Abstract
BACKGROUND
Although materials for secondary cranial reconstruction have evolved with time, the overall approach in terms of bone flap/implant reconstruction after necessary delay has remained constant.
OBJECTIVE
To present our cases series of 50 consecutive secondary cranial reconstruction patients and to describe a multidisciplinary cranioplasty approach developed to reduce morbidity, to minimize infection, and to improve aesthetic appearance.
METHODS
Standard technique teaches us to place the bone flap and/or alloplastic implant directly over the dura or dural protectant after scalp flap re-elevation. However, this procedure is fraught with high complication rates, including infection. While raising the previously incised scalp flap overlying the full-thickness calvarial defect, the dissection is performed within the loose areolar tissue plane beneath the galea aponeurosis, thus leaving vascularized pericranium intact over the dura.
RESULTS
A total of 50 consecutive patients were treated by the senior author encompassing 46 cranioplasties using the pericranial-onlay approach, along with 4 isolated temporal soft tissue reconstructions with liquid poly-methyl-methacrylate. Of the 46 cranioplasties (> 5 cm2), only 1 autologous bone flap developed deep infection necessitating bone flap removal (1 of 46, 2.17%; 95% confidence interval, 0.003–11.3). None of the alloplastic custom implants placed have developed any infection requiring removal.
CONCLUSION
This multidisciplinary approach illustrated in our case series, including our “pericranial-onlay” technique described here for the first time, has the potential to improve patient outcomes, to decrease perioperative morbidity, and to minimize costs associated with postoperative infections after secondary cranial reconstruction.
Keywords: Cranial reconstruction, Craniofacial surgery, Cranioplasty, Pericranial onlay, Pterional custom cranial implant, Skull defect, Temporal soft tissue reconstruction
Secondary cranial reconstruction, often referred to as a cranioplasty, is indicated for cerebral protection, cosmetic appearance restoration, and treatment of the syndrome of the trephined.1–4 A secondary (or delayed) cranioplasty is required for those patients requiring staged reconstruction. This includes patients undergoing decompressive hemicraniectomy for life-threatening conditions such as traumatic brain injury, acute subdural hematoma, intracerebral hemorrhage, and severe ischemic stroke. Several options currently exist for reconstructing a large (> 5 cm2) calvarial defect.5 Historically, bone flaps harvested from dogs was first described in 1670,6 and autologous bone flaps, as opposed to xenogeneic, have since remained the gold standard.7–11 However, because of the morbidity associated with large bone donor sites and occasional bone flap resorption/warping, numerous materials have been developed for secondary cranial reconstruction. The outbreak of World War II resulted in a large number of cranial defects, and the evolution of acrylic resins led to the first use of poly-methyl-methacrylate (PMMA) in humans in 1940.11–18 Since then, PMMA has become the most frequently used alloplastic material for cranial reconstruction.7,8,19–22 It is also associated with decreased rates of exposure and infection compared with bone flaps.23,24
These defects pose a special reconstructive challenge, primarily because of both the skeletal deficit and the corresponding soft tissue deficiency after interval scalp contraction, temporalis malposition/foreshortening, and encephalomalacia with overlying dead space. This is especially deforming in areas with complex convexities such as the pterional region. This area has been shown to have a higher incidence of complications.23 In situations when the bone flap requires removal, there are absent fixation points for the temporalis muscle, leading to muscle foreshortening with caudal displacement and temporal deformity. In these cases, freehand molding of acrylic implants is possible,25 but it is time consuming and technically demanding and may require exposure of the lateral orbit. With the advent of computer-aided design and manufacturing, both metal and acrylic cranial implants can now be designed and manufactured to precisely fit the patient’s defect.26,27
Although the materials and manufacturing have steadily evolved, common techniques for cranial bone flap and implant placement have remained constant. In fact, Booth and Curtis11 described in 1893 secondary cranial reconstruction as simply “elevating the pericranium and scalp flap up with an elevator” and then 19 days later “slipping an aluminum plate under the scalp flap above the dura.” Now, nearly 120 years later, standard techniques still include elevating a full-thickness scalp flap and placing the bone flap or implant directly onto the previously exposed dura or previously placed dural substitute. This is associated with dural inflammation28,29 and exposes the meninges/implant materials to bacterial contamination, especially in cases after bone flap osteomyelitis. At the same time, bone flap fixation has also evolved.30,31 This traditional teaching is to reincise and reelevate the full-thickness scalp flap, similar to the initial neurological procedure, which mandates a dissection along the epidural space. Drawbacks include significant blood loss, potential for dural injury, and unnecessarily exposing the field and materials to bacterial contamination.32 Therefore, we present here a novel approach that preserves the vascularized pericranium as a protective layer against infection. It uses the same horizontal boundaries of the previous scalp incision as a “delayed scalp flap” but divides the full-thickness scalp flap into 2 segments: a lower segment containing a vascularized “pericranial-onlay” and an upper segment consisting of a new galea fasciocutaneous (partial-thickness) scalp flap based on the superficial temporal vessels. This in turn provides a “vascularized sandwich” encasing and protecting the replaced bone flap or implant. This is similar to the mind-set of a plastic surgeon faced with an exposed, infected implant in the setting of alloplastic breast reconstruction, which uses the creation of a “neo-subpectoral pocket.” With the osetomyelitic bone flap acting like an infected implant within the epidural space, one places the bone flap or cranial implant at a second stage using a separate pocket from the previous one and surrounds the area with vascularized tissue as similar to the pericranial onlay being introduced here.33
In this scenario, scalp closure over the bone flap or implant is also critical and can easily be complicated by excessive tension, especially in cases when large scalp concavities exist and when tissue mobility is limited from scalp contraction, scar, and/or irradiation. Therefore, some surgeons use staged tissue expansion, which requires additional time, expense, and potential morbidity.32 Skin dehiscence, whether small or large, is paramount. It allows inoculation of bacteria to the underlying bone flap and/or implant, which may lead to irreversible infection and eventual removal. Therefore, we outline our multidisciplinary approach and case series to suggest decreased morbidity, improved final outcomes, and the ability to avoid unnecessary tissue expansion.
METHODS
The “SCALP” is a complex, reliable, highly vascularized, 5-layered structure extending from skin level down to pericranium. The skin itself has a rich network of dermal-subdermal plexus vessels providing perfusion and therefore does not depend on the deep layers, especially in instances when a previous skin incision has been made. We dissect cautiously under loupe magnification and with fine-needlepoint electrocautery along the loose areolar plane, experiencing minimal blood loss. We then elevate a brand new fasciocutaneous scalp flap consisting of only galeal fascia and skin, leaving behind an intact “pericranial onlay” covering the epidural space. This not only creates a new dissection plane far away from the dura (which in most instances has had infectious contamination) but also alleviates the dreaded and difficult epidural dissection.
All craniums are shaved completely at surgery to ensure proper symmetry. A solid line is drawn over the previous incision, and a dotted line outlines the calvarial defect. This is important for teaching residents and fellows because the calvarial defect varies and does not match up to the scalp incision. Furthermore, all efforts should be made to use the previous incision because this serves as a surgical delay for the flap and contributes to enhanced temporal artery perfusion. When possible, incisions should not be made or used in areas directly over the bone flap and/or implant material.
Local anesthetic diluted 1:1 with injected saline (0.5% lidocaine with epinephrine 1:200 000) is then injected as tumescence along the incisions and areas of planned dissection (Figure 1). Starting at the anterior midline portion of the marked incision, the cutaneous layer of the scalp is divided with a scalpel down to the deep dermis. Fine-tip electrocautery on a low setting is then used to continue the incision through the scalp, extending full thickness through the galea and pericranium. The surrounding calvarium needs to be exposed to allow rigid fixation and to help minimize tension at the time of closing. Once the limits of the cranial defect are identified posteriorly, medially, and anteriorly, a new flap dissection plane is started beginning at the distal segment. The needlepoint electrocautery is directed superficially, creating a new plane within the loose areolar tissue, below the galeal fascia and above the pericranium (see Video 1, Supplemental Digital Content 1, http://youtu.be/PXY7uRYyTp8, which demonstrates the technique). As the dissection proceeds past the boundaries of the cranial defect, the dissection then changes back to a subpericranial plane in the anteromedial area near the frontal bandeau. Thus, the cranial defect remains covered by a vascularized segment of pericranium (ie, onlay), which remains perfused inferolaterally from its temporal attachments and the superficial temporal system. In addition, any areas of scarred, devitalized pericranium along the calvarial margins are removed to enhance plating exposure. The exposed calvarium allows placement of rigid fixation with titanium plates and 4-mm screws (Figure 2). Attention is given to the forehead region, and all plates are placed in hair-bearing regions if possible. Of note, our operative times have also been reduced by 1 team preplating the bone flap or implant on the back table while the other team completes the soft tissue dissection.
FIGURE 1.
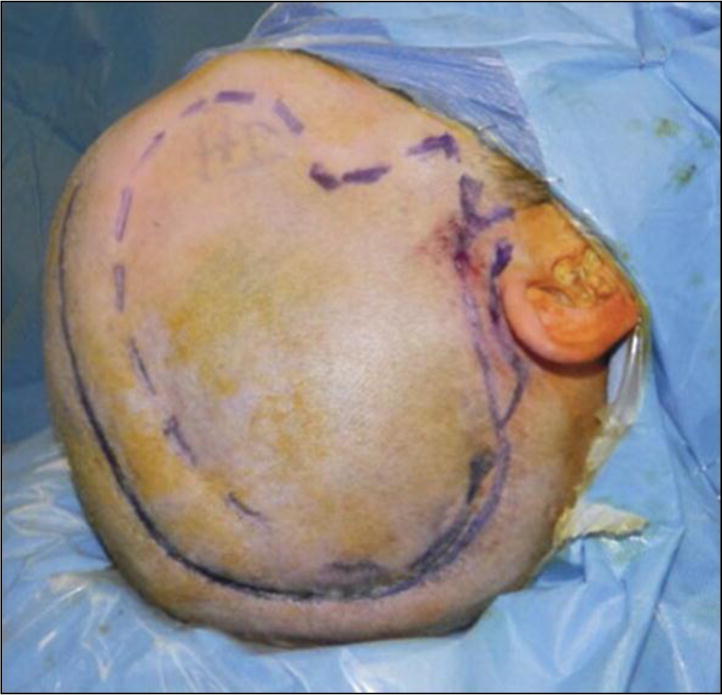
Skin markings used to denote the previous neurosurgical incision (solid line) and the large calvarial defect (dotted line) after infected bone flap removal.
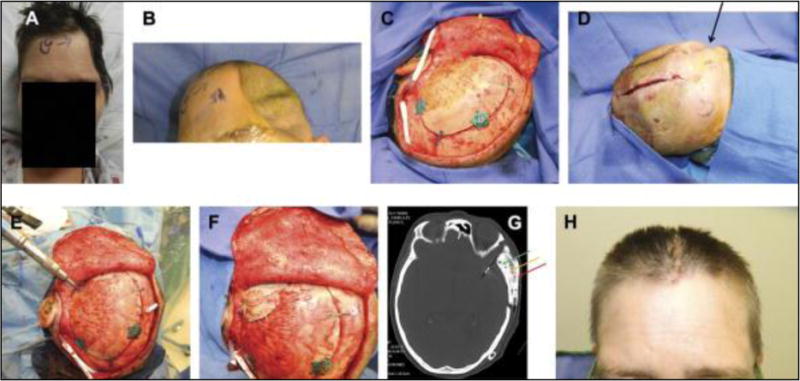
FIGURE 2.
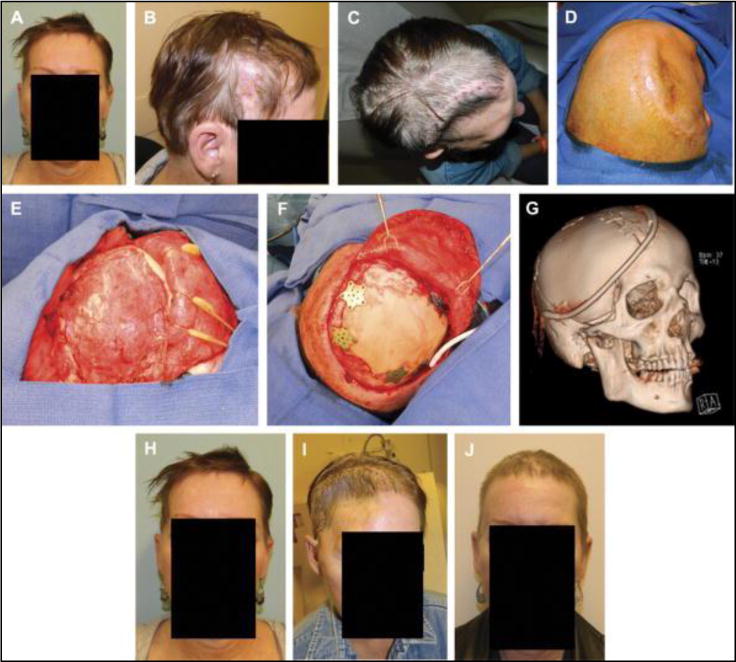
Frontal view (A) and right profile view (B) of the original presentation with bone flap osteomyelitis. Aerial view (C) of the patient two weeks after removal of infected bone flap. Cephalad (D) on-table view before secondary cranial reconstruction 4 months after the bone flap removal. Photograph of vascularized pericranial-onlay dissection covering the dura (E) before insetting of the custom cranial implant with plates and screws (F). Postoperative 3-dimensional computed tomographic scan demonstrating the relative size of the implant, drain placement, and large amount of accompanying soft tissue reconstruction required with temporalis reinsertion (G). Frontal view photographs at the time of bone flap infection (H), at 3 months after bone flap removal (I), and at 6 months after reconstruction demonstrating acceptable contour and temporal symmetry (J).
At this point, the cranial defect is now ready for repair with any of the common elements, including autologous bone flaps, titanium mesh, split calvarial bone grafts, and/or alloplastic implant. However, unlike autologous bone, alloplastic custom cranial implants (CCIs) do not undergo resorption and/or warping and therefore are our preferred medium. CCIs also provide a full-thickness calvarial reconstruction, unlike titanium mesh, and may avoid unnecessary dead space underneath. In addition, in some instances, certain CCIs need to be shaped intraoperatively to obtain a precise fit. Because of this latter property, we prefer PMMA over polyetheretherketone. We have found that solid PMMA implants are more easily contoured intraoperatively with a cutting burr set at 20 000 RPM (standard, 40 000 RPM), which prevents unwanted melting of the material (Figure 3).
FIGURE 3.
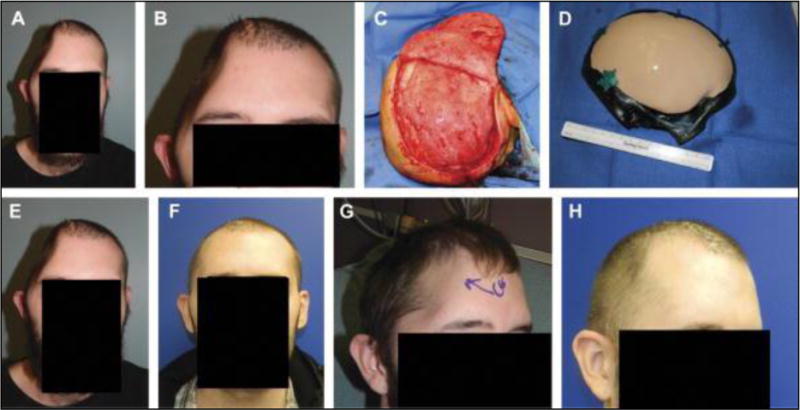
Frontal view (A) and magnified view (B) of a hemicraniectomy patient at 1 month after infected bone flap removal. Right-sided bird’s eye view of pericranial onlay after careful dissection under loupe magnification and needlepoint electrocautery (C). Intraoperative preplating of a custom cranial implant by way of a sterile host bone model (D). Frontal view after bone flap removal (E) and the appearance at 2 months after reconstruction (F). Comparative right oblique views before and after reconstruction (G and H). Submental view demonstrates no signs of temporal hollowing (I).
If soft tissue deformities coexist at the time of secondary cranioplasty, additional materials are used for simultaneous correction. We prefer liquid PMMA for soft tissue reconstruction and/or for filling gaps between bone flap/implant and surrounding calvarium. Consideration is required, however, of the additional expense, skill, and time necessary for fabrication. For pterional reconstruction in particular, it is often necessary to separate the adhered temporalis muscle from the dura before plating the bone flap or implant. This is done at the caudal extent of the defect and is required to allow proper bone flap/implant positioning. Transposing the temporalis is important because it prevents trapping the muscle under the cranioplasty and consequent pain during mastication. In complex cases with simultaneous temporal hollowing, soft tissue reconstruction with liquid PMMA is fixated within the fossa with screws used for stabilization.25 After the implant is secure, the temporal muscle should be draped and attached to the temporal implant back into near-anatomic position. Tacking drill holes, fixation plates, and/or titanium mesh can be used strategically with permanent sutures to resuspend the temporalis muscle (Figure 4). This maneuver helps to improve aesthetic outcomes and decreases one’s risk for temporal muscle wasting or hollowing.
FIGURE 4.
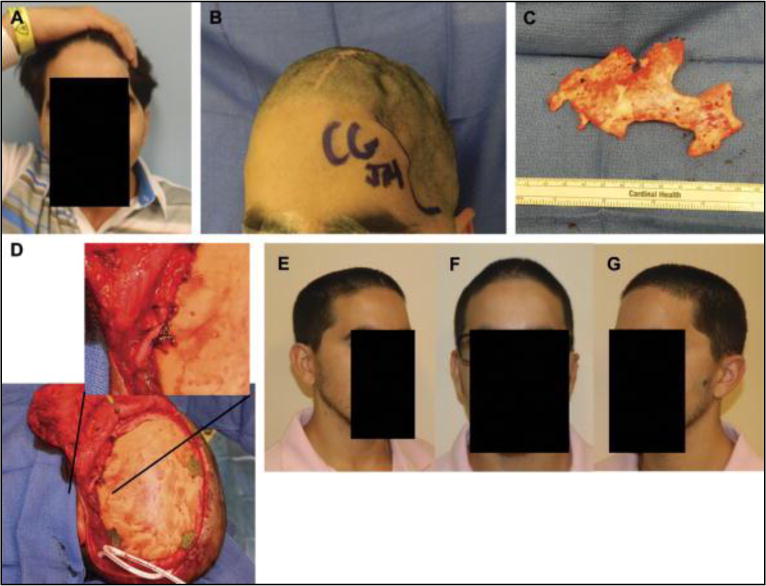
Preoperative photographs at 1 year after autologous bone flap cranioplasty with severe resorption (A and B). Intraoperative photograph of thin, friable bone flap after removal with near-complete resorption (C). Secondary alloplastic cranial reconstruction required temporalis mobilization and fixation to the implant using a titanium plate and permanent suture fixation, allowing near-anatomical reconstruction (D). Right oblique, frontal, and left oblique views at 2 months showing acceptable contour and temporal symmetry (E–G).
To prevent unwanted fluid collections and dead space from accumulating around the bone flap or implant material, we place 2 postauricular 15F round, fluted drains. The first drain is placed in the infratemporal area above the temporalis, which happens to be a gravity-dependent location. The second drain is placed at the farthest extent of the contralateral and posterior undermining.
In preparation for scalp closure, steps should be taken to ensure tension-free approximation and to minimize all risk of wound dehiscence. The outer edge of the native scalp is everted onstretch with a double skin hook retractor. Needle-tip electrocautery, set at a low setting of 10 to 15, is used to score the galeal fascia in repeated lines parallel to the incision, on the stable scalp side only, to improve closure without tension. These scoring lines are placed 1 to 2 cm a part, and sufficiency energy isused to visualize and obtain subcutaneous fat herniation through the fascia. Again, one should note that on the scalp flap itself, the interscalp dissection (between the pericranium and the galeal aponeurosis) acts in theory as a “component release,” further decreasing tension at the time of closure. This is represented by the fact that none of the patients presented here required any type of staged tissue expansion.
The scalp must be closed meticulously in 3 layers. The galea is approximated with 3-0 delayed, absorbable monofilament suture in an interrupted buried fashion. Monofilament sutures have a delayed rate of absorption as opposed to the braided type, which is critical in areas of delayed wound healing such as irradiation and malnutrition. Next, a deep dermal running subcuticular suture is placed using 3-0 dissolvable braided suture. The skin edges are then aligned tension free with 3-0 nylon in an interrupted fashion with precise dermal ridge alignment and wound eversion. Of note, we do not offer this pericranial-onlay technique to active smokers because of concerns about small vessel perfusion via the dermal-subdermal plexus, those with previous irradiation, and patients with genetic disorders affecting wound healing such Ehlers-Danlos syndrome.
RESULTS
A 2-year, retrospective chart review for all consecutive outcomes related to secondary cranial reconstructions completed between July 2011 and June 2013 was performed following institutional review board approval from the Johns Hopkins University School of Medicine. Forty-six consecutive cranioplasties (> 5 cm2) were identified, along with 4 simultaneous temporal soft tissue reconstructions for temporal hollowing deformities (n = 50 total patients). Patients who reported being active smokers, had undergone previous free tissue transfer for concomitant scalp reconstruction, were diagnosed with genetic wound healing disorders, or presented with an open, complicated scalp wound at time of surgery were excluded. To date, only 1 autologous bone flap has developed significant deep infection necessitating bone flap removal, in a patient who had presented with a cerebellar abscess (1 of 46, 2.17%). None of the alloplastic implants since placement have developed any infection requiring removal. Most notably, none of the galea-fasciocutaneous scalp flaps have demonstrated any signs of flap necrosis, skin ischemia, and/or wound dehiscence with at least 6 months of follow-up on the majority of patients (25 of 46, 54%).
In this case series, the corresponding adverse event rate was 2.17% (95% confidence interval, 0.003–11.3). With the use of an α of 0.05, the rate is statistically significantly lower than 7. The adverse event rate with this technique is potentially as low as 0% and unlikely to be higher than 11.3%. Tables 1 and 2 show a detailed description of our complex patient cohort and demonstrate the wide array of materials used for secondary cranial reconstruction.
TABLE 1.
Study Cohort of 50 Consecutive Craniofacial Reconstruction Patientsa
| Case | Patient Age, y | Sex | Diagnosis | Date of Operation | Implant Material |
|---|---|---|---|---|---|
| 1 | 76 | F | Intracranial meningioma | July 2011 | Titanium mesh |
| 2 | 44 | M | Acquired skull defect in the left temporoparietal region | August 2011 | CCI-PEEK |
| 3 | 37 | F | Calvarial defect after craniotomy for middle cerebral artery stroke | September 2011 | ABF |
| 4 | 33 | M | Infection after benign brain tumor resection | January 2012 | CCI-PEEK |
| 5 | 42 | M | Left frontotemporal bone deformity after trauma | March 2012 | L-PMMA with screws |
| 6 | 41 | M | Infection after subarachnoid hemorrhage and middle cerebral artery aneurysm clipping | March 2012 | ABF |
| 7 | 37 | F | Infection after superficial temporal artery to middle cerebral artery bypass to treat bilateral moyamoya disease | March 2012 | CCI-PMMA |
| 8 | 46 | M | Cranial revision to improve acquired head deformity | May 2012 | CCI-PMMA |
| 9 | 23 | F | Calvarial mass in contact with the dura | July 2012 | Titanium mesh |
| 10 | 70 | M | Recurrent infection after right frontal craniotomy | July 2012 | CCI-PEEK |
| 11 | 17 | M | Incisional pain and poor wound healing after craniotomy for bilateral trigeminal neuralgia | August 2012 | Titanium mesh |
| 12 | 24 | M | Right-sided hemicraniotomy after an acute subdural hematoma | August 2012 | CCI-PMMA |
| 13 | 43 | F | Methicillin-resistant Staphylococcus aureus infection after craniotomy | August 2012 | ABF |
| 14 | 9 | M | Large hemicraniectomy defect after severe bone flap resorption and sagittal synostosis | September 2012 | CCI-PMMA |
| 15 | 46 | M | Removal of malpositioned cranial hardware | December 2012 | CCI-PMMA |
| 16 | 54 | F | Temple region deformity after pterional craniotomy and infection | December 2012 | CCI-PMMA |
| 17 | 44 | F | Infection after left-sided craniotomy for aneurysm clipping | January 2013 | CCI-PMMA |
| 18 | 25 | M | Infection after craniotomy to treat a right-sided subdural hematoma | January 2013 | CCI-PMMA |
| 19 | 62 | F | Left temporal acquired head deformity | January 2013 | CCI-PMMA |
| 20 | 66 | M | Hemicraniectomy after right-sided subdural hematoma | March 2013 | ABF |
| 21 | 52 | M | Craniotomy after subarachnoid hemorrhage with right-sided middle cerebral artery aneurysm and stroke | March 2013 | CCI-PMMA |
| 22 | 62 | M | Cranial deformity after right frontal meningioma resection | April 2013 | ABF |
| 23 | 49 | F | Interosseous hemangioma in the left orbit, temporal bone, and greater sphenoid region | April 2013 | Titanium mesh |
| 24 | 39 | M | Persistent left-sided calvarial defect and head deformity after trauma | May 2013 | CCI-PMMA |
| 25 | 64 | F | Persistent calvarial deformity on left side after decompressive hemicraniectomy for a ruptured middle cerebral artery aneurysm | May 2013 | ABF |
| 26 | 70 | M | Acquired head deformity after a large right sphenoid skull base tumor | May 2013 | CCI-PEEK |
| 27 | 42 | F | Concave head deformity after removal of infected bone flap | May 2013 | CCI-PMMA |
| 28 | 70 | F | Infection with concave head deformity over full-thickness calvarial defect | May 2013 | CCI-PMMA |
| 29 | 57 | M | Asymmetry of the left frontal temporal parietal area | May 2013 | L-PMMA with screws |
| 30 | 82 | F | Acquired head deformity after bone flap infection | June 2013 | L-PMMA with screws |
| 31 | 25 | F | Bone flap osteomyelitis with calvarial defect | June 2013 | Titanium mesh |
| 32 | 26 | M | Head deformity after severe bone flap resorption | June 2013 | CCI-PMMA |
| 33 | 64 | M | Extruding frontal bone PMMA implant placed in 1976 | June 2013 | SCBG |
| 34 | 69 | F | Acquired calvarial deformity after emergent decompressive craniectomy for ruptured aneurysm | July 2013 | ABF |
| 35 | 67 | F | Acquired calvarial deformity after removal of infected bone flap | July 2013 | CCI-PMMA |
| 36 | 48 | F | Acquired calvarial deformity after removal of infected bone flap | August 2013 | ABF |
| 37 | 37 | F | Acquired calvarial deformity after removal of infected bone flap after tumor resection | August 2013 | CCI-PMMA |
| 38 | 44 | M | Acquired temporal deformity after titanium mesh cranioplasty | August 2013 | L-PMMA with screws |
| 39 | 69 | F | Calvarial defect after tumor resection | August 2013 | Titanium mesh |
| 40 | 53 | F | Infection after left-sided craniotomy for aneurysm clipping | August 2013 | SCBG |
| 41 | 52 | F | Acquired calvarial deformity after infected bone flap removal after tumor resection | August 2013 | Titanium mesh, L-PMMA with screws |
| 42 | 67 | F | Calvarial defect after tumor resection | August 2013 | CCI-PMMA |
| 43 | 26 | M | Calvarial defect following emergent decompressive craniectomy for traumatic intracranial hemorrhage | August 2013 | CCI-PMMA |
| 44 | 44 | F | Calvarial defect after tumor resection | September 2013 | Titanium mesh |
| 45 | 23 | M | Acquired head deformity after infection following craniotomy for aneurysm clipping | September 2013 | CCI-PMMA |
| 46 | 32 | M | Acquired calvarial deformity after emergent decompressive craniectomy for traumatic intracranial hemorrhage | September 2013 | CCI-PEEK |
| 47 | 76 | M | Acquired calvarial deformity after removal of infected bone flap | September 2013 | CCI-PMMA |
| 48 | 41 | M | Bilateral frontal bone defect after fibrous dysplasia resection | September 2013 | Titanium mesh, L-PMMA onlay |
| 49 | 45 | M | Acquired calvarial deformity following infected bone flap removal after tumor resection and irradiation | October 2013 | CCI-PMMA |
| 50 | 32 | F | Acquired calvarial deformity following infected bone flap removal after tumor resection | October 2013 | CCI-PMMA |
Forty-six patients required full-thickness, second-stage cranial reconstruction using a wide spectrum of autologous bone and alloplastic materials listed here: ABF, autologous bone flap; CCI, custom cranial implant; L-PMMA, liquid poly-methyl-methacrylate; PEEK, polyetheretherketone; PMMA, poly-methyl-methacrylate (prefabricated, solid form); SCBG, split-calvarium bone graft.
TABLE 2.
Summary of Materials Used in This Consecutive Case Series (n = 50)a
| Cranioplasty Materials | Case | Adverse Events, n |
|---|---|---|
| Split-calvarium bone grafts | 2 | 0 |
| Autologous bone flap | 7 | 1b |
| Autologous bone flap + PMMA | 2 | 0 |
| Titanium mesh | 5 | 0 |
| Titanium mesh + L-PMMA with screw fixation | 1 | 0 |
| Titanium mesh + L-PMMA onlay | 1 | 0 |
| PMMA CCI | 22 | 0 |
| PMMA + L-PMMA | 1 | 0 |
| L-PMMA with screw fixation | 4 | 0 |
| PEEK CCI | 5 | 0 |
| Total | 50 | 1c |
CCI, custom cranial implant; L-PMMA, liquid poly-methyl-methacrylate; PEEK, polyetheretherketone; PMMA, poly-methyl-methacrylate (prefabricated, solid form).
Fifty consecutive craniofacial reconstructions were performed in 50 subjects (4 subjects required isolated soft tissue reconstruction for temporal hollowing). Among the 46 cranioplasties, there was only 1 adverse event in a patient who had undergone autologous bone flap reconstruction.
In this cranioplasty group (n = 46), the corresponding adverse event rate was 2.17% (95% confidence interval, 0.003–11.3). With the use of an α of 0.05, the rate is statistically significantly lower than 7.0% (P = .03). The adverse event rate using this technique is potentially as low as 0% and unlikely to be higher than 11.3%. The isolated bone flap requiring removal for infection occurred in a patient with a recent history of cerebellar abscess.
DISCUSSION
By separating the scalp components (galea-skin flap from the pericranium), this unique dissection allows a partial release and advancement of tissues helping to reduce tension. Interestingly, a similar technique is commonly used in plastic surgery for abdominal wall reconstruction that is referred to commonly as a component release.33 Our new approach can be used for all types of secondary cranial reconstruction regardless of the choice of autologous or alloplastic material.
Overall, deep infection remains the major leading complication after secondary cranioplasty, with reported rates between 21% and 40%.5,23,34–36 In 2011, Frederick et al5 presented a large series of 109 secondary alloplastic implant reconstructions with a reinfection rate of 40%, with methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus being the most common causes. In fact, in that same year, a meta-analysis conducted by Yadla et al36 convincingly showed that these cranial infections occur regardless of surgery timing (early vs late), implant materials, and method of flap preservation. Common complications reported in this study included seizure, hematoma formation, seroma formation, hardware exposure, and cerebrospinal fluid leak.23,34,36–38 Perhaps this is high-level evidence to support our hypothesis that well-vascularized tissue placed on either side of the bone flap and/or implant will aid in reducing the high infection rate, decrease intraoperative bleeding, and prevent cortical irritation leading to postoperative seizures.
Walcott et al39 found that patients who undergo second-stage cranial reconstruction after a decompressive craniotomy for stroke and patients who have had a prior reoperation were found to be at higher risk for complications. The authors postulated that both patient groups represent populations that likely have a combination of poor wound healing progression and systemic nutritional deficiencies secondary to neurological impairment. In such populations with high risk factors, it is important to consider the use of an approach such as this one to decrease morbidity, to optimize tissue vascularity and wound healing, and, at the same time, to improve aesthetic outcomes.
In an effort to minimize these complications while capitalizing on the benefits of CCIs, we describe this pericranial-onlay cranioplasty technique (developed in 2011). It has since evolved to include autologous bone flaps and split-thickness calvarial grafts as well. It entails elevating a new fasciocutaneous scalp flap, placing each construct between the vascularized pericranial onlay and partial-thickness scalp flap, and reconstructing the soft tissue with intricate mobilization and complex closure.
In addition, for those patients with temporal hollowing and/or bone flap warping, temporal augmentation is performed either simultaneously or at a later stage. Over this time period, 50 consecutive craniofacial reconstructions were performed (Table 1). In this cohort, a wide range of materials were used, including autologous bone flap, split calvarial grafts, titanium mesh, titanium mesh with liquid PMMA onlay, and a wide range of CCIs, including polyetheretherketone and PMMA. We believe that through a multidisciplinary approach, encompassing the pericranial-onlay technique and other modifications detailed here, one can decrease overall infection rates, reduce subdural inflammation caused by the implant material, and minimize surgical blood loss, cerebrospinal fluid leaks, and wound dehiscence, all of which alleviate risk for hardware/implant/bone flap exposure and further surgery. Of note, this is based on comparison with previously reported outcome studies such as those demonstrated in Table 3.
TABLE 3.
Summary of Complication Rates and Outcomes in the Literature
There are several potential advantages to this approach. The greatest benefit is the possible reduction in the number of postoperative complications, mainly related to infection and bleeding. This is achieved by providing complete flap encasement of the bone flap or cranial implant with vascularized tissue. Traditionally, the implant has been placed either directly onto the dura or onto some type of dural protectant. To the best of our knowledge, this is the first description and series with this type of pericranial-onlay technique. In our experience, the risk of deep intracranial infections requiring removal and reoperation (reported to be up to 40%5,23,35–43) may be significantly decreased. Any infections that occur in this setting are most likely to be from superficial contamination rather than meninges or brain parenchyma because the additional tissue plane in this instance further isolates the intracranial space. This also eliminates the possibility of dural injury, subdural hematoma formation, and noninfectious meningitis resulting from alloplastic irritation.
Since its original introduction, our total estimated blood loss has been drastically reduced. Average total blood loss ranges between 100 and 200 cm3 per case. This benefit most likely results from the tumescent local anesthesia and the departure of violating the epidural space to re-elevate the same, original full-thickness scalp flap. Instead, dissection is carried out with needlepoint electrocautery set at a low-energy setting and under loupe magnification carefully within the avascular, loose areolar tissue plane.
We believe that our approach presented here for soft tissue reconstruction will also yield improved aesthetic results. Tension is a major factor in wound dehiscence, infection, and incisional breakdown. By extensively undermining the surrounding scalp and releasing the scalp components, tension is minimized. We, in contrast to common practice, therefore propose wide undermining to both the contralateral and posterior sides, in addition to using galeal scoring carefully along the stable scalp side in a parallel fashion. This may seem counterintuitive, but wound tension is minimized even in the presence of increased cranial circumference with reconstruction, which undoubtedly requires additional tissue stretch. Furthermore, component separation of the galea fascia from the pericranium underneath allows mobilization and release in 2 separate tissue planes, thus increasing the amount of recruited tissue. Additionally, because this is being done in instances of secondary reconstruction, the original flap incision is often made weeks to months earlier. The original scalp incision is thereby inadvertently serving as a valuable “delay” based on the superficial temporal system. This “delay” produces relative ischemia to the periphery and improves vascularity of the flap’s pedicle.43
To obtain optimal aesthetic results, a liquid PMMA implant material may also be used to reconstruct all areas of soft tissue temporal deformity (ie, hollowing) at the time of closing or in instances when there is inconsistent bone-to-bone orbone-to-implant interfaces. Transformation of the PMMA from liquid to solid is exothermic, but the heat generated can be limited with cool irrigation at the time of placement. For the first scenario, oblique screws are placed (commonly 8 mm long) into the underlying pterional bone flap or custom implant, leaving 4- to 5-mm screw length exposed to provide rigid support for the liquid PMMA. As the liquid hardens, the scalp flap is reflected back, and this implant material fills in cavities to camouflage all soft tissue irregularities. This is a modification of the original technique published by Gordon and Yaremchuk25 in 2011 and is valuable in instances when the temporalis muscle is deficient or there is temporal fat pad wasting (Figure 5). For the latter scenario, a small amount of liquid PMMA is applied to the irregular interfaces. Careful attention should be paid to prevent dripping of the material into the intracranial space. Of note, in contrast to liquid PMMA, the solid PMMA implants used within this study are form stable and undergo zero contraction with respect to time. In addition, because the chemical reaction has already occurred before shipping by the manufacturer, there is no exothermia during placement, alleviating any type of concern for excessive heat generation. Furthermore, although various other synthetics have been introduced over the past few decades, none has been shown superior to PMMA with respect to infectious complications, and the other synthetics are associated with increased costs.23,24
FIGURE 5.
Preoperative frontal view (A) and left oblique view (B) after infected bone flap removal 6 months earlier. Secondary autologous cranial reconstruction with the patient’s own bone flap (C). On-table view of the persistent temporal deformity (D) and simultaneous temporal soft tissue reconstruction using strategic screw placement (E). Left oblique bird’s eye view (F) of hardened poly-methyl-methacrylate (PMMA) placed for concomitant soft tissue deformity correction and temporal symmetry. Axial computed tomographic scan showing left-side bone flap (green arrow), liquid PMMA with screw fixation (yellow arrow), and overlying drain (red arrow) (G). The patient at 3 months with no signs of temporal deformity (H).
Finally, a meticulous 3-layer soft tissue closure is used with interrupted sutures, ensuring correct tissue alignment. Closing subcutaneous tissue improves apposition of the skin edges, prevents inversion of the wound, and results in a finer scar. This is vital when the surgical incision lies over an implant or bone flap because both are at high risk for bacterial contamination in the setting of poor wound healing and skin dehiscence. Closed-suction drains allow dead space evacuation and prevent fluid from accumulating around the bone flap or alloplastic CCI, which in theory reduces the chance of bacterial inoculation. Of note, closed-suction drains have been studied in areas of alloplastic reconstruction and were found not to affect the overall rate of infectious complications.44 Drains for our patients are normally removed by postoperative day 3. The average hospital length of stay for our patients is between 2 and 3 days.
This technique has several potential disadvantages. Obtaining the correct dissection plane between the galea and pericranium is technically challenging and requires careful dissection under loupe magnification (Figure 6). The success of creating this pericranial onlay is partly predicated on the initial pericranial dissection during craniectomy. Taking care to preserve this tissue layer by reflecting it with the scalp flap, without too many breeches, can assist future reconstruction, which is the impetus for our suggestion describing a multidisciplinary approach. In addition, because of the heightened learning curve, performing this technique may lead to prolonged operative times, potential flap necrosis, and associated anesthetic risk. However, with surgeon experience, we believe that no significant difference in operating time exists between our approach with the pericranial onlay and the traditional techniques used today.
FIGURE 6.
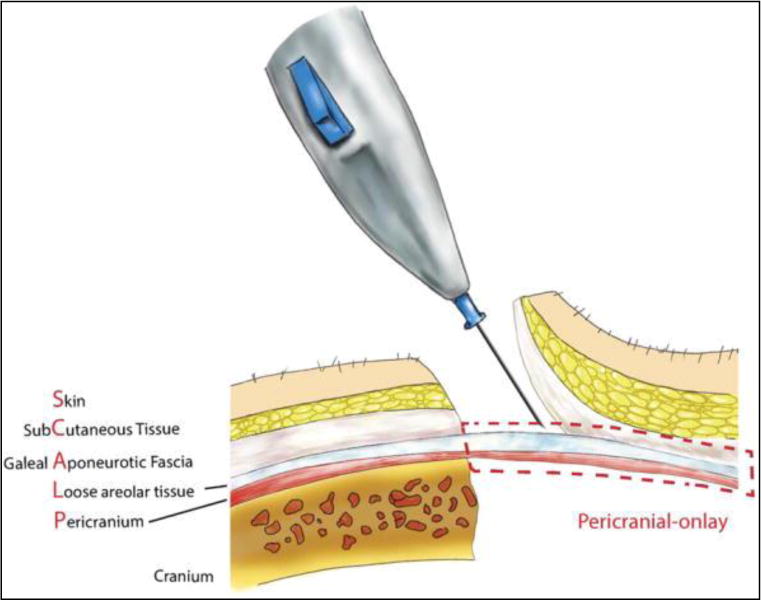
Anatomic illustration demonstrating the pericranial-onlay dissection.
CONCLUSION
Our multidisciplinary approach presented here, including the pericranial-onlay cranioplasty technique, has the potential to improve patient outcomes, to decrease perioperative morbidity, and to minimize costs associated with postoperative infectious complications after secondary cranial reconstruction. This may represent a paradigm shift in the manner for which secondary bone flap and implant-based cranial reconstruction is performed.
ABBREVIATIONS
- CCI
custom cranial implant
- PMMA
poly-methyl-methacrylate
Footnotes
Disclosure
Dr Lim is a consultant and speaker for and has received an honorarium from Stryker. The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.neurosurgery-online.com).
References
- 1.Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47(3):238–241. doi: 10.1016/s0090-3019(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 2.Grantham EC, Landis HP. Cranioplasty and the post-traumatic syndrome. J Neurosurg. 1948;5(1):19–22. doi: 10.3171/jns.1948.5.1.0019. [DOI] [PubMed] [Google Scholar]
- 3.Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53(3):288–292. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 4.Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47(3):231–237. doi: 10.1016/s0090-3019(96)00376-x. [DOI] [PubMed] [Google Scholar]
- 5.Frederick M, Mittermiller P, Hoffman W. Alloplastic cranioplasty outcomes in previously infected sites; Scientific Program, 90th Annual Meeting of the American Association of Plastic Surgeons; April 2011; Available at: http://meeting.aaps1921.org/abstracts/2011/9.cgi. Accessed July 18, 2013. [Google Scholar]
- 6.Pankratiev BE. Dead bone grafts to repair skull defects. Ann Surg. 1933;97(3):321–326. doi: 10.1097/00000658-193303000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prolo DJ, Oklund SA. The use of bone grafts and alloplastic materials in cranioplasty. Clin Orthop Relat Res. 1991;(268):270–278. [PubMed] [Google Scholar]
- 8.Gladstone HB, McDermott MW, Cooke DD. Implants for cranioplasty. Otolaryngologic Clin N Am. 1995;28(2):381–400. [PubMed] [Google Scholar]
- 9.Jackson IT, Adham M, Bite U, Marx R. Update on cranial bone grafts in craniofacial surgery. Ann Plast Surg. 1987;18(1):37–40. doi: 10.1097/00000637-198701000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Recon Surg. 1986;77(6):888–904. [PubMed] [Google Scholar]
- 11.Booth JA, Curtis BF. I, report of a case of tumor of the left frontal lobe of the cerebrum; operation; recovery. Ann Surg. 1893;17(2):127–139. doi: 10.1097/00000658-189301000-00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerster A. Heteroplasty for defect of skull. Trans Am Surg Assoc. 1895;13:485–486. [Google Scholar]
- 13.Morestin H. Les transplantations cartilagineuses dans la chirurgie réparatrice. Soc Chir Bull Mem. 1915;41:1994–2046. [Google Scholar]
- 14.Cornioly C. A propos de cranioplastie. Rev Med Suisse Romande. 1929;49:677–693. [Google Scholar]
- 15.Geib F. Vitallium skull plates. JAMA. 1941;117:8–12. [Google Scholar]
- 16.Farrington PR. Closure of a defect of the skull with tantalum. Rocky Mountain Med J. 1945;42:842–844. [PubMed] [Google Scholar]
- 17.Gordon DS, Blair GA. Titanium cranioplasty. Br Med J. 1974;2(5917):478–481. doi: 10.1136/bmj.2.5917.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolf J, Walker A. Cranioplasty: collective review. Int Abs Surg. 1945;81:1–23. [Google Scholar]
- 19.Gosain AK, Persing JA. Biomaterials in the face: benefits and risks. J Craniofac Surg. 1999;10(5):404–414. doi: 10.1097/00001665-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lara WC, Schweitzer J, Lewis RP, Odum BC, Edlich RF, Gampper TJ. Technical considerations in the use of polymethylmethacrylate in cranioplasty. J Long Term Eff Med Implants. 1998;8(1):43–53. [PubMed] [Google Scholar]
- 21.Luparello D, Bruschi S, Verna G, et al. Cranioplasty with polymethylmethacrylate: the clinico-statistical considerations [in Italian] Minerva Chir. 1998;53(6):575–579. [PubMed] [Google Scholar]
- 22.Blum KS, Schneider SJ, Rosenthal AD. Methyl methacrylate cranioplasty in children: long-term results. Pediatr Neurosurg. 1997;26(1):33–35. doi: 10.1159/000121158. [DOI] [PubMed] [Google Scholar]
- 23.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14(2):144–153. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hammon WM, Kempe LG. Methyl methacrylate cranioplasty. 13 years experience with 417 patients. Acta Neurochir. 1971;25(1):69–77. doi: 10.1007/BF01808863. [DOI] [PubMed] [Google Scholar]
- 25.Gordon CR, Yaremchuk MJ. Temporal augmentation with methyl methacrylate. Aesth Surg J. 2011;31(7):827–833. doi: 10.1177/1090820X11417425. [DOI] [PubMed] [Google Scholar]
- 26.Chim H, Schantz JT. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Recon Surg. 2005;116(6):1726–1741. doi: 10.1097/01.prs.0000182386.78775.cd. [DOI] [PubMed] [Google Scholar]
- 27.Dean D, Min KJ, Bond A. Computer aided design of large-format prefabricated cranial plates. J Craniofac Surg. 2003;14(6):819–832. doi: 10.1097/00001665-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Rengachary SS, Benzel EC, AANS Publications Committee . Calvarial and Dural Reconstruction. Park Ridge, IL: American Association of Neurological Surgeons; 1998. [Google Scholar]
- 29.Yamanaka Y, Karuppaiah K, Abu-Amer Y. Polyubiquitination events mediate polymethylmethacrylate (PMMA) particle activation of NF-kappaB pathway. J Biol Chem. 2011;286(27):23735–23741. doi: 10.1074/jbc.M111.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SC, Pelofsky S. Adaptation of rigid fixation to cranial flap replacement. Neurosurgery. 1991;29(3):417–418. doi: 10.1097/00006123-199109000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Francel PC, Persing JA. Microplating and screw systems for cranial bone fixation. Neurosurgery. 1993;32(4):683–686. [Google Scholar]
- 32.Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008;122(6):195e–208e. doi: 10.1097/PRS.0b013e3181858eee. [DOI] [PubMed] [Google Scholar]
- 33.Bennett SPH, Fitoussi AD, Berry B, et al. Management of exposed, infect implant-based breast reconstruction and strategies for salvage. J Plast Recon Aesth Surg. 2011;64(10):1270–1277. doi: 10.1016/j.bjps.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez OM. Inception and evolution of the components separation technique: personal recollections. Clin Plast Surg. 2006;33(2):241–246. vi. doi: 10.1016/j.cps.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Stephens FL, Mossop CM, Bell RS, et al. Cranioplasty complications following wartime decompressive craniectomy. Neurosurg Focus. 2010;28(5):E3. doi: 10.3171/2010.2.FOCUS1026. [DOI] [PubMed] [Google Scholar]
- 36.Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurg. 2011;68(4):1124–1129. doi: 10.1227/NEU.0b013e31820a5470. discussion 1130. [DOI] [PubMed] [Google Scholar]
- 37.Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26(6):E9. doi: 10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 38.Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010;112(5):1120–1124. doi: 10.3171/2009.6.JNS09133. [DOI] [PubMed] [Google Scholar]
- 39.Walcott BP, Kwon CS, Sheth SA, et al. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013;118(4):757–762. doi: 10.3171/2013.1.JNS121626. [DOI] [PubMed] [Google Scholar]
- 40.Rish BL, Dillon JD, Meirowsky AM, et al. Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery. 1979;4(5):381–385. doi: 10.1227/00006123-197905000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Poetker DM, Pytynia KB, Meyer GA, Wackym PA. Complication rate of transtemporal hydroxyapatite cement cranioplasties: a case series review of 76 cranioplasties. Otol Neurotol. 2004;25(4):604–609. doi: 10.1097/00129492-200407000-00031. [DOI] [PubMed] [Google Scholar]
- 42.Jaberi J, Gambrell K, Tiwana P, Madden C, Finn R. Long-term clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J Oral Maxillofac Surg. 2013;71(2):e81–e88. doi: 10.1016/j.joms.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 43.Lauzon G. Transfer of a large, single temporo-occipital flap for treatment of baldness. Plast Recon Surg. 1979;63(3):369–371. doi: 10.1097/00006534-197903000-00013. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy CM, Disa JJ, Pusic AL, Mehrara BJ, Cordeiro PG. The effect of closed-suction drains on the incidence of local wound complications following tissue expander/implant reconstruction: a cohort study. Plast Recon Surg. 2007;119(7):2018–2022. doi: 10.1097/01.prs.0000260586.55628.29. [DOI] [PubMed] [Google Scholar]


