Abstract
Redox homeostasis is a fundamental requirement for the maintenance of metabolism, energy generation, and growth in Saccharomyces cerevisiae. The redox cofactors NADH and NADPH are among the most highly connected metabolites in metabolic networks. Changes in their concentrations may induce widespread changes in metabolism. Redox imbalances were achieved with a dedicated biological tool overexpressing native NADH-dependent or engineered NADPH-dependent 2,3-butanediol dehydrogenase, in the presence of acetoin. We report that targeted perturbation of the balance of cofactors (NAD+/NADH or, to a lesser extent, NADP+/NADPH) significantly affected the production of volatile compounds. In most cases, variations in the redox state of yeasts modified the formation of all compounds from the same biochemical pathway (isobutanol, isoamyl alcohol, and their derivatives) or chemical class (ethyl esters), irrespective of the cofactors. These coordinated responses were found to be closely linked to the impact of redox status on the availability of intermediates of central carbon metabolism. This was the case for α-keto acids and acetyl coenzyme A (acetyl-CoA), which are precursors for the synthesis of many volatile compounds. We also demonstrated that changes in the availability of NADH selectively affected the synthesis of some volatile molecules (e.g., methionol, phenylethanol, and propanoic acid), reflecting the specific cofactor requirements of the dehydrogenases involved in their formation. Our findings indicate that both the availability of precursors from central carbon metabolism and the accessibility of reduced cofactors contribute to cell redox status modulation of volatile compound formation.
INTRODUCTION
Aroma and flavor profiles are the most important characteristics defining wine character and quality. The aromas of wines involve a wide variety of volatile compounds, some of which originate from the grapes (varietal aromas), whereas others are secondary products synthesized during the fermentation (fermentative aromas) and aging (postfermentative aromas) of wine. The aroma molecules produced by Saccharomyces cerevisiae, including, among others, aldehydes, higher alcohols, medium-chain fatty acids (MCFAs), long-chain fatty acids, ethyl fatty acid esters, and acetate esters, give fruity and floral notes to wine (1, 2). These volatile compounds are formed through a complex and dynamic metabolic process during fermentation, and many factors, such as grape variety, the nature of precursors (mainly amino acids), yeast strain, must treatments, and fermentation conditions, including oxygen, temperature, micronutrients, and nitrogen availability in the must, have been reported to control their production (1, 3–12).
The redox status of yeast cells, which largely contributes to the regulation of microbial activity, may be an additional parameter involved in qualitative and quantitative modulation of the fermentative aroma profile. Maintenance of the balance between the pyridine nucleotide pairs NAD+/NADH and NADP+/NADPH, the main contributors to redox metabolism, is a fundamental requirement for sustained metabolism and growth in S. cerevisiae. These cofactors, which are involved in more than 300 reactions in yeast, are among the most highly connected molecules in metabolic networks (13, 14). NADH is predominantly associated with the catabolism of glucose and the generation of energy produced through glycolysis (cytosol) and the tricarboxylic acid (TCA) cycle (mitochondria). The major reaction of fermentative metabolism, namely, ethanol production, is a redox-neutral process that does not result in NADH accumulation. However, excess NADH is generated through biomass synthesis, and most of the excess NADH is further oxidized by glycerol dehydrogenase to maintain redox homeostasis (15, 16). NADPH is the cofactor used as a reducing agent in a large number of anabolic reactions, including the biosynthesis of amino acids, lipids, and nucleotides, as well as a protective agent against oxidative stress (17). During fermentation, NADPH is mainly provided in the cytosol by the oxidative steps of the pentose phosphate pathway and by the NADP-dependent acetaldehyde dehydrogenase Ald6p, which is involved in the formation of acetate (18). Overall, any modification of the balance between the oxidized and reduced forms of redox cofactors is expected to have far-reaching effects on the functional metabolic network. In agreement with this finding, altered metabolite patterns were identified in previous studies that focused on the impact of redox perturbations on fermentation kinetics and the formation of products from central carbon metabolism, such as glycerol, acetate, and succinate (19–24).
The formation of aroma compounds may be affected directly by changes in the redox balance of NAD(P)+ and NAD(P)H, because their synthesis involves a wide range of dehydrogenases (25, 26). The aroma profiles also may be modified indirectly through the effects of redox on the availability of precursors, most of which are derived from central carbon metabolism (25, 26). Thus, modulations of the redox status of cells by changes in the redox potential of the medium (27) and replacement of the main route of NAD+ generation (glycerol production) by alternative pathways (28) have been found to result in variations in the production of volatile molecules. However, the approaches used in those studies are not specific to a single cofactor or interfere with different metabolic pathways, impairing the ability to assess the metabolic basis of the impact of NAD(P)H availability on the formation of aromatic compounds.
The objective of this study was to gain a better understanding of the effects of an NADPH or NADH imbalance separately on the synthesis of key yeast aroma compounds during wine fermentation (higher alcohols, fatty acids, ethyl esters, and ethyl acetates). To this end, fermentations on chemically defined fermentation medium, as found in grape must, were performed using strains overexpressing native NADH-dependent or engineered NADPH-dependent butanediol dehydrogenase (Bdh). On the basis of the amount of added acetoin, this biological tool enables specific modulation of the NAD(P)+/NAD(P)H balance (21, 22, 23). This experimental design allowed the study of the metabolic changes involved in the maintenance of redox balance in response to increased NAD(P)H demand. Compared to those of the yeast without acetoin, the aroma profiles of the engineered strains with acetoin showed that the resulting redox imbalance widely affected the synthesis of volatile compounds during fermentation. The metabolic features that trigger the responses of yeast to modifications of the NAD(P)H demands were further investigated by analyzing the changes in the levels of each compound in association with their biosynthetic pathways.
MATERIALS AND METHODS
Strains and culture conditions.
The strains used in this study were constructed from S. cerevisiae strain 59A, a haploid derivative of the wine yeast strain Lalvin EC1118 (29). The 59A-NADH-Bdh and 59A-NADPH-Bdh strains were obtained previously by overexpressing the BDH1 gene encoding the NADH-dependent 2,3-butanediol dehydrogenase (Bdh1p) or a mutated version of this gene encoding a NADPH-dependent Bdh1p (21, 30). The S. cerevisiae strains were grown in yeast extract-peptone-dextrose (YPD) medium (1% Bacto yeast extract, 2% Bacto peptone, and 2% glucose).
Fermentation conditions.
Batch fermentations were performed in 1-liter fermentors equipped with fermentation locks to maintain anaerobiosis at 24°C, with permanent stirring (500 rpm). The fermentation experiments were performed in synthetic medium 250 (SM250), which contains 100 g liter−1 glucose, 100 g liter−1 fructose, 6 g liter−1 malic acid, 6 g liter−1 citric acid, and 250 mg liter−1 nitrogen (pH 3.3), as well as vitamins and oligoelements found in grape juice (30). Ergosterol (3.75 g liter−1), oleic acid (0.125%), and Tween 80 (12.5%) were provided as anaerobic growth factors. Nitrogen (250 g liter−1) was supplied as one of the various mixtures of amino acids and NH4Cl (31, 32). Fermentation experiments using strains 59A, 59A-NADH-Bdh, and 59A-NADPH-Bdh were performed in synthetic medium supplemented or not supplemented with 200 mM acetoin at the beginning of the fermentation. The cells were precultured first at 28°C in 50-ml flasks containing YPD medium, with agitation (150 rpm), and then at 28°C in 50-ml flasks containing SM250, with agitation (150 rpm). The pregrown cultures were used to inoculate the fermentor at 1 × 106 CFU/ml. The fermentation experiments were performed in triplicate. Ten samples were taken throughout the fermentation process.
Analytical techniques.
Cell growth was monitored by cell counting with an electronic particle counter (Multisizer 3 Coulter; Beckman Coulter) fitted with a probe with a 100-mm aperture. Cell dry weight (DW) was measured by filtering 10 ml of the culture through a preweighed nitrocellulose filter (pore size, 0.45 μm; Millipore). The filter was washed with 20 ml of distilled water and dried at 105°C for 48 h. Biomass composition was determined as described by Celton et al. (22, 23).
The concentrations of glucose, fructose, glycerol, ethanol, acetate, succinate, and pyruvate were measured by high-performance liquid chromatography (HPLC) (HPLC 1260 Infinity system; Agilent Technologies, Santa Clara, CA, USA), using a Rezex ROA organic acid H+ column (8%, 300 by 7.8 mm; Phenomenex). The compounds were eluted with 0.005 N H2SO4, at a flow rate of 0.6 ml/min. Dual detection was performed with an Agilent refractometer and a UV detector for peak detection and quantification. Analysis was performed using the Agilent EZChrom software package.
Acetoin and 2,3-butanediol were analyzed using a gas chromatography (GC) system equipped with a flame ionization detector (FID). Acetoin and 2,3-butanediol were extracted with chloroform as described previously (23). A Hewlett-Packard 6890 series gas chromatograph was used, with a BP20 column (30 m by 0.53 mm by 1.0 μm; SGE Analytical Science). The temperature was maintained at 80°C for 2 min, increased to 200°C at 10°C/min, and maintained at 200°C for 5 min. The carrier gas was H2, at 3 ml/min. Two microliters of the organic phase was injected in the split mode, and the split flow rate was 12.3 ml/min.
The volatile aroma compounds were extracted according to the method described by Rollero et al. (5). Samples were prepared for analysis by GC-mass spectrometry (MS) with the addition of deuterated internal standards at 100 μg liter−1 to each 5-ml sample and two extractions with 1 ml of dichloromethane. The organic extracts were dried over anhydrous sodium sulfate and concentrated under nitrogen flux. The extracts were analyzed with a Hewlett Packard 6890 gas chromatograph (Agilent Technologies) coupled to a HP 5973 mass spectrometer.
Statistical analysis.
The statistical analyses were performed using R software (version 3.1.1) (33). To obtain a general overview of the final production of volatile compounds, a principal-component analysis (PCA) was performed using the FactoMineR package (34). Each variable was then tested through one-way analysis of variance (ANOVA), using the condition (strains overexpressing NADH-dependent or NADPH-dependent Bdh, with 0 or 200 mM acetoin) as a factor to detect the global effect. Because effects were significant with a P value threshold of 0.05, all pairwise comparisons for two conditions were assessed using Tukey's honestly significant difference (HSD) test. In the figures and tables, conditions sharing the same letter are not significantly different, with a P value threshold of 0.05. For each parameter, the normality of the residual distributions and the homogeneity of the variance were assessed using standard diagnostic graphics; no violation of the assumptions was detected. To describe the temporal production of volatile compounds, a local regression was fitted to smooth the production of compounds over time (in hours), expressed as released CO2 (in grams per liter) to provide biological meaning, using the locfit package (35). For each compound and each condition, a 95% confidence interval was approximated by using the estimated predictions and standard errors. Conditions for which the confidence intervals do not intersect can be considered significantly different.
RESULTS
Quantification of NADPH/NADH perturbations.
To assess the effects of a redox imbalance on the formation of aroma compounds during fermentation, we used strains overexpressing NADH- and NADPH-dependent Bdh systems to generate specific increases in the NADH and NADPH demands. To this end, fermentations using strains 59A, 59A-NADH-Bdh, and 59A-NADPH-Bdh were conducted in synthetic medium supplemented or not supplemented with 200 mM acetoin. Considering the impact of the nitrogen source on the redox status of cells (36), nitrogen was supplied in the form of a mixture of ammonium and amino acids, as found in grape juice.
The 59A-NADPH-Bdh strain was as efficient as the 59A-NADH-Bdh strain, and 99% of the acetoin provided was consumed, corresponding to the oxidation of 198 mM NAD(P)H (see Fig. S1A in the supplemental material). These strains consumed 20 to 25% more acetoin than strain 59A. The NADPH perturbation imposed by the Bdh system throughout the fermentation (59.5 mmol g DW−1) corresponded to a 13.8-fold greater NADPH demand than that required for anabolism, as determined from the biomass composition (see Fig. S1B in the supplemental material).
The analysis of the NADH perturbation revealed that the amounts of NADH consumed by the overexpressed native NADH-dependent Bdh enzyme equaled 1.5 and 61.7 mmol g DW−1 in the absence and presence of added acetoin, respectively. The NADH perturbation generated by the consumption of 200 mM acetoin corresponded to a 3.6-fold greater amount of NADH being reoxidized by the glycerol pathway (10 to 17 mmol g DW−1 in the absence or presence of added acetoin), which plays a key role in the maintenance of the NAD+/NADH balance during fermentation (see Fig. S1C in the supplemental material). These results show disturbance levels that are similar to those obtained with the same strains grown on minimal medium with ammonium (23).
Effects of NAD(P)H perturbations on metabolites from central carbon metabolism and on growth.
A large number of aroma compounds are synthesized using precursors from central carbon metabolism. Therefore, we investigated the effects of an increased demand for NAD(P)H on growth and the formation of central carbon metabolism intermediates. A previous study reported that up to 200 mM acetoin did not affect the growth rate and biomass formation of strain 59A (23). In this study, during fermentation under wine-making conditions, yeast growth was slightly affected by redox perturbations generated by the addition of 200 mM acetoin, as shown by small decreases in the final populations of strains 59A (20%), 59A-NADH-Bdh (21%), and 59A-NADPH-Bdh (11.7%), in the growth rates, and in the maximal fermentation rates, compared with the fermentations without added acetoin (see Fig. S2 in the supplemental material). It is difficult to assess, from these experiments, the direct effects of acetoin toxicity on growth and fermentation, apart from the redox effect. Indeed, the activity of the native NADH-dependent 2,3-butanediol dehydrogenase resulted in a substantial redox perturbation in strain 59A grown in the presence of acetoin, which was slightly smaller than that obtained with strain 59A-NADH-Bdh (see Table S1 in the supplemental material). To clarify the outcomes, we focused on the effects of 59-NADH-Bdh and 59A-NADPH-Bdh perturbation.
At the end of the fermentation, when all of the sugars were consumed by yeasts, modification of the NADH or NADPH demand substantially affected the production patterns of ethanol, glycerol, acetate, and, to a lesser extent, succinate (Table 1). As expected from the role of glycerol in the maintenance of homeostasis, the NADH perturbation triggered an important decrease in glycerol formation (36%). Ethanol production was also decreased, consistent with competition for the use of NADH between glycerol-3-phosphate dehydrogenase, alcohol dehydrogenase, and Bdh. As a consequence of the limited conversion of acetaldehyde to ethanol, a marked increase (5-fold) in the acetate concentration was observed. Finally, succinate production was increased (38%) in response to the NADH perturbation, suggesting that this compound was synthesized mainly by the TCA oxidative branch, which generates NADH. This increase may be related to the decreased biomass obtained with the NADH perturbation, because the excess α-ketoglutarate not incorporated into the biomass via amino acid biosynthesis may have been redirected toward the formation of succinate.
TABLE 1.
Effects of redox perturbation of NADH and NADPH levels on extracellular metabolite production at the end of fermentations and on biomassa
| Acetoin added (mM) for indicated strain | Glycerol (mmol/g DW) | Ethanol (g/liter) | Acetate (mmol/g DW) | Succinate (mmol/g DW) | Pyruvate (mmol/g DW) | Biomass (g/liter) |
|---|---|---|---|---|---|---|
| 59A-NADH-Bdh | ||||||
| 0 | 16.5 ± 0.19 Bb | 97.9 ± 0.59 B | 2.89 ± 0.11 A | 0.95 ± 0.09 B | 0.17 ± 0.03 A | 3.98 ± 0.07 C |
| 200 | 10.6 ± 0.23 A | 92.2 ± 1.85 A | 14.7 ± 0.43 C | 1.04 ± 0.08 B | 0.40 ± 0.03 B | 3.11 ± 0.08 A |
| 59A-NADPH-Bdh | ||||||
| 0 | 19.0 ± 0.51 C | 98.1 ± 1.69 B | 3.39 ± 0.08 A | 0.96 ± 0.05 B | 0.22 ± 0.06 A | 3.86 ± 0.15 C |
| 200 | 16.9 ± 0.32 B | 96.2 ± 0.68 B | 9.08 ± 0.16 A | 0.59 ± 0.02 A | 0.30 ± 0.02 AB | 3.38 ± 0.04 B |
The experiments were performed in SM250 containing 100 g/liter glucose and 100 g/liter fructose. Concentrations were measured at the end of fermentations, after the exhaustion of sugars.
Different letters denote significant differences between the treatments at P < 0.05, as determined by ANOVA followed by Tukey's test.
Glycerol production was not markedly affected by NADPH oxidation, i.e., only an 11% decrease was obtained with the addition of acetoin. In contrast, the formation of acetate was substantially increased (1.6-fold) in the presence of 200 mM acetoin, and succinate production tended to decrease. The mechanisms underlying this behavior likely reflect redirection of the carbon fluxes at the pyruvate node toward acetate formation through the NADPH-dependent acetaldehyde dehydrogenase, to regenerate the NADPH consumed by the overproduced 59A-NADPH-Bdh.
Effects of NADPH and NADH perturbations on aroma compounds. (i) Principal-component analysis.
We investigated, at the end of fermentation, the impact of increased demand for cofactors (NADH and NADPH) on aroma compounds that are synthesized through pathways involving several aldehyde/alcohol dehydrogenases that are sensitive to NAD(P)+/NAD(P)H ratios (37). To overcome the biomass variations for the different conditions and to compare the cellular metabolic activities, the concentrations of the aroma compounds (higher alcohols, acetate esters, ethyl esters, and medium-chain fatty acids) are expressed in millimoles or micromoles per gram of dry weight (Table 2). Fermentations with the 59A-NADH-Bdh and 59A-NADPH-Bdh strains grown without added acetoin were used as controls for analysis of the effects of NADH and NADPH perturbations, respectively, on aroma compound formation. Overall, the cofactor perturbations substantially affected the formation of many of the volatile compounds detected (Table 2). Furthermore, the extent of the metabolic variations was greater in response to a NADH imbalance than to modification of the NADPH demand.
TABLE 2.
Effects of redox perturbation of NADH and NADPH levels on volatile aroma compound productiona
| Aroma compound | 59A-NADH-Bdh |
59A-NADPH-Bdh |
||
|---|---|---|---|---|
| Without acetoin | With 200 mM acetoin | Without acetoin | With 200 mM acetoin | |
| Higher alcohols (mmol/g DW) | ||||
| Propanol | 0.203 ± 0.013 Ab | 0.634 ± 0.031 C | 0.215 ± 0.019 A | 0.366 ± 0.026 B |
| Isobutanol | 0.352 ± 0.054 | 0.411 ± 0.036 | 0.378 ± 0.048 | 0.397 ± 0.041 |
| Isoamyl alcohol | 1.224 ± 0.066 B | 0.772 ± 0.093 A | 1.366 ± 0.140 B | 1.167 ± 0.006 B |
| Methionol | 0.011 ± 0.001 B | 0.003 ± 0.0003 A | 0.012 ± 0.003 B | 0.012 ± 0.002 B |
| Phenylethanol | 0.448 ± 0.019 B | 0.227 ± 0.016 A | 0.474 ± 0.033 B | 0.421 ± 0.038 B |
| Acetates (μmol/g DW) | ||||
| Propyl acetate | 0.339 ± 0.038 A | 1.170 ± 0.095 C | 0.342 ± 0.056 A | 0.688 ± 0.086 B |
| Isobutyl acetate | 0.578 ± 0.014 A | 0.938 ± 0.060 C | 0.589 ± 0.017 A | 0.764 ± 0.049 B |
| Isoamyl acetate | 63.2 ± 1.24 B | 39.8 ± 2.09 A | 60.9 ± 2.08 B | 70.4 ± 2.30 C |
| 2-Methylbutyl acetate | 0.618 ± 0.057 | 0.378 ± 0.084 | 0.393 ± 0.144 | 0.569 ± 0.130 |
| Phenylethyl acetate | 4.73 ± 1.34 AB | 2.53 ± 0.42 A | 4.81 ± 0.96 AB | 5.36 ± 0.53 B |
| Ethyl esters (μmol/g DW) | ||||
| Ethyl acetate | 10.5 ± 0.57 A | 17.5 ± 1.39 C | 12.4 ± 2.1 AB | 14.9 ± 0.51 BC |
| Ethyl propionate | 0.337 ± 0.010 A | 0.939 ± 0.181 B | 0.352 ± 0.020 A | 0.822 ± 0.217 B |
| Ethyl isobutyrate | 0.034 ± 0.007 AB | 0.018 ± 0.003 A | 0.039 ± 0.008 B | 0.023 ± 0.004 A |
| Ethyl butanoate | 0.872 ± 0.025 | 0.765 ± 0.064 | 0.864 ± 0.091 | 0.769 ± 0.034 |
| Ethyl hexanoate | 0.991 ± 0.056 B | 0.524 ± 0.064 A | 0.953 ± 0.137 B | 0.544 ± 0.037 A |
| Ethyl octanoate | 1.82 ± 0.09 B | 0.939 ± 0.116 A | 1.89 ± 0.39 B | 1.09 ± 0.05 A |
| Ethyl decanoate | 1.47 ± 0.09 AB | 0.829 ± 0.384 A | 1.94 ± 0.04 B | 0.974 ± 0.314 A |
| Ethyl dodecanoate | 0.523 ± 0.229 AB | 0.165 ± 0.044 A | 0.933 ± 0.348 B | 0.085 ± 0.083 A |
| Acids (μmol/g DW) | ||||
| Propanoic acid | 0.741 ± 0.241 A | 2.10 ± 0.29 B | 0.740 ± 0.012 A | 1.02 ± 0.11 A |
| Isobutyric acid | 1.17 ± 0.06 A | 2.71 ± 0.48 B | 1.39 ± 0.14 A | 2.54 ± 0.31 B |
| Butyric acid | 0.818 ± 0.049 | 0.856 ± 0.116 | 0.860 ± 0.026 | 0.963 ± 0.175 |
| Isovaleric acid | 0.361 ± 0.022 C | 0.048 ± 0.006 A | 0.390 ± 0.010 C | 0.196 ± 0.013 B |
| 2-Methylbutanoic acid | 0.210 ± 0.010 C | 0.054 ± 0.0007 A | 0.222 ± 0.008 C | 0.122 ± 0.008 B |
| Hexanoic acid | 2.57 ± 1.3 | 1.65 ± 0.57 | 2.52 ± 1.51 | 1.51 ± 0.73 |
| Octanoic acid | 1.53 ± 0.26 | 1.13 ± 0.36 | 1.29 ± 0.28 | 0.892 ± 0.189 |
| Decanoic acid | 0.355 ± 0.082 | 0.283 ± 0.084 | 0.301 ± 0.060 | 0.197 ± 0.075 |
| Dodecanoic acid | 0.045 ± 0.0003 | 0.021 ± 0.016 | 0.044 ± 0.007 | 0.018 ± 0.001 |
Concentrations were measured at the end of fermentations, after the exhaustion of sugars.
Different letters denote significant differences between the treatments at P < 0.05, as determined by ANOVA followed by Tukey's test.
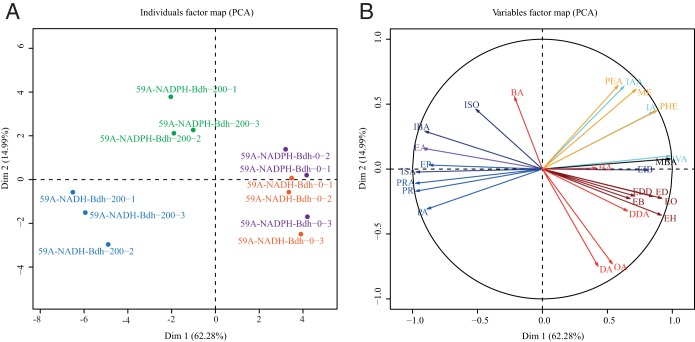
A principal-component analysis (PCA) was performed to obtain a general overview of the effects of redox modifications on aroma compound formation (Fig. 1). The final volatile compounds (Table 2) produced by the 59A-NADH-Bdh and 59A-NADPH-Bdh strains, grown with or without 200 mM acetoin, were used as quantitative variables. The two first axes accounted for 70% of the total variation and independently separated the fermentations with NADH or NADPH imbalances from the 59A-NADH-Bdh and 59A-NADPH-Bdh cultures without acetoin, which were clustered together. The 59A-NADH-Bdh strain grown in the presence of acetoin was differentiated along the first axis based on higher levels of production of propanol and isobutanol and their derivatives, as well as decreased synthesis of medium-chain fatty acids and their ethyl esters. This axis also contributed to the discrimination of the condition with NADPH perturbation, albeit to a lesser extent, and, together with the second axis, was mainly defined by the following variables: isoamyl alcohol, isoamyl acetate, methionol, and 2-phenyl ethyl acetate. We then further analyzed the specific responses of volatile compounds to changes in the NAD(P)H demand.
FIG 1.
Principal-component analysis of strain 59A overexpressing native NADH-dependent butanediol dehydrogenase (59A-NADH-Bdh) and strain 59A engineered to overexpress NADPH-dependent butanediol dehydrogenase (59A-NADPH-Bdh), supplemented with 200 mM acetoin (-200) or not (-0), for 26 volatile aroma compounds, based on mean values calculated from 3 replicates (-1, -2, and -3). (A) Individuals factor map. (B) Variables factor map. On the x axis is the percentage of variation explained by principal component 1 (Dim 1) (62.28%); on the y axis is the percentage of variation explained by principal component 2 (Dim 2) (14.99%). ISO, isobutanol; IBA, isobutyric acid; BA, butyric acid; EA, ethyl acetate; EP, ethyl propionate; ISA, isobutyl acetate; PRA, propyl acetate; PR, propanol; PA, propanoic acid; DA, decanoic acid; OA, octanoic acid; DDA, dodecanoic acid; EH, ethyl hexanoate; EB, ethyl butanoate; ED, ethyl decanoate; EO, ethyl octanoate; EDD, ethyl dodecanoate; EIB, ethyl isobutyrate; MBA, 2-methylbutanoic acid; PEA, phenyl ethyl acetate; ME, methionol; PHE, phenylethanol; IAA, isoamyl acetate; IA, isoamyl alcohol; IVA, isovaleric acid; HA, hexanoic acid.
(ii) Propanol and derivatives.
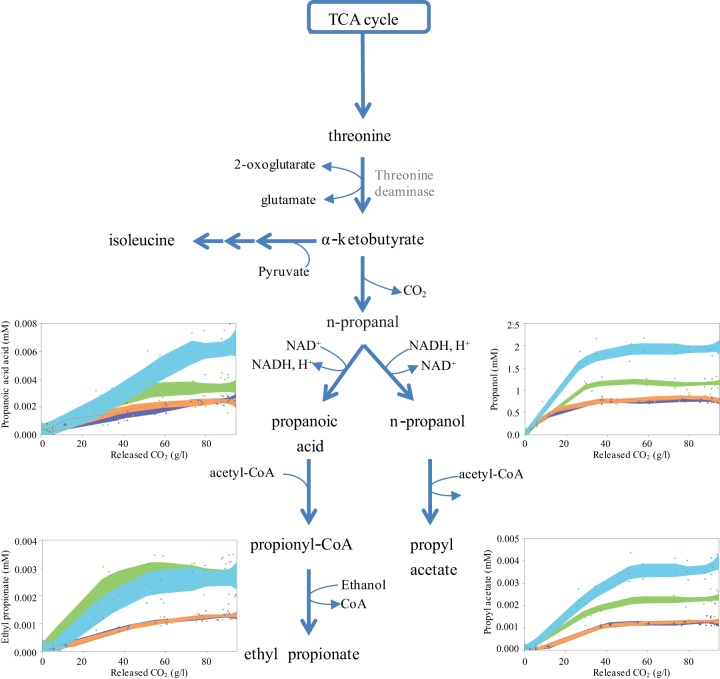
During fermentation in the absence of acetoin, no differences in propanol production between the two modified strains were observed. In the presence of 200 mM acetoin, propanol production by the 59A-NADH-Bdh and 59A-NADPH-Bdh strains was increased by 60% and 40%, respectively (Fig. 2). The profiles of all of the propyl derivatives showed the same trend, consistent with an overall increase in the metabolic flux through the threonine degradation pathway. This change may be related to the decreased production of biomass observed during fermentation with redox perturbation, compared with that observed under the conditions without acetoin. The excess threonine that was not incorporated into biomass via protein biosynthesis was likely redirected toward the formation of propanol and its derivatives. Analysis of the dynamics of propanol and propyl acetate formation during fermentation revealed that it stopped at the end of growth, as reported previously for propanol (4), when the assimilable nitrogen was exhausted, which supports the role of these compounds as part of the threonine degradation pathway. Interestingly, the kinetics of propanoic acid production differed depending on the cofactor involved in the redox perturbation. The formation of this metabolite by the 59A-NADPH-Bdh strain grown with 200 mM acetoin stopped at the end of growth, whereas the 59A-NADH-Bdh strain continuously produced propanoic acid throughout the fermentation in the presence of acetoin, resulting in higher production levels (Table 2). Thus, the NAD-dependent oxidation of oxobutanoate to propanoic acid likely contributed to the regeneration of NADH when the demand for this cofactor increased.
FIG 2.
Concentrations of propanol, propanoic acid, and corresponding esters following changes in the NAD(P)H demand. The values represent the averages of three biological replicates. The metabolite concentrations obtained with the 59-NADH-Bdh strain in the presence of 200 mM acetoin (blue) or 0 mM acetoin (orange) and with the 59A-NADPH-Bdh strain in the presence of 200 mM acetoin (green) or 0 mM acetoin (violet) are shown.
(iii) Medium-chain fatty acids and ethyl esters.
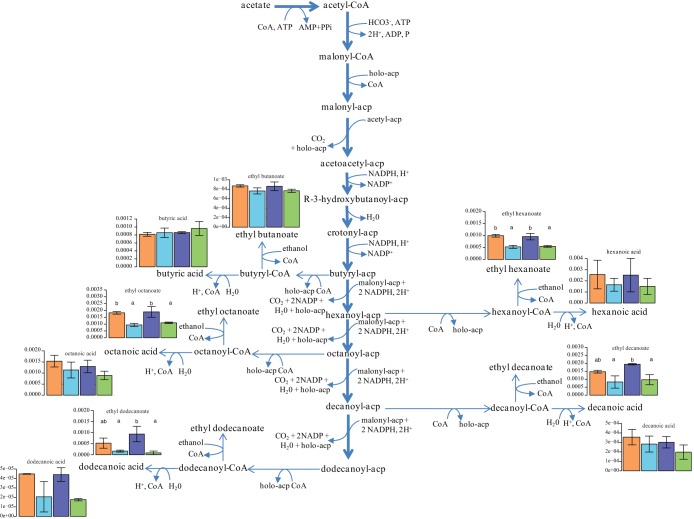
We then focused on the production of medium-chain fatty acids (MCFAs) and their ethyl ester derivatives. The levels of formation of both butanoic acid, the shortest MCFA, and ethyl butanoate were almost unchanged by a redox imbalance (Fig. 3). The further synthesis of MCFAs consists of two-carbon-unit elongations of the carbon chain, provided by the active malonyl coenzyme A (malonyl-CoA), which requires NADPH. In agreement with the cofactor requirements for MCFA elongation, the formation of MCFAs (hexanoic, octanoic, and decanoic acids) was reduced by 30%, on average, as a result of increased NADH or NADPH demand. Similarly, fatty acid esters, which are derived from the combination of MCFAs with ethanol, exhibited similar patterns in relation to the redox status. The formation of ethyl hexanoate, ethyl octanoate, and ethyl decanoate decreased by 50% with changes in the NAD(P)H demand, and ethyl dodecanoate synthesis decreased by 68% and 90% as a consequence of increased oxidation of NADH and NADPH, respectively.
FIG 3.
Average contents (in micromoles per gram of DW) of medium-chain fatty acids and ethyl esters produced by the 59A-NADH-Bdh and 59A-NADPH-Bdh strains in response to redox perturbation (200 mM acetoin), at the end of the fermentation. Conditions with the same letter do not differ significantly, as determined by Tukey's test (P < 0.05). The metabolite concentrations obtained with the 59A-NADH-Bdh strain in the presence of 200 mM acetoin (blue) or 0 mM acetoin (orange) and with the 59A-NADPH-Bdh strain in the presence of 200 mM acetoin (green) or 0 mM acetoin (violet) are shown. PPi, inorganic pyrophosphate; acp, acyl carrier protein.
(iv) Higher alcohols and derivative compounds from the Ehrlich pathway.
Higher alcohols and short-chain fatty acids are synthetized from α-keto acids that are produced as intermediates in amino acid catabolism by the Ehrlich pathway or that originate from central carbon metabolism (26). These volatile compounds are formed by decarboxylation and reduction or oxidation of their precursors, involving many dehydrogenases. Consequently, both the availability of α-keto acids and the redox status of the cells determine their production levels (37). Acetate esters are formed through the condensation of higher alcohols with acetyl coenzyme A (acetyl-CoA).
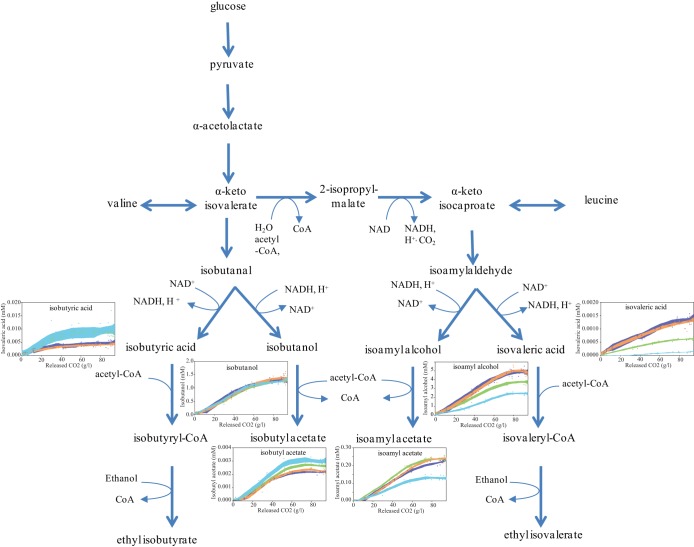
The concentrations of most fusel alcohols, acids, and corresponding esters were affected by increases in the oxidation of NADH or NADPH. Nevertheless, different patterns of formation according to the intracellular redox status were observed, depending on the compound considered (Fig. 4). Decreases in the total formation of branched volatile compounds derived from α-ketoisocaproate (KIC) (i.e., isoamyl alcohol, isoamyl acetate, and isovaleric acid) were found in response to NADH (30%) and NADPH (20%) modifications (Fig. 4). The KIC node was the most affected. Decreased oxidation of KIC to isoamyl alcohol by the 59A-NADH-Bdh and 59A-NADPH-Bdh strains in the presence of acetoin (44% and 28%, respectively) was observed. Isovaleric acid production by the 59A-NADH-Bdh and 59A-NADPH-Bdh strains was also reduced with increasing acetoin concentrations (87% and 50%, respectively). This overall decrease in the synthesis of metabolites originating from KIC, irrespective of the requirements for oxidized and reduced cofactors, suggests modulation of the availability of precursors for KIC biosynthesis according to the intracellular redox status. In contrast, the direct conversion of α-ketoisovalerate (KIV) to volatile compounds, particularly isobutanol, was markedly less modified by the redox demand. The 2-fold increase in the formation of isobutyric acid triggered by both 59A-NADH-Bdh and 59A-NADPH-Bdh perturbations likely reflected decreased availability of reduced cofactors. In addition, the increases in isobutyl acetate formation and, more generally, in acetate esters as a consequence of redox modifications were in agreement with the greater acetate availability observed under these conditions.
FIG 4.
Profiles of the production of isobutanol, isobutyric acid, isobutyl acetate, isoamyl alcohol, isovaleric acid, and isoamyl acetate following changes in NADH and NADPH demands. The metabolite concentrations obtained with the 59A-NADH-Bdh strain in the presence of 200 mM acetoin (blue) or 0 mM acetoin (orange) and with the 59A-NADPH-Bdh strain in the presence of 200 mM acetoin (green) or 0 mM acetoin (violet) are shown.
Finally, the formation of phenylethanol, phenyl ethyl acetate, and methionol displayed different responses according to the nature of the redox perturbation (Table 2). The production of these compounds strongly decreased with increased NADH demand (50%, 44%, and 72% for phenylethanol, phenyl ethyl acetate, and methionol, respectively). In contrast, their formation was almost unaffected by a disturbance in the NADPH balance, except for the slight increase in phenyl ethyl acetate formation.
DISCUSSION
Changes in the redox status of S. cerevisiae cells have a wide range of effects on the formation of intermediates of central carbon metabolism and volatile compounds (23, 27, 28) (Tables 1 and 2) and therefore may serve as convenient leverage for the modulation of aroma compound production (e.g., through aeration of the medium prior to fermentation). However, implementation of such an approach requires a better understanding of the mechanisms underlying the responses of yeast to variations in the redox balance that may result from oxygenation. In this study, we showed that the production of most metabolites during fermentation responded in similar ways to changes in NADH and NADPH availability, although the phosphorylated nicotinamide cofactors triggered smaller variations. These shared behaviors may involve different underlying mechanisms, however, according to the nature of the cofactors. This is the case with acetate production, which increases as a consequence of the limited conversion of acetaldehyde to ethanol with increased NADH demand. Conversely, the greater acetate formation when 59A-NADPH-Bdh cells were grown in the presence of 200 mM acetoin reflects greater acetaldehyde dehydrogenase activity to regenerate NADPH. Focusing on volatile compounds, we demonstrated that changes in the formation of these molecules arising from specific targeted perturbation of the cofactor balances (NAD+/NADH or NADP+/NADPH) were triggered by differences in both the availability of precursors from central carbon metabolism and the accessibility of reduced cofactors. The relative contributions of these factors vary according to the chemical classes of the compounds, the metabolic routes involved in their synthesis, and possibly the nature of the cofactor.
Our data revealed in particular that increases in the oxidation of NADH or NADPH favored the production of all of the volatile compounds derived from the precursor α-ketobutyrate (Fig. 2). Combined increases in propanol and its acetate (propyl acetate) and propanoic acid and its ethyl ester (ethyl propionate) were observed, irrespective of the cofactors (whether oxidized or reduced) required for their synthesis. This α-keto acid intermediate originates exclusively from the deamination of a nitrogen source, namely, threonine, via the Ehrlich pathway and is not synthesized directly through central carbon metabolism, in contrast to other keto acids involved in the production of higher alcohols (38). Consequently, the synthesis of propanol and its derivatives is controlled by the nitrogen availability in the medium and is achieved solely during the growth phase (4, 27). Accordingly, we found that the global increase in the formation of these volatile molecules as a result of redox imbalances is related to increased availability of α-ketobutyrate within the cells, in agreement with reduced use of threonine for anabolism. Small decreases in biomass formation were observed during fermentations with redox perturbations, and these decreases may be due to a toxic effect of acetoin, which is added as an electron acceptor to modulate the NAD(P)H demand (39).
In yeast, α-ketoisovalerate (KIV) may be directly catabolized through the Ehrlich pathway to isobutanol, isobutyric acid, or isobutyl acetate or elongated to α-ketoisocaproate, the precursor for the synthesis of isoamyl alcohol, isoamyl acetate, and isovalerate (26). Increased demand for either NADH or NADPH resulted in global decreases (40% and 19%, respectively) in the formation of volatile compounds derived from KIV and KIC (Fig. 4). This effect, caused by both NADH and NADPH modifications, cannot be imputed directly to changes in cofactor availability, because the dehydrogenases involved in the Ehrlich pathway are described as being specific for NAD+/NADH (26). Both KIV and acetoin originate from the metabolic precursor α-acetolactate. In the Bdh-overexpressing strains without acetoin, it is possible that the formation of acetoin, the Bdh substrate, is favored at the expense of KIV, as suggested by Celton et al. (22). The same redirection of α-acetolactate toward acetoin formation at the expense of KIV in the engineered strains with acetoin likely explains the decrease in the formation of volatile compounds from KIV.
Interestingly, the formation of isoamyl derivatives was more affected by redox perturbations than was that of isobutyl compounds. This finding is consistent with decreased flux toward KIC formation. The first step in KIC synthesis from KIV consists of the condensation of acetyl-CoA and KIV, a reaction that is catalyzed by α-isopropylmalate synthase (40). In yeast, the intracellular levels of acetyl-CoA range from 5 to 10 μM (41), close to the Km of α-isopropylmalate synthase for acetyl-CoA (i.e., 9 μM) (42). The decrease in the acetyl-CoA synthase flux in the Bdh-engineered strains grown in the presence of acetoin (22) likely results in decreased acetyl-CoA availability within the cells and, as demonstrated through previous data, limitations in KIC formation. In agreement with these observations, the formation of isobutanol and that of isoamyl alcohol were differently disturbed by the lipid concentrations in the medium, environmental factors that are known to exert direct effects on acetyl-CoA metabolism (4, 5).
In contrast, increased oxidation of NAD(P)H was accompanied by general decreases in both medium-chain fatty acids (MCFAs) and ethyl esters, irrespective of the nature of the cofactor (Fig. 3). MCFA formation involves the carboxylation of acetyl-CoA to malonyl-CoA, which then serves as a two-carbon donor in a cyclic series of reactions involving condensation, reduction, and dehydration steps (43, 44) catalyzed by multifunctional fatty acid synthases (FASs). The NADPH specificity of FASs excludes direct regulation of MCFA production by cofactor availability. As noted for isoamyl derivatives (see above), the most likely explanation for the decrease in MCFA formation observed with increased NAD(P)H demand involves limitation of the intracellular pool of the acetyl-CoA precursor. The modulation of ethyl ester synthesis, which involves condensation between ethanol and fatty acyl-CoA, likely reflects the limited formation of MCFAs. Moreover, downregulation of the EEB1 gene, encoding the main acyl-CoA-ethanol O-acyltransferase, which is responsible for ethyl ester formation (45), in response to increased NAD(P)H oxidation also may contribute to the reduced production of this family of volatile compounds (22).
In addition to modifying the availability of precursors, which modulates the formation of all compounds derived through the same metabolic route or belonging to the same chemical class, changes in the intracellular concentrations of NADH and NADPH affect the synthesis of specific volatile molecules. This was true with respect to the formation of propanoic acid by NADH-dependent Bdh-engineered strains, which increased during the stationary phase, likely to regenerate reduced cofactors. This response, which is specific to modification of the NADH demand, suggests that NADH-dependent dehydrogenases are involved in the formation of propanoic acid. Similarly, the NADH availability affected the formation of two specific fusel alcohols (namely, methionol and phenylethanol), which was reduced with increased NADH demand but remained unchanged with modifications to the intracellular NADPH pool. The latter observation differs from previous results showing that an increase in the NADPH demand results in an increase in phenylethanol formation. The activation of many genes involved in the methionine formation pathway (22), consistent with increased methionol formation during growth on yeast nitrogen base (YNB) medium, was not observed under our conditions. One possible explanation could be that a complex nitrogen source, with all of the amino acids required for growth, was used in this study, resulting in inhibition of the precursors for phenylethanol and methionol synthesis by phenylalanine and methionine. In fact, Aro3p, which catalyzes the first step of phenylethanol formation from pentose phosphate intermediates, undergoes feedback inhibition by l-phenylalanine (46, 47), whereas genes involved in the methionine pathway are negatively repressed by methionine (48).
These results demonstrate that variations in the redox state of yeasts could change the final concentrations of some important volatile compounds, derived from the same metabolic pathway or belonging to the same chemical family, that are implicated in the fruity character of wine. The changes in the levels of aroma compounds were found to be closely linked to the effects of the redox status on the availability of acetyl-CoA, an intermediate of central carbon metabolism, and on precursors of α-keto acids. Understanding the links between cell redox status and the concentrations of volatile compounds in wine has important implications with respect to wine-making parameters (such as oxygen) and their effects on wine quality. Moreover, the results of this study indicate that the nitrogen composition of the medium could influence the redox balance in yeast cells during alcoholic fermentation, which may induce the development of an industrial organic nitrogen mixture to affect the redox status and to optimize yeast performance in terms of aroma compound production.
From a microbiological standpoint, the results presented here can serve as the basis for interpreting the effects of differences in internal NAD(P)+/NAD(P)H ratios on aroma compound production in Saccharomyces cerevisiae and wine yeast strains in general. Furthermore, in the future, this information may facilitate the screening and phenotyping of natural yeasts, in terms of their abilities to produce volatile aroma compounds.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02429-15.
REFERENCES
- 1.Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS. 2005. Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res 11:139–173. doi: 10.1111/j.1755-0238.2005.tb00285.x. [DOI] [Google Scholar]
- 2.Lambrechts MG, Pretorius IS. 2001. Yeast and its importance to wine aroma: a review. S Afr J Enol Vitic 21:97–129. [Google Scholar]
- 3.Roland A, Vialaret J, Razungles A, Rigou P, Schneider R. 2010. Evolution of S-cysteinylated and S-glutathionylated thiol precursors during oxidation of Melon B. and Sauvignon blanc musts. J Agric Food Chem 58:4406–4413. doi: 10.1021/jf904164t. [DOI] [PubMed] [Google Scholar]
- 4.Mouret JR, Perez M, Angenieux M, Nicolle P, Farines V, Sablayrolles JM. 2014. Online-based kinetic analysis of higher alcohol and ester synthesis during winemaking fermentations. Food Bioprocess Technol 7:1235–1245. doi: 10.1007/s11947-013-1089-5. [DOI] [Google Scholar]
- 5.Rollero S, Bloem A, Camarasa C, Sanchez I, Ortiz-Julien A, Sablayrolles JM, Dequin S, Mouret JR. 2015. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl Microbiol Biotechnol 99:2291–2304. doi: 10.1007/s00253-014-6210-9. [DOI] [PubMed] [Google Scholar]
- 6.Barrajón-Simancas N, Giese E, Arévalo-Villena M, Ubeda J, Briones A. 2011. Amino acid uptake by wild and commercial yeasts in single fermentations and co-fermentations. Food Chem 127:441–446. doi: 10.1016/j.foodchem.2010.12.151. [DOI] [PubMed] [Google Scholar]
- 7.Carrau FM, Medina K, Farina L, Boido E, Dellacassa E. 2010. Effect of Saccharomyces cerevisiae inoculum size on wine fermentation aroma compounds and its relation with assimilable nitrogen content. Int J Food Microbiol 143:81–85. doi: 10.1016/j.ijfoodmicro.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Carrau FM, Medina K, Farina L, Boido E, Henschke PA, Dellacassa E. 2008. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res 8:1196–1207. doi: 10.1111/j.1567-1364.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 9.Fleet G. 2008. Wine yeasts for the future. FEMS Yeast Res 8:979–995. doi: 10.1111/j.1567-1364.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 10.Nicolini G, Larcher P, Pangrazzi P, Bontempo L. 2004. Changes in the contents of micro- and trace-elements in wine due to winemaking treatments. Vitis 43:41–45. [Google Scholar]
- 11.Torrea D, Varela C, Ugliano M, Ancin-Azpilicueta C, Leigh Francis I, Henschke PA. 2011. Comparison of inorganic and organic nitrogen supplementation of grape juice: effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem 127:1072–1083. doi: 10.1016/j.foodchem.2011.01.092. [DOI] [PubMed] [Google Scholar]
- 12.Varela C, Torrea D, Schmidt SA, Ancin-Azpilicueta C, Henschke PA. 2012. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem 135:2863–2871. doi: 10.1016/j.foodchem.2012.06.127. [DOI] [PubMed] [Google Scholar]
- 13.Forster J, Famili I, Fu P, Palsson BO, Nielsen J. 2003. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res 13:244–253. doi: 10.1101/gr.234503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen J. 2003. It is all about metabolic fluxes. J Bacteriol 185:7031–7035. doi: 10.1128/JB.185.24.7031-7035.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overkamp KM, Bakker BM, Kötter P, Van Tuijl A, de Vries S, Van Dijken JP, Pronk JT. 2000. In vivo analysis of the mechanisms for oxidation of cytosolic NADH by Saccharomyces cerevisiae mitochondria. J Bacteriol 182:2823–2830. doi: 10.1128/JB.182.10.2823-2830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dijken JP, Sheffers WA. 1986. Redox balances in the metabolism of sugar by yeast. FEMS Microbiol Rev 32:199–224. doi: 10.1111/j.1574-6968.1986.tb01188.x. [DOI] [Google Scholar]
- 17.Herrero H, Ros J, Bellí G, Cabiscol E. 2008. Redox control and oxidative stress in yeast cells. Biochim Biophys Acta 1780:1217–1235. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Saint-Prix F, Bönquist L, Dequin S. 2004. Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: the NADP+-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology 150:2209–2220. doi: 10.1099/mic.0.26999-0. [DOI] [PubMed] [Google Scholar]
- 19.Remize F, Cambon B, Barnavon L, Dequin S. 2003. Glycerol formation during wine fermentation is mainly linked to Gpd1p and is only partially controlled by the HOG pathway. Yeast 20:1243–1253. doi: 10.1002/yea.1041. [DOI] [PubMed] [Google Scholar]
- 20.Heux S, Cachon R, Dequin S. 2006. Cofactor engineering in Saccharomyces cerevisiae: expression of a H2O-forming NADH oxidase and impact on redox metabolism. Metab Eng 8:303–314. doi: 10.1016/j.ymben.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Ehsani M, Fernandez MR, Biosca JA, Dequin S. 2009. Reversal of coenzyme specificity of 2,3-butanediol dehydrogenase from Saccharomyces cerevisiae and in vivo functional analysis. Biotechnol Bioeng 104:381–389. doi: 10.1002/bit.22391. [DOI] [PubMed] [Google Scholar]
- 22.Celton M, Sanchez I, Goelzer A, Fromion V, Camarasa C, Dequin S. 2012. A comparative transcriptomic, fluxomic and metabolomic analysis of the response of Saccharomyces cerevisiae to increases in NADPH oxidation. BMC Genomics 13:317. doi: 10.1186/1471-2164-13-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celton M, Goelzer A, Camarasa C, Fromion V, Dequin S. 2012. A constraint-based model analysis of the metabolic consequences of increased NADPH oxidation in Saccharomyces cerevisiae.. Metab Eng 14:366–379. doi: 10.1016/j.ymben.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Lages NF, Oldiges M, Vemuri GN. 2009. Metabolic impact of redox cofactor perturbations in Saccharomyces cerevisiae. Metab Eng 11:253–261. doi: 10.1016/j.ymben.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Lilly M, Bauer FF, Styger G, Lambrechts MG, Pretorius IS. 2006. The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavor profiles of wine and distillates. FEMS Yeast Res 6:726–743. doi: 10.1111/j.1567-1364.2006.00057.x. [DOI] [PubMed] [Google Scholar]
- 26.Hazelwood LA, Daran JM, van Maris AJA, Pronk JT, Dickinson JR. 2008. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fariña L, Medina K, Urruty M, Boido E, Dellacassa E, Carrau F. 2012. Redox effect on volatile compound formation in wine during fermentation by Saccharomyces cerevisiae. Food Chem 134:933–939. doi: 10.1016/j.foodchem.2012.02.209. [DOI] [PubMed] [Google Scholar]
- 28.Jain VK, Divol B, Prior BA, Bauer FF. 2012. Effect of alternative NAD+-regenerating pathways on the formation of primary and secondary aroma compounds in a Saccharomyces cerevisiae glycerol-defective mutant. Appl Microbiol Biotechnol 93:131–141. doi: 10.1007/s00253-011-3431-z. [DOI] [PubMed] [Google Scholar]
- 29.Ambroset C, Petit M, Brion C, Sanchez I, Delobel P, Guérin C, Chiapello H, Nicolas P, Bigey F, Dequin S, Blondin B. 2011. Deciphering the molecular basis of wine yeast fermentation traits using a combined genetic and genomic approach. G3 (Bethesda) 1:263–281. doi: 10.1534/g3.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehsani M, Fernández MR, Biosca JA, Julien A, Dequin S. 2009. Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl Environ Microbiol 75:3196–3205. doi: 10.1128/AEM.02157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bely M, Sablayrolles JM, Barre P. 1990. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J Ferment Bioeng 70:246–252. doi: 10.1016/0922-338X(90)90057-4. [DOI] [Google Scholar]
- 32.Crépin L, Nidelet T, Sanchez I, Dequin S, Camarasa C. 2012. Sequential use of nitrogen compounds by Saccharomyces cerevisiae during wine fermentation: a model based on kinetic and regulation characteristics of nitrogen permeases. Appl Environ Microbiol 78:8102–8111. doi: 10.1128/AEM.02294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 34.Husson F, Josse J, Le S, Mazet J. 2014. FactoMineR: multivariate exploratory data analysis and data mining with R: R package version 1.27. Agrocampus West, Rennes, France: http://CRAN.R-project.org/package=FactoMineR. [Google Scholar]
- 35.Loader C. 2013. locfit: local regression, likelihood and density estimation: R package versions 1.5 to 9.1. Merck, Kenilworth, NJ: http://CRAN.R-project.org/package=locfit. [Google Scholar]
- 36.Albers E, Larsson C, Liden G, Niklasson C, Gustafsson L. 1996. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoondermark-Stolk SA, Tabernero M, Chapman J, ter Schure EG, Theo Verrips C, Verkleij AJ, Boonstra J. 2005. Bat2p is essential in Saccharomyces cerevisiae for fusel alcohol production on the non-fermentable carbon source ethanol. FEMS Yeast Res 5:757–766. doi: 10.1016/j.femsyr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Sluis C, Pahardjo YSP, Smit BA, Kroon PJ, Hartmans S, Ter Schure EG, Tramper J, Wijffels RH. 2002. Concomitant extracellular accumulation of alpha-keto acids and higher alcohols by Zygosaccharomyces rouxii. J Biosci Bioeng 93:117–124. doi: 10.1016/S1389-1723(02)80002-0. [DOI] [PubMed] [Google Scholar]
- 39.Roustan JL, Sablayrolles JM. 2002. Modification of the acetaldehyde concentration during alcoholic fermentation and effects on fermentation kinetics. J Biosci Bioeng 93:367–375. doi: 10.1016/S1389-1723(02)80069-X. [DOI] [PubMed] [Google Scholar]
- 40.Roeder PR, Kohlhaw GB. 1980. Alpha-isopropylmalate synthase from yeast: a zinc metalloenzyme. Biochim Biophys Acta 613:482–487. doi: 10.1016/0005-2744(80)90103-5. [DOI] [PubMed] [Google Scholar]
- 41.Lian J, Si T, Nair N, Zhao H. 2014. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab Eng 24:139–149. doi: 10.1016/j.ymben.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Ulm EH, Böhme R, Kohlhaw G. 1972. α-Isopropylmalate synthase from yeast: purification, kinetic studies, and effect of ligands on stability. J Bacteriol 110:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tehlivets O, Scheuringer K, Kohlwein SD. 2007. Fatty acid synthesis and elongation in yeast. Biochim Biophys Acta 1771:255–270. doi: 10.1016/j.bbalip.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM. 2010. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol 3:165–177. doi: 10.1111/j.1751-7915.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saerens SM, Verstrepen KJ, Van Laere SD, Van Dijck P, Delvaux FR, Thevelein JM. 2006. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J Biol Chem 281:4446–4456. doi: 10.1074/jbc.M512028200. [DOI] [PubMed] [Google Scholar]
- 46.Braus GH. 1991. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: a model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol Rev 55:349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paravicini G, Schmidhetni T, Braus G. 1989. Purification and properties of the 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase (phenylalanine-inhibitable) of Saccharomyces cerevisiae. Eur J Biochem 186:361–366. doi: 10.1111/j.1432-1033.1989.tb15217.x. [DOI] [PubMed] [Google Scholar]
- 48.Thomas D, Surdin-Kerjan Y. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61:503–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.