Abstract
Staphylococcus aureus biofilm infections of indwelling medical devices are a major medical challenge because of their high prevalence and antibiotic resistance. As fibrin plays an important role in S. aureus biofilm formation, we hypothesize that coating of the implant surface with fibrinolytic agents can be used as a new method of antibiofilm prophylaxis. The effect of tissue plasminogen activator (tPA) coating on S. aureus biofilm formation was tested with in vitro microplate biofilm assays and an in vivo mouse model of biofilm infection. tPA coating efficiently inhibited biofilm formation by various S. aureus strains. The effect was dependent on plasminogen activation by tPA, leading to subsequent local fibrin cleavage. A tPA coating on implant surfaces prevented both early adhesion and later biomass accumulation. Furthermore, tPA coating increased the susceptibility of biofilm infections to antibiotics. In vivo, significantly fewer bacteria were detected on the surfaces of implants coated with tPA than on control implants from mice treated with cloxacillin. Fibrinolytic coatings (e.g., with tPA) reduce S. aureus biofilm formation both in vitro and in vivo, suggesting a novel way to prevent bacterial biofilm infections of indwelling medical devices.
INTRODUCTION
Modern medicine uses increasing numbers of indwelling medical devices. Over 5 million implants and 150 million vascular catheters are used each year in the United States alone (1, 2). This leads to a rising challenge of infections associated with indwelling medical devices, which already constitute 60 to 70% of hospital-acquired infections (2). These infections are typically caused by microorganisms that grow in biofilms, three-dimensional communities of bacteria covered in an extracellular matrix and attached to an implant surface (3). Biofilms damage surrounding tissues, trigger inflammation, interfere with device function, and might further seed other body sites with bacteria (4). Importantly, biofilms are inherently resistant to antibiotics and host immune defenses, and removal of the device is frequently indispensable for successful treatment (4). Efforts to prevent biofilm formation, including the use of antibacterial coating and materials surfaced with nanostructures, have had some success (4–6), but other efficient methods to prevent biofilm formation are still urgently needed.
One of the leading bacteria in biofilm infections is Staphylococcus aureus. It forms biofilms on vascular catheters, peritoneal dialysis catheters, joint prostheses, pacemaker/defibrillator leads, prosthetic heart valves, vascular grafts, orthopedic fixation devices, and other implants (5, 7). All of the above-mentioned infections occur inside the human body; therefore, host factors play an essential role in their development. Local coagulation induced by S. aureus coagulases promotes bacterial attachment to the surfaces of implants (8–10). After an initial adhesion phase, bacteria divide, deposit an extracellular matrix, and develop complex three-dimensional biofilm structures. Recently, several studies have demonstrated that, at this stage, fibrin deposits act as a central structural component of the S. aureus biofilm matrix (11–14). Intriguingly, certain S. aureus strains secrete large amounts of staphylokinase, a bacterial plasminogen activator (15–17). Release of staphylokinase induces local activation of fibrinolysis, resulting in less fibrin deposition on the implant surface and reduced bacterial attachment and biofilm formation (11). Therefore, we hypothesize that precoating of the surfaces of indwelling medical devices with plasminogen activators might reduce the risk of attachment of free-floating bacteria with subsequent biofilm formation.
In the present study, we analyzed the anti-S. aureus biofilm effect of precoating implants with tissue plasminogen activator (tPA). Our data demonstrate that tPA precoating induced local fibrinolysis at the implant surface and efficiently prevented S. aureus biofilm formation both in vitro and in vivo.
MATERIALS AND METHODS
Mice.
Female NMRI mice, 6 to 8 weeks old (Charles River Laboratories), were housed in the animal facility of the Department of Rheumatology and Inflammation Research, University of Gothenburg. Mice were kept under standard temperature and light conditions and fed laboratory chow and water ad libitum. The ethics committee of animal research of Gothenburg approved this study.
Bacteria and growth conditions.
S. aureus strain LS-1 was used in most of the assays. The other laboratory strains used were SH1000, Newman, RN6390, V329 (kindly provided by Pietro Speziale, University of Pavia), and SA113 (= CCUG41582; kindly provided by Edward R. B. Moore, Culture Collection of the University of Gothenburg). Strains LS-1, SH1000, and V329 do not secrete staphylokinase; Newman and RN6390 secrete moderate quantities of staphylokinase; and SA113 secretes large quantities of staphylokinase. V329 is a typical strain forming protein-dependent biofilms, while SA113 is a typical polysaccharide biofilm former (18). Additionally, three congenic strains differing in staphylokinase secretion, previously described LS-1EP, LS-1sak, and LS-1spa-sak (16, 17), were used. Thirteen randomly selected clinical S. aureus isolates from biofilm-related infections were taken from a previously described collection (19).
Bacteria were grown at 37°C in tryptic soy broth (TSB) with shaking. Stock cultures were stored in 10% glycerol at −70°C and checked for purity before experiments by being streaked onto blood agar plates.
tPA coating.
Ninety-six-well polystyrene plates with a MaxiSorp surface (Nunc; Thermo Scientific) were coated by being filled to 100 μl/well with 10 μg/ml tPA (Actilyse; Boehringer Ingelheim) in 100 mM carbonate buffer, pH 9.6, and incubated for 18 to 20 h at 4°C. Control wells were coated with buffer only. Afterwards, wells were washed three times with phosphate-buffered saline (PBS) at 200 μl/well and used for assays. An analogous procedure was used to coat eight-well μslides with ibiTreat surface (ibidi), with coating and washing volumes of 300 and 500 μl, respectively, and 13-mm Thermanox coverslips (Nunc) placed in the wells of a 24-well plate with volumes of 500 and 750 μl. In some experiments, coating with human high-molecular-weight urokinase plasminogen activator (uPA; Medac) or bovine serum albumin (BSA; Sigma-Aldrich) was performed analogously.
Biofilm formation assays.
A microplate method (20) was used for biofilm formation assays. Bacteria from an overnight TSB culture were diluted 100× in fresh biofilm medium. The biofilm medium used, if not indicated otherwise, was TSB with 50% human plasma (heparinized with Li-heparin, collected from healthy donors, and heat inactivated at 56°C for 30 min) and 0.25% glucose. In some experiments, pure TSB with 0.25% glucose was used instead. In some experiments, aprotinin (125 μg/ml; Sigma-Aldrich) or plasminogen activator inhibitor 1 (PAI-1; 10 μg/ml; Molecular Innovations) was added to the medium. Culture plates were filled with a bacterial suspension (100 μl) and incubated at 37°C for 24 h (or another time, as indicated), the medium was removed, the wells were washed with PBS (200 μl) to remove nonadherent bacteria, and the plates were dried at 60°C for 2 h. The biofilms in the wells were stained for 5 min with 0.5% crystal violet (80 μl), rinsed under running tap water, and dried overnight, and stain bound to the biofilm was dissolved by the addition of 33% acetic acid (80 μl). The resulting solution was diluted 20×, the optical density at 570 nm (OD570) of 100 μl was measured with a SpectraMax 340PC348 microplate reader (Molecular Devices), and the OD570 of blanks (wells filled originally with uninfected medium) was subtracted, and the OD570 of the undiluted solution was calculated. As crystal violet binds to bacterial cells and the extracellular matrix, this measurement reflected the total biofilm biomass. All assays were done in triplicate in two to four separate experiments. For clinical isolates, the assay was run in triplicate and mean values were calculated for each strain.
Confocal microscopy of biofilms.
Biofilms were grown for 24 h in 300 μl of medium in eight-well μslides. The wells were washed with PBS, stained for 15 min with 300 μl of 6.7 μM Syto9 dye (Molecular Probes, Life Technologies), washed, and filled with fluorescence mounting medium (ibidi). Biofilms were visualized with an LSM 700 confocal fluorescence microscope equipped with ZEN2009 software for capturing images (Carl Zeiss Microscopy). Images were acquired from four random sites in the well and analyzed with the ISA-2 software (ISA3D) (21).
Electron microscopy of biofilms.
Biofilms grown on 13-mm Thermanox polyester coverslips in 300 μl of medium in the wells of a 24-well plate were visualized with a scanning electron microscope as described previously (11).
Plasmin activity measurement.
Biofilm supernatants were centrifuged for 10 min at 3,000 × g at 4°C to remove the debris and assayed for plasmin activity with a previously described assay (22). Assays were performed in triplicate in two separate experiments.
MIC and MBEC determination.
The MICs and minimal biofilm eradication concentrations (MBECs) of four different antibiotics (vancomycin, ciprofloxacin, rifampin, and cloxacillin) were assayed as described previously (23). MICs were determined by the microdilution method, and MBECs were determined with a metabolic activity assay based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye by viable cells. Determination was performed in duplicate.
Primary adhesion assay.
Bacteria from an overnight culture were centrifuged, resuspended in PBS, and diluted in the biofilm medium to a final OD600 of 0.2 of 100 μl measured with the microplate reader. One hundred microliters of this mixture per well was added to a 96-well plate and incubated at 37°C for 30 min. Afterwards, the medium was aspirated, the wells were washed three times with PBS, and the amount of adherent material was measured either by drying and staining with crystal violet as for the biofilm assay or by filling the wells with 200 μl of PBS, sonicating them for 3 min at 45 kHz in an ultrasonic bath (USC300TH; VWR International), and counting the CFU by serial dilution on horse blood agar (detection limit, 2 × 103 CFU/well). Assays were performed in triplicate in three separate experiments.
In vivo biofilm models.
tPA-coated polyester coverslips (13 mm; Thermanox) were cut in half and placed in TSB with 50% human plasma (500 μl) with S. aureus LS-1 in the wells of a 24-well plate to give the bacteria an opportunity to adhere to the surface. After 2 h of incubation at 37°C, the coverslips were washed three times with PBS and inserted into subcutaneous pockets made on the shaved backs of NMRI mice (n = 8). A coverslip coated with tPA was inserted into one flank of each mouse, and one coated with buffer was inserted into the other flank. The pockets were closed with metal clips, and a biofilm was allowed to develop for 3 days. The mice were then euthanized; the implants were retrieved, washed with PBS, placed in 1 ml of PBS, and sonicated for 5 min at 45 kHz in an ultrasonic bath; and the CFU in the biofilm were counted by serial dilution on horse blood agar. The results of two experiments were pooled.
Since washing steps before implantation may cause a lower bacterial burden on the tPA-coated coverslips than on the buffer-coated controls prior to introduction into the animals, a modified protocol was used to study the effect of tPA coating on S. aureus biofilm infection in more clinically relevant settings. S. aureus LS-1 was cultured overnight in TSB medium and then diluted 1:20 in PBS with 50% heat-inactivated mouse plasma. A 25-μl volume of a bacterial solution was added on top of the tPA-coated coverslips and incubated in 37°C for 4 h in a humid chamber. Without a washing step, the infected coverslips were then implanted subcutaneously into the flanks of mice as described above (one side with the tPA coating and the other side with the control). Mice were either not treated (n = 18) or treated with an antibiotic (n = 13) for 3 days. On day 3 after implantation, the coverslips were collected for analysis of the bacterial loads on the surfaces of implants. Cloxacillin (Cloxacillinnatr; Stragen) dissolved in sterile PBS was used for the antimicrobial treatments. The mice were injected with 0.2 ml of the solution (0.5 mg/g of body weight) intraperitoneally twice a day, starting at 12 h after implantation.
Statistical analysis.
Statistical significance was assessed with the Mann-Whitney test for continuous variables between two groups. Differences among biofilms formed by clinical isolates and differences among biofilms formed on implants in vivo were analyzed with the Wilcoxon matched-pair signed-rank test. Two-tailed P values were used, and values of <0.05 were considered significant. Prism 6.3 software (GraphPad Software) was used for statistical calculations.
RESULTS
tPA coating prevents S. aureus biofilm formation on polystyrene material.
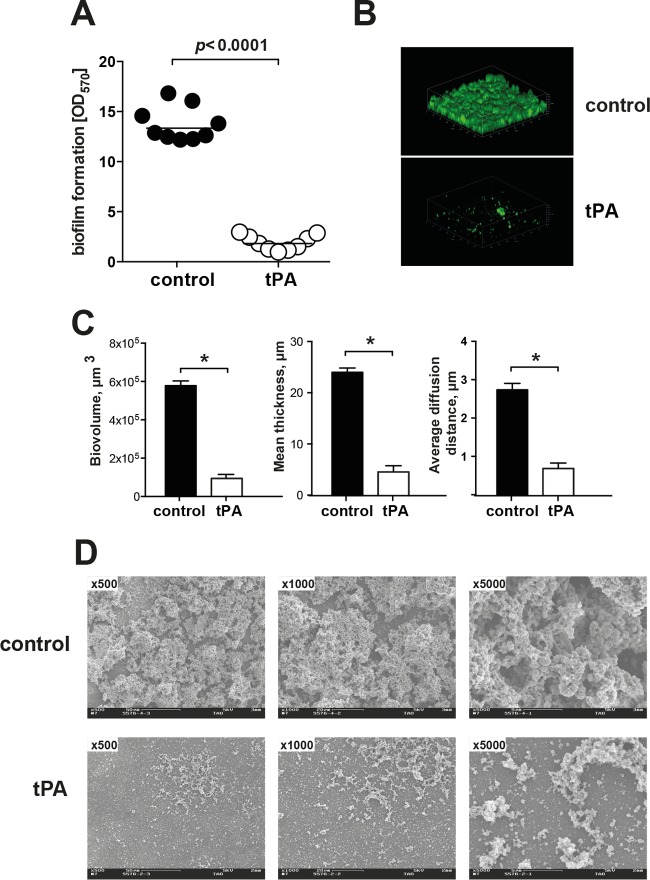
Coating of polystyrene culture wells with tPA efficiently inhibited biofilm formation by S. aureus LS-1 in TSB with 50% human plasma (Fig. 1A). This was confirmed when biofilms were directly visualized with a confocal microscope. Instead of the thick, robust biofilm structure in the control well, only small bacterial clumps appeared in the tPA-coated well (Fig. 1B). Image analysis showed that structures on the tPA-coated surfaces had a smaller total biovolume, were thinner, and were composed of much smaller cell clumps (Fig. 1C) than those of the control group. Scanning electron microscopy images also showed much less biofilm formation on the tPA-coated surface than on the surface without pretreatment (Fig. 1D).
FIG 1.
Coating of polystyrene surfaces with tPA reduces S. aureus biofilm formation. (A) Biofilm formation on tPA-coated or buffer-treated polystyrene surfaces by S. aureus LS-1 after overnight culture in TSB with 50% heparinized human plasma. A microplate colorimetric assay was used. (B) Confocal microscopy images of S. aureus LS-1 biofilms on buffer-treated and tPA-coated polystyrene surfaces after overnight culture in TSB with 50% heparinized human plasma. (C) Biovolume, thickness, and diffusion distance (proxy for clump size) of biofilm masses formed by S. aureus LS-1 on buffer-treated or tPA-coated polystyrene surfaces after overnight culture in TSB with 50% heparinized human plasma. (D) Representative scanning electron microscopy images of S. aureus LS-1 biofilms on buffer-treated (upper row) or tPA-coated (lower row) polyester surfaces after overnight culture in TSB with 50% heparinized human plasma. Magnifications: ×500, ×1,000, and ×5,000. Data are presented as the mean and the standard error of the mean. *, P < 0.05.
The same antibiofilm effect was achieved when coating with other plasminogen activators, e.g., urokinase, was tested (see Fig. S1 in the supplemental material). A similar difference was also observed when tPA-coated wells were compared to BSA-coated wells (see Fig. S2 in the supplemental material), suggesting that the antibiofilm activity of tPA was not due to unspecific effects of protein surface coating.
Local plasminogen activation by tPA coating mediates antibiofilm effects.
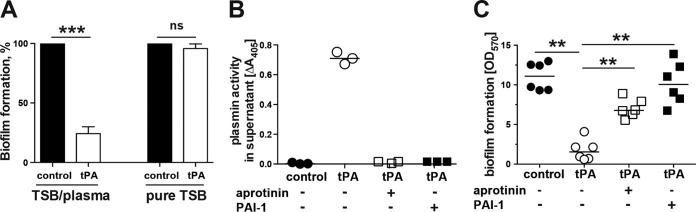
When TSB-glucose medium without plasma was used for biofilm culture, no inhibition by tPA coating was observed (Fig. 2A). This indicates that certain plasma components are crucial for the inhibitory effect of tPA coating. Indeed, supernatant in the wells coated with tPA had markedly increased plasmin activity (Fig. 2B), showing that surface-bound tPA activates plasminogen from plasma to induce local fibrinolysis. Importantly, when PAI-1 (an efficient tPA inhibitor) or aprotinin (a plasmin inhibitor) were added at concentrations resulting in complete inhibition of fibrinolysis, the antibiofilm effect of tPA coating was abrogated and biofilms were successfully formed (Fig. 2C).
FIG 2.
The preventive effect of tPA coating against S. aureus biofilm formation was mediated by plasminogen activation. (A) Biofilm formation (percent) by S. aureus LS-1 on tPA-coated polystyrene surfaces after overnight culture in TSB with 50% human plasma or pure TSB. A microplate colorimetric assay was used. Biofilm formation on a buffer-treated polystyrene surface (control) was assigned a value of 100%. (B) Plasmin activity in biofilm culture supernatants from control and tPA-coated surfaces. (C) Effect of inhibition of tPA (PAI-1) and plasmin (aprotinin) on biofilm formation on tPA-coated versus buffer-treated (control) surfaces. Data are presented as the mean and the standard error of the mean or as a scatter dot plot with the mean indicated by a horizontal bar. ns, not significant; **, P < 0.01; ***, P < 0.001.
tPA coating prevents primary adhesion and delays biofilm formation.
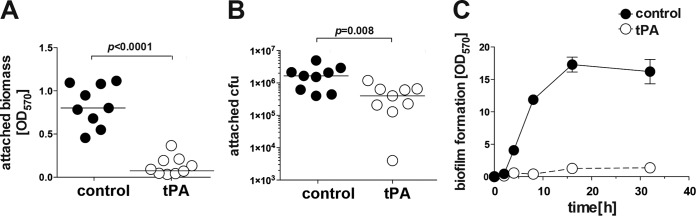
To study whether tPA coating affects the early stage of biofilm formation, the mass and the number of attached S. aureus bacteria on the surface were analyzed after 30 min of exposure of culture wells to a bacterial suspension (Fig. 3). Both the biomass (Fig. 3A) and the number of bacteria (Fig. 3B) attached to the tPA-treated surface after this initial short exposure were greatly reduced.
FIG 3.
tPA coating prevents S. aureus attachment to polystyrene surfaces. Shown are the attached biomass (A) and CFU counts of attached bacteria (B) on buffer-treated or tPA-coated polystyrene surfaces after 30 min of incubation with S. aureus LS-1 in TSB with 50% heparinized human plasma. (C) Differences in the kinetics of biofilm formation on buffer-treated or tPA-coated polystyrene surfaces after 32 h of culture of S. aureus LS-1 in TSB with 50% heparinized human plasma.
Also, a striking difference in biofilm formation kinetics was found (Fig. 3C). Biofilm development on the tPA-coated surface was delayed up to 32 h after bacteria contacted the surface, confirming that bacterial adhesion was already blocked by the tPA coating in the initial phase.
tPA coating is effective against biofilm formation by various S. aureus strains.
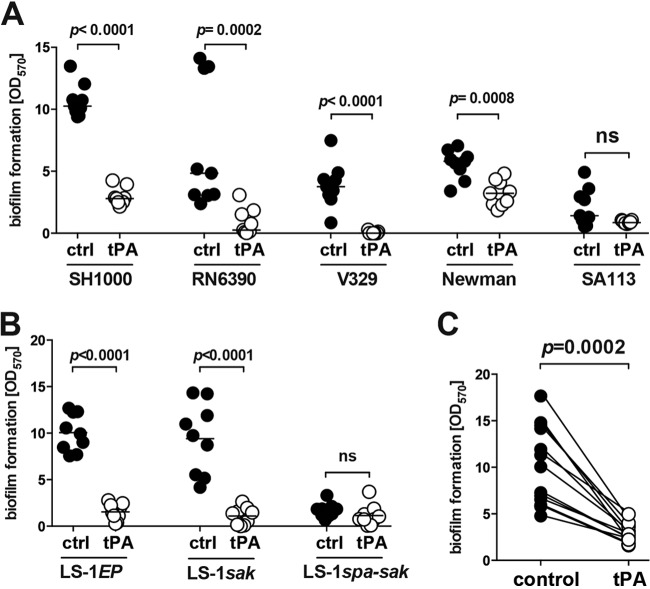
To assess whether the results observed are applicable to other S. aureus isolates, we tested tPA coating on various laboratory S. aureus strains and clinical isolates from biofilm-associated infections (Fig. 4). tPA coating reduced biofilm formation by nearly all of the laboratory strains tested (Fig. 4A). Interestingly, tPA coating had a weak effect on strain SA113, a poor biofilm former that secretes vast quantities of staphylokinase.
FIG 4.
tPA coating reduces biofilm formation by both laboratory strains and clinical S. aureus isolates on polystyrene surfaces. Biofilm formation on tPA-coated and buffer-treated (control [ctrl]) polystyrene surfaces by laboratory S. aureus strains (SH1000, RN6390, V329, Newman, and SA113) (A), three congenic S. aureus strains differing in the level of expressed Sak (EP, no expression; sak, moderate expression; spa-sak, high expression) (B), and 13 S. aureus clinical isolates from biofilm-related infections (C) after overnight culture in TSB with 50% heparinized human plasma. A microplate colorimetric assay was used. ns, not significant.
To further study how staphylokinase secretion interacts with the antibiofilm effect of tPA coating, three congenic strains secreting different amounts of staphylokinase were used (Fig. 4B). Coating with tPA efficiently blocked biofilm formation by a strain secreting no staphylokinase (LS-1EP) or a moderate amount (LS-1sak), while it had no additional effect on a strain overexpressing staphylokinase (LS-1spa-sak), which failed to form a biofilm because of staphylokinase hypersecretion (Fig. 4B).
The efficacy of tPA coating was not limited to laboratory strains. When clinical S. aureus isolates from biofilm-related infections were tested, all of them showed inhibited biofilm formation on tPA-coated surfaces (Fig. 4C).
tPA coating exposes S. aureus to antibiotics.
Bacteria in biofilms are known to be protected against antibiotics because of various mechanisms, e.g., poor penetration by antibiotics. Indeed, all four of the antibiotics tested in this study (vancomycin, ciprofloxacin, rifampin, and cloxacillin) lost their efficacy when S. aureus LS-1 was grown as a biofilm (Table 1). The MICs of the antibiotics tested were low (≤1 mg/liter). However, when a biofilm was formed, all four antibiotics failed to eradicate the bacteria inside the biofilm matrix even at a concentration of 1,024 μg/ml. In contrast, bacteria on a tPA-coated surface were eradicated by significantly lower concentrations of antibiotics (4 to 128 μg/ml).
TABLE 1.
Impact of tPA coating on the MICs and MBECs of different antibiotics for planktonic cells and biofilms of S. aureus LS-1
| Antibiotic | Planktonic cell MIC (mg/liter) | Biofilm MBEC (mg/liter) |
|
|---|---|---|---|
| Control | tPA coated | ||
| Vancomycin | 1 | >1,024 | 8 |
| Ciprofloxacin | 1 | >1,024 | 16 |
| Rifampin | 0.008 | >1,024 | 4 |
| Cloxacillin | 0.5 | >1,024 | 128 |
tPA coating prevents biofilm-related infection in a mouse model.
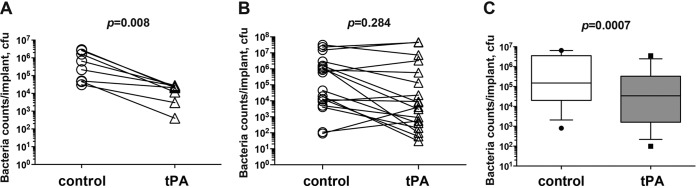
To examine the effect of tPA coating in vivo, S. aureus-inoculated coverslips were washed and then implanted subcutaneously into mice and the bacterial loads on the coverslips were analyzed after 3 days. This led to biofilm formation on the control (buffer-treated) implant surface, which could be seen by the naked eye during the surgical procedure, but bacteria failed to form a biofilm on tPA-coated implants. In line with this, the count of viable bacteria attached to the implant was reduced more than 20-fold by tPA coating (Fig. 5A).
FIG 5.
tPA coating reduces S. aureus biofilm formation in vivo. (A) The buffer-treated and tPA-coated coverslips were infected with S. aureus LS-1 for 2 h and washed with PBS and then implanted subcutaneously into both flanks of NMRI mice (n = 8). The coverslips were collected 3 days after implantation. The bacterial loads on the coverslips were analyzed as CFU counts. The buffer-treated and tPA-coated coverslips were infected with S. aureus LS-1 for 4 h and implanted subcutaneously into both flanks of NMRI mice (B and C). Mice were either not treated (n = 18) (B) or treated with cloxacillin (n = 13) twice a day starting at 12 h after implantation (C). On day 3, the coverslips were collected to analyze the bacterial loads (CFU counts) on the implant surfaces. Data are presented as dot plots (panels A and B) or medians (center lines), interquartile ranges (boxes), and 80% central ranges (whiskers) (C). The Wilcoxon matched-pair signed-rank test was used.
Since washing before implantation may cause a lighter bacterial burden on the tPA-coated coverslips prior to their introduction into the animals, unwashed S. aureus-infected coverslips were implanted into mice and the CFU counts on the coverslips were analyzed after 3 days (Fig. 5B). Twelve of 18 tPA-coated implants had fewer bacteria attached to their surfaces than the control coverslips (P = 0.09). No significant difference in the CFU counts on the coverslips was observed between the groups (P = 0.284).
To assess the effect of tPA coating combined with antibiotic treatment on S. aureus biofilm infection, mice implanted with S. aureus-infected coverslips with a tPA coating were treated with cloxacillin for 3 days and the bacterial loads on the coverslips were analyzed (Fig. 5C). Viable S. aureus bacteria were found on both tPA-coated and control coverslips 3 days after cloxacillin treatment. Twelve of 13 tPA-coated implants had fewer bacteria attached to their surfaces than the control coverslips on the collateral side (P < 0.0001). Also, the CFU counts were four times as low in the tPA-coated group than in the control group (P = 0.0007).
DISCUSSION
In this study, we demonstrated that a tPA coating on the surfaces of implants prevents S. aureus biofilm formation under culture conditions containing plasma. This preventive effect was mediated through plasminogen activation by surface-bound tPA. tPA coating also significantly enhanced the susceptibility of S. aureus biofilms to various antibiotics. Importantly, in vivo tPA coating combined with antibiotic treatment significantly reduced the amount of bacteria attached to the implant surface.
Growth medium with human plasma added is a more physiological setting in which to study biofilm formation than a pure bacterial culture medium is, since it more closely resembles real-life conditions. The preventive effect of a tPA coating on biofilm formation became apparent only when human plasma was used, suggesting that plasma proteins are crucial for both biofilm matrix formation and the preventive effect of a tPA coating. Indeed, human plasma proteins are known to immediately coat implant surfaces and mediate bacterial attachment when medical devices are implanted (5, 7). Among those proteins are host fibrinogen and fibrin, to which S. aureus binds with bacterial surface proteins (24). Moreover, S. aureus induces local coagulation by secreting two coagulases that convert fibrinogen into fibrin nets as an anchoring place for staphylococci and thus enhance bacterial adhesion to the implant surface (8, 9, 25, 26). A tPA coating on implants activated host plasminogen into plasmin and created fibrinolytic activity directly on the implant surface, where it can cleave the fibrin deposited by infecting bacteria. Plasmin is a broad-spectrum protease, so it potentially also cleaves other proteins involved in biofilm formation, further strengthening the antibiofilm effect. As tPA has increased activity in the presence of fibrin (27), tPA coating might selectively induce increased fibrinolysis in response to abundant fibrin, which is one of the essential structural components of the S. aureus biofilm matrix (11). tPA coating displayed a robust ability to reduce biofilm formation by clinical isolates from biofilm-related infections, whereas it had no effect on staphylokinase-overexpressing strains, which are usually not associated with biofilm infections (11). This is conceivably due to the fact that staphylokinase-overexpressing strains have already exerted their full effect of local plasminogen activation, which is similar to the mechanism of action of tPA coating. An alternative explanation is that staphylokinase inhibits the activity of a tPA coating on an implant surface. Indeed, staphylokinase was shown to reduce plasmin formation by tPA or uPA (28). Theoretically, coagulation inhibitors might achieve an antibiofilm effect partly similar to that of tPA, but classic anticoagulants (vitamin K antagonists, heparin, hirudin) cannot inhibit fibrin deposition induced by S. aureus (9), and heparin even increases its biofilm formation (29).
The first hours after implantation are crucial for biofilm infection and its prevention, as they represent the window of opportunity for the immune system and antibiotics to eradicate free-floating bacteria before they develop a resistant biofilm (4). Unfortunately, fibrin-rich S. aureus biofilms appear rapidly, which might decrease the efficacy of antibiotic prophylaxis (30) and blunt phagocytosis (9, 11, 31, 32). Importantly, tPA coating prevents the first step of biofilm formation, the adhesion of S. aureus to the implant surface, thereby expanding the window of opportunity and allowing more efficient pathogen clearance. Because of poor biofilm formation, S. aureus on a tPA-coated surface remained vulnerable to immune system attack and susceptible to antibiotics, which was apparent in the mouse implant infection model, where tPA coating and antibiotics displayed a synergistic effect against biofilm infection.
The first animal model used in this study was a simple replication of our in vitro system, since the free-floating bacteria were washed away and there were already fewer bacteria on a tPA-coated plastic surface than on control coverslips before implantation. In contrast, in the later animal experiments, the same dose of S. aureus (irrespective of free-floating bacteria or biofilm-forming bacteria) on tPA-coated and control coverslips were implanted into mice, which ensures the clinical relevance of this study. Interestingly, no significant difference in CFU counts was observed in the absence of antibiotic treatment (Fig. 5B). In this case, only a trend toward lower CFU counts on tPA-coated implants than on contralateral buffer-treated implants was seen. In animal experiments, large inocula are typically used to infect implants, which might overwhelm the bacterial killing capacity of the host immune system. Therefore, the effect of tPA coating on biofilm formation can be masked.
In the presence of antibiotics, however, representative of the clinical situation, tPA coating did result in lighter bacterial loads on the implants. We failed to show a full preventive effect, i.e., eradication of the bacteria on implants, of combining tPA coating and antibiotic treatment, which might be due to the late start of the antibiotic treatment (12 h after implantation) or to the slow inactivation of tPA in vivo. Nevertheless, the current study presents proof of the concept that a plasminogen activator coating on the implant surface might be a novel concept in the prevention of biofilm formation. Many practical details, e.g., efficient coating techniques, the type of plasminogen activator, and the starting time of antibiotic treatment, need to be addressed in future studies.
As a new treatment concept, tPA coating might provide efficient protection during the early, most crucial phase of bacterial attachment after implantation. There are, however, some foreseeable challenges. Endogenous PAI-1 in the physiological nanogram quantities found in plasma did not interfere with the efficacy of tPA coating in our experiments (data not shown). However, inside a human body, implants are continuously exposed to PAI-1, which might eventually lead to loss of tPA activity. This notion is supported by our observation that larger (microgram) PAI-1 quantities totally abrogated the protective effect of tPA coating on biofilm formation in vitro. Indeed, we have previously shown that PAI-1 levels are greatly increased in both blood and local infected organs in S. aureus infections (33). There is, however, the possibility of bypassing the inhibitory effect of PAI-1 by using other fibrinolytic agents, e.g., a PAI-1-resistant variant of tPA (tenecteplase), bacterial plasminogen activators (staphylokinase, streptokinase), or other mammalian activators (desmoteplase) (27). The good availability of various plasminogen activators in combination with different coating methods might lead to the fast development of new prophylactic methods for biofilm-related infections on indwelling medical devices.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yvonne Josefsson, Kanita Cukur, and Bengt R. Johansson for their help with electron microscopy.
We do not have any commercial associations that might pose a conflict of interest.
Funding Statement
This work was supported by the Swedish Medical Research Council (grants D0275001 and D0275002 to T. Jin), the Swedish Medical Society (grants SLS-496741 and SLS-402871 to T. Jin), the Stiftelsen Clas Groschinskys Minnesfond (grants M1566 and M14099 to T. Jin), the Royal Society of Arts and Sciences in Gothenburg (grant to T. Jin and M. Na), the Wilhelm and Martina Lundgren Foundation (grant to T. Jin, M. Na, J. Kwiecinski, and A. Jarneborn), the Scandinavian Society for Antimicrobial Chemotherapy Foundation (grant SLS-501701 to T. Jin), Rune och Ulla Amlövs Stiftelse för Neurologisk och Reumatologisk Forskning (grant 2015-00056 to T. Jin), Adlerbertska Forskningsstiftelsen (grant to T. Jin and M. Na) and the Research Foundation Flanders (FWO-Vlaanderen) (grant to M. Peetermans and P. Verhamme). The funders had no role in study design, data collection and interpretation or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02803-15.
REFERENCES
- 1.Ahmed S, Darouiche RO. 2015. Anti-biofilm agents in control of device-related infections. Adv Exp Med Biol 831:137–146. doi: 10.1007/978-3-319-09782-4_9. [DOI] [PubMed] [Google Scholar]
- 2.Bryers JD. 2008. Medical biofilms. Biotechnol Bioeng 100:1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gristina AG. 1987. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues LR. 2011. Inhibition of bacterial adhesion on medical devices. Adv Exp Med Biol 715:351–367. doi: 10.1007/978-94-007-0940-9_22. [DOI] [PubMed] [Google Scholar]
- 5.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. 2012. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Kiedrowski MR, Horswill AR. 2011. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 8.Vanassche T, Peetermans M, Van Aelst LN, Peetermans WE, Verhaegen J, Missiakas DM, Schneewind O, Hoylaerts MF, Verhamme P. 2013. The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. J Infect Dis 208:92–100. doi: 10.1093/infdis/jit130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanassche T, Verhaegen J, Peetermans WE, Van Ryn J, Cheng A, Schneewind O, Hoylaerts MF, Verhamme P. 2011. Inhibition of staphylothrombin by dabigatran reduces Staphylococcus aureus virulence. J Thromb Haemost 9:2436–2446. doi: 10.1111/j.1538-7836.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- 10.Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E. 4 June 2015. An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device-related infections. J Infect Dis doi: 10.1093/infdis/jiv319. [DOI] [PubMed] [Google Scholar]
- 11.Kwiecinski J, Peetermans M, Liesenborghs L, Na M, Bjornsdottir H, Zhu X, Jacobsson G, Johansson BR, Geoghegan JA, Foster TJ, Josefsson E, Bylund J, Verhamme P, Jin T. 1 July 2015. Staphylokinase control of Staphylococcus aureus biofilm formation and detachment through host plasminogen activation. J Infect Dis doi: 10.1093/infdis/jiv360. [DOI] [PubMed] [Google Scholar]
- 12.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. 2015. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis 211:641–650. doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiecinski J, Kahlmeter G, Jin T. 2015. Biofilm formation by Staphylococcus aureus isolates from skin and soft tissue infections. Curr Microbiol 70:698–703. doi: 10.1007/s00284-014-0770-x. [DOI] [PubMed] [Google Scholar]
- 14.Peetermans M, Vanassche T, Liesenborghs L, Claes J, Vande Velde G, Kwiecinksi J, Jin T, De Geest B, Hoylaerts MF, Lijnen RH, Verhamme P. 2014. Plasminogen activation by staphylokinase enhances local spreading of S. aureus in skin infections. BMC Microbiol 14:310. doi: 10.1186/s12866-014-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin T, Bokarewa M, McIntyre L, Tarkowski A, Corey GR, Reller LB, Fowler VG Jr. 2003. Fatal outcome of bacteraemic patients caused by infection with staphylokinase-deficient Staphylococcus aureus strains. J Med Microbiol 52:919–923. doi: 10.1099/jmm.0.05145-0. [DOI] [PubMed] [Google Scholar]
- 16.Kwiecinski J, Jacobsson G, Karlsson M, Zhu X, Wang W, Bremell T, Josefsson E, Jin T. 2013. Staphylokinase promotes the establishment of Staphylococcus aureus skin infections while decreasing disease severity. J Infect Dis 208:990–999. doi: 10.1093/infdis/jit288. [DOI] [PubMed] [Google Scholar]
- 17.Kwiecinski J, Josefsson E, Mitchell J, Higgins J, Magnusson M, Foster T, Jin T, Bokarewa M. 2010. Activation of plasminogen by staphylokinase reduces the severity of Staphylococcus aureus systemic infection. J Infect Dis 202:1041–1049. doi: 10.1086/656140. [DOI] [PubMed] [Google Scholar]
- 18.Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. 2009. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials 30:3158–3166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson G, Dashti S, Wahlberg T, Andersson R. 2007. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand J Infect Dis 39:6–13. doi: 10.1080/00365540600810026. [DOI] [PubMed] [Google Scholar]
- 20.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 21.Beyenal H, Donovan C, Lewandowski Z, Harkin G. 2004. Three-dimensional biofilm structure quantification. J Microbiol Methods 59:395–413. doi: 10.1016/j.mimet.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Kwiecinski J, Josefsson E, Jin T. 2011. Fibrinolysis is down-regulated in mouse collagen-induced arthritis, but its normalization does not alleviate the course of disease. Inflamm Res 60:1021–1029. doi: 10.1007/s00011-011-0363-0. [DOI] [PubMed] [Google Scholar]
- 23.Kwiecinski J, Eick S, Wojcik K. 2009. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int J Antimicrob Agents 33:343–347. doi: 10.1016/j.ijantimicag.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akiyama H, Ueda M, Kanzaki H, Tada J, Arata J. 1997. Biofilm formation of Staphylococcus aureus strains isolated from impetigo and furuncle: role of fibrinogen and fibrin. J Dermatol Sci 16:2–10. doi: 10.1016/S0923-1811(97)00611-7. [DOI] [PubMed] [Google Scholar]
- 26.Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. 2005. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci 38:197–205. doi: 10.1016/j.jdermsci.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Baruah DB, Dash RN, Chaudhari MR, Kadam SS. 2006. Plasminogen activators: a comparison. Vascul Pharmacol 44:1–9. doi: 10.1016/j.vph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Jin T, Bokarewa M, Zhu Y, Tarkowski A. 2008. Staphylokinase reduces plasmin formation by endogenous plasminogen activators. Eur J Haematol 81:8–17. doi: 10.1111/j.1600-0609.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 29.Shanks RM, Sargent JL, Martinez RM, Graber ML, O'Toole GA. 2006. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant 21:2247–2255. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 30.Dastgheyb S, Hammoud S, Ketonis C, Liu AY, Fitzgerald K, Parvizi J, Purtill J, Ciccotti M, Shapiro IM, Otto M, Hickok NJ. 2015. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob Agents Chemother 59:2122–2128. doi: 10.1128/AAC.04579-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guggenberger C, Wolz C, Morrissey JA, Heesemann J. 2012. Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLoS Pathog 8:e1002434. doi: 10.1371/journal.ppat.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loof T, Goldmann O, Naudin C, Morgelin M, Neumann Y, Pils M, Foster S, Medina E, Herwald H. 2015. Staphylococcus aureus induced clotting of plasma is an immune evasion mechanism to persist within the fibrin network. Microbiology 161(Pt 3):621–627. doi: 10.1099/mic.0.000019. [DOI] [PubMed] [Google Scholar]
- 33.Jin T, Bokarewa M, Tarkowski A. 2005. Urokinase-type plasminogen activator, an endogenous antibiotic. J Infect Dis 192:429–437. doi: 10.1086/431600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.