Abstract
Background and Purpose
Stroke induced neuroinflammation and white-matter damage are associated with neurological deficits. Whether D-4F, an apolipoprotein A-I mimetic peptide, treatment of stroke decreases neuroinflammation and white-matter damage and improves functional outcome has not been investigated.
Methods
Adult male C57BL/6 mice were subjected to permanent middle cerebral artery occlusion (MCAo) and were orally administered saline as a vehicle control and different doses of D-4F (2, 4, 8, 16 or 32mg/kg) starting at 2h after MCAo and daily until sacrifice at 7 days after MCAo. D-4F-treatment did not alter the blood levels of high density lipoprotein, total-cholesterol, triglyceride, blood-brain barrier leakage and infarction volume compared to control group.
Results
D-4F (16mg/kg) treatment of stroke significantly improved functional outcome, increased the white-matter density and the number of oligodendrocyte-progenitor cells in the ischemic boundary zone of the ipsilateral striatum, and increased myelin basic protein, insulin-like growth factor-1, but decreased inflammatory factor toll-like receptor-4 and tumor necrosis factor alpha expression in the ischemic brain 7 days after MCAo (p<0.05, n=11/group). The neurite/axonal outgrowth in primary cultured neurons was significantly increased when treated with D-4F (100ng/ml) and insulin-like growth factor-1 (100ng/ml) compared with the non-treatment control. Inhibition of IGF1 significantly attenuated D-4F or insulin-like growth factor-1 treatment induced axonal outgrowth. D-4F-treatment did not increase oligodendrocyte-progenitor cell proliferation but decreased oligodendrocyte-progenitor cell death.
Conclusions
D-4F-treatment initiated 2h after MCAo decreases neuroinflammation and white-matter damage and improves functional outcome after stroke. D-4F-induced increase in insulin-like growth factor-1 may contribute to D-4F-induced neurite/axonal outgrowth after stroke.
Keywords: white matter, neuroinflammation, axon, stroke, insulin-like growth factor-1
Introduction
Increasing high density lipoprotein (HDL) functionality may have important implications for treatment and prevention of cerebral inflammation and white matter (WM) damage after stroke1. D-4F is an 18-amino acid peptide that mimics the tertiary structure of apolipoprotein A-I, and is easily absorbed without liver toxicity. Previous studies show that orally administered D-4F does not increase serum levels of HDL, but does enhance HDL function, such as, increased pre-beta HDL formation, increased cholesterol efflux, conversion of pro-inflammatory and dysfunctional HDL to anti-inflammatory and functional HDL2–4, and alteration of HDL particle size distribution and metabolism5. However, the effect of D-4F-treatment of stroke on reducing WM-damage and improving neurological functional recovery after stroke has not been investigated.
Macrophages/microglia were activated after stroke and produced pro-inflammatory cytokines, proteolytic enzymes, and growth factors that alter survival of neurons and axonal growth6–9. Toll-Like receptors (TLRs) and tumor necrosis factor-alpha (TNFα) are implicated in the stroke induced inflammatory process. The expression of TLR4 is upregulated in ischemic stroke, at least during the subacute stage, which increases neuroinflammation and exacerbates stroke injury10–12. Insulin-like growth factor-1 (IGF1) expression is regulated by TNFα, and has antiinflammatory properties and an opposite effect to TNFα13, 14. Increasing IGF1 promotes neuroprotection and decreases WM-damage after stroke7. In this study, we investigate the hypothesis that D-4F-treatment reduces inflammation, improves WM-damage and functional recovery after stroke in mice, at least partially via the IGF1 signaling pathway.
Materials and Methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Animal model and experimental groups
Adult male C57BL/6 mice (3~4 month old, Jackson Laboratory, Bar Harbor, ME) were subjected to permanent right middle cerebral artery occlusion (MCAo) using a 6-0 nylon filament method, as previously described15. The animals were randomly divided into three sets: 1. mice (n=11/group) were randomly orally administered different doses of D-4F (BioMatik, Cambridge, ON, Canada) 2, 4, 8, 16 or 32mg/kg and saline as vehicle control starting at 2h after MCAo and daily for a total of 7 days. These mice were used for functional test, blood biochemistry and lesion volume measurement, histochemical and immunohistochemical staining; 2. mice (n=12/group) were orally administered saline or D-4F 16mg/kg for 7 days and used for blood-brain barrier (BBB) leakage measurement (n=6/group) and Western blot (WB) and real time-PCR (RT-PCR) assays (n=6/group). All survival animals were sacrificed 7 days after MCAo. Total 120 mice were used and 18 mice died; the mortality rate is 15% in this study.
Functional test
To evaluate neurological functional deficits and recovery after stroke, modified neurological severity score (mNSS) and left foot-fault test were performed before MCAo, and at 1, 3 and 7 days after MCAo15.
Blood biochemical measurement
Blood was collected from the tail vein 7 days after MCAo, and the level of HDL, total cholesterol (T-CH) and triglyceride were measured using CardioChek P•A analyzer (Polymer Technology System Inc. Indianapolis, IN, USA) following the manufacture’s instruction.
BBB-leakage measurement
Evans blue dye extravasation was used for quantitation of BBB leakage 7 days after MCAo16.
Histochemical/histoimmunostaining and infarct volume measurement
Mice brains were fixed embedded in paraffin and were cut into seven equally spaced (1mm) coronal blocks. For calculation of lesion volume, a series of adjacent 6 μm-thick sections were cut from each block and stained with hematoxylin and eosin17. Every 10th coronal section was cut from the center of the lesion (bregma −1mm to +1mm), and a total of 5 sections was used for staining of Bielshowsky silver (BS, an axon marker), Luxol Fast Blue (LFB, a myelin marker), SMI31 (a marker of phosphorylated-neurofilament, 1:1000, Covance) and platelet-derived growth factor receptor alpha (PDGFRα, a marker of oligodendrocyte progenitor cells-OPCs, 1:100; Chemicon) were employed17.
For immunostaining quantitative measurement, five sections with each section containing 8 fields of view within the cortex and striatum from the ischemic boundary zone (IBZ, which is adjacent to the ischemic core) were digitized using a 40× objective (Olympus BX40) with a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID computer imaging analysis system (Imaging Research, St. Catharines, Canada). The following were calculated: 1) the percentage of BS+, LFB+ or SMI31+ -area in the WM-bundles IBZ; 2) the number of PDGFRα+ -cells in the IBZ of the ipsilateral-striatum.
WB assay
The IBZ brain tissue and the equal volumes of homologous contralateral-tissue were extracted for WB assay. Total protein was isolated with TRIzol (Invitrogen) and the protein samples were heated in 1% SDS for about 20min at 60°C to recover the protein activity. The samples were normalized for total protein content in WB assay. Specific proteins were visualized using a SuperSignal West Pico chemiluminescence kit (Pierce). The following primary antibodies against myelin basic protein (MBP, a myelin marker, 1: 200; Dako, Carpinteria, CA), IGF1 (1: 1000, Abcam), TLR4 (1:500, Santa Cruz), and TNFα (1: 1000, Abcam), and β-actin (1: 2000; Santa Cruz) were used for WB assay.
RT-PCR analysis
Total RNA was isolated using a standard protocol. Quantitative PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA) using 3-stage program parameters provided by the manufacturer. Each sample was tested in triplicate, and analysis of relative gene expression data using the 2−ΔΔCT method. The following primers for RT-PCR were designed using Primer Express software (ABI). IGF1: FWD: TGGATGCTCTTCAGTTCGTG, REV: TGGTAGATGGGGGCTGATAC; TLR4: Fwd: TAT TTT GTG ATT CTG GTG ATT; Rev: GTT TCG CTT TAT TTT TGT AATG; TNFα: FWD: TACTCCCAGGTTCTCTTCAAGG; REV: GAGGTTGACTTTCTCCTGGTA. GAPDH: FWD: AGA ACA TCA TCC CTG CAT CC; REV: CAC ATT GGG GGT AGG AAC AC.
Primary cortical neuron (PCN) culture and axonal outgrowth measurement
To investigate whether D-4F-treatment increases neurite-outgrowth and to further elucidate whether IGF1 mediates D-4F-induced neurite-outgrowth after stroke, a primary PCN culture and neurite-outgrowth measurements were employed7. Briefly, PCNs were isolated from E15 C57BL/6 mice and cultured with 8-well slide chambers. On day in vitro 3 (DIV3), to mimic ischemia in vivo, the PCN cultures were subjected to 3h of oxygen and glucose deprivation (OGD) in DMEM with serum and glucose free media. The PCNs were then cultured with neurobasal medium plus 2% B-27, 2mM GlutaMax, and 1% antibiotic-antimycotic. After 24h, the PCNs were divided into 5 groups as follows: 1) non-treatment as control; 2) +D-4F (100ng/ml); 3) +IGF1 (recombinant mouse IGF1 protein, 100ng/ml, Abcam, Cat# ab9861); 4) +D-4F + IGF1-inhibitor (AG1024, 10μM, Calbiochem, Cat#121767); 5) +IGF1+IGF1-inhibitor. AG1024 is a cell-permeable, reversible, substrate competitive, and specific inhibitor of IGF-1 and insulin receptor tyrosine kinase activity18. The PCNs were allowed to incubate for another 24h before being immunostained for TUJ1 (a phenotypic marker of neural cells, 1:1000, Covance) with Cy3 and photographed using a 10× objective fluorescent microscope (Zeiss). The average length of the 20 longest neurites in each well (n=6wells/group) was measured using MCID software.
To further investigate whether D-4F-treatment increases axonal-outgrowth, and whether IGF1 mediates D-4F-treatment induced axonal-outgrowth, a microfluidic axonal growth model (Standard Neuron Device, catalog #SND450, Xona Microfluidics) was also employed19. Briefly, microchambers were affixed to poly-D-lysine-coated (Sigma-Aldrich) dishes (35mm, Corning) and the PCNs were plated at a density of 6×105 cells/chamber in DMEM with 5% FBS. PCNs were changed to neuronal-growth media after 24h incubation. On DIV3, culture medium containing 20μM 5-fluorodeoxyuridine was used to kill astrocytes. The PCN cultures were then divided into the same 5 groups as above, and subjected to the above neurite-outgrowth measurements. PCNs were allowed to grow for DIV5 and axonal-outgrowth length was measured (n=6wells/group)19.
Premature oligodendrocyte cell line culture
To investigate the effect of D-4F on OPC proliferation and survival, an immortalized mouse premature oligodendrocyte cell line (N20.1; generously provided by Dr. Anthony Campagnoni, UCLA) culture was employed6. N20.1 cells were cultured in DMEM/F12 (Invitrogen) media with 10% FBS and 100μg/mL G418. Two hours of OGD was induced and cells were treated with: (1) non-treatment for control; (2) D-4F (100ng/ml) for 48h (n = 6wells/group). Lactate dehydrogenase (LDH), as a cell death assay, and MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, as a cell proliferation assay, were performed.
For the LDH assay, the CytoTox 96 non-radioactive cytotoxicity assay kit (Promega) was used. Data are presented as percentage of LDH level in the media to total LDH both in the media and cells. For MTS assay, CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega) was used. Absorbance was recorded at 490nm6.
Statistical Analysis
All measurements were performed by experimenters blinded to each group and condition. Results are expressed as the mean ± SE. Significance of difference between animal groups was determined by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for blood chemistry, lesion volume, functional evaluation, and neurite/axonal outgrowth; two-way ANOVA was used for analysis of WB and RT-PCR data; Student t-test was used for BBB-leakage, WM-density and OPC-number measurements. Correlation between the BS+-axon density and mNSS was tested by Pearson’s correlation coefficients.
Results
D-4F-treatment effect on blood lipids and lipoproteins, lesion volume, BBB-leakage and functional outcome 7 days after stroke
There were no significant differences in the blood levels of HDL, T-CH and triglyceride (Fig. 1A), and infarction volume (Fig. 1B) among animals in the control or MCAo mice treated with 2, 4, 8, 16 and 32mg/kg of D-4F daily for 7 days. However, treatment of stroke with 16mg/kg D-4F significantly improved neurological functional outcome 7 days as tested by mNSS, and at 3 and 7 days as tested by foot-fault after stroke (p<0.05), respectively. Therefore, the 16mg/kg D-4F-treatment was selected for further measurement of BBB-leakage, WM-density, and WB/RT-PCR assay. No significant difference in BBB-leakage was observed between D-4F-treatment and MCAo alone groups 7 days after stroke (Fig. 1C).
Fig. 1.
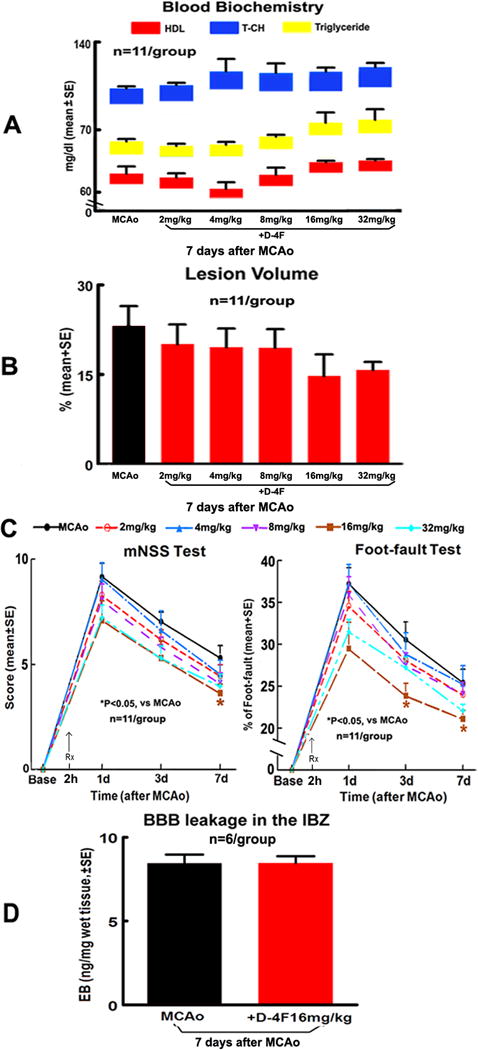
Dose-effect responses of D-4F on blood level of HDL, T-CH and triglyceride, lesion volume, BBB-leakage and functional outcome 7 days after stroke. Blood levels of HDL (red), T-CH (blue) and triglyceride (yellow, A), infarction volume (B), functional outcome (C) and BBB-leakage (D).
D-4F treatment decreased WM-damage and increased OPC number in the ischemic brain after stroke
To test whether D-4F-treatment decreases WM-damage after stroke, the WM-change and OPC number measurements were performed using histochemical- or histoimmuno-staining. The density of BS+-axon/LFB+-myelin/SMI31+-phosphorylated neurofilament in the WM-bundles in the IBZ of ipsilateral hemispheres and the number of PDGFRα+-OPCs in the IBZ of ipsilateral striatum were significantly increased (Fig. 2A–D, p<0.05, n=11/group) in D-4F-treatment mice compared with non-treatment MCAo control mice. Using Pearson’s correlation coefficients analysis, we found that the functional outcome (mNSS) was significantly and negatively correlated with BS+-axon density (Fig. 2A, r = −0.835, p<0.05).
Fig. 2.
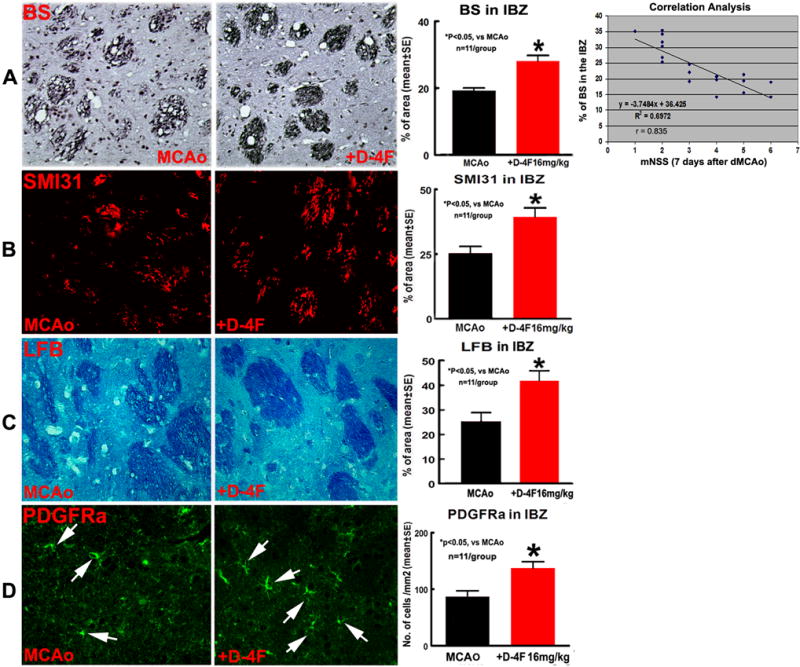
D-4F-treatment decreased WM-damage and increased OPC number in the ischemic brain 7 days after stroke. BS-immunostaining and correlation analysis between mNSS and BS+axon density (A); SMI31- (B), LFB- (C) and PDGFRα- (D) immunostaining and quantitative data.
To confirm the immunostaining data, MBP protein level was measured by WB-assay and was confirmed increased by D-4F-treatment (Fig. 3A, p<0.05, n=6/group). These data indicate that increasing axonal density may contribute to D-4F-induced neurological functional outcome after stroke.
Fig. 3.
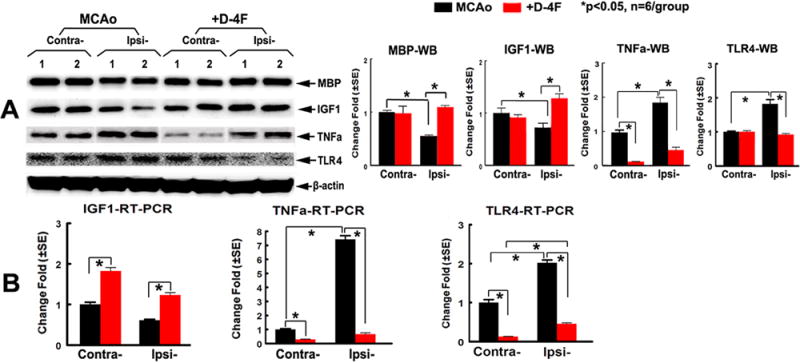
D-4F-treatment decreased inflammatory factors (TNFα/TLR4) but increased MBP and IGF1 expression in the ischemic brain 7 days after stroke. Western-blot (A) and RT-PCR (B) and quantitative data.
D-4F-treatment of stroke decreased inflammatory factors (TLR4 and TNFα) but increased IGF1 expression in the ischemic brain after stroke
To test the mechanism by which D-4F-treatment reduced WM-damage after stroke, we measured IGF1/TLR4/TNFα gene and protein expression. Our data show that IGF1 protein/mRNA levels are decreased but TNFα/TLR4 protein/mRNA levels significantly increased in the ipsilateral compared with the contralateral-brains in MCAo control mice (Fig. 3A–B, p<0.05, n=6/group). D-4F-treatment significantly increased IGF1, but decreased TNFα/TLR4 protein/mRNA levels in the ischemic brain. D-4F-treatment also significantly increased IGF1 but decreased TNFα/TLR4 mRNA levels in the contralateral-brain, when compared to MCAo control mice (Fig. 3B, p<0.05, n=6/group).
D-4F treatment increased PCN neurite and axon outgrowth, and decreased OPC death; IGF1 mediates D-4F treatment induced neurite/axon growth after stroke
To confirm the in vivo findings, in vitro neurite/axonal outgrowth and OPC proliferation/survival studies were performed using PCN/axonal or N20.1 cell culture models. The optimal doses of D-4F (100ng/ml) used for PCN and OPC cultures were selected from our pilot study of D-4F dose-effect (5, 50, 100, 250 and 500ng/ml) on the MTS assay (data not shown). We found that PCN neurite-outgrowth significantly increased after treatment with D-4F or IGF1 compared with the non-treatment PCNs 24h after OGD (Fig. 4A, p<0.05, n=6/group). Inhibition of IGF significantly attenuated D-4F and IGF treatment induced neurite-outgrowth. Similarly, the axonal-outgrowth in axon cultures treated both with D-4F and IGF1 significantly increased compared with the non-treatment axons 48h after treatment (Fig. 4B, p<0.05, n=6/group). Inhibition of IGF significantly attenuated D-4F and IGF induced axonal-outgrowth. D-4F-treatment did not increase OPC proliferation but significantly decreased OPC death measured by MTS and LDH assays, respectively (Fig. 4C), which is consistent with the myelin protective effect observed in vivo.
Fig. 4.
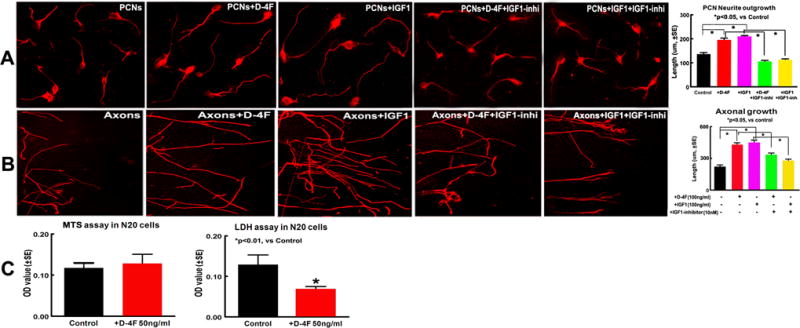
D-4F-treatment increased PCN neurite/axonal outgrowth and IGF1 mediated D-4F-treatment induced neurite/axonal outgrowth; D-4F-treatment did not increase OPC proliferation but decrease OPC death after stroke. Neurite-outgrowth (A), axonal-outgrowth (B), OPC MTS and LDH (C) assays and quantitative data.
Discussion
In this study, we found that D-4F-treatment of stroke initiated 2h after the ischemic onset did not change blood levels of lipid and lipoprotein, infarct volume and BBB-leakage in the ischemic brain, but significantly decreased TLR4 and TNFα gene and protein levels in the ischemic brain and improved neurological functional outcome 7 days after stroke. D-4F-treatment patients with coronary heart disease does not change plasma lipid or lipoprotein levels, but improves the HDL anti-inflammatory index20. D-4F-treatment also had no effect on infarct size in mice with myocardial infarction21. LDL receptor-null (LDLR−/−) mice treated with D-4F exhibited no significant differences in plasma lipids and lipoproteins, but had significantly reduced inflammation and cognitive impairment22, 23. D-4F inhibits inflammatory properties (TNFα and IL-1β) in the brain of APPSwe-PS1 Delta E9 mice and improves cognitive function24. These data are consistent with our findings in the present study.
After an ischemic stroke, there is a prolonged inflammatory response and a secondary phase of WM-damage that may be more amenable to treatment than the acute injury8, 9. WM is composed of bundles of myelinated axons. WM-damage defined as axonal degeneration and loss of myelin, induces disturbance of nerve impulse transport between neurons, and evokes serious neurological functional deficits after stroke25. OPCs are immature forms of oligodendrocytes. OPC may proliferate and differentiate into mature oligodendrocytes and thereby, help to decrease the burden of axonal injury. Enhanced proliferation, migration and differentiation of OPCs are seen in the peri-infarct region and in the subventricular zone after stroke26. OPCs are the key source of myelin production, and thus are essential for repair of damaged WM after stroke, and play an important role in promotion of functional recovery after brain injury.27 Stroke induced cerebral inflammatory processes adversely impact WM, including axon and myelin structural integrity, which is associated with long-term neurological functional deficits after stroke28, 29. In this study, we found that the decreased axonal-damage was significantly and negatively correlated with the functional outcome. D-4F-treatment of stroke increased axonal and myelin integrity as well as increased the numbers of oligodnedrocytes/OPCs in the ischemic brain. D-4F also increased PCN neurite/axonal outgrowth and OPC survival in vitro. These data support the hypothesis that by decreasing WM-damage, D-4F-treatement induced functional outcome after stroke.
IGF1 is an important growth factor that promotes neuronal and oligodendrocytes differentiation, proliferation, myelination and neurite-outgrowth, reduces apoptosis and sustains cell survival both in the developing brain and throughout life30–35. IGF1 also reduces gray matter and WM-damage after stroke, and upregulates OPC numbers via suppression of apoptosis33, 36, 37. Conversely, one might predict that inhibition of IGF1 signaling via blockade of the IGF1 receptor would potentially increase the severity of ischemic brain injury. TNFα markedly decreased IGF1 mRNA and protein leading to a reduction in bioactive IGF1 in myoblasts, skeletal muscle and vascular smooth muscle cell38. Activation of TNFα signaling can inhibit IGF1 signaling and thus elevate inflammatory cytokines in skeletal muscle in vivo13, 14. In the present study, we found that D-4F significantly decreased TNFα but increased IGF1 level and decreased WM-damage in the ischemic brain after stroke, and in vitro study shows that both D-4F and IGF1 treatment significantly increased neurite/axon outgrowth, whereas, inhibition of IGF1 attenuated D-4F or IGF1-induced axonal growth. D-4F-treatment did not increase OPC proliferation but significantly decreased OPC death. These data suggested that the increase of IGF1 may contribute to D-4F-treatment induced neurite and axonal outgrowth after stroke in mice.
Limitations: 1. we did not test whether D-4F improves functional outcome in non-stroke animals, we only tested the D-4F-treatment effect in young (3–4 month old) male stroke mice, since female mice are a more complex preclinical model because of the hormonal changes associated with the estrus cycle. In addition, the therapeutic response to D-4F may differ between young and old subjects. Thus, the effects of D-4F on females and elderly animals, both male and female, warrant further investigation. 2. We only evaluated the D-4F-treatment effect on decreasing WM-damage in the ischemic brain, whether D-4F regulates gray-matter warrants investigation. 3. Given the experimental design of acute 2h post-stroke treatment, we cannot distinguish between WM-protective and WM-restorative effects. Clearly, D-4F did not decrease lesion volume, but, it may protect WM. 4. Previous studies have found that D-4F-treatment significantly decreases brain arteriole inflammation and cognitive impairment. However, the scrambled D-4F-treatment did not induce the beneficial effects23. The scrambled D-4F control was not employed in this study, therefore our current findings have not yet been demonstrated to be specific to D-4F.
In summary, we are the first to report that D-4F-treatment initiated 2h after stroke significantly decreased neuroinflammation and WM-damage, and improved functional outcome 7 days after MCAo. IGF1 may mediate D-4F-treatment induced neurite/axonal outgrowth.
Acknowledgments
The authors thank Cynthia Roberts1, Yisheng Cui1, Qinge Lu1 and Sutapa Santra1 for technical assistance.
Sources of Funding
This work was supported by National Institute on Aging R01 AG037506 (Michael Chopp), National Institute of Neurological Disorders and Stroke R01 NS083078 (Jieli Chen) and R01 NS092917-01 (Xu Cui), and American Heart Association grants 14GRNT20460026 (Jieli Chen) and 12SDG9300009 (Xu Cui).
Footnotes
Subject Codes: Treatment [10155]; Ischemic Stroke[10178]
Disclosures
None.
References
- 1.Crisby M, Bronge L, Wahlund LO. Low levels of high density lipoprotein increase the severity of cerebral white matter changes: Implications for prevention and treatment of cerebrovascular diseases. Curr Alzheimer Res. 2010;7:534–539. doi: 10.2174/156720510792231694. [DOI] [PubMed] [Google Scholar]
- 2.Imaizumi S, Navab M, Morgantini C, Charles-Schoeman C, Su F, Gao F, et al. Dysfunctional high-density lipoprotein and the potential of apolipoprotein a-1 mimetic peptides to normalize the composition and function of lipoproteins. Circ J. 2011;75:1533–1538. doi: 10.1253/circj.cj-11-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navab M, Anantharamaiah GM, Reddy ST, Fogelman AM. Apolipoprotein a-i mimetic peptides and their role in atherosclerosis prevention. Nature clinical practice. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. Hdl and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nature reviews. Cardiology. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 5.Chajek-Shaul T, Hayek T, Walsh A, Breslow JL. Expression of the human apolipoprotein a-i gene in transgenic mice alters high density lipoprotein (hdl) particle size distribution and diminishes selective uptake of hdl cholesteryl esters. Proc Natl Acad Sci U S A. 1991;88:6731–6735. doi: 10.1073/pnas.88.15.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui X, Chopp M, Zacharek A, Karasinska JM, Cui Y, Ning R, et al. Deficiency of brain atp-binding cassette transporter a-1 exacerbates blood-brain barrier and white matter damage after stroke. Stroke. 2015;46:827–834. doi: 10.1161/STROKEAHA.114.007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43:1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moxon-Emre I, Schlichter LC. Evolution of inflammation and white matter injury in a model of transient focal ischemia. J Neuropathol Exp Neurol. 2010;69:1–15. doi: 10.1097/NEN.0b013e3181c3ce6c. [DOI] [PubMed] [Google Scholar]
- 10.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 11.Ning R, Chopp M, Yan T, Zacharek A, Zhang C, Roberts C, et al. Tissue plasminogen activator treatment of stroke in type-1 diabetes rats. Neuroscience. 2012;222:326–332. doi: 10.1016/j.neuroscience.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, et al. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anwar A, Zahid AA, Scheidegger KJ, Brink M, Delafontaine P. Tumor necrosis factor-alpha regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation. 2002;105:1220–1225. doi: 10.1161/hc1002.105187. [DOI] [PubMed] [Google Scholar]
- 14.Frost RA, Nystrom GJ, Lang CH. Tumor necrosis factor-alpha decreases insulin-like growth factor-i messenger ribonucleic acid expression in c2c12 myoblasts via a jun n-terminal kinase pathway. Endocrinology. 2003;144:1770–1779. doi: 10.1210/en.2002-220808. [DOI] [PubMed] [Google Scholar]
- 15.Liu XS, Zhang ZG, Zhang RL, Gregg SR, Meng H, Chopp M. Comparison of in vivo and in vitro gene expression profiles in subventricular zone neural progenitor cells from the adult mouse after middle cerebral artery occlusion. Neuroscience. 2007;146:1053–1061. doi: 10.1016/j.neuroscience.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiology of disease. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. Journal of the neurological sciences. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 18.Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, et al. The microrna-17–92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoa-i mimetic peptide d-4f in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baotic I, Ge ZD, Sedlic F, Coon A, Weihrauch D, Warltier DC, et al. Apolipoprotein a-1 mimetic d-4f enhances isoflurane-induced enos signaling and cardioprotection during acute hyperglycemia. American journal of physiology. 2013;305:H219–227. doi: 10.1152/ajpheart.00850.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buga GM, Frank JS, Mottino GA, Hakhamian A, Narasimha A, Watson AD, et al. D-4f reduces eo6 immunoreactivity, srebp-1c mrna levels, and renal inflammation in ldl receptor-null mice fed a western diet. J Lipid Res. 2008;49:192–205. doi: 10.1194/jlr.M700433-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Buga GM, Frank JS, Mottino GA, Hendizadeh M, Hakhamian A, Tillisch JH, et al. D-4f decreases brain arteriole inflammation and improves cognitive performance in ldl receptor-null mice on a western diet. J Lipid Res. 2006;47:2148–2160. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Handattu SP, Garber DW, Monroe CE, van Groen T, Kadish I, Nayyar G, et al. Oral apolipoprotein a-i mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of alzheimer’s disease. Neurobiology of disease. 2009;34:525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS neuroscience & therapeutics. 2014;20:603–612. doi: 10.1111/cns.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Experimental brain research. 2001;138:384–392. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Frontiers in cellular neuroscience. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasi F, Wei Y, Balkaya M, Tikka S, Mandeville JB, Waeber C, et al. Recognition memory impairments after subcortical white matter stroke in mice. Stroke. 2014;45:1468–1473. doi: 10.1161/STROKEAHA.114.005324. [DOI] [PubMed] [Google Scholar]
- 29.Scantlebury N, Mabbott D, Janzen L, Rockel C, Widjaja E, Jones G, et al. White matter integrity and core cognitive function in children diagnosed with sickle cell disease. Journal of pediatric hematology/oncology. 2011;33:163–171. doi: 10.1097/MPH.0b013e3182036f33. [DOI] [PubMed] [Google Scholar]
- 30.Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of igf-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS ONE. 2014;9:e91427. doi: 10.1371/journal.pone.0091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman EL, Sullivan KA, Kim B, Russell JW. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiology of disease. 1997;4:201–214. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Segura LM, Cardona-Gomez GP, Chowen JA, Azcoitia I. Insulin-like growth factor-i receptors and estrogen receptors interact in the promotion of neuronal survival and neuroprotection. Journal of neurocytology. 2000;29:425–437. doi: 10.1023/a:1007125626308. [DOI] [PubMed] [Google Scholar]
- 33.Russell JW, Cheng HL, Golovoy D. Insulin-like growth factor-i promotes myelination of peripheral sensory axons. J Neuropathol Exp Neurol. 2000;59:575–584. doi: 10.1093/jnen/59.7.575. [DOI] [PubMed] [Google Scholar]
- 34.Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocrine reviews. 2005;26:916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 35.Kooijman R. Regulation of apoptosis by insulin-like growth factor (igf)-i. Cytokine & growth factor reviews. 2006;17:305–323. doi: 10.1016/j.cytogfr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Cao Y, Gunn AJ, Bennet L, Wu D, George S, Gluckman PD, et al. Insulin-like growth factor (igf)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23:739–747. doi: 10.1097/01.WCB.0000067720.12805.6F. [DOI] [PubMed] [Google Scholar]
- 37.Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Progress in neurobiology. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Burton C, Song X, McNamara L, Langella A, Cianetti S, et al. An apoa-i mimetic peptide increases lcat activity in mice through increasing hdl concentration. International journal of biological sciences. 2009;5:489–499. doi: 10.7150/ijbs.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]


