Abstract
An outbreak of type emm59 invasive group A Streptococcus (iGAS) disease was declared in 2008 in Thunder Bay District, Northwestern Ontario, 2 years after a countrywide emm59 epidemic was recognized in Canada. Despite a declining number of emm59 infections since 2010, numerous cases of iGAS disease continue to be reported in the area. We collected clinical information on all iGAS cases recorded in Thunder Bay District from 2008 to 2013. We also emm typed and sequenced the genomes of all available strains isolated from 2011 to 2013 from iGAS infections and from severe cases of soft tissue infections. We used whole-genome sequencing data to investigate the population structure of GAS strains of the most frequently isolated emm types. We report an increased incidence of iGAS in Thunder Bay compared to the metropolitan area of Toronto/Peel and the province of Ontario. Illicit drug use, alcohol abuse, homelessness, and hepatitis C infection were underlying diseases or conditions that might have predisposed patients to iGAS disease. Most cases were caused by clonal strains of skin or generalist emm types (i.e., emm82, emm87, emm101, emm4, emm83, and emm114) uncommonly seen in other areas of the province. We observed rapid waxing and waning of emm types causing disease and their replacement by other emm types associated with the same tissue tropisms. Thus, iGAS disease in Thunder Bay District predominantly affects a select population of disadvantaged persons and is caused by clonally related strains of a few skin and generalist emm types less commonly associated with iGAS in other areas of Ontario.
INTRODUCTION
Group A Streptococcus (GAS) causes a wide variety of diseases ranging in severity from uncomplicated pharyngitis to life-threatening necrotizing fasciitis and streptococcal toxic shock syndrome (1). More than 240 GAS emm types are recognized based on the sequence of the hypervariable 5′ end of gene emm, encoding M protein, a major GAS virulence factor with antiphagocytic properties (2–4). Beginning in 2006, Canada experienced an epidemic of invasive GAS (iGAS) infections caused by strains of the previously rare emm59 type (5). A hypervirulent clone, which later disseminated to several areas of the United States, was shown to be responsible for hundreds of cases countrywide (6–10). Invasive type emm59 strains were frequently isolated from soft tissue infections and were recovered in higher percentages from patients with distinctive risk factors, including alcohol abuse, homelessness, hepatitis C virus (HCV) infection, and intravenous drug use (IVDU) (5, 6). In the province of Ontario, most emm59 iGAS infections occurred in Thunder Bay, the most populous municipality in the Northwestern area of the province (population, approximately 110,000), and the regional center of the Thunder Bay District (TBD), which extends over an area of 103,720 square kilometers.
The number of emm59 iGAS cases in Canada, particularly in Ontario, has been in continuous decline since 2010 (8). However, TBD public health authorities continue to observe rates of iGAS disease incidence that are approximately 2 to 4 times the provincial average. Associations between strains of specific emm types and tropism for different tissues have long been described (11–13). Markers of niche specificity defining skin, throat, and generalist (i.e., those commonly isolated from both skin and throat infections) GAS strains have been proposed based on the arrangement of the emm and emm-like chromosomal region and on the presence of different variants of the mga, rofA, or nra loci (encoding standalone transcriptional regulators), the pilus-encoding FCT loci, and the sof gene (encoding a serum opacity factor) (13).
To understand in more detail the sustained high incidence of iGAS disease in Thunder Bay, we evaluated available clinical and epidemiological data. We also used genomics to investigate the population structure of GAS strains causing disease in the area. We report that, for the period from 2011 to 2013, most iGAS cases occurred in a specific, vulnerable population and were mostly caused by clonally related strains of a few skin and generalist emm types which were infrequently identified in other areas of the province.
MATERIALS AND METHODS
Clinical data collection.
Clinical data were collected for the period from 2001 to 2013. Mandatory reporting by the diagnosing laboratories and chart reviews of suspected cases presenting at Thunder Bay Regional Health Sciences Centre identified iGAS disease cases in the TBD. Communicable disease control staff of the Thunder Bay District Health Unit (TBDHU) conducted follow-up chart reviews on all cases. In the spring of 2008, the TBDHU noticed an increase in the number of iGAS cases and implemented an enhanced surveillance protocol (at the district level, no enhanced surveillance protocol was implemented in the province of Ontario), which is still ongoing and which included periodic chart reviews, to identify cases missed through the routine mandatory reporting system, as well as additional data collection on identified cases. The clinical and public health information collected included the patient's name, age and place of residence, disease presentation, and underlying diseases or conditions that might have predisposed patients to invasive disease (alcohol abuse; chronic underlying organ system disease, e.g., chronic lung, heart, or renal disease; diabetes mellitus; HCV infection; HIV infection; homelessness; history of illicit drug use; immunocompromised status; postpartum infection; surgical and nonsurgical wound infections; or none identified, unknown, and other). The iGAS incidence rates for the metropolitan region of Toronto/Peel were provided by the Toronto Invasive Bacterial Diseases Network, an active surveillance program for iGAS disease (population under surveillance, approximately 5.5 million; sensitivity of the active surveillance is estimated to be 95%; an annual audit is performed in all participant laboratories to identify any cases that may have been missed through the regular reporting process). The iGAS incidence rates for the province of Ontario take into account all iGAS cases identified in Thunder Bay and Toronto/Peel, and the rates were calculated by Public Health Ontario. iGAS disease is reportable in Ontario. Provincial iGAS surveillance is passive, and the population under surveillance is approximately 13.3 million. We received only aggregated data and did not have access to individual patient clinical data for either Toronto/Peel or the province of Ontario. Irrespective of geographical area, iGAS disease cases met the following criteria: (i) acute illness in association with isolation of GAS from a normally sterile site or (ii) isolation of GAS from a nonsterile site (e.g., skin, sputum) in the presence of confirmed or probable streptococcal toxic-shock syndrome and/or soft tissue necrosis (including necrotizing fasciitis), meningitis, or death (14). Normally sterile sites included blood; cerebrospinal, pleural, peritoneal, pericardial, or joint fluid (including bursa); deep aspirates; tissue specimens; or swabs obtained during surgery. We also recorded in a separate database all laboratory-confirmed cases of GAS soft tissue infection (including wounds and cellulitis, but not superficial skin samples) occurring in Thunder Bay (most of which required hospitalization) that did not meet the definition for iGAS.
Strain collection, culture conditions, DNA preparation, and emm typing.
Isolates available to the study were collected during 2011 to 2013, a period during which the TBDHU recorded 66 iGAS disease cases and 64 additional GAS cases of laboratory-confirmed soft tissue infections, most of which required hospitalization, that did not meet the definition for invasive disease. Public Health Ontario laboratories received one isolate for each of the 66 iGAS strains and one isolate for 54 of the 64 additional GAS cases. Of these 120 strains, 50 iGAS strains and 52 GAS strains from severe soft tissue infection were available to this study. Available strain metadata were limited to geographic location descriptors, date of collection, and anatomical source of the isolate (see Table S1 in the supplemental material). Strains were cultured at 37°C with 5% CO2 on Columbia blood agar plates containing 5% sheep blood or in Todd-Hewitt broth supplemented with 0.2% yeast extract. GAS species was confirmed by beta-hemolysis on sheep blood agar, grouping of carbohydrate antigen, large colony size, and bacitracin susceptibility (15). DNA was prepared from overnight cultures using the QIAamp DNA mini kit (Qiagen, Toronto, ON, Canada). emm typing was performed by PCR and sequencing, as previously described (16).
Whole-genome sequencing (WGS), closure of reference genomes, bioinformatics, and phylogenetic analysis.
One strain of each of the emm types most frequently recovered in Thunder Bay (emm82, emm83, emm87, emm101, and emm114) was randomly chosen, and its genome sequenced to closure using single-molecule real-time sequencing (Pacific Biosciences, Menlo Park, CA, USA), Illumina sequencing (Illumina, San Diego, CA, USA), and optical mapping (OpGen Technologies, Madison, WI, USA). The genomes of the remaining 97 GAS strains were sequenced using Illumina technology. Sequence read archive accession numbers are provided in Table S1 in the supplemental material. Multilocus sequence-typing (MLST) and the presence of genes potentially conferring antibiotic resistance were determined directly from the WGS data using SRST2 (17). Single-nucleotide polymorphisms (SNPs) and short insertion/deletions were identified against reference genomes using VAAL (18). Whole-genome SNPs were used to construct neighbor-joining phylogenetic trees using SplitsTree4 (19). Genome visualizations were created using BRIG (20) and edited using Adobe Illustrator. Detailed methods are presented in the supplemental material.
Nucleotide sequence accession numbers.
New genome sequences have been deposited in GenBank under accession numbers CP007561, CP007562, CP007560, CP010450, and CP010449.
RESULTS
The incidence of iGAS disease is higher in Thunder Bay than in the metropolitan region of Toronto/Peel and the province of Ontario.
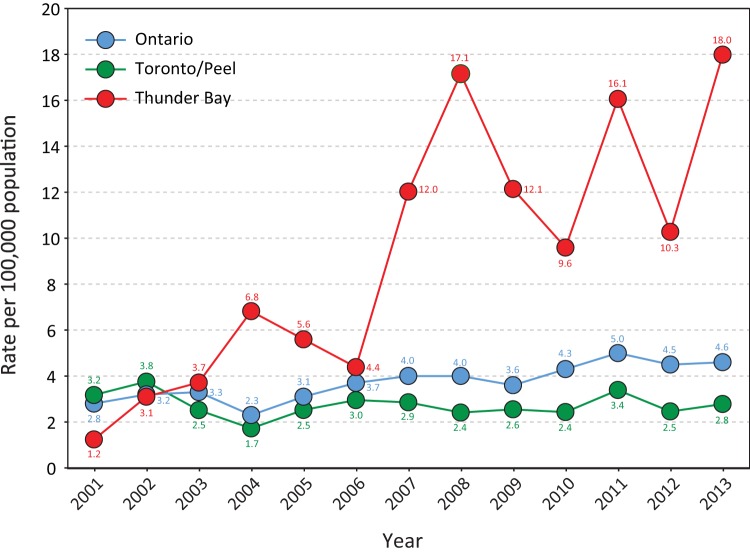
While the incidence of iGAS disease in Thunder Bay had been comparable to that observed in the metropolitan region of Toronto/Peel and similar to the Ontario provincial average pre-2006, retrospective investigations revealed an ongoing increase in iGAS incidence in Thunder Bay since early 2007 (Fig. 1). A community outbreak of emm59 iGAS disease was declared in Thunder Bay in May 2008. It lasted until July 2009, at which time the number of emm59 invasive cases significantly decreased. The increased surveillance implemented during the emm59 outbreak permitted the prospective evaluation of all iGAS cases recovered since 2008. Extensive chart reviews revealed levels of iGAS disease well above those observed in Toronto/Peel and also higher than the average for the province of Ontario continuing to 2013 (Fig. 1).
FIG 1.
Reported incidence rates of invasive group A streptococcal disease per 100,000 population in Thunder Bay, in the metropolitan region of Toronto/Peel, and in the province of Ontario, from 2001 to 2013. The incidence of iGAS disease in Thunder Bay sharply increased in 2007, and it peaked in 2008 coincidentally with the declaration of a local outbreak of emm59 invasive disease. An extensive chart review revealed incidence rates of iGAS disease in Thunder Bay that were 3 to 6 times those in Toronto/Peel and the provincial average between 2010 and 2013, years in which the isolation of emm59 strains was minimal.
In the period from 2008 to 2013, 14%, 21%, 37%, and 28% of iGAS cases in Thunder Bay occurred in individuals aged 0 to 19, 20 to 39, 40 to 59, and ≥60 years, respectively. Males accounted for 60% of the cases. A substantial number of the cases (41%) occurred among First Nations populations, which make up 8.2% of the total Thunder Bay population according to the Statistics Canada 2006 census. Most cases occurred in a specific population (Table 1). Common identified underlying diseases or conditions that might have predisposed patients to invasive disease included IVDU and/or polysubstance abuse (opiates, inhalation drugs, nonpotable intoxicant substances), HCV infection, underhousing/homelessness, and alcohol abuse. During the emm59 outbreak and its immediate aftermath, a larger proportion of cases were IVDU patients or those infected with HCV than in the period from 2011 to 2013 (Table 1).
TABLE 1.
Clinical characteristics of invasive GAS disease, Thunder Bay District Health Unit, 2008-2013
| Condition/comorbiditya | No. (%) of patients by year |
Significant differences between time periodsb (P) | |
|---|---|---|---|
| 2008-2010 (n = 61) | 2011-2013 (n = 66) | ||
| First Nations | 27 (44) | 27 (41) | No (0.60) |
| IVDUc | 15 (25) | 9 (14) | No (0.29) |
| Hepatitis C | 11 (18) | 6 (9) | Yes (0.01) |
| Homeless | 8 (11) | 6 (13) | No (0.52) |
| Alcohol abuse | 19 (31) | 25 (38) | No (0.07) |
Conditions and comorbidities are not mutually exclusive.
Chi-square test.
IVDU, intravenous drug usage.
Unusual emm types were responsible for the majority of iGAS disease in Thunder Bay in 2011 to 2013.
In 2008 to 2009, type emm59 strains were the most frequently identified strains among iGAS cases in Thunder Bay (37.8%), followed by type emm1 strains (18.9%) (Table 2), after which time type emm59 strains essentially disappeared from the area (1 case in 2011). During the years 2011 to 2013, the six most prevalent emm types in Thunder Bay among iGAS disease cases were emm87, emm82, emm1, emm101, emm83, and emm114 (12.3%, 10.8%, 9.2%, 9.2%, 9.2%, and 7.7%, respectively) (Fig. 2A). In contrast, the emm types isolated from iGAS disease cases in metropolitan Toronto/Peel were significantly different (chi-square test, P < 0.001). The most frequently isolated emm type in Toronto/Peel and in the rest of Ontario was emm1 (26.3% and 20.6%, respectively), followed by emm89 (12.2% and 13.3%, respectively), emm3 (10.3% and 12.3%, respectively), emm12 (9.2%, and 7.3%, respectively), and emm28 (4.6% and 6.9%, respectively). In all three geographical areas, iGAS strains were most often isolated from blood. However, we observed decreased isolation from blood and increased isolation from synovial fluid in Thunder Bay in comparison to Toronto/Peel and the rest of Ontario (Fig. 2B). Interestingly, most strains causing iGAS disease in Thunder Bay belonged to either the skin (i.e., emm83 and emm101) or generalist (i.e., emm82, emm87, and emm114) groups, with a relatively small percentage of strains with tropism for throat (Fig. 2C).
TABLE 2.
Number of GAS isolates recovered from cases of invasive disease, Thunder Bay District Health Unit, 2008-2013
| emm type | No. of isolates by year |
Total no. | |||||
|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | ||
| 1 | 5 | 2 | 1 | 2 | 0 | 4 | 14 |
| 3 | 1 | 0 | 3 | 0 | 0 | 0 | 4 |
| 4 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| 6 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| 11 | 0 | 0 | 0 | 0 | 0 | 4 | 4 |
| 12 | 1 | 0 | 0 | 0 | 0 | 2 | 3 |
| 18 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 22 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 28 | 0 | 0 | 0 | 2 | 0 | 1 | 3 |
| 41 | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| 44 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| 49 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 53 | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| 59 | 11 | 3 | 0 | 1 | 0 | 0 | 15 |
| 68 | 1 | 0 | 0 | 0 | 0 | 3 | 4 |
| 73 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 75 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| 77 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 80 | 0 | 0 | 0 | 0 | 1 | 2 | 3 |
| 82 | 0 | 0 | 0 | 2 | 4 | 1 | 7 |
| 83 | 0 | 0 | 0 | 6 | 0 | 0 | 6 |
| 87 | 0 | 0 | 0 | 4 | 4 | 0 | 8 |
| 89 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 101 | 0 | 1 | 4 | 1 | 3 | 2 | 11 |
| 114 | 0 | 1 | 3 | 2 | 1 | 2 | 9 |
| 115 | 0 | 2 | 1 | 0 | 0 | 0 | 3 |
| 118 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| NTa | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Total | 25 | 12 | 14 | 25 | 16 | 25 | 117 |
NT, nontypeable.
FIG 2.
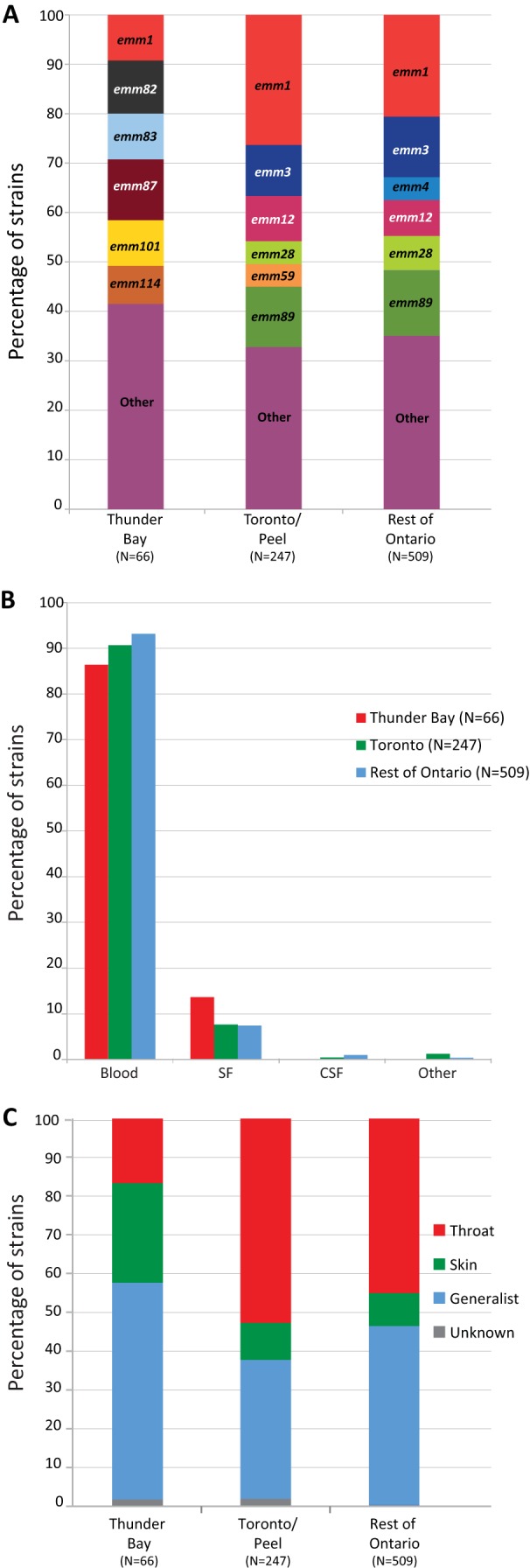
(A) emm type distribution of isolates causing iGAS disease in Thunder Bay, in metropolitan Toronto/Peel, and in the rest of Ontario during the period of 2011 to 2013. Strains of emm1 GAS were among the top 6 emm types responsible for iGAS disease in all geographical areas. The other top 5 emm types (emm82, emm83, emm87, emm101, and emm114) associated with iGAS disease in Thunder Bay were not observed among the top 6 emm types causing disease in the metropolitan region of Toronto/Peel or in the rest of Ontario. There were significant differences in the emm type distribution between Thunder Bay and Toronto/Peel (chi-square test, P < 0.0001). (B) Source of isolation of iGAS strains from Thunder Bay, Toronto/Peel, and the rest of Ontario. Most strains were isolated from blood in all three geographical areas. However, proportionally fewer strains were isolated from blood, and more were isolated from synovial fluid (SF), in Thunder Bay. CSF, cerebrospinal fluid; other, other sources included pleural fluid and undetermined autopsy tissue. (C) Tissue tropism of iGAS strains isolated from 2011 to 2013 in Thunder Bay, Toronto/Peel, and the rest of Ontario. Significant differences were observed for skin, throat, and generalist groups (defined according to references 11–13) between Thunder Bay and Toronto/Peel plus the rest of Ontario (chi-square test, P < 0.05). Unknown, nontypeable strains.
Overabundance of soft tissue infections in Thunder Bay correlated with circulating strains belonging to skin and generalist emm types.
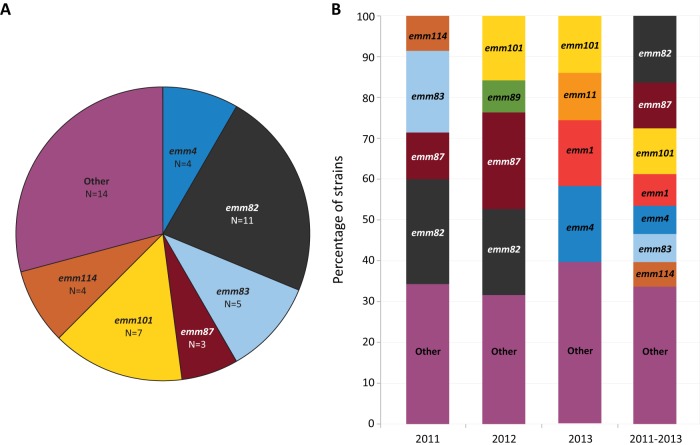
Figure 3A shows that the emm type distribution of strains recovered from cases of severe GAS tissue infections that did not meet the definition for iGAS disease was, overall, similar to the distribution observed among iGAS disease cases during the period from 2011 to 2013. Most of these strains also belonged to either the skin or generalist groups. When isolates recovered from iGAS disease and cases of severe soft tissue in Thunder Bay were taken together, the most frequently identified types in 2011 were emm82 (23.1%), emm83 (20.5%), and emm87 (10.3%). In 2012, the most frequently identified types were emm87, emm82, and emm101 (25%, 22.2%, and 16.7%, respectively). Strains of types emm4, emm1, and emm101 predominated in 2013 (18.2%, 15.9%, and 13.6%, respectively). Thus, for the period from 2011 to 2013, we observed a similar emm type distribution among strains in this extended collection compared to the collection of iGAS organisms, with the exception of type emm4 strains, which were mostly associated with severe soft tissue infections not meeting the definition of iGAS disease (Fig. 2A and 3B).
FIG 3.
(A) emm distribution of strains isolated in Thunder Bay from severe skin and soft tissue infections that did not meet the criteria for iGAS disease during 2011 to 2013. The emm type distribution closely resembles the overall distribution of emm types associated with iGAS disease in Thunder Bay during the period. (B) emm type distribution of an extended GAS collection comprising strains causing invasive disease and severe skin and soft tissue infections that did not meet the criteria for iGAS disease during 2011 to 2013. In 2011, the 4 most frequently identified emm types in Thunder Bay were emm82 (23.1%), emm83 (20.5%), emm87 (10.3%), and emm114 (7.7%). In 2012, the most frequently identified emm types were emm87, emm82, emm101, and emm89 (25%, 22.2%, 16.7%, and 8.3%, respectively). Strains of emm4, emm1, emm101, and emm11 predominated in 2013 (18.2%, 15.9%, 13.6%, and 11.4%, respectively). When the 3 years are taken together, the most frequently isolated emm types were emm82 (16%), emm101 (10.9%), emm87 (9.2%), emm1 (7.6%), emm4 (6.7%), emm114 (5.9%), and emm83 (5%).
DNA sequence analysis of reference genomes.
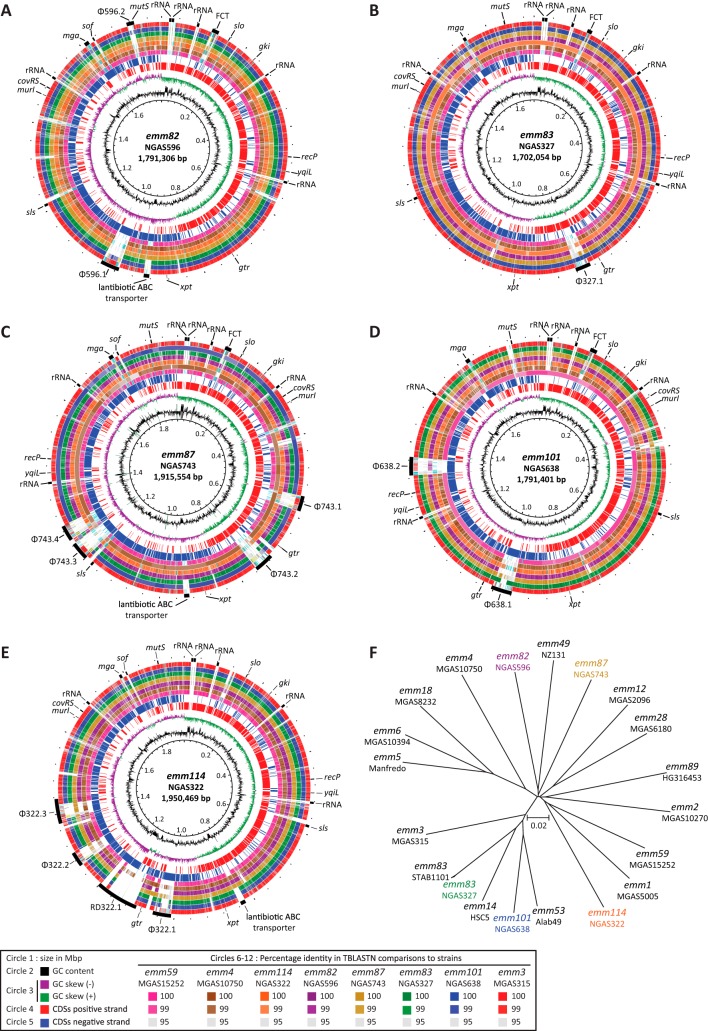
We first sequenced to closure the genomes of one arbitrarily chosen strain each of emm82, emm83, emm87, emm101, and emm114. The genomes were circular chromosomes of 1,791,306 bp (NGAS596, emm82), 1,702,054 bp (NGAS327, emm83), 1,915,554 bp (NGAS743, emm87), 1,791,401 bp (NGAS638, emm101), and 1,950,469 bp (NGAS322, emm114) (Fig. 4A through E, respectively). The differences in genome size were mainly explained by the number of prophages and other mobile genetic elements, such as integrative-conjugative elements present in each genome (Fig. 4A through E and Fig. S1 in the supplemental material). Whole-genome SNP-based phylogenies of newly sequenced genomes and all complete GAS genomes available in GenBank revealed that strains of emm82, emm87, and emm114 types were not closely related to any other sequenced GAS strains (Fig. 4F). However, strains of types emm83 and emm101 were found in a discrete branch of the phylogenetic tree that also contains emm53 strain Alab49 and emm14 strain HSC5 (Fig. 4F) (13, 21, 22). Type emm83 strain NGAS327 clustered tightly with emm83 strain STAB1101, whose genome was published while this article was in preparation (23). Some of the newly sequenced strains had large rearrangements which occurred at repetitive elements, such as prophages and rRNA operons (see Fig. S2 in the supplemental material). Symmetrical inversions in bacterial organisms have been suggested to correlate with enhanced bacterial fitness, as they occur most frequently in clinical isolates (24, 25). Due to the presence of bacteriophages, though, some of the GAS genomes sequenced here have asymmetrical inversions around the origin of replication (Fig. 4A through E and Fig. S2 in the supplemental material). The biological role of these inversions, if any, remains to be investigated.
FIG 4.
Genome atlases and phylogenetic relationships of GAS strains whose genomes were sequenced to closure. Strains NGAS596 (emm82) (A), NGAS327 (emm83) (B), NGAS743 (emm87) (C), NGAS638 (emm101) (D), and NGAS322 (emm114) (E). Data from innermost to outermost circles in the atlases are described in the figure itself, with the exception of the outermost circle, which depicts genome landmarks such as prophages, genes used on the GAS multilocus-sequence typing scheme, and other genes of interest. RD322.1 is an integrative-conjugative element. GC (guanine-cytosine) skew, or (G−C)/(G+C), averaged over a moving window of 10,000 bp. (F) Neighbor-joining tree constructed using 68,511 single-nucleotide polymorphisms identified among all closed GAS genomes available in GenBank against the genome of emm59 reference strain MGAS15252. CDS, coding DNA sequence.
Population structure of the most common GAS emm types causing disease in Thunder Bay, 2011 to 2013.
We next sequenced the genomes of 96 GAS strains isolated in Thunder Bay during 2011 to 2013 representing 22 different emm types. Only one MLST sequence type (ST) was found among strains of the same emm type, with the exception of emm83 strains which belonged to two STs (see Table S1 in the supplemental material). Antibiotic resistance gene profiles were consistent among strains of the same serotype, with the exception of type emm83, for which antibiotic resistance gene patterns differed between the different MLST STs (see Table S2 in the supplemental material). We next performed whole-genome SNP analysis of emm59, emm82, emm83, emm87, emm101, emm114, and emm4 strains, which together represented 62% of the total GAS strains isolated in Thunder Bay during 2011 to 2013. Only 16 core genome SNPs, on average, separated the three emm59 strains (one from an iGAS case, and two from severe soft tissue infections) from the archetypical emm59 Canadian epidemic strain MGAS15252, indicating that these newer emm59 strains are bona fide members of the emm59 epidemic clone that caused numerous cases of iGAS disease in Canada since 2006 (6). Thunder Bay emm59 strains differed between themselves by, on average, 13 SNPs. Limited diversity was also observed among emm82 isolates, which, on average, differed from each other by only 9 core genome SNPs (Fig. 5A). On the other hand, emm83 strains differed from each other, on average, by 123 core genome SNPs. Two clearly differentiated clades matching MLST STs were identified by phylogenetic analysis. However, intraclade variation was minimal (Fig. 5B). Strains of the other emm types also had minimal diversity (Fig. 5C through E): emm87 strains differed from one another by 11 core genome SNPs, on average, while emm101 strains were separated by, on average, 6 core genome SNPs. On average, 3 core genome SNPs separated emm114 strains from one another. Finally, on average, Thunder Bay emm4 strains differed from the emm4 MGAS10750 reference strain (26) by 175 core genome SNPs. However, Thunder Bay emm4 strains were very closely related, differing from each other by an average of only 2 SNPs (Fig. 5F). We did not find evidence of recombination events between strains of the same emm type in this data set. Thus, excepting emm83 strains, for which two clearly distinct clones were identified, we conclude that strains of the most frequently identified skin and generalist emm types causing disease in Thunder Bay have each descended from single progenitor organisms that were introduced into the community.
FIG 5.
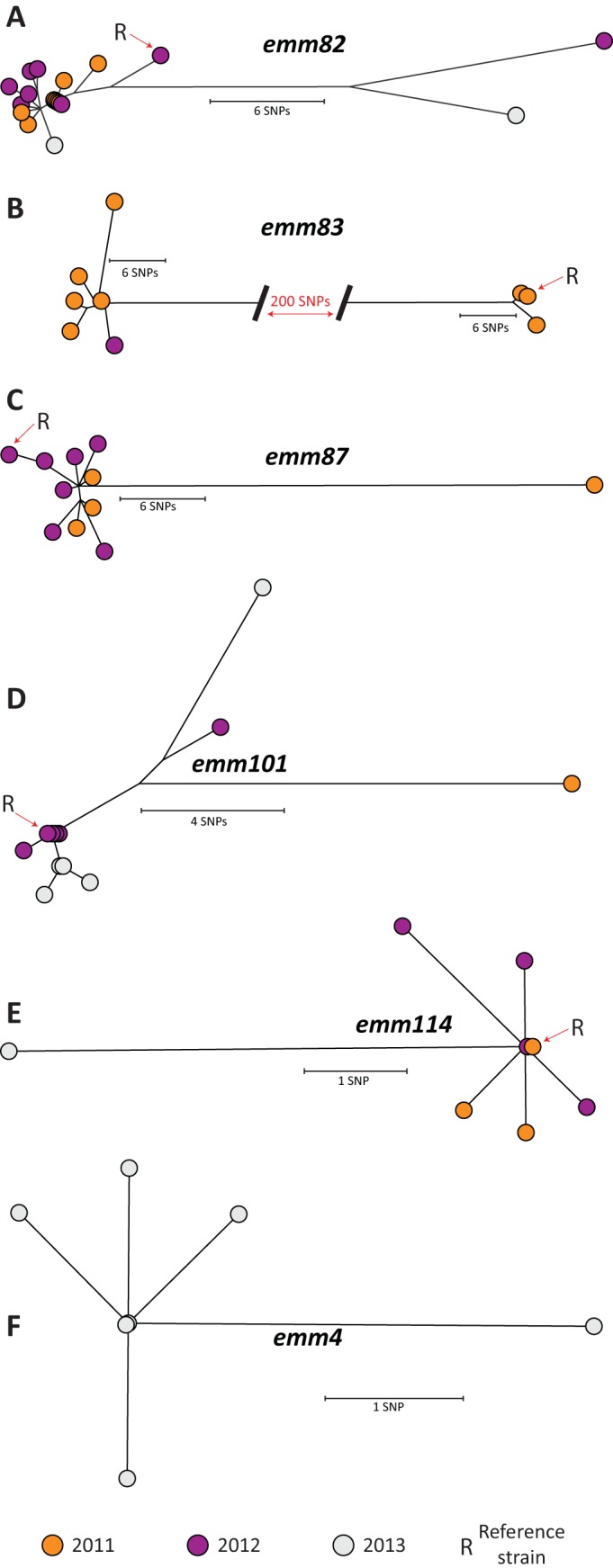
Inferred phylogenetic relationship among GAS strains recovered in Thunder Bay from invasive disease cases and from cases of severe soft tissue infection. Neighbor-joining phylogenetic trees for each of emm82 (A), emm83 (B), emm87 (C), emm101 (D), emm114 (E), and emm4 (F) strains were constructed using nonredundant, whole-genome single-nucleotide polymorphism (SNP) loci (emm82, 56 SNPs; emm83, 259 SNPs; emm87, 59 SNPs; emm101, 29 SNPs; emm114, 12 SNPs; emm4, 7 SNPs) identified against their corresponding reference genomes. Strains from 2011 are depicted in orange, those from 2012, in purple, and those from 2013, in gray. See the supplemental material for details about methods.
DISCUSSION
In 2008, Thunder Bay experienced a marked increase in iGAS notifications, followed by the declaration of a local outbreak of emm59 iGAS that was inscribed in the context of a large Canadian emm59 epidemic characterized by a high rate of skin and soft tissue infections (5, 6, 8). Retrospective analysis found that the incidence of iGAS disease in Thunder Bay had been greater than the provincial average since 2007, while in previous years it had been comparable to that observed in the metropolitan region of Toronto/Peel and in line with rates for the province of Ontario. High rates of skin and soft tissue infections were also observed in 2008 to 2009 among local emm59 cases. Alcohol abuse, homelessness, HCV infection, and illicit drug use were found to be underlying diseases or conditions that might have predisposed patients to invasive disease, suggesting that the increased incidence of iGAS was and continues to be focused on a very specific population.
GAS skin and soft tissue infections can be consequential and even disabling in adults who live under poor environmental and hygienic conditions or who share certain lifestyles, such as those observed for the population at risk in Thunder Bay (27). For example, pioneering studies in Australia's Northern Territory identified higher incidences of invasive, skin and soft tissue infections among Aboriginal compared to non-Aboriginal patients (28). However, non-Aboriginal people with GAS bacteremia were as likely as Aboriginal people to have risk factors for infection (28). Similarly, as reported in Arizona, Native Americans were at increased risk for iGAS infections, although risks factors were, overall, similar among the different populations studied (29). Prospective surveillance in Thunder Bay demonstrated that the incidence of iGAS disease remained high in the area after the emm59 local outbreak subsided. In addition to iGAS disease, we continued to observe a striking overabundance of skin and soft tissue infections that did not meet the definition of iGAS disease but which were severe and, for the most part, required hospitalization. Interestingly, most of these soft tissue-related GAS infection cases in Thunder Bay were detected through enhanced chart reviews. We believe it is likely that hospitals in Ontario might be failing to recognize a considerable proportion of these severe GAS cases, thus underreporting the true burden of GAS illness provincewide. In addition to soft tissue infections, we also observed (within the TBDHU) several cases of extensive peritonsillar deep abscesses and deep pharyngeal abscesses. Since, historically, these types of infections have not merited documentation in reportable disease registries, we have excluded them from our analysis. Thus, the incidence of GAS disease in Thunder Bay is likely higher than reported here.
The association of certain GAS emm types with particular tissue tropisms has long been described (13, 22). Here, we show that the majority of skin and soft tissue infections in TBD since 2011 were caused by GAS strains of a few skin and generalist emm types (Fig. 3B). Similar results were observed among Australian remote Aboriginal communities (30) and in Ethiopia (31). Thorough comparisons (underway in our laboratories) of the newly generated genomes for 3 generalist and 2 skin emm types to genomes available in public databases hold the promise of enhancing our understanding of GAS tissue tropism and its role in the different diseases caused by this organism. In Australia, it was suggested that a major risk factor for GAS bacteremia in Aboriginal people in the Northern Territory was high levels of exposure to a wide array of GAS (28). Our data for 2011 to 2013 do not show such a broad diversity of emm types but suggest that clonal replacement is a key factor associated with continuing high GAS incidence in Thunder Bay. Although waxing and waning of GAS clones causing disease are well-recognized events (32), the driving mechanisms are poorly understood. Some of the possible reasons for a decrease in the reported incidence of a particular clone include a decrease in virulence, an increase in host defense (herd immunity), and/or serotype replacement by a more fit clone (33, 34). It has been suggested that at least one of the at-risk groups of patients identified here (IVDU patients) may benefit from a greater natural immunity due to repeated infection with GAS over time, rendering the individuals more immunologically primed to respond to such infections (35). It may be possible that, as host immunity against a particular offending emm type increases among the population at risk, introduction of strains of potentially more fit emm types by colonized individual(s) leads to their clonal expansion in the community.
Seminal studies of Army recruits have found that nasal/throat carriage is critical to the ability of an individual to spread GAS (36, 37). A model in which iGAS strains originate from the local nasal/throat carriage strains has been proposed, in which cyclical outbreaks of invasive infection coincide or follow recent outbreaks of pharyngitis (38–40). Because nasal/throat cultures were not obtained in Thunder Bay, we cannot relate our results to the extent of nasal/throat carriage. However, one of the frequently identified emm types identified here, emm87, commonly causes pharyngitis in market economy countries (http://www.cdc.gov/streplab/emmtype-proportions.html41). Type emm82 and type emm114 strains have also been identified among pharyngitis cases (42). However, it is unlikely that throat carriage is the only reservoir. For example, characterization of GAS strains associated with mixed skin and soft tissue infections in Northern Saskatchewan showed that invasive cases of emm82 and emm101 greatly exceeded the number of pharyngeal isolates in the area (http://www.narp.ca/pdf/publications/MCDONALD.pdf43). Moreover, during the Canadian emm59 epidemic, pharyngeal emm59 strains were identified (in very low numbers) only in 2009, 3 years after the sudden increase in invasive emm59 cases (6, 40). Similar results of low incidence of pharyngitis have been observed in Australia among Aboriginal communities with skin and soft tissue infections (28, 44). GAS carriage in throat and skin in Thunder Bay needs to be further studied in order to elucidate infection sources and local transmission patterns.
The diversity of emm types present in Thunder Bay suggests that an effective emm-based component vaccine against GAS infections would need to be modifiable and allow emm substitutions to be implemented as required. In this regard, a new 30-valent M-protein-based vaccine has been shown in preliminary trials to offer cross-protection against most of the emm types reported here in Thunder Bay (45). However, the rapid fluctuations in the prevalence of the different emm types (for example, our unpublished observations show emergence of invasive emm53, an emm type for which cross-protection offered by the new vaccine was minimal [45], in 2015 in Thunder Bay) suggests that vaccine substitutions may not be achievable in relevant time frames in all cases. Given the severity and rapid progression of iGAS disease, prompt detection and rapid medical intervention remain the most effective control measures available to reduce morbidity and mortality. Continued monitoring of iGAS in Thunder Bay to identify further resurgences of infection in this vulnerable population is warranted.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff at PHO Genome Core, and Dax Torti (Donelly Center, University of Toronto) for Illumina sequencing of our samples. We thank Leah Vanderploeg and other public health nurses at TBDHU for conducting chart reviews and follow-up interviews. We thank Michael Whelan (PHO) for sharing epidemiological iGAS data for Ontario.
This study was funded by Public Health Ontario.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02201-15.
REFERENCES
- 1.Olsen RJ, Musser JM. 2010. Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol 5:1–31. doi: 10.1146/annurev-pathol-121808-102135. [DOI] [PubMed] [Google Scholar]
- 2.Lancefield RC. 1928. The antigenic complex of Streptococcus haemolyticus: I. demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. J Exp Med 47:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott JR, Pulliam WM, Hollingshead SK, Fischetti VA. 1985. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A 82:1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manjula BN, Acharya AS, Fairwell T, Fischetti VA. 1986. Antigenic domains of the streptococcal Pep M5 protein: localization of epitopes crossreactive with type 6 M protein and identification of a hypervariable region of the M molecule. J Exp Med 163:129–138. doi: 10.1084/jem.163.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell GJ, Lovgren M, St Jean T, Hoang L, Patrick DM, Horsman G, Van Caeseele P, Sieswerda LE, McGeer A, Laurence RA, Bourgault AM, Low DE. 2010. Epidemic of group A Streptococcus M/emm59 causing invasive disease in Canada. Clin Infect Dis 51:1290–1297. doi: 10.1086/657068. [DOI] [PubMed] [Google Scholar]
- 6.Fittipaldi N, Beres SB, Olsen RJ, Kapur V, Shea PR, Watkins ME, Cantu CC, Laucirica DR, Jenkins L, Flores AR, Lovgren M, Ardanuy C, Linares J, Low DE, Tyrrell GJ, Musser JM. 2012. Full-genome dissection of an epidemic of severe invasive disease caused by a hypervirulent, recently emerged clone of group A Streptococcus. Am J Pathol 180:1522–1534. doi: 10.1016/j.ajpath.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Fittipaldi N, Olsen RJ, Beres SB, Van Beneden C, Musser JM. 2012. Genomic analysis of emm59 group A Streptococcus invasive strains, United States. Emerg Infect Dis 18:650–652. doi: 10.3201/eid1804.111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fittipaldi N, Tyrrell GJ, Low DE, Martin I, Lin D, Hari KL, Musser JM. 2013. Integrated whole-genome sequencing and temporospatial analysis of a continuing group A Streptococcus epidemic. Emerg Microbes Infect 2:e13. doi: 10.1038/emi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CC, Olsen RJ, Fittipaldi N, Morman ML, Fort PL, Neuwirth R, Majeed M, Woodward WB, Musser JM. 2014. Spread of virulent group A Streptococcus type emm59 from Montana to Wyoming, USA. Emerg Infect Dis 20:679–681. doi: 10.3201/eid2004.130564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen RJ, Fittipaldi N, Kachroo P, Sanson MA, Long SW, Como-Sabetti KJ, Valson C, Cantu C, Lynfield R, Van Beneden C, Beres SB, Musser JM. 2014. Clinical laboratory response to a mock outbreak of invasive bacterial infections: a preparedness study. J Clin Microbiol 52:4210–4216. doi: 10.1128/JCM.02164-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wannamaker LW. 1970. Differences between streptococcal infections of the throat and of the skin (second of two parts). N Engl J Med 282:78–85. doi: 10.1056/NEJM197001082820206. [DOI] [PubMed] [Google Scholar]
- 12.Wannamaker LW. 1970. Differences between streptococcal infections of the throat and of the skin. I N Engl J Med 282:23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- 13.Bessen DE, Lizano S. 2010. Tissue tropisms in group A streptococcal infections. Future Microbiol 5:623–638. doi: 10.2217/fmb.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies HD, McGeer A, Schwartz B, Green K, Cann D, Simor AE, Low DE. 1996. Invasive group A streptococcal infections in Ontario, Canada: Ontario group A streptococcal study group. N Engl J Med 335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 15.Facklam R, Washington J. 1991. Streptococcus and related catalase-negative gram-positive cocci, p 238–257. In Manual of clinical microbiology. ASM Press, Washington, DC. [Google Scholar]
- 16.Beall B, Gherardi G, Lovgren M, Facklam RR, Forwick BA, Tyrrell GJ. 2000. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 146(Part 5):1195–1209. doi: 10.1099/00221287-146-5-1195. [DOI] [PubMed] [Google Scholar]
- 17.Inouye M, Dashnow H, Raven L, Schultz MB, Pope BJ, Tomita T, Zobel J, Holt KE. 2014. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nusbaum C, Ohsumi TK, Gomez J, Aquadro J, Victor TC, Warren RM, Hung DT, Birren BW, Lander ES, Jaffe DB. 2009. Sensitive, specific polymorphism discovery in bacteria using massively parallel sequencing. Nat Methods 6:67–69. doi: 10.1038/nmeth.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 20.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Port GC, Paluscio E, Caparon MG. 2013. Complete Genome sequence of emm type 14 Streptococcus pyogenes strain HSC5. Genome Announc 1:e00612-13. doi: 10.1128/genomeA.00612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bessen DE, Kumar N, Hall GS, Riley DR, Luo F, Lizano S, Ford CN, McShan WM, Nguyen SV, Dunning Hotopp JC, Tettelin H. 2011. Whole-genome association study on tissue tropism phenotypes in group A Streptococcus. J Bacteriol 193:6651–6663. doi: 10.1128/JB.05263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano N, Vincent P, Auger G, Cariou ME, Moullec S, Lagente V, Ygout JF, Kayal S, Faili A. 2015. Full-length genome sequence of type M/emm83 group A Streptococcus pyogenes strain STAB1101, isolated from clustered cases in Brittany. Genome Announc 3:e01459-14. doi: 10.1128/genomeA.01459-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen JA, Heidelberg JF, White O, Salzberg SL. 2000. Evidence for symmetric chromosomal inversions around the replication origin in bacteria. Genome Biol 1:RESEARCH0011. doi: 10.1186/gb-2000-1-6-research0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes D. 2000. Evaluating genome dynamics: the constraints on rearrangements within bacterial genomes. Genome Biol 1:REVIEWS0006. doi: 10.1186/gb-2000-1-6-reviews0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM. 2006. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci U S A 103:7059–7064. doi: 10.1073/pnas.0510279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glezen WP, Lindsay RL, DeWalt JL, Dillon HC Jr. 1972. Epidemic pyoderma caused by nephritogenic streptococci in college athletes. Lancet 1:301–303. [DOI] [PubMed] [Google Scholar]
- 28.Carapetis JR, Walker AM, Hibble M, Sriprakash KS, Currie BJ. 1999. Clinical and epidemiological features of group A streptococcal bacteremia in a region with hyperendemic superficial streptococcal infection. Epidemiol Infect 122:59–65. doi: 10.1017/S0950268898001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoge CW, Schwartz B, Talkington DF, Breiman RF, MacNeill EM, Englender SJ. 1993. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome: a retrospective population-based study. JAMA 269:384–389. doi: 10.1001/jama.1993.03500030082037. [DOI] [PubMed] [Google Scholar]
- 30.McDonald MI, Towers RJ, Fagan P, Carapetis JR, Currie BJ. 2007. Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiol infect 135:1398–1405. doi: 10.1017/S0950268807008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tewodros W, Kronvall G. 2005. M protein gene (emm type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol 43:4369–4376. doi: 10.1128/JCM.43.9.4369-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, Sturdevant DE, Graham MR, Vuopio-Varkila J, Hoe NP, Musser JM. 2005. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis 192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 33.Beres SB, Carroll RK, Shea PR, Sitkiewicz I, Martinez-Gutierrez JC, Low DE, McGeer A, Willey BM, Green K, Tyrrell GJ, Goldman TD, Feldgarden M, Birren BW, Fofanov Y, Boos J, Wheaton WD, Honisch C, Musser JM. 2010. Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc Natl Acad Sci U S A 107:4371–4376. doi: 10.1073/pnas.0911295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, Kristinsson KG, Gottfredsson M, Vuopio J, Raisanen K, Caugant DA, Steinbakk M, Low DE, McGeer A, Darenberg J, Henriques-Normark B, Van Beneden CA, Hoffmann S, Musser JM. 2014. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci U S A 111:E1768-1776. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamagni TL, Neal S, Keshishian C, Hope V, George R, Duckworth G, Vuopio-Varkila J, Efstratiou A. 2008. Epidemic of severe Streptococcus pyogenes infections in injecting drug users in the United Kingdom, 2003 to 2004. Clin Microbiol Infect 14:1002–1009. doi: 10.1111/j.1469-0691.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- 36.Hamburger M Jr, Green MJ. 1946. The problem of the dangerous carrier of hemolytic streptococci; observations upon the role of the hands, of blowing the nose, of sneezing, and of coughing in the dispersal of these microorganisms. J Infect Dis 79:33–44. doi: 10.1093/infdis/79.1.33. [DOI] [PubMed] [Google Scholar]
- 37.Hamburger M Jr, Green MJ, Hamburger VG. 1945. The problem of the dangerous carrier of hemolytic streptococci; spread of infection by individuals with strongly positive nose cultures who expelled large numbers of hemolytic streptococci. J Infect Dis 77:96–108. doi: 10.1093/infdis/77.2.96. [DOI] [PubMed] [Google Scholar]
- 38.Hoe NP, Vuopio-Varkila J, Vaara M, Grigsby D, De Lorenzo D, Fu YX, Dou SJ, Pan X, Nakashima K, Musser JM. 2001. Distribution of streptococcal inhibitor of complement variants in pharyngitis and invasive isolates in an epidemic of serotype M1 group A Streptococcus infection. J Infect Dis 183:633–639. doi: 10.1086/318543. [DOI] [PubMed] [Google Scholar]
- 39.Shea PR, Beres SB, Flores AR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, Serrano-Gonzalez M, Fittipaldi N, Ayers SD, Webb P, Willey BM, Low DE, Musser JM. 2011. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc Natl Acad Sci U S A 108:5039–5044. doi: 10.1073/pnas.1016282108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, Serrano-Gonzalez M, Fittipaldi N, Beres SB, Flores AR, Low DE, Willey BM, Musser JM. 2011. Group A Streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002 to 2010. Emerg Infect Dis 17:2010–2017. doi: 10.3201/eid1711.110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. 2009. StrepLab: emm types as proportions of total disease isolates in six global regions. http://www.cdc.gov/streplab/emmtype-proportions.html.
- 42.Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, Cederlund E, Patel D, Rippe J, Li Z, Sakota V. 2009. Seven-year surveillance of North American pediatric group A streptococcal pharyngitis isolates. Clin Infect Dis 49:78–84. doi: 10.1086/599344. [DOI] [PubMed] [Google Scholar]
- 43.Northern Antibiotic Resistance Partnership. 2008. Characterization of Streptococcus pyogenes and Staphylococcus aureus isolates associated with mixed skin and soft tissue infections in northern Saskatchewan. http://www.narp.ca/pdf/publications/MCDONALD.pdf.
- 44.McDonald MI, Towers RJ, Andrews RM, Benger N, Currie BJ, Carapetis JR. 2006. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 43:683–689. doi: 10.1086/506938. [DOI] [PubMed] [Google Scholar]
- 45.Dale JB, Penfound TA, Chiang EY, Walton WJ. 2011. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against nonvaccine serotypes of group A streptococci. Vaccine 29:8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.