Abstract
Hematopoietic stem cell transplantation (HSCT) is a lifesaving expensive medical procedure. Hence, more transplants are performed in more affluent countries. The impact of economic factors on patient outcome is less defined. We analyzed retrospectively a defined cohort of 102,549 patients treated with an allogeneic (N = 37,542; 37%) or autologous (N = 65,007; 63%) HSCT. They were transplanted by one of 404 HSCT centers in 25 European countries between 1999 and 2006. We searched for associations between center-specific microeconomic or country-specific macroeconomic factors and outcome. Center patient-volume and center program-duration were significantly and systematically associated with improved survival after allogeneic HSCT (HR 0·87; 0·84–0·91 per 10 patients; p < 0·0001; HR 0·90;0·85–0·90 per 10 years; p < 0·001) and autologous HSCT (HR 0·91;0·87–0·96 per 10 patients; p < 0·001; HR 0·93;0·87–0·99 per 10 years; p = 0·02). The product of Health Care Expenditures by Gross National Income/capita was significantly associated in multivariate analysis with all endpoints (R2 = 18%; for relapse free survival) after allogeneic HSCT. Data indicate that country- and center-specific economic factors are associated with distinct, significant, systematic, and clinically relevant effects on survival after HSCT. They impact on center expertise in long-term disease and complication management. It is likely that these findings apply to other forms of complex treatments.
Keywords: Hematopoietic stem cell transplantation, Macroeconomics, Microeconomics, Center effect, Patient volume, Program duration, Outcome, Survival, GNI/cap, HCE/cap, HDI, Risk assessment
Highlights
-
•
Hematopoietic stem cell transplants (HSCTs) are expensive; economics plays a role on use and outcome.
-
•
102,549 patients treated with HSCT in 404 European centers between 1999 and 2006 were investigated.
-
•
Center program duration, patient volume, accreditation and country economics were associated with survival.
-
•
Effects were significant, clinically relevant but distinct for allogeneic and autologous HSCT.
Hematopoietic stem cell transplantation (HSCT) is lifesaving but expensive; it's more frequently used in richer countries. We asked whether economics impact on outcome. For 102,549 patients treated with an allogeneic or autologous HSCT in 404 European centers between 1999 and 2006 survival was significantly better in centers with longer program duration and a higher patient volume. Survival was better in economically advantaged countries. Data indicate distinct, significant, systematic, and clinically relevant effects on long-term disease and complication management by country- and center-specific economic factors. It is likely that these effects apply to other forms of complex treatments.
1. Introduction
The close relationship between the economy of individual countries and the extent of their medical activities has long been accepted as reality but has become a topic of research only in the last decade (Waitzkin 2003). The relevance of macroeconomics in health provision has recently been highlighted by the World Health Organization (WHO), with more solid organ and hematopoietic stem cell transplants (HSCT) performed in more affluent countries (White et al., 2014, Gratwohl et al., 2015). Allogeneic HSCT represents one role model of a low volume, high cost, but lifesaving medical procedure (Copelan, 2006, Majhail et al., 2013, Khera et al., 2012). There is a strong association of country-specific economic factors with its use. Extensive studies have indicated significant correlations between transplant rates, e.g. the number of transplants compared to the number of inhabitants, and macroeconomic indices such as Gross National Income/capita (GNI/cap) or the availability of an unrelated donor registry. For a functioning national transplant network, a country must have a minimum size and a minimum of resources, teams require a minimum of support, donors must be available and patients have to have access to the transplant (Gratwohl et al., 2015, Gratwohl et al., 2010a, Gratwohl et al., 2010b).
It is intuitive that country-specific macroeconomic factors could have an impact on outcome as well. The vast numbers of well recognized patient-, disease-, donor- and transplant technique associated risk factors hamper simple comparisons (Copelan, 2006, Giebel et al., 2010, Gratwohl et al., 2009). There is as well a potential independent role of center-specific microeconomic factors at the level of the individual team. Complex medical procedures require the close cooperation of multiple persons and institutions, training, competency and experience; in short, team expertise. The role of “minimal center size” or “patient/hospital volume” has been discussed for many years, with conflicting data (Loberiza et al., 2005, Gratwohl et al., 1989, Frassoni et al., 2000, Matsuo et al., 2000, Giebel et al., 2013, Klingebiel et al., 2010, Horowitz et al., 1992, Taylor et al., 2013).The topic of “center experience” is not restricted to HSCT but a matter of debate in many fields of medicine. Data suggest that minimum numbers of specific practice are required to perform complex medical procedures safely; again, results have been conflicting (Hunsicker et al., 1993, Ozhathil et al., 2011, Guba, 2014, Birkmeyer et al., 2003, Lüchtenborg et al., 2013). Hence, relatively arbitrary thresholds have been set in accreditation standards (Jones et al., 2006, http://www.jacie.org/standards/6th-edition-2015, n.d). However, patient interest groups, health policy makers, competent authorities and other stakeholders are increasingly asking for objective measures of patient safety and outcome. They expect transparency and fair systems of comparisons between centers (Horowitz et al., 1992, Logan et al., 2008).
We previously identified JACIE accreditation as a center-specific factor after allogeneic HSCT and found indications for an effect of patient volume (Gratwohl et al. 2014). We used this well-defined large cohort of patients to investigate the multifaceted relationship between potential center- and country-specific economic factors and long-term outcome after the less complex autologous or the more complex allogeneic HSCT.
2. Methods
2.1. Study design
This retrospective observational analysis was based on a previously published cohort. It consists of patients transplanted between January 1st 1999 and December 31st 2006 and reported by 404 teams (see appendix) to the European Society for Blood and Marrow Transplantation (EBMT) database (www.ebmt.org) (Gratwohl et al. 2014). The analysis was initiated on January 1st 2013; when all analyses were completed, patient's survival data were updated as of January 1st, 2015. Last follow-up time was used as endpoint. Endpoints in all analyses were overall survival, relapse, non-relapse mortality and relapse free survival. They served as indicators for team expertise in complication management (non-relapse mortality), and as indicators for team expertise in disease management (relapse incidence). Relapse incidence and non-relapse mortality were taken as competing risks. All data were censored at 8 years post HSCT to provide for a homogeneous observation period.
All EBMT teams are required to obtain patients' consent and to have internal review board approval for their transplant programs and for data transfer to EBMT. The present study was released by the Ethics Committee Nordwest- and Zentralschweiz (www.eknz.ch).
2.2. Patient population
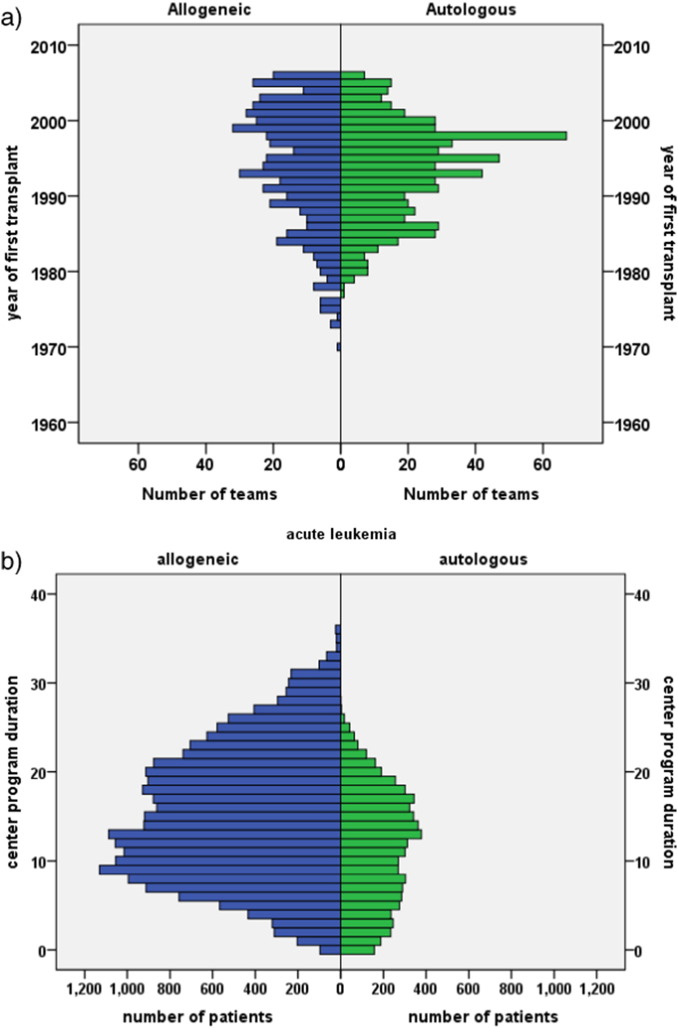
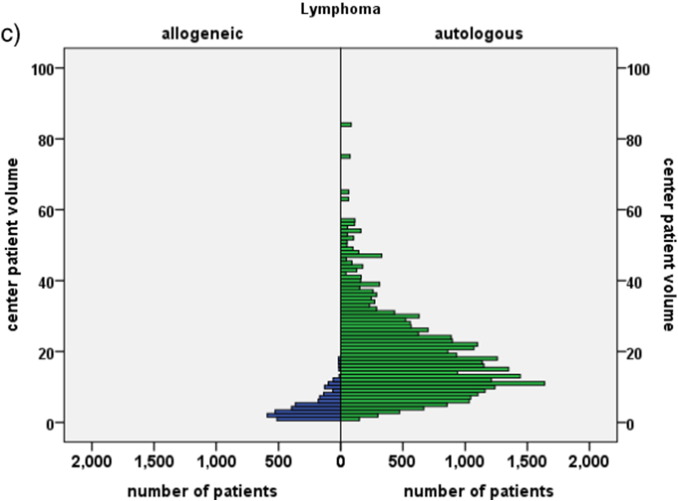
The cohort was restricted to 102,549 patients, 59% males, with a first allogeneic (N = 37,542; 37%) or autologous HSCT (N = 65,007; 63%) for an acquired hematological malignancy from 1999 to 2006 (Table 1). This corresponds to 93% of all patients transplanted during this time frame by the participating teams with these indications (see appendix). The cohort was heterogeneous; there was an increase in acute and a decrease in chronic myeloid leukemia and an increase in EBMT risk score over time (Gratwohl et al. 2009). Allogeneic HSCT was preferentially used for acute leukemias (N = 21,991; 78% allogeneic), chronic leukemias (N = 7486; 83% allogeneic) and myelodysplastic/myeloproliferative disorders (N = 3864; 94% allogeneic); autologous HSCT was preferentially used for lymphoma (N = 32,358; 91% autologous) and plasma cell disorders (N = 24,500; 95% autologous) (Table 1; Fig. 1). There were significant differences between centers regarding program duration (Fig. 1a, b; supplementary Fig. 1a), and patient volume (Fig. 1c; supplementary Fig. 1b), and between accredited and non accredited centers (Gratwohl et al. 2014).
Table 1.
Patient characteristics
Demographics of 102,549 HSCT (allogeneic 37,542; 37% and autologous 65,007; 63%) between 1999 and 2006 in Europe.
| Allogeneic HSCT | Autologous HSCT | Total | |
|---|---|---|---|
| N centers | 299 | 401 | 404 |
| JACIE⁎ accredited | 119 | 133 | 135 |
| JACIE⁎ not accredited | 180 | 268 | 269 |
| N Patients | 37,542 | 65,007 | 102,549 |
| Male % | 21,797 (58.2%) | 38,089 (58.7%) | 59,886 (58.5%) |
| Age | |||
| Median (years) | 39·2 | 53·4 | 49·1 |
| < 20 years | 7326 (20%) | 2240 (3%) | 9566 (9%) |
| 20–40 years | 12,055 (32%) | 11,800 (18%) | 23,855 (23%) |
| 40–60 years | 15,563 (41%) | 33,973 (52%) | 49,536 (48%) |
| > 60 years | 2598 (7%) | 16,994 (26%) | 19,592 (19%) |
| Disease | |||
| Acute leukemia | 21,991 (59%) | 6361 (10%) | 28,352 (27%) |
| Chronic leukemia | 7486 (20%) | 1556 (2%) | 9042 (9%) |
| MDS/MPS | 3864 (10%) | 232 (< 1%) | 4096 (4%) |
| Lymphoma | 3307 (9%) | 32,358 (50%) | 35,665(35%) |
| PCD | 894 (2%) | 24,500 (38%) | 25,394 (25%) |
| Year Transplant | |||
| 1999–2002 | 17,589 (47%) | 29,368 (45%) | 46,957 (46%) |
| 2003–2006 | 19,953 (53%) | 35,639 (55%) | 55,592 (54%) |
| 0–I | 5444(15%) | 3755 (6%) | 9199 (9%) |
| II–III | 16,680 (44%) | 35,623 (55%) | 52,303 (51%) |
| IV–V | 13,352 (36%) | 25,629 (39%) | 38,981 (38%) |
| VI–VII | 2066 (5%) | 0 | 2066(2%) |
JACIE = Joint Accreditation Committee of the International Society for Cellular Therapy and the European Society for Blood and Marrow Transplantation (www.jacie.org).
Fig. 1.
Distribution of center program duration and patient volume.
The figure depicts the diversity of the patient population and the heterogeneity in center program duration in years and in patient volume according to main disease indication for the 102,549 patients with an allogeneic (N = 37,542; 37%) or autologous (N = 65,007; 63%) hematopoietic stem cell transplant in Europe between 1999 and 2006.
a. Program duration.
The graph shows the number of teams (frequency) beginning their program to perform allogeneic HSCT (left part, blue) or autologous HSCT (right part, green) by the year of first transplant from 1970 to 2006.
b. Disease specific program duration.
The graph illustrates numbers of patients (frequency) by treatment modality (allogeneic HSCT, blue; autologous HSCT, green) and a selected main indication (acute leukemia) according to the program duration in years for this indication of their respective transplant team. Note that program duration for allogeneic HSCT was longer than for autologous HSCT, as illustrated above in Fig. 1a; Numbers of allogeneic HSCT for acute leukemia were higher than numbers of autologous HSCT.
For other main disease indications, see supplementary Fig. 1.
c. Center patient volume.
The graph depicts the number of patients (frequency) according to treatment modality (allogeneic HSCT, blue; autologous HSCT, green) and main indication (lymphoma) by the number of patients treated for this disease by their respective transplant team in their respective transplant year. Note that the number of patients transplanted with an allogeneic HSCT is much lower than the number of patients transplanted with an autologous HSCT for this indication; that fewer centers performed allogeneic HSCT for this indication and with lower patient numbers.
For other main disease indications, see supplementary Fig. 1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Definitions of selected economic factors
Economic factors were defined at the center (microeconomic) and country (macroeconomic) level as follows. Center program duration was defined by the numbers of years since the first transplant. Years were counted separately for the combination of each main indication and transplant type (allogeneic versus autologous HSCT) from the first transplant in the center up to the transplant of the individual patients included in the study (Fig. 1b; supplementary Fig. 1a). Center patient volume was defined by the number of HSCT by transplant type for each main indication in the respective year of each of the transplants (Fig. 1c; supplementary Fig. 1b). Center accreditation was defined by having obtained JACIE accreditation (www.jacie.org) by 2012 at the latest. The year 2012 was chosen on purpose; it corresponds to the previous analysis (Gratwohl et al. 2014).
For the analysis of country-specific macroeconomic factors, each country constituted one observation in the data set. As covariates, we used its HDI (Human Development Index), GNI/cap, Health Care expenditures/capita (HCE/cap), team density (defined as number of transplant teams per 10 million inhabitants) and transplant rates (defined as number of transplants per 10 million inhabitants). Information on population data and GNI/cap and HCE/cap were obtained from the World Bank (www.worldbank.org). Information on HDI was obtained from the United Nations Human Development Report (http://hdr.undp.org/en/statistics/hdi).
2.4. Statistical analysis
The focus of the statistical approach was the interaction between center- and country-specific macroeconomic factors, and their possible effects on outcome. All analyses were adjusted for the established key risk factors related to patient, disease, donor and transplant technique (Gratwohl et al. 2009). Covariates in all models were main disease (acute leukemia, chronic leukemia, myelodysplastic −/myeloproliferative syndrome, lymphoma and plasma cell disorders) and conditioning (reduced intensity conditioning/standard conditioning) as stratum. Included were age in four categories (< 20 years, 20–40 years, 40–60 years and > 60 years) and donor relationship for allogeneic HSCT (HLA-id sibling donor, matched unrelated donor, mismatched related and unrelated donor) as independent factors. EBMT risk score and calendar year were used as continuous variables. “Center” was included in all analyses as cluster. Patient volume and program duration were included as continuous variables.
Survival over an 8 year period was modeled using the center-specific factors (program duration, patient volume, and accreditation status) as well as established treatment, disease, patient and donor risks factors. An extended COX proportional hazards model was used. For an analysis at the country level, hazards between countries were analyzed together with country-specific macroeconomic factors and searched for a relation with transplant outcome. Country-specific hazard ratios were adjusted for established treatment, disease, patient and donor risks factors and for patient volume, program duration and center accreditation. The dependent variable for each country (the variable to be tested for association with the country-specific macroeconomic factors) is the Hazard Ratio of that country in a model containing all patient- and center-specific covariates. The hazard ratios for each country are the country-specific properties quantifying the excess risk or benefit for the four endpoints, in each country compared to the average risk among all countries. Ordinary least squares regressions as well as parametric and non-parametric correlation coefficients were used for associations between country-specific macroeconomic factors and the country-specific hazard ratios, again for all four outcome endpoints.
Country-specific macroeconomic factors varied over time but with minor changes in the ranking of the individual countries over time. Regression analyses confirmed the close association of the 2012 value with the mean value over time (HDI: R2 = 0·967; GNI/cap: R2 = 0·966; HCE/cap: R2 = 0·977; population: R2 = 0·998; supplementary Fig. 2). We decided therefore to use the year 2012 for data presentation, for comparability reasons with the selection of the year 2012 as cut off for accreditation.
3. Results
At the time of the analysis, 61,645 patients were alive, 40,904 had died. The probability of overall survival at 8 years was 48%, of relapse free survival 36%, of relapse incidence 45% and of non-relapse mortality 19%. Of note, overall survival declined from 76% at 1 year to 63% at 3 years, and 56% at five years. Outcome differed significantly between allogeneic and autologous HSCT with higher non-relapse mortality (30% vs 13%) and lower relapse incidence (33% vs 53%) after allogeneic HSCT (overall survival 43% vs 51%; relapse free survival 37% vs 35%). Overall survival improved significantly over time for allogeneic HSCT (per 10 years HR 0·70; 0·64–0.77; p < 0·001) and autologous HSCT (per 10 years HR 0·69; 0·63–0·76; p < 0·001).
Survival was significantly influenced by EBMT risk score. Overall survival decreased for each score point (allogeneic HSCT HR 1·21; 1·19–1·23; p < 0·001; autologous HSCT HR 1·17; 1·15–1·19; p < 0·001) due to increasing relapse incidence (allogeneic p < 0·001; autologous p < 0·001) and non-relapse mortality (allogeneic p < 0·001; autologous p < 0·001) (Table 2, Table 3).
Table 2.
Allogeneic HSCT.
Probability of overall survival (OS), relapse free survival (RFS), relapse incidence (RI), and non-relapse mortality (NRM) after HSCT depending on center specific economic factors.
Numbers represent hazard ratios (HR), adjusted for all other risk factors by stratification (see Methods section for details).
| OS | RFS | RI | NRM | |
|---|---|---|---|---|
| Accreditation | ||||
| JACIE − | 1 | 1 | 1 | 1 |
| JACIE + | 0·93 [0.87–0.99] | 0·95 [0.90–1.00] | 1·00 [0.93–1·06] | 0·91 [0·83–0·99] |
| Center patient volume | ||||
| Per 10 patients | 0·87 [0·84–0·91] | 0·92 [0·88–0·96] | 0·98 [0·92–1·04] | 0·86 [0·82–0·91] |
| 0–4 patients | 1 | 1 | 1 | 1 |
| 5–9 patients | 0·90 | 0·93 | 0·96 | 0·90 |
| 10–14 patients | 0·86 | 0·91 | 0·94 | 0·88 |
| 15–19 patients | 0·84 | 0·90 | 0·97 | 0·84 |
| > 20 patients | 0·78 | 0·86 | 0·95 | 0·76 |
| Center program duration | ||||
| Per 10 years | 0·90 [0·85–0·90] | 0·92 [0·87–0·96] | 0·93 [0·88–0·99] | 0·90 [0·83–0·97] |
| 0–4 years | 0·91 | 0·93 | 0·88 | 0·97 |
| 5–9 years | 0·87 | 0·89 | 0·88 | 0·90 |
| 10–14 years | 0·84 | 0·86 | 0·82 | 0·90 |
| 15–19 years | 0·81 | 0·83 | 0·85 | 0·81 |
| EBMT risk score | ||||
| Per score point | 1·21 [1·19–1·23] | 1·18 [1·16–1·20] | 1·16 [1·14–1·19] | 1·20 [1·17–1·22] |
Multiplier of the hazard ratio (interaction term in model) for the difference in speed of improvement between accredited and non-accredited centers.
EBMT risk score (score points 0–7 for allogeneic, 0–5 for autologous HSCT: age of patient: < 20 years = 0, 20–40 years = 1, > 40 = 2; disease stage: early = 0, intermediate = 1, advanced = 2; time interval from diagnosis to transplant: < 1 year = 0, > 1 year = 1; allogeneic HSCT only: donor type: HLA id sibling = 0; other donor = 1; donor recipient gender combination: all other = 0, female donor for male recipient = 1).
Table 3.
Autologous HSCT.
Probability of overall survival (OS), relapse free survival (RFS), relapse incidence (RI), and non-relapse mortality (NRM) after HSCT depending on center specific economic factors.
Numbers represent hazard ratios (HR), adjusted for all other risk factors by stratification (see Methods section for details).
| OS | RFS | RI | NRM | |
|---|---|---|---|---|
| Accreditation | ||||
| JACIE − | 1 | 1 | 1 | 1 |
| JACIE + | 1·08 [1·00–1·15] | 1·04 [0·99–1·11] | 1·07 [0·99–1·17] | 0·93 [0·80–1·07] |
| Center patient volume | ||||
| Per 10 patients | 0·91 [0·87–0·96] | 0·93 [0·89–0·97] | 0·92 [0·87–0·98] | 0·96 [0·87–1·07] |
| 0–4 patients | 1 | 1 | 1 | 1 |
| 5–9 patients | 0·96 | 0·96 | 0·96 | 0·93 |
| 10–14 patients | 0·97 | 0·95 | 0·95 | 0·97 |
| 15–19 patients | 0·94 | 0·93 | 0·94 | 0·90 |
| > 20 patients | 0·84 | 0·87 | 0·86 | 0·92 |
| Center program duration | ||||
| Per 10 years | 0·93 [0·87–0·99] | 0·92 [0·87–0·98] | 0·92 [0·84–0·98] | 0·99 [0·88–1·11] |
| 0–4 years | 1 | 1 | 1 | 1 |
| 5–9 years | 0·98 | 0·93 | 0·92 | 0·96 |
| 10–14 years | 0·97 | 0·91 | 0·89 | 0·99 |
| 15–19 years | 0·91 | 0·80 | 0·88 | 0·95 |
| EBMT risk score | ||||
| Per score point | 1·17 [1·15–1·19] | 1·14 [1·12–1·16] | 1·10 [1·08–1·13] | 1·30 [1·26–1·34] |
Multiplier of the hazard ratio (interaction term in model) for the difference in speed of improvement between accredited and non-accredited centers.
EBMT risk score (score points 0–7 for allogeneic, 0–5 for autologous HSCT: age of patient: < 20 years = 0, 20–40 years = 1, > 40 = 2; disease stage: early = 0, intermediate = 1, advanced = 2; time interval from diagnosis to transplant: < 1 year = 0, > 1 year = 1; allogeneic HSCT only: donor type: HLA id sibling = 0; other donor = 1; donor recipient gender combination: all other = 0, female donor for male recipient = 1).
3.1. Center and country-specific economic factors
We observed a close interaction between center and country specific economic factors but with distinct differences between allogeneic and autologous HSCT. Centers in higher income countries were more likely to be accredited (allogeneic HSCT −.678; p < 0.000; autologous HSCT −.693; p < 0.000) and to have a longer disease specific experience (allogeneic HSCT −.886; p < 0.000; autologous HSCT −.577; p = 0.001). Centers in higher income countries were more likely to have a higher patient volume of allogeneic (−.678; p < 0.000) but not of autologous HSCT (−.138; p = 0.482) (Spearman's rank test, factor vs GNI/cap/HDI).
3.1.1. Center-specific microeconomic risk factors and outcome after HSCT
Overall survival was significantly higher in JACIE accredited centers (N = 119 vs 180; HR 0·93; 0·87–0·99; p = 0·03) for patients transplanted with an allogeneic HSCT due to decreased non-relapse mortality (Table 2). Results confirmed previous findings with a now substantially longer follow up of a minimum of 8 years. We found no significant effects of JACIE accreditation after autologous HSCT (Table 3).
We showed a systematic and significant increase in overall and relapse free survival and a systematic decrease in non-relapse mortality and relapse incidence with patient volume, with a decrease in the hazard ratio, quantified by steps of 10 patients (HR 0·87; 0·84–0·91; p < 0·001 for overall survival in allogeneic HSCT (Fig. 2a); HR 0·91; 0·87–0·96; p < 0·001 in autologous HSCT) (Table 2, Table 3). The same systematic and significant increases in overall and relapse free survival were observed with increasing disease-specific center program duration, quantified in steps of 10 years (HR 0·90; 0·85–0·90; p < 0·001 for overall survival in allogeneic (Fig. 2b); HR 0·93; 0·87–0·99; p < 0·02 in autologous HSCT). Significant effects were seen on both non-relapse mortality and relapse incidence after allogeneic HSCT, only on relapse incidence after autologous HSCT (Table 2, Table 3).
Fig. 2.
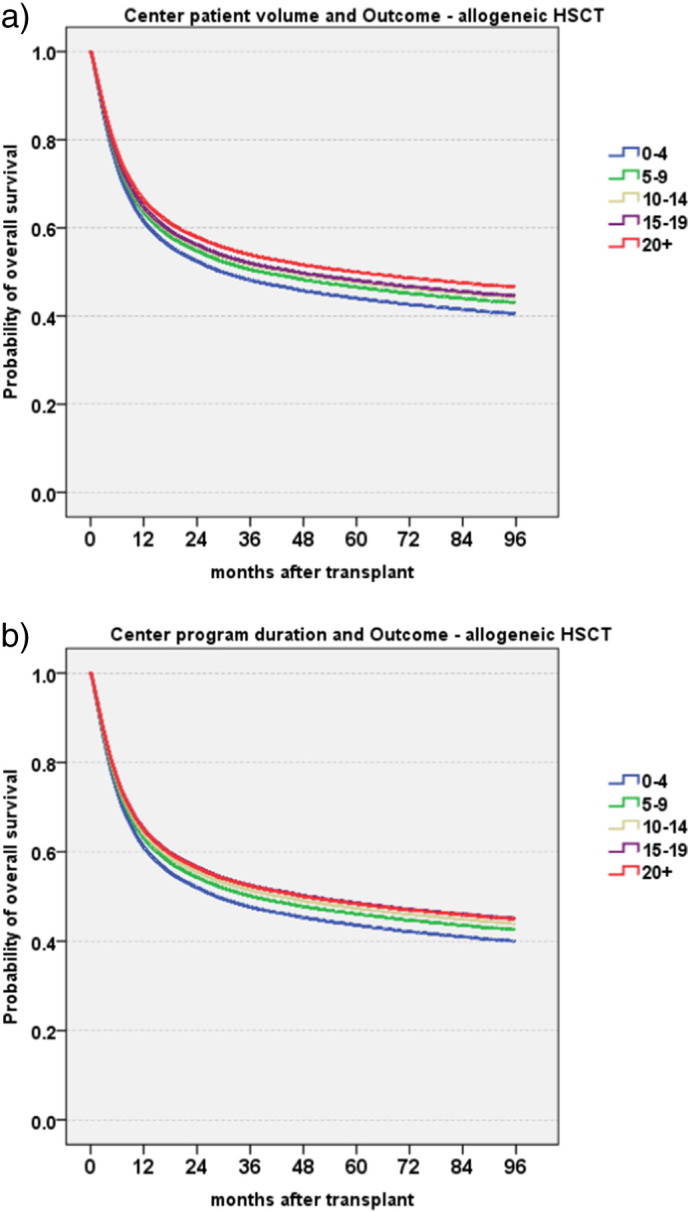
Center specific microeconomic aspects and overall survival.
The graphs illustrate Cox model estimates of overall survival of 37,542 patients with an allogeneic HSCT by center specific microeconomic aspects (for definitions see Methods section). The model integrates year of transplant, years of experience, size of the center, main disease indication, EBMT risk score, conditioning and accreditation status as factors.
a. Patient volume.
The graph shows Cox model estimates of overall survival depending on patient volume in absolute numbers. Numbers give numbers of patients treated for the respective disease in the year of the transplant at the patients' center (0–4 patients blue, 5–9 patients green, 10–14 patients yellow, 15–19 patients purple, 20 patients and > 20 patients red).
b. Program duration.
The graph shows Cox model estimates of overall survival depending on the centers program duration in years for. Numbers give numbers of years of program duration at the time of the transplant (0–4 years blue, 5–9 years green, 10–14 years yellow, 15–19 years purple, 20 years and > 20 years red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Country-specific macroeconomic factors and outcome
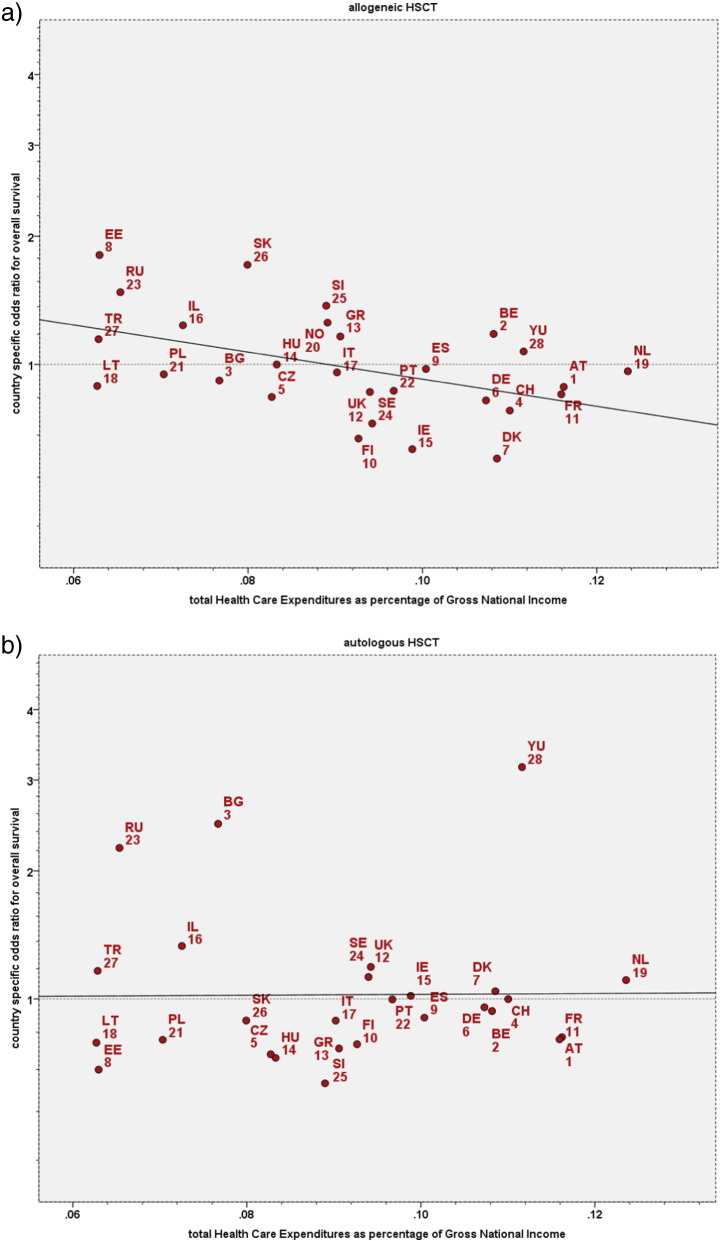
Integrating these center specific microeconomic factors at the individual patient level, outcome over the 8 year period of observation differed significantly between individual countries, independent of, but adjusted for patient, donor, and disease and center-specific risk factors. The effects differed for the individual macroeconomic factors by type of HSCT and the endpoint assessed. After allogeneic HSCT, overall survival and relapse free survival were significantly higher, and non-relapse mortality and relapse incidence lower in countries with higher economic indices. Relapse free survival, was significantly associated with GNI/cap (R2 16%; p = 0·009), HDI (R2 21%; p = 0·012), HCE/cap (R2 20%; p = 0·016) or higher transplant rates (R2 16%; p = 0·017) in univariate analysis. All macroeconomic factors were closely related with each other; in multivariate analysis, the product of HCE by GNI/cap (R2 = 18%; p = 0·004) (Fig. 3a) remained the sole significant variable. In contrast, no significant associations between country-specific macroeconomic factors and outcome could be found after autologous HSCT (Fig. 3b).
Fig. 3.
Country specific macroeconomic aspects and overall survival: impact of total Health Care Expenditures as a fraction of GNI/cap.
The graphs depict the regression analyses of the country specific risk of death (inverse of overall survival) after HSCT against the respective countries Health Care Expenditures as a fraction of its GNI/cap. Country specific hazard ratios were adjusted for established treatment, disease, patient and donor risks factors as well as for center size, center experience and center accreditation (for details see Methods section).
3a. Allogeneic HSCT.
The graph depicts the regression analysis of country specific hazards after allogeneic HSCT against the Health Care Expenditures as a fraction of GNI/cap.
Spearman rank test: R2 = 0·21; p = 0·01.
Abbreviations correspond to the respective country codes.
b. Autologous HSCT.
The graph depicts the regression analysis of country specific hazards after autologous HSCT against the Health Care Expenditures as a fraction of GNI/cap.
Spearman R2 = 0·03 (3% explained variance); p = 0·38.
Abbreviations correspond to the respective country codes.
Of interest, there was no significant correlation between the hazard ratios of the individual countries for allogeneic and autologous HSCT, with one exception. Countries with lower levels of non-relapse mortality after allogeneic HSCT had a lower non-relapse mortality after autologous HSCT as well (R2 = 25%; p = 0·011).
4. Discussion
Country- and center-specific economic factors were significantly associated with outcome in this large cohort of patients treated with an allogeneic or autologous HSCT. Survival was significantly and consistently better when the transplant was performed in a center with a higher patient volume and with longer program duration. It was significantly better after allogeneic HSCT when the transplant was performed in an accredited team and in a country with more resources. The effects on outcome were systematic and clinically relevant but there were distinct differences between allogeneic and autologous HSCT. The observed better overall and relapse free survival after allogeneic HSCT was accompanied by a similar systematic reduction in relapse incidence and non-relapse mortality. In contrast, improved survival after autologous HSCT with increasing patient volume or program duration was restricted to a systematic reduction in relapse incidence; there were no significant associations with non-relapse mortality, with accreditation or with country specific macroeconomic factors.
These differences between allogeneic and autologous HSCT warrant some explanations, as the systematic pattern of the effects renders a simple chance finding unlikely. They help as well to understand the findings. Allogeneic HSCT is a highly complex procedure with non-relapse mortality as the main cause of failure, autologous HSCT is less complex but with a high risk of relapse (Copelan, 2006, Gratwohl et al., 2009). HSCT is not an isolated procedure; outcome depends on the pre-transplant history, the patient and donor selection and includes long-term post-transplant follow-up. Only half of the mortality occurs within the first year after HSCT. Success requires expertise in disease and complication management and the close collaboration of multiple individuals at various levels and over a long time period. Longer program duration and a higher patient volume can improve expertise in disease management, as shown by the reduced relapse rate and improved survival for all patients. Longer program duration, a higher patient volume and standardized patient management (indicated by the JACIE accreditation) can improve expertise in complication management as shown by the reduced non-relapse mortality after the more complex allogeneic HSCT with its inherently higher non-relapse mortality. These microeconomic center effect findings fit with the macroeconomic country observations. The impact of more resources for the health care system in a given country (indicated by the proportion of HCE/cap of the respective GNI/cap) and of the network infrastructure (as indicated by the HDI) became visible only after the more complex allogeneic HSCT with its higher non-relapse mortality over a long time period. More resources are required to achieve sufficient expertise for the team, and to maintain the pre- and post-transplant networks for individual patients (Majhail et al. 2012). This is probably best reflected by the lower long-term survival of patients transplanted in the years 1999 to 2006 in a center that failed to strive for accreditation at least in 2012.
The present findings fit into ongoing general discussions on quality assurance, safety issues, center comparisons and minimum numbers of patients required for specific complex treatments (Horowitz et al., 1992, Taylor et al., 2013, Hunsicker et al., 1993, Ozhathil et al., 2011, Guba, 2014, Birkmeyer et al., 2003, Lüchtenborg et al., 2013). Our data indicate that there is no threshold, rather a systematic impact of both, experience in years and experience in numbers. “Center effects” therefore should not be reduced to just one aspect, such as patient volume, learning curve or surgical skills (Guba, 2014, Birkmeyer et al., 2003). Microeconomic center-specific effects comprise program duration, patient volume, center accreditation as well as training programs, standardization and individual skills; they are complemented by the respective country-specific factors. Data show as well that team expertise in disease and complication management is required for optimal outcome (Majhail et al. 2012).Measuring day 30 or day 100 mortality alone is an insufficient measure of quality assurance when long-term disease free survival presents the most valid endpoint. As evidenced by this study, individual center- or country-specific economic factors may impact variably according to and depending on the complexity of the procedure (Greenfield et al., 2014, Apperley et al., 2000). Our results potentially apply to solid organ transplantation and other fields of complex medical care as well.
Our study has some limitations. We describe associations, not causal effects. A higher patient volume may not necessarily improve survival and a long-established institution still can have poor results. We tried to adjust for key patient-, donor- and treatment-related factors. We cannot exclude that patient risk profiles differed significantly between centers or countries and might have accounted for some of the differences. We have no explanation why the country specific hazards were favorable in some after allogeneic but not autologous, or vice versa or after both. Further studies are required, not to address “who is better”, but rather to dissect potential reasons for the differences, and what measures are required to improve clinical practice, education, competency and infrastructure in centers with poorer outcomes to make them as good as those centers with the best outcomes. We could not identify significant associations of economic factors with non-relapse mortality after autologous HSCT. The relative low non-relapse mortality is an insufficient explanation per se; we observed a significant reduction of non-relapse mortality over time and found a positive correlation in country specific non-relapse mortality for autologous and allogeneic HSCT. The larger, accredited teams showed a trend for lower non-relapse mortality but higher relapse incidence; they might have a shorter waiting time, associated with a slightly higher risk profile of their patients (Frassoni et al. 2000).
The results of this report have implications. Outcome comparisons directed to patients will need to integrate country- and center-specific micro- and macroeconomic factors for an informative evaluation of the disease-specific experience of their respective transplant center. Transplant organizations and transplant centers will have to consider concentration on specific disease entities. The prospect of every transplant team embarking on or continuing HSCT for all potential indications should be reappraised by transplant communities and health service commissioners, and concentration of clinical practice may improve outcomes in a defined population or region. Those looking to establish new centers should embark on transplant activity if they have sufficient resources for infrastructure and a clear perspective to achieve adequate activity and accreditation over a rapid and pre-defined time period. Professional organizations such as EBMT and JACIE are challenged to support emerging teams in less privileged countries via training and partnership programs to achieve these goals. Small centers in high income countries and close to existing larger enters, in contrast, might need to reconsider their activities. Competent authorities should be aware of these results which might not be restricted to HSCT. They should foster, in cooperation with the transplant organizations, regulations and legislations, and facilitate regional task distribution (Jones et al. 2006).
In conclusion, these results show that macroeconomic factors are systematically and significantly associated with outcome after HSCT. Clinical HSCT practice should accommodate for the needs of individual patients with specific disease states, with the appropriate stem cell procedure, delivered at experienced transplant centers, thereby providing better outcomes regarding long-term survival, quality of life and cost-effectiveness, compared to any other non-transplant strategy. We recommend that micro- and macroeconomic factors should be integrated into clinical transplant practice for both, individual patients and for the cooperative working between regional transplant centers, to improve outcomes through better patient selection and increased expertise in disease- and complication management.
Funding
The study was funded by the European Group for Blood and Marrow Transplantation EBMT and the European Leukemia Net ELN. EBMT is supported by grants from the corporate members: Amgen Europe, ViroPharma Europe, Celegene International SARL, Genzyme Europe B.V., Gilead Sciences Europe Ltd., Miltenyi Biotec GmbH, Schering-Plough International Inc., Bristol Myers Squibb, CaridianBCT Europe NV, Cephalon Europe, F. Hoffmann-La Roche Ltd., Fresenius Biotech GmbH, Therakos Inc., Alexion Europe, Chugai Sanofi–Aventis, Merck Sharp and Dohme, Novartis, Pfizer, and Pierre Fabre Médicament.
Authorship and disclosures
AG and RB designed the study concept. E McG, CRdE and HB were responsible for the collection and quality control of the data and the organization of the datasets. AS, AR and JAS were responsible for the JACIE accreditation part. PD, NK, PL, MM and AN were responsible for the adequacy of the data within the working parties of the EBMT. RB and TS performed the statistical analysis. JP is responsible for the EBMT activity survey office. MG advised the macroeconomic aspects. AG and RB had access to all data and wrote the manuscript. All authors have contributed significantly to the manuscript, seen the final version and approved it.
Writing of the manuscript was the sole responsibility of the authors. There are no conflicts of interest to declare.
Acknowledgments
The authors acknowledge the cooperation of the participating teams and their staff ; the JACIE Accreditation Office in Barcelona, the JACIE Board and Executive Committee, the JACIE Medical Directors, the JACIE Accreditation Committee, all JACIE inspectors and EBMT registered transplant programs that worked hard to prepare and achieve JACIE accreditation; the EBMT presidents and the working party chairs; the EBMT statistical office in Leiden; the EBMT Co-ordination offices in Barcelona, Paris and London , the Austrian Registry (ASCTR), the Czech BMT Registry, the French Registry (SFGM-TC), the German Registry (DRST), the Italian Registry (GITMO), the Dutch Registry (HOVON), the Spanish BMT Registry (GETH), the Swiss Registry (SBST), the Turkish BMT Registry and the British Registry (BSBMT). JFA acknowledges the support of the NIHR Biomedical Research Centre at Imperial College Healthcare NHS Trust and JS the support of Sheffield Hospitals Charity.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.11.021.
Appendix A. Supplementary data
Supplementary material
Supplementary figures
References
- Waitzkin H. Report of the WHO Commission on Macroeconomics and Health: a summary and critique. Lancet. 2003;361:523–526. doi: 10.1016/S0140-6736(03)12491-9. [DOI] [PubMed] [Google Scholar]
- White S.L., Hirth R., Mahíllo B. The global diffusion of organ transplantation: trends, drivers and policy implications. Bull. World Health Organ. 2014;92:826–835. doi: 10.2471/BLT.14.137653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A., Pasquini M.C., Aljurf M. For the Worldwide Network of Blood and Marrow Transplantation WBMT. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2:e91–100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- Copelan E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Majhail N.S., Mau L.W., Denzen E.M., Arneson T.J. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone Marrow Transplant. 2013;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera N., Zeliadt S.B., Lee S.J. Economics of hematopoietic cell transplantation. Blood. 2012;120:1545–1551. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Baldomero H., Aljurf M. Worldwide Network of Blood and Marrow Transplantation. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303 doi: 10.1001/jama.2010.491. (1617–24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A., Schwendener A., Baldomero H. Changes in the use of hematopoietic stem cell transplantation: a model for diffusion of medical technology. Haematologica. 2010;95:637–643. doi: 10.3324/haematol.2009.015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel S., Labopin M., Ehninger G. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Association of Human Development Index with rates and outcomes of hematopoietic stem cell transplantation for patients with acute leukemia. Blood. 2010;116:122–128. doi: 10.1182/blood-2010-01-266478. [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Stern M., Brand R. European Group for Blood and Marrow Transplantation and the European Leukemia Net. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115:4715–4726. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- Loberiza F.R., Zhang M.J., Lee S.J. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–2987. doi: 10.1182/blood-2004-10-3863. [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Hermans J., Lyklema A., Zwaan F.E. Bone marrow transplantation for leukaemia in Europe. Folia Haematol Int Mag Klin Morphol Blutforsch. 1989;116:353–360. [PubMed] [Google Scholar]
- Frassoni F., Labopin M., Powles R. Effect of centre on outcome of bone-marrow transplantation for acute myeloid leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 2000;355:1393–1398. doi: 10.1016/s0140-6736(00)02137-1. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Hamajima N., Morishima Y., Harada M. Hospital capacity and post-transplant survival after allogeneic bone marrow transplantation: analysis of data from the Japan Society for Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2000;26:1061–1067. doi: 10.1038/sj.bmt.1702681. [DOI] [PubMed] [Google Scholar]
- Giebel S., Labopin M., Mohty M. The impact of center experience on results of reduced intensity: allogeneic hematopoietic SCT for AML. An analysis from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2013;48:238–242. doi: 10.1038/bmt.2012.131. [DOI] [PubMed] [Google Scholar]
- Klingebiel T., Cornish J., Labopin M. Pediatric Diseases and Acute Leukemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115 doi: 10.1182/blood-2009-03-207001. (3437–46) [DOI] [PubMed] [Google Scholar]
- Horowitz M.M., Przepiorka D., Champlin R.E. Should HLA-identical sibling bone marrow transplants for leukemia be restricted to large centers? Blood. 1992;79:2771–2774. [PubMed] [Google Scholar]
- Taylor D.S., Dharmar M., Urquhart-Scott E. Relationship between pediatric blood and marrow transplant center volume and day + 100 mortality: pediatric blood and marrow transplant consortium experience. Bone Marrow Transplant. 2013;48:514–522. doi: 10.1038/bmt.2012.192. [DOI] [PubMed] [Google Scholar]
- Hunsicker L.G., Edwards E.B., Breen T.J., Daily O.P. Effect of center size and patient-mix covariates on transplant center-specific patient and graft survival in the United States. Transplant. Proc. 1993;25:1318–1320. [PubMed] [Google Scholar]
- Ozhathil D.K., Li Y.F., Smith J.K. Impact of center volume on outcomes of increased-risk liver transplants. Liver Transpl. 2011;17:1191–1199. doi: 10.1002/lt.22343. [DOI] [PubMed] [Google Scholar]
- Guba M. Center volume, competition, and outcome in German liver transplant centers. Transplant. Res. 2014;3:6. doi: 10.1186/2047-1440-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmeyer J.D., Stukel T.A., Siewers A.E., Goodney P.P., Wennberg D.E., Lucas F.L. Surgeon volume and operative mortality in the United States. N. Engl. J. Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- Lüchtenborg M., RiazSP C.V.H. High procedure volume is strongly associated with improved survival after lung cancer surgery. J. Clin. Oncol. 2013;31:3141–3146. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- Jones R.B., Anasetti C., Appel P. Committee on Hematopoietic Cell Therapy Quality Outcomes. ASBMT committee report: white paper on measurement of quality outcomes. Biol. Blood Marrow Transplant. 2006;12:594–597. doi: 10.1016/j.bbmt.2006.04.001. [DOI] [PubMed] [Google Scholar]
- http://www.jacie.org/standards/6th-edition-2015. (Last accessed August 4, 2015)
- Logan B.R., Nelson G.O., Klein J.P. Analyzing center specific outcomes in hematopoietic cell transplantation. Lifetime Data Anal. 2008;14:389–404. doi: 10.1007/s10985-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A., Brand R., McGrath E. Joint Accreditation Committee (JACIE) of the International Society for Cellular Therapy and the European Group for Blood and Marrow Transplantation, and the European Leukemia Net. Use of the quality management system "JACIE" and outcome after hematopoietic stem cell transplantation. Haematologica. 2014;99:908–915. doi: 10.3324/haematol.2013.096461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhail N.S., Rizzo J.D., Lee S.J. Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT); SociedadeBrasileira de Transplante de MedulaOssea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Hematol. Oncol. Stem Cell Ther. 2012;5:1–30. doi: 10.5144/1658-3876.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield D., Civil M., Donnison A. A mechanism for revising accreditation standards: a study of the process, resources required and evaluation outcomes. BMC Health Serv. Res. 2014;14:571. doi: 10.1186/s12913-014-0571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperley J.F., Brand R., Gratwohl A., Hermans J., Niederwieser D., Frassoni F. Is center size an accurate surrogate marker of quality of patient care in stem cell transplant units? Blood. 2000;96:846A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary figures