Abstract
Rationale
Alcohol use disorders are associated with deficits in adaptive behavior. While some behavioral impairments that are associated with alcohol use disorders may predate exposure to drugs of abuse, others may result directly from exposure to drugs of abuse, including alcohol. Identifying a causal role for how alcohol exposure leads to these impairments will enable further investigation of the neurobiological mechanisms by which it acts to dysregulate adaptive behavior.
Objectives
In the present study, we examined the effects of chronic intermittent ethanol exposure (CIE) on the use of reward-paired cues to guide consummatory behaviors in a mouse model, and further, how manipulations of mGluR2/3 signaling – known to be dysregulated after chronic alcohol exposure – may alter the expression of this behavior.
Methods
Adult male C57B/6J mice were trained to self-administer 10% ethanol and exposed to CIE via vapor inhalation. After CIE exposure, mice were trained in a Pavlovian task wherein a cue (tone) was paired with the delivery of a 10% sucrose unconditioned stimulus. The use of the reward-paired cue to guide licking behavior was determined across training. The effect of systemic mGluR2/3 manipulation on discrimination between cue-on and cue-off intervals was assessed by administration of the mGluR2/3 agonist LY379268 or the antagonist LY341495 prior to a testing session.
Results
Exposure to CIE resulted in reductions in discrimination between cue-on and cue-off intervals, with CIE exposed mice exhibiting significantly lower consummatory behavior during reward-paired cues than Air controls. In addition, systemic administration of an mGluR2/3 agonist restored the use of reward-paired cues in CIE exposed animals without impacting behavior in Air controls. Conversely, administration of an mGluR2/3 antagonist mimicked the effects of CIE on cue-guided licking behavior, indicating that mGluR2/3 signaling can bidirectionally regulate the ability to use reward-paired cues to guide behavior.
Conclusions
Together, these data suggest that chronic ethanol exposure drives impairments in the ability to use reward-paired cues to adaptively regulate behavior, and that mGluR2/3 represent a therapeutic target for restoration of these deficits in behavioral control in the alcoholic.
Keywords: Alcohol, metabotropic glutamate receptors, cue-mediated behavior
Introduction
Individuals with alcohol use disorders often show impairments in the ability to flexibly regulate their behavior (Hildebrandt et al. 2006; Sjoerds et al. 2013; Sjoerds et al. 2014; Sanchez-Roige et al. 2014; Brevers et al. 2014). In human populations, it remains unclear whether innate differences in cognitive control and behavioral flexibility predispose individuals to the development of alcohol use disorders, or whether ethanol exposure itself results in these deficits. The use of animal models has suggested that preexisting differences may in part predict the development of addiction-related behaviors (Flagel et al. 2007; Barker et al. 2012; Saunders and Robinson 2013; Barker et al. 2014), while other behavioral deficits may result directly from exposure to drugs of abuse (Gourley et al. 2013; DePoy et al. 2013). These findings suggests that while initiation of drug seeking and taking behavior may result from innate risk factors, ongoing drug use is exacerbated by drug-induced deficits in adaptive regulation of behavior (c.f., Jentsch & Taylor, 1999).
The use of reward-predictive cues to guide behavior is critical for optimal performance and behavioral efficiency. However, over- or under- reliance on these stimulus-outcome contingencies can result in maladaptive reward seeking behaviors. Enhanced cue reactivity may result in a transition from outcome mediated behavior to stimulus-response behaviors that are no longer sensitive to changes in outcome contingency or outcome value. Indeed, Pavlovian approach, in which animals are trained to associate a cue with reward delivery, is predictive of the development of habitual ethanol seeking behaviors that are associated with the development of uncontrolled, addictive like alcohol seeking (Barker et al. 2014). However, the failure to use outcome-predictive cues to guide actions can lead to inappropriate reward-seeking behavior when a reward is known to be unavailable. While innate differences in the use of reward-predictive cues to mediate behavior may be related to the development of inflexible ethanol seeking (Bartholow et al. 2010; Kareken et al. 2010; Barker et al. 2012), how ethanol exposure acts to alter cue-mediated behavior is largely unclear. A growing body of literature suggests that chronic exposure to ethanol may result in aberrant use of environmental cues to guide behavior with certain paradigms suggest that alcohol exposure can facilitate the use of cues to drive behavior (DePoy et al. 2013), while others suggest that ethanol exposure reduces the abilities of cues to invigorate behavior (Depoy et al. 2014). The mixed nature of these results suggests that these effects appear to be dependent on the duration and timing of ethanol exposure and the precise behavior assayed (Depoy et al. 2014).
Glutamate signaling is known to be critical for the expression of adaptive reward seeking behavior, including habitual behavior (Corbit et al. 2014b; Corbit et al. 2014a) and Pavlovian approach (Mead and Stephens 2003). Chronic exposure to ethanol has been shown to dysregulate glutamatergic signaling within the limbic corticostriatal circuits that mediate adaptive reward seeking behavior. In particular, chronic intermittent ethanol (CIE) exposure resulted in deficits in behavioral flexibility that were associated with alterations in mGluR2 expression in prefrontal projection neurons (Meinhardt et al. 2013). These deficits could be rescued by overexpression of mGluR2 in infralimbic projections, suggesting a causal role for dependence-induced alterations in mGluR2 signaling in the development of maladaptive reward seeking. CIE has been shown to produce alterations in glutamate in the nucleus accumbens, resulting in elevations in extracellular glutamate that persisted beyond withdrawal (Griffin III et al. 2013) and may mediate uncontrolled ethanol seeking. Together with a larger literature implicating glutamate signaling in addiction in general (Kalivas 2009) and in alcohol use disorders (Olive 2009) in particular, these findings suggest that abnormalities in mGluR2/3 signaling arising from chronic alcohol exposure may drive impairments in adaptive reward-seeking behavior.
In the present study, we used a vapor inhalation model to investigate how chronic ethanol exposure impacted the ability to use reward-paired cues to guide consummatory behavior. Given evidence that chronic ethanol exposure results in dysregulation in glutamatergic signaling in the limbic corticostriatal circuits that mediate appetitive behavior, we assessed a role for mGluR2/3 signaling in restoration of adaptive reward-seeking behavior.
Methods
Subjects
All subjects were adult male (>70 days) C57B/6J mice (Jackson Labs). Mice were group-housed in the Medical University of South Carolina vivarium and maintained at free-feeding weights throughout the duration of the experiment. Mice were maintained on a reverse light-dark cycle (12h light:12h dark) and all behavioral experiments were performed during the dark cycle.
Operant conditioning chambers
Mice were trained in standard self-administration boxes from Med Associates (St. Albans, VT) with 2 plexiglas walls and 2 metal walls. The floors were metal grid floors and the boxes had two retractable levers that were extended during instrumental training sessions. One was assigned as the ‘active’ lever that would result in reinforcer deliver. On the same wall, sucrose or ethanol were delivered by syringe pump to a well in a magazine equipped with a lickometer to measure consummatory behavior.
Ethanol self-administration and chronic intermittent ethanol (CIE) exposure
Prior to CIE, mice were trained to self-administer ethanol using a post-prandial consumption paradigm to facilitate acquisition. For this procedure, mice received limited access to food and water. Specifically, 1 hour prior to beginning the self-administration sessions, mice received 15 min access standard homecage chow and the water bottles were removed from the cages until after the self-administration sessions. Weights were monitored and additional food was provided after the behavior session to maintain free-feeding weights. In these sessions, mice were trained to respond (lever press) on a fixed ratio 1 (FR1) schedule for access to a 10% unsweetened ethanol solution in 30 min sessions; i.e., each depression of the active lever resulted in delivery of 20 ul of the ethanol solution. After establishing baseline responding (i.e., mice were consistently earning at least 10 ethanol reinforcers across 2 sessions; 5-10 sessions), mice were returned to ad libitum food and water access and responding was maintained on a FR1 schedule for 5 sessions. Mice were either assigned to the ethanol vapor group or served as air exposed controls (AIR) based on response rates during instrumental performance. CIE mice underwent two cycles of ethanol exposure that involved for 16 hr/day in the vapor chambers for 4 consecutive days. Eight hrs of withdrawal separated each exposure and the two cycles were separated by 5 days of operant self-administration on an FR1 schedule identical to the 30 min sessions prior to CIE. After the second CIE cycle, mice received one additional day of FR1 responding to compare pre and post CIE self-administration levels. The use of this self-administration procedure allowed us to assess whether this CIE exposure paradigm resulted in escalation of ethanol self-administration. On the days that mice went into chambers for exposure, each mouse received equivalent injections of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) to promote higher blood ethanol concentrations. Blood ethanol concentration was confirmed to be within the 150-250 mg/dl range throughout the exposure period.
Pavlovian training
Mice were exposed to a cue-outcome pairing in which a previously neutral cue (tone) was associated with the delivery of a 10% sucrose reinforcer across 8 sessions. In these sessions, mice were placed in the same operant conditioning boxes described previously, however, no levers were available. Prior to training, mice received a single 15-min session of magazine training during which 20 ul of 10% sucrose was delivered on a fixed interval 60-s schedule. Mice then received eight 30-min Pavlovian training sessions. During these sessions, the tone was played on a fixed interval schedule (120 s off, 60 s on). Twenty ul of sucrose was delivered during each “cue-on” interval on a random time schedule so that delivery could not be predicted and no reinforcers were delivered during “cue-off” periods. No response was required to initiate reinforcer delivery and all reinforcer delivery was noncontingent on behavior. Licks at the magazine during the cue-on and cue-off intervals were measured to assess appetitive behavior during these intervals.
Reversal of CIE-induced deficit with an mGluR2/3 agonist
The role mGluR2/3 signaling in the expression of adaptive reward seeking was assessed by administering the mGluR2/3 agonist LY379268 (Tocris) in a subset of the animals trained in the Pavlovian training paradigm described above. Of this cohort, animals were assigned to receive either drug or vehicle (saline) based on ethanol exposure and licking behavior during training. Drug was dissolved in sterile saline and was administered via i.p. injection at a dose of 1mg/kg min 30 prior to the test session. The test session was identical to a training session except for the administration of the mGluR2/3 agonist.
Mimic of the CIE-induced effect with an mGluR2/3 antagonist
To assess whether mGluR2/3 antagonism would mimic the effects of CIE on discrimination, a separate cohort of mice were trained in the Pavlovian training paradigm described above across 6 training sessions. After training, mice were matched by responding and assigned to receive i.p. injections of either saline or the mGluR2/3 antagonist LY341495 (Tocris) at 1mg/kg 30 min prior to testing sessions. On the following day, mice were returned to identical testing conditions except that mice received the opposite drug (i.e., mice that received LY341495 now received saline) at the same time point.
Assessment of mGluR2 expression
To investigate whether mGluR2 protein expression was downregulated in the nucleus accumbens shell after two weeks of CIE, a separate cohort of mice underwent ethanol self-administration and CIE exposure following the protocol described above. Mice were sacrificed after two weeks of CIE via rapid decapitation under isoflurane anesthesia. Nucleus accumbens shell was microdissected from tissue, sonicated in LDS, and stored at −20 °C until Western blot analysis. After transfer, total protein was assessed using Swift Membrane Stain (G-Biosciences). Membranes were probed using a selective mouse anti-mGluR2 antibody (Abcam; 1:1000) and visualized using a HRP-conjugated goat anti-mouse secondary antibody (Southern Biotech; 1:5000) with BioRad high-sensitivity ECL. mGluR2 protein was normalized to total protein to control for loading.
Analysis
All statistical analysis was performed using SPSS. The effects of CIE on ethanol self-administration were analyzed by a repeated measures ANOVA with session as a repeated measure and CIE exposure (CIE vs Air) as a between subjects variable. The effect of CIE on licking behavior was analyzed by repeated measures ANOVA with session and cue (cue-on vs cue-off) as repeated measures and CIE exposure (CIE vs Air) as a between subjects variable. To test the ability of an mGluR2/3 agonist to reverse CIE-induced deficits, licking behavior was analyzed via repeated measures ANOVA with cue (cue-on vs cue-off) as repeated measures and CIE exposure (CIE vs Air) and drug administration (LY379268 versus saline) as between subjections effects. To determine whether mGluR2/3 antagonism mimicked CIE-induced deficits in discrimination, licking behavior was analyzed by repeated measures with cue (cue-on vs cue-off) and drug (saline versus LY341495) as repeated measures. Protein expression was analyzed using t test. For all experiments, α = 0.05, and data is presented as mean +/− SEM.
Results
Blood ethanol levels
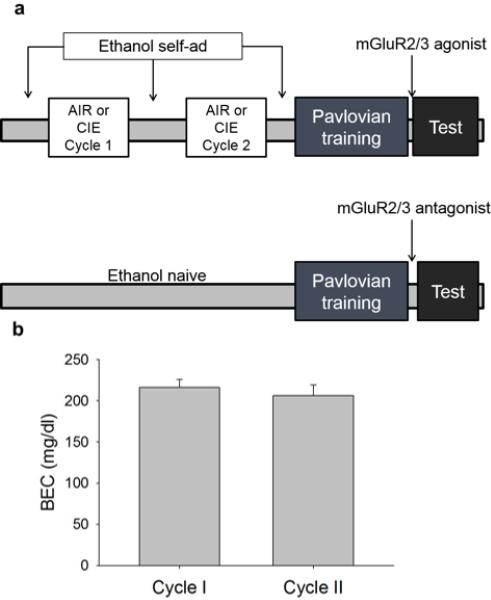
Blood ethanol levels were targeted to 200 mg/dl across two separate sessions. For mice used in Pavlovian training sessions, the mean of session one was 216.1, and session two was 206.2 mg/dl (Fig 1b). To investigate whether escalation of self-administration was observed, we compared g/kg ethanol consumption on the first day after each cycle of CIE (days 1 and 6) to an equivalent session prior to CIE exposure (day - 5). No main effect of session (p > 0.2), CIE exposure (p > 0.8) or CIE × session interaction (p > 0.5) was observed, indicating that self-administration did not escalate after only 2 cycles of CIE exposure. For mice used for mGluR2 expression experiments, average blood ethanol concentration was 187.1 mg/dl.
Fig. 1.
Time-line of behavioral training and chronic intermittent ethanol exposure (CIE). (a) For experiments in which mice were exposed to CIE, they were trained to self-administer ethanol prior to CIE. After the final self-administration session, mice were trained in a Pavlovian conditioning paradigm to associate a cue (tone) with delivery of a sucrose reinforcer. To investigate whether mGluR2/3 antagonism mimicked the effects of CIE, a cohort of ethanol-naïve mice was trained in a Pavlovian conditioning paradigm. The effect of mGluR2/3 agonism or antagonism on cue-mediated behavior was assessed by administering LY379268 or LY341495, respectively, prior to a test session. (b) Shown is the mean BEC after the end of each cycle of CIE exposure for mice trained in Pavlovian procedures. There was no significant difference in the mean BEC between cycle 1 and 2.
Effect of CIE on adaptive reward seeking
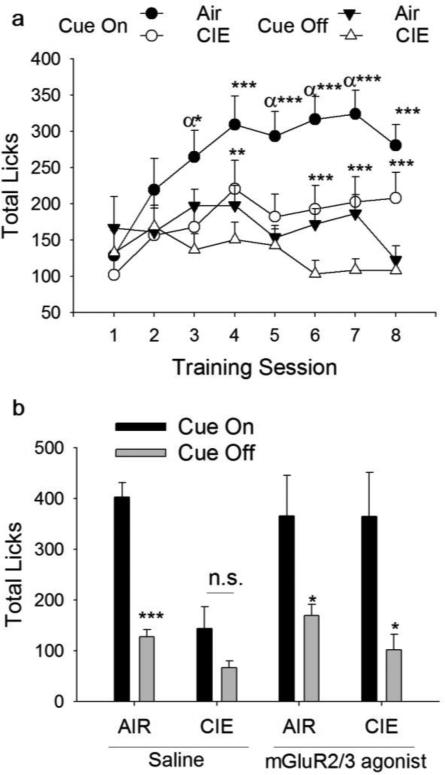
To determine how CIE impacted the ability to use cues to guide reward seeking behavior, mice were trained in a Pavlovian association paradigm in which cue presentation predicted the delivery of the sucrose reinforcer (Fig 2a; n = 23 air, 24 CIE). Assessment of licking behavior across eight days of training indicated an interaction between CIE and cue presentation on licking behavior (F1,45 = 4.026, p = 0.05), as well as a session by CIE interaction (F1,45 = 2.247, p = 0.05). Main effects of session (F1,45 = 3.573, p < 0.01), cue (F1,45 = 34.684, p < 0.001) and ethanol (F1,45 = 3.962, p = 0.05) were also observed. While a significant session × cue interaction (F1,45 = 7.712, p < 0.001) was observed, there was no session × cue × CIE effect (p > 0.4) consistent with increased discrimination between the cue-on and cue-off sessions for both Air controls and CIE exposed mice. Post-hoc analyses indicated that CIE only impacted licking during the tone (p < 0.05) as no effect of CIE was observed on licking behavior during the inter-tone interval (p > 0.1). Follow-up analyses on the main effect of session indicated that cue-on licking generally increased across training for Air exposed animals (p < 0.05), but licking did not increase during the cue-off interval. In contrast, for CIE exposed mice, licking during the cue-on interval increased after day 2 of training, but no significant increases in licking during the cue-on interval were observed after subsequent training (p's > 0.1). No increase in licking during cue-off intervals was observed in CIE-exposed animals either. Rather, the only significant differences across days observed for cue-off licking in CIE-exposed mice were a reduction in responding on day 7 compared to days 4-6. Notably, no measurable residual sucrose was present in any training session for any animal, suggesting that there was no CIE-induced deficit in sucrose consumption.
Fig. 2.
Administration of an mGluR2/3 agonist reverses CIE-induced deficits in cue-guided behavior. (a) Chronic ethanol exposed mice were trained to associate a cue (tone) with delivery of a reinforcer (sucrose) for eight sessions. CIE exposed animals exhibited deficits in discrimination between cue-on and cue-off intervals (p = 0.05), suggesting impairments in the use of reward-paired cues to guide behavior. An effect of CIE was observed only on responding during the cue-on intervals, no difference was observed during the cue-off intervals between Air and CIE exposed mice. n = 23 (Air) and 24 (CIE). Cue-on vs cue-off; *p < 0.05, **p < 0.01, ***p < 0.001; Air cue-on vs CIE cue-on responding, αp < 0.05. Values represent mean ± SEM. (b) To probe the ability of an mGluR 2/3 agonist to reverse CIE-induced deficits, LY379268 was administered 30 min prior to a test session. Under saline conditions, CIE exposed mice did not discriminate between cue-on and cue-off intervals (p > 0.05) while Air controls responded at a significantly higher rate (p < 0.001). Administration of the mGluR2/3 agonist resulted in discrimination between cue-on and cue-off intervals in CIE animals (p < 0.05). Additionally, administration of the mGluR2/3 agonist normalized licking behavior in CIE mice such that licking during the cue-on intervals was equivalent to that observed in Air exposed controls. n = 7 (Air, Saline), 8 (Air, LY379268), 6 (CIE, Saline), 7 (CIE, LY379268), *p < 0.05, **p < 0.01, ***p < 0.001
Effect of an mGluR2/3 agonist on cue-guided reward seeking
To determine if administration of an mGluR2/3 agonist can alter the ability of CIE exposed mice to use cues to guide consummatory behavior, one cohort of mice received an i.p. injection of the mGluR2/3 agonist LY379268 or saline vehicle 30 min prior to a standard Pavlovian training session (Fig 2b; n = 7 Air-Saline; 8 Air-LY379268; 6 CIE-Saline; 7 CIE-LY379268). A repeated measures ANOVA indicated a CIE × treatment × cue interaction (F1,24 = 4.544, p = 0.04). Post-hoc analyses indicate that under saline conditions, CIE exposed mice did not discriminate between cue-on and cue-off sessions (p > 0.05), while AIR exposed mice licked significantly more during cue-on intervals than cue-off (p < 0.001). In addition, licking behavior during cue-on intervals was significantly lower for CIE animals than AIR controls (p < 0.001). In contrast, both AIR and CIE mice receiving the mGluR2/3 agonist LY379268 discriminated between cue-on and cue-off intervals (p < 0.05 for each group). Further, licking behavior during the cue-on interval was identical for AIR and CIE animals that received the mGluR2/3 agonist, indicating that administration of LY379268 restored the ability of reward-paired cues to drive consummatory behavior in CIE exposed animals. No significant effect of LY379268 was observed on licking during the cue-off intervals for AIR (p > 0.1) or CIE mice (p > 0.3). In contrast, CIE exposed mice administered LY379268 showed significantly higher licking behavior during the cue-on interval than mice receiving saline (p = 0.05), while AIR exposed animals did not differ (p > 0.6). Again, no sucrose residue was present in the well after the test session, suggesting that mGluR2/3 agonism did not reduce sucrose consumption under these testing conditions.
Effect of CIE on mGluR2 expression in the NAcS
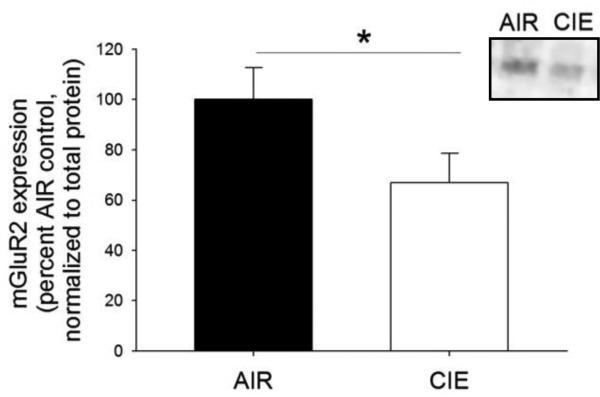
To determine if two weeks of CIE exposure and responding for ethanol on an FR1 schedule would result in reductions in mGluR2 protein expression in the NAcS, tissue was dissected from a separate cohort of mice and analyzed by Western blot analysis (n=8/group). This analysis revealed that CIE exposure resulted in a reduction in mGluR2 expression in the NAcS (p < 0.05; Fig 3).
Fig. 3.
Two weeks of CIE resulted in reductions of mGluR2 protein expression in the nucleus accumbens shell. *p < 0.05.
Effect of an mGluR2/3 antagonist on cue-guided licking behavior
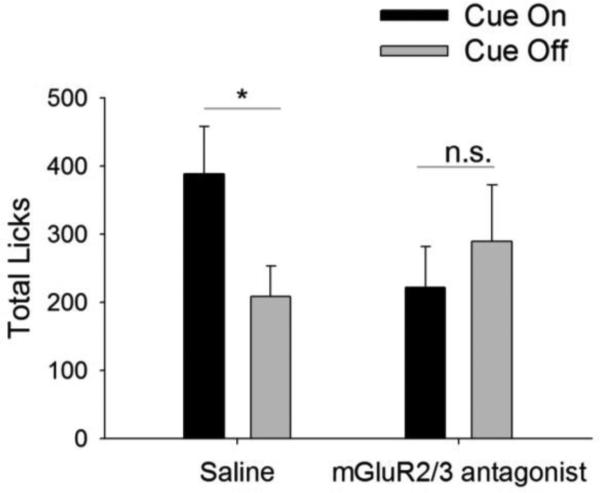
To assess the effects of reduced mGluR2/3 signaling on licking behavior, a separate cohort of mice received an i.p. injection of the mGluR2/3 antagonist LY341495 or saline vehicle (Fig 4; n = 7). A repeated measures ANOVA indicated a drug × cue interaction (F1,6 = 19.157, p = 0.005) but no main effects of drug (p > 0.5) or cue (p > 0.4) were observed. Post-hoc analyses indicated that mice discriminated between cue-on and cue-off intervals when receiving a saline injection (p < 0.05), but failed to discriminate between these intervals when administered the mGluR2/3 antagonist (p > 0.48). As with administration of the mGluR2/3 agonist LY379268, no sucrose residue was present in the well after testing, again indicating that mGluR2/3 antagonism by LY341495 did not reduce sucrose consumption under these parameters.
Fig. 4.
Administration of an mGluR2/3 antagonist mimics the effects of CIE on cue-guided behavior. While alcohol-naïve mice discriminated between cue-on and cue-off intervals when receiving a saline injection (p < 0.05), administration of the mGluR2/3 antagonist LY341495 prior to the test session resulted in loss of this ability to discriminate between cue-on and cue-off intervals, mimicking chronic ethanol effects on cue-guided behavior. n = 7. *p < 0.05
Discussion
In the present study, we observed that chronic ethanol exposure results in impairments in the ability to use reward-predictive cues to guide behavior. Following CIE exposure, mice exhibited less discrimination between cue-on and cue-off intervals in a task where cue presentation is predictive of reward delivery, suggesting that CIE resulted in impairments in cue-driven adaptive reward seeking. Interestingly, in a single test session, acute systemic administration of an mGluR2/3 agonist restored discrimination between cue-on and cue-off intervals such that CIE exposed mice exhibited patterns of consummatory behavior similar to that observed with the Air control mice. To assess a bidirectional role for mGluR2/3 signaling in discrimination between cue-on and cue-off intervals, we assessed whether administration of an mGluR2/3 antagonist to control mice could replicate the effect of CIE on consummatory behavior. Indeed, there was a loss of discrimination when an mGluR2/3 antagonist was administered prior to testing, while the same mice were capable of using reward-paired cues to guide behavior when receiving a saline injection. Together, these data indicate that CIE results in deficits in the use of cues to guide consummatory behavior that can be reversed by mGluR2/3 agonism, or mimicked by mGluR2/3 antagonism.
Of particular interest is the finding that manipulations of mGluR2/3 signaling acutely regulated discrimination between cue-off and cue-on intervals, suggesting that the deficit observed in CIE mice or in Air-exposed mice receiving an mGluR2/3 antagonist may not be related to a deficit in acquisition of stimulus-outcome information, but potentially an impairment in the use of that contingency to guide behavior. This deficit may be related to alterations in memory retrieval. In all cases, both CIE exposed and Air control mice consumed all of the sucrose available to them. While this observation does not preclude effects of CIE or mGluR2/3 signaling on motivation, it does suggests that these manipulations are not having gross effects on sucrose consumption in this paradigm. Additionally, CIE-exposed mice did not exhibit general deficits in behavior or reward consumption as they lever pressed for, and consumed, equivalent ethanol reinforcers as Air-exposed controls, indicating that a general motor impairment does not mediate this effect. Furthermore, it is unlikely that mGluR2/3 effects on locomotor behavior can explain the increased discrimination between cue-on and cue-off intervals as mGluR2/3 agonism has been reported to decrease, rather than increase, locomotor behavior in non-drug exposed animals (Arndt et al. 2014). Because the experimental design of the present study involved measurement of consummatory behavior that is mediated by reward-paired cues, these findings cannot be directly compared to previous studies that suggest differences in other cue-mediated behaviors, including Pavlovian approach and Pavlovian-to-instrumental behavior. However, we believe our observations of CIE-induced impairments in the acquisition of reward-paired cues to drive behavior complement findings from other groups showing deficits in the ability of reward-paired cues to invigorate instrumental behavior in a Pavlovian-to-instrumental paradigm task when tested after ethanol exposure (DePoy et al., 2014). Notably, in this model, the expression of cue-mediated behavior was not impaired when these behaviors are acquired prior to chronic alcohol exposure (DePoy et al., 2014).
In contrast to the results reported here, data from rats that had been chronically exposed to ethanol via access to a liquid diet containing 7% ethanol indicated no difference in licking discrimination between cue and inter-cue intervals (Ripley et al. 2004). However, the CIE vapor inhalation model --- in which animals experience continuing ethanol exposure and withdrawal --- has been shown to induce considerably different behavioral effects than 24 hour access liquid diet models, including differences in acquisition of a new response (DePoy et al., 2013) and reinforcer devaluation (Lopez et al. 2014). For these reasons, it is critical to consider the specific neurobiological and behavioral effects of these exposure paradigms, and how they may differentially model alcohol consumption and associated deficits in individuals with alcohol use disorders.
Though our findings do not address the precise neuroanatomical substrates at which mGluR2/3 signaling acts to restore cue-guided reward seeking, this effect is likely mediated through actions within the limbic corticostriatal circuitry that guides cue-mediated behavior. It has recently been observed that CIE-induced reductions in mGluR2 expression on infralimbic projection neurons is related to impairments in flexible regulation of ethanol seeking behavior under extinction conditions (Meinhardt et al., 2013). CIE exposure has also been shown to dysregulate NAc function, including producing elevations in extracellular glutamate that persisted beyond withdrawal (Griffin III et al. 2013). Indeed glutamatergic dysregulation in the NAc has been identified as a potential cause of uncontrolled alcohol consumption. While the precise mechanism by which this hyperglutamatergic state develops remains unclear, one possibility is that this results from a lack of negative feedback from presynaptic mGluR2 receptors. In support of this, mGluR2 receptor expression has been shown to be downregulated on prefrontal projections to the NAc after chronic alcohol exposure (Meinhardt et al. 2013). Our own data indicate a downregulation in mGluR2 at the time-point when animals would begin training on the Pavlovian procedure. This down-regulation of mGluR2 in the prefrontal-accumbens pathway likely impacts addictive alcohol-seeking and –taking behavior as pharmacological regulation of mGluR2/3 signaling in the NAc has also been shown to impact home cage drinking of ethanol (Griffin III et al. 2013), as well as ethanol reinforcement and stress-induced ethanol seeking (Kufahl et al. 2011). Furthermore, consistent with a reduction of mGluR2 expression and a general enhancement in extracellular glutamate, the ability of the mGluR2/3 agonist to reduce ethanol consumption and seeking was found to be greater in ethanol dependent animals.
A number of recent studies have identified glutamate receptors as potential targets for pharmacotherapeutic treatment of addictive disorders. Drugs targeting metabotropic receptors, including the Group II mGluRs, have been considered particularly appealing as their application appears to have considerably fewer side effects than pharmacological regulation of ionotropic glutamate receptors. mGluR2/3 receptors are primarily expressed presynaptically where it is thought they provide a negative feedback signal to reduce glutamate release at the synapse. Importantly, ethanol dependence has been shown to be associated with downregulation of mGluR2 expression in the prefrontal cortex in human alcoholics (Meinhardt et al., 2013). Though causality cannot be determined in this population, these data together suggest that dysregulation of metabotropic glutamate receptor expression may be related to the development of deficits in behavioral flexibility observed in alcohol use disorders. Together with findings from human populations, the current findings join a growing body of evidence suggesting that systemic manipulation of mGluR2/3 signaling can rescue ethanol-induced impairments in behavior, and support further investigation into the pharmacotherapeutic potential of this target in addictive behavior.
Acknowledgments
The authors would like to thank Laura Ralston for her expert technical assistance. This work was supported by NIH grants AA007474 and AA023141 (JMB) and AA010761 (LJC).
References
- Arndt DL, Arnold JC, Cain ME. The effects of mGluR2/3 activation on acute and repeated amphetamine-induced locomotor activity in differentially reared male rats. Exp Clin Psychopharmacol. 2014;22:257–65. doi: 10.1037/a0035273. doi: 10.1037/a0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR. Low prefrontal PSA-NCAM confers risk for alcoholism-related behavior. Nat Neurosci. 2012;15:1356–8. doi: 10.1038/nn.3194. doi: 10.1038/nn.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Zhang H, Villafane JJ, et al. Epigenetic and pharmacological regulation of 5HT3 receptors controls compulsive ethanol seeking in mice. Eur J Neurosci. 2014;6:999–1008. doi: 10.1111/ejn.12477. doi: 10.1111/ejn.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Lust SA, Tragesser SL. Specificity of P3 Event-Related Potential Reactivity to Alcohol Cues in Individuals Low in Alcohol Sensitivity. Psychol Addict Behav. 2010;24:220–228. doi: 10.1037/a0017705. doi: 10.1037/a0017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, et al. Impaired decision-making under risk in individuals with alcohol dependence. Alcohol Clin Exp Res. 2014;38:1924–31. doi: 10.1111/acer.12447. doi: 10.1111/acer.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014a;39:1893–901. doi: 10.1038/npp.2014.37. doi: 10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci. 2014b;8:301. doi: 10.3389/fnbeh.2014.00301. doi: 10.3389/fnbeh.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110:14783–8. doi: 10.1073/pnas.1308198110. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoy L, Daut R, Wright T, et al. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Biol. 2014 doi: 10.1111/adb.12131. doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007:599–607. doi: 10.1007/s00213-006-0535-8. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Gordon J, Taylor JR. Cytoskeletal determinants of stimulus-response habits. J Neurosci. 2013;33:11811–6. doi: 10.1523/JNEUROSCI.1034-13.2013. doi: 10.1523/JNEUROSCI.1034-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Haun HL, Hazelbaker CL, et al. Increased Extracellular Glutamate In the Nucleus Accumbens Promotes Excessive Ethanol Drinking in Ethanol Dependent Mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.256. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H, Brokate B, Hoffmann E, et al. Conditional responding is impaired in chronic alcoholics. J Clin Exp Neuropsychol. 2006;28:631–45. doi: 10.1080/13803390590949520. doi: 10.1080/13803390590949520. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50:267–76. doi: 10.1016/j.neuroimage.2009.11.076. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–73. doi: 10.1038/npp.2011.174. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC, Chandler LJ. Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol. 2014 doi: 10.1016/j.alcohol.2014.09.002. doi: 10.1016/j.alcohol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Involvement of AMPA Receptor GluR2 Subunits in Stimulus – Reward Learning: Evidence from Glutamate Receptor gria2 Knock-Out Mice. October. 2003;23:9500–9507. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, et al. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–806. doi: 10.1523/JNEUROSCI.4062-12.2013. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Borlikova G, Lyons S, Stephens DN. Selective deficits in appetitive conditioning as a consequence of ethanol withdrawal. Eur J Neurosci. 2004;19:415–25. doi: 10.1111/j.0953-816x.2003.03114.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S, Baro V, Trick L, et al. Exaggerated Waiting Impulsivity Associated with Human Binge Drinking, and High Alcohol Consumption in Mice. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.151. doi: 10.1038/npp.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.02.008. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, et al. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry. 2013;3:e337. doi: 10.1038/tp.2013.107. doi: 10.1038/tp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, van den Brink W, Beekman ATF, et al. Response inhibition in alcohol-dependent patients and patients with depression/anxiety: a functional magnetic resonance imaging study. Psychol Med. 2014;44:1713–25. doi: 10.1017/S0033291713002274. doi: 10.1017/S0033291713002274. [DOI] [PubMed] [Google Scholar]