dinoflagellate Alexandrium minutum. Harmful Algae. 2011;12:55–69. doi: 10.1016/j.hal.2011.08.012. [DOI] [Google Scholar]
- 22.Johnson JG, Morey JS, Neely MG, Ryan JC, Van Dolah FM. Transcriptome remodeling associated with chronological aging in the dinoflagellate, Karenia brevis. Mar Genomics. 2012;5:15–25. doi: 10.1016/j.margen.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Sui Z, Chang L, Kang K, Ma J, Kong F, et al. Transcriptome de novo assembly sequencing and analysis of the toxic dinoflagellate Alexandrium catenella using the Illumina Platform. Gene. 2014;537(2):285–93. doi: 10.1016/j.gene.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 24.Morey JS, Monroe EA, Kinney AL, Beal M, Johnson JG, Hitchcock GL, et al. Transcriptomic response of the red tide dinoflagellate, Karenia brevis, to nitrogen and phosphorus depletion and addition. BMC Genomics. 2011;12(1):346. doi: 10.1186/1471-2164-12-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudela RM, Gobler CJ. Harmful dinoflagellate blooms caused by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae. 2012;14:71–86. doi: 10.1016/j.hal.2011.10.015. [DOI] [Google Scholar]
- 26.Kim CS, Lee SG, Lee CK, Kim HG, Jung J. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J Plankton Res. 1999;21(11):2105–15. doi: 10.1093/plankt/21.11.2105. [DOI] [Google Scholar]
- 27.Gárate-Lizárraga I, López-Cortes DJ, Bustillos-Guzman JJ, Hernández-Sandoval F. Blooms of Cochlodinium polykrikoides (Gymnodiniaceae) in the gulf of California, Mexico. Rev Biol Trop. 2004;52(Suppl 1):51–8. [PubMed] [Google Scholar]
- 28.Ebenezer V, Ki J-S. Evaluation of the sub-lethal toxicity of Cu, Pb, bisphenol A and polychlorinated biphenyl to the marine dinoflagellate Cochlodinium polykrikoides. Algae. 2012;27(1):63–70. doi: 10.4490/algae.2012.27.1.063. [DOI] [Google Scholar]
- 29.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36(suppl 1):D480–4. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 32.Kriedemann P, Graham R, Wiskich J. Photosynthetic dysfunction and in vivo changes in chlorophyll a fluorescence from manganese-deficient wheat leaves. Aust J Agr Res. 1985;36(2):157–69. doi: 10.1071/AR9850157. [DOI] [Google Scholar]
- 33.Kalaji HM, Bosa K, Kościelniak J, Żuk-Gołaszewska K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot. 2011;73:64–72. doi: 10.1016/j.envexpbot.2010.10.009. [DOI] [Google Scholar]
- 34.Ebenezer V, Ki J-S. Biocide sodium hypochlorite decreases pigment production and induces oxidative damage in the harmful dinoflagellate Cochlodinium polykrikoides. Algae. 2014;29(4):311–9. doi: 10.4490/algae.2014.29.4.311. [DOI] [Google Scholar]
- 35.Guo R, Ki J-S. Evaluation and validation of internal control genes for studying gene expression in the dinoflagellate Prorocentrum minimum using real-time PCR. Eur J Protistol. 2011;48(3):199–206. doi: 10.1016/j.ejop.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45–5. [DOI] [PMC free article] [PubMed]
- 37.Zhang Y, Zhang S-F, Lin L, Wang D-Z. Comparative transcriptome analysis of a toxin-producing dinoflagellate Alexandrium catenella and its non-toxic mutant. Mar Drugs. 2014;12(11):5698–718. doi: 10.3390/md12115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer JM, Rödelsperger C, Eichholz K, Tillmann U, Cembella A, McGaughran A, et al. Transcriptomic characterisation and genomic glimps into the toxigenic dinoflagellate Azadinium spinosum, with emphasis on polykeitde synthase genes. BMC Genomics. 2015;16(1):27. doi: 10.1186/s12864-014-1205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Coninck DI, Janssen CR, De Schamphelaere KA. An investigation of the inter-clonal variation of the interactive effects of cadmium and Microcystis aeruginosa on the reproductive performance of Daphnia magna. Aquat Toxicol. 2013;140:425–31. doi: 10.1016/j.aquatox.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Guidelines for drinking-water quality: recommendations, vol. 1. Geneva; WHO, 2004.
- 41.Baumgarten S, Bayer T, Aranda M, Liew YJ, Carr A, Micklem G, Voolstra CR. Integrating microRNA and mRNA expression profiling in Symbiodinium microadriaticum, a dinoflagellate symbiont of reef-building corals. BMC Genomics. 2013;14:704. [DOI] [PMC free article] [PubMed]
- 42.Ponmani T, Guo R, Ki J-S. Analysis of the genomic DNA of the harmful dinoflagellate Prorocentrum minimum: a brief survey focused on the non-coding RNA gene sequences. J Applied Phycology. 2015: doi:10.1007/s10811-10015-10570-10810.
- 43.Lee D-H, Mittag M, Sczekan S, Morse D, Hastings J. Molecular cloning and genomic organization of a gene for luciferin-binding protein from the dinoflagellate Gonyaulax polyedra. J Biol Chem. 1993;268(12):8842–50. [PubMed] [Google Scholar]
- 44.Li L, Hastings JW. The structure and organization of the luciferase gene in the photosynthetic dinoflagellate Gonyaulax polyedra. Plant Mol Biol. 1998;36(2):275–84. doi: 10.1023/A:1005941421474. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto OK, Robertson DL, Fagan TF, Hastings JW, Colepicolo P. Different regulatory mechanisms modulate the expression of a dinoflagellate iron-superoxide dismutase. J Biol Chem. 2001;276(23):19989–93. doi: 10.1074/jbc.M101169200. [DOI] [PubMed] [Google Scholar]
- 46.Morse D, Milos PM, Roux E, Hastings JW. Circadian regulation of bioluminescence in Gonyaulax involves translational control. Proc Natl Acad Sci. 1989;86(1):172–6. doi: 10.1073/pnas.86.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weake VM, Workman JL. Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet. 2010;11(6):426–37. doi: 10.1038/nrg2781. [DOI] [PubMed] [Google Scholar]
- 48.Sormani R, Masclaux-Daubresse C, Daniele-Vedele F, Chardon F. Transcriptional regulation of ribosome components are determined by stress according to cellular compartments in Arabidopsis thaliana. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X-D, Xie L, Wei Y, Zhou X, Jia B, Liu J, et al. Abiotic stress resistance, a novel moonlighting function of ribosomal protein RPL44 in the halophilic fungus Aspergillus glaucus. Appl Environ Microbiol. 2014;80(14):4294–300. doi: 10.1128/AEM.00292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Contreras-Porcia L, Dennett G, González A, Vergara E, Medina C, Correa JA, et al. Identification of copper-induced genes in the marine alga Ulva compressa (Chlorophyta) Marine Biotechnol. 2011;13(3):544–56. doi: 10.1007/s10126-010-9325-8. [DOI] [PubMed] [Google Scholar]
- 51.MacQuarrie KL, Fong AP, Morse RH, Tapscott SJ. Genome-wide transcription factor binding: beyond direct target regulation. Trends Genet. 2011;27(4):141–8. doi: 10.1016/j.tig.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn S, Young ET. Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics. 2011;189(3):705–36. doi: 10.1534/genetics.111.127019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hook SE, Osborn HL, Gissi F, Moncuquet P, Twine NA, Wilkins MR, et al. RNA-Seq analysis of the toxicant-induced transcriptome of the marine diatom, Ceratoneis closterium. Mar Genomics. 2014;16:45–53. doi: 10.1016/j.margen.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Sudo E, Itouga M, Yoshida-Hatanaka K, Ono Y, Sakakibara H. Gene expression and sensitivity in response to copper stress in rice leaves. J Exp Bot. 2008;59(12):3465–74. doi: 10.1093/jxb/ern196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong B, Zhang W, Chen L, Lin K-F, Guo M-J, Wang W-L, et al. Effects of Pb (II) exposure on Chlorella protothecoides and Chlorella vulgaris growth, malondialdehyde, and photosynthesis-related gene transcription. Environ Toxicol. 2014;29(11):1346–54. doi: 10.1002/tox.21865. [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Lan H, Fang H, Huang X, Zhang H, Huang J. Quantitative Proteomic Analysis of the Rice (Oryza sativa L.) Salt Response. PLoS ONE. 2015;10(3):e0120978. doi: 10.1371/journal.pone.0120978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu J, Patzoldt WL, Radwan O, Tranel PJ, Clough SJ. Effects of photosystem-II-interfering herbicides atrazine and bentazon on the soybean transcriptome. The Plant Genome. 2009;2(2):191–205. doi: 10.3835/plantgenome2009.02.0010. [DOI] [Google Scholar]
- 58.Wu S, Zhang H, Yu X, Qiu L. Toxicological responses of Chlorella vulgaris to dichloromethane and dichloroethane. Environ Eng Sci. 2014;31(1):9–17. doi: 10.1089/ees.2013.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez-Mendoza D, Escoboza-Garcia F, Santamria JM, Zapata-Perez O. Copper stress on photosynthesis of black mangle (Avicennia germinans) An Acad Bras Cienc. 2013;85(2):665–70. doi: 10.1590/S0001-37652013000200013. [DOI] [PubMed] [Google Scholar]
- 60.Küpper H, Šetlík I, Spiller M, Küpper FC, Prášil O. Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J Phycol. 2002;38(3):429–41. doi: 10.1046/j.1529-8817.2002.t01-1-01148.x. [DOI] [Google Scholar]
- 61.Blot N, Mella-Flores D, Six C, Le Corguillé G, Boutte C, Peyrat A, et al. Light history influences the response of the marine cyanobacterium Synechococcus sp. WH7803 to oxidative stress. Plant Physiol. 2011;156(4):1934–54. doi: 10.1104/pp.111.174714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun HP, Binder S, Brennicke A, Eubel H, Fernie AR, Finkemeier I, et al. The life of plant mitochondrial complex I. Mitochondrion. 2014;2014(19):295–313. doi: 10.1016/j.mito.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Jacoby RP, Li L, Huang S, Pong Lee C, Millar AH, Taylor NL. Mitochondrial Composition, Function and Stress Response in Plants. J Integr Plant Biol. 2012;54(11):887–906. doi: 10.1111/j.1744-7909.2012.01177.x. [DOI] [PubMed] [Google Scholar]
- 64.Garcia L, Welchen E, Gonzalez DH. Mitochondria and copper homeostasis in plants. Mitochondrion. 2014;19:269–74. doi: 10.1016/j.mito.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 65.Guo R, Ebenezer V, Ki J-S. PmMGST3, a novel microsomal glutathione S-transferase gene in the dinoflagellate Prorocentrum minimum, is a potential biomarker of oxidative stress. Gene. 2014;546(2):378–85. doi: 10.1016/j.gene.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 66.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141(2):391–6. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ponmani T, Guo R, Suh YS, Ki J-S. Molecular characterisation and expression analysis of a novel calreticulin (CRT) gene in the dinoflagellate Prorocentrum minimum. Mol Biol Rep. 2015;42(3):681–8. doi: 10.1007/s11033-014-3815-0. [DOI] [PubMed] [Google Scholar]
- 68.Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013. [DOI] [PMC free article] [PubMed]
- 69.Patwari P, Lee RT. Thioredoxins, mitochondria, and hypertension. Am J Pathol. 2007;170(3):805–8. doi: 10.2353/ajpath.2007.061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahsan MK, Lekli I, Ray D, Yodoi J, Das DK. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxid Redox Signal. 2009;11(11):2741–58. doi: 10.1089/ars.2009.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poole LB, Hall A, Nelson KJ. Overview of peroxiredoxins in oxidant defense and redox regulation. Current Protocols in Toxicology 2011:Unit7.9. [DOI] [PMC free article] [PubMed]
- 72.Herve C, de Franco P, Groisillier A, Tonon T, Boyen C. New members of the glutathione transferase family discovered in red and brown algae. Biochem J. 2008;412:535–44. doi: 10.1042/BJ20071464. [DOI] [PubMed] [Google Scholar]
- 73.de Franco P-O, Rousvoal S, Tonon T, Boyen C. Whole genome survey of the glutathione transferase family in the brown algal model Ectocarpus siliculosus. Mar Genomics. 2008;1(3):135–48. doi: 10.1016/j.margen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 75.Poynton RA, Hampton MB. Peroxiredoxins as biomarkers of oxidative stress. Biochimica et Biophysica Acta (BBA)-General Subjects. 2014;1840(2):906–12. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Dietz K-J. Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal. 2011;15(4):1129–59. doi: 10.1089/ars.2010.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ritter A, Dittami SM, Goulitquer S, Correa JA, Boyen C, Potin P, et al. Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol. 2014;14(1):116. doi: 10.1186/1471-2229-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lovazzano C, Serrano C, Correa JA, Contreras-Porcia L. Comparative analysis of peroxiredoxin activation in the brown macroalgae Scytosiphon gracilis and Lessonia nigrescens (Phaeophyceae) under copper stress. Physiol Plant. 2013;149(3):378–88. doi: 10.1111/ppl.12047. [DOI] [PubMed] [Google Scholar]
- 79.Sordet C, Contreras-Porcia L, Lovazzano C, Goulitquer S, Andrade S, Potin P, et al. Physiological plasticity of Dictyota kunthii (Phaeophyceae) to copper excess. Aquat Toxicol. 2014;150:220–8. [DOI] [PubMed]


