Abstract
Background
A number of neurological and neurodegenerative diseases share impaired cognition as a common symptom. Therefore, the development of clinically applicable therapies to enhance cognition has yielded significant interest. Previously, we have shown that activation of lysophosphatidic acid receptors (LPARs) via gintonin application potentiates synaptic transmission by the blockade of K+ channels in the mature hippocampus. However, whether gintonin may exert any beneficial impact directly on cognition at the neural circuitry level and the behavioral level has not been investigated.
Methods
In the current study, we took advantage of gintonin, a novel LPAR agonist, to investigate the effect of gintonin-mediated LPAR activation on cognitive performances. Hippocampus-dependent fear memory test, synaptic plasticity in the hippocampal brain slices, and quantitative analysis on synaptic plasticity-related proteins were used.
Results
Daily oral administration of gintonin for 1 wk significantly improved fear memory retention in the contextual fear-conditioning test in mice. We also found that oral administration of gintonin for 1 wk increased the expression of learning and memory-related proteins such as phosphorylated cyclic adenosine monophosphate-response element binding (CREB) protein and brain-derived neurotrophic factor (BDNF). In addition, prolonged gintonin administration enhanced long-term potentiation in the hippocampus.
Conclusion
Our observations suggest that the systemic gintonin administration could successfully improve contextual memory formation at the molecular and synaptic levels as well as the behavioral level. Therefore, oral administration of gintonin may serve as an effective noninvasive, nonsurgical method of enhancing cognitive functions.
Keywords: fear conditioning, ginseng, long-term potentiation, lysophosphatidic acid receptor, synaptic plasticity
1. Introduction
Cognitive enhancement has yielded significant interest as disrupted learning and memory is one of several widely shared deficits found in many neurological and neurodegenerative diseases. So far, a few manipulations have been proposed to enhance cognitive function [1]. Lysophosphatidic acids (LPAs) are extracellular phospholipidic molecules that bind to G-protein coupled LPA receptors (LPARs) [2]. LPARs-associated signaling plays a number of roles in the early development of central nervous system [3]. Recent studies have revealed that activation of LPARs in adult brain exerts a significant role in intellectual processing. For example, a study using intrahippocampal LPA infusion to activate Rho pathway showed enhanced long-term spatial memory in water maze test [4]. These studies suggest that the activation of LPARs could contribute to enhanced cognitive performances.
Recently, we have shown that a newly identified active component of ginseng, gintonin, enhances synaptic transmission in mature hippocampal synapses via the activation of LPARs [5]. Gintonin, a subset of glycolipoproteins, consists of LPAs and other protein complexes with highly abundant acidic amino acids [6], [7]. Recent investigations suggest that gintonin is a potential candidate to mediate the beneficial actions of ginseng [8]. Previous studies showed that the application of gintonin induces a transient Ca2+ increase through the binding of LPARs (LPAR1-6) in oocytes preparation [6], [9]. We have shown that gintonin-mediated LPAR activation increases the neuronal excitability and slightly depolarizes the resting membrane potential of the hippocampal pyramidal neurons [5]. These observations strongly suggest that gintonin-mediated LPAR activation possibly may modulate synaptic plasticity and/or learning processes.
Here, we aimed to investigate the effect of systemic gintonin administration on cognitive function. We administered gintonin daily by oral gavage to mice for 7 d and evaluated their memory employing the contextual fear-conditioning test. We also examined whether chronic gintonin administration enhanced long-term synaptic plasticity and accompanied by synaptic plasticity-related proteins in the hippocampus.
2. Materials and methods
2.1. Study participants
Male DBA/2 mice (22–25 g) were used in all experiments (Charles River, Gapyeong, Republic of Korea). Mice were 7 wks old at the time of arrival and maintained for at least 1 wk before the start of the experiments. They were housed in a controlled vivarium on a 12-h light/dark cycle with controlled temperature (22 ± 1°C) and humidity (50 ± 10%). They were allowed free access to food and water. Behavior testing was conducted during the light cycle. Among strains of mice available, the DBA/2 mouse strain was chosen due to their poor performance in the contextual fear conditioning [10]. All experiments were approved by the Institutional Animal Care and Use Committee of Konkuk University, Seoul, Korea (KU14154).
2.2. Gintonin preparation
Gintonin was extracted from Panax ginseng without ginseng saponin as previously described [7]. We used the similar median effective dose (ED50) concentrations, that exerted LPA receptor activation in Xenopus oocyte preparation in a previous study [7]. Crude gintonin was dissolved in vehicle solution for oral administration at the concentration (50 mg/kg) that was previously shown to have behavioral effect in vivo [5]. For acute treatment, gintonin was dissolved in dimethyl sulfoxide (DMSO; final concentration of DMSO, 0.1%).
2.3. Administration of gintonin and experimental procedures
To test the effect of gintonin on cognitive function, mice were randomly separated into two groups: vehicle (saline, n = 8) and gintonin (50 mg/kg, n = 7). After 5 d of handling, vehicle or gintonin was administered daily by oral gavage for 7 d. The experimenters were blind to the administration condition. The mice were sacrificed the day after the final treatment; 30 min after the last testing on the behavioral test (see Fig. 1).
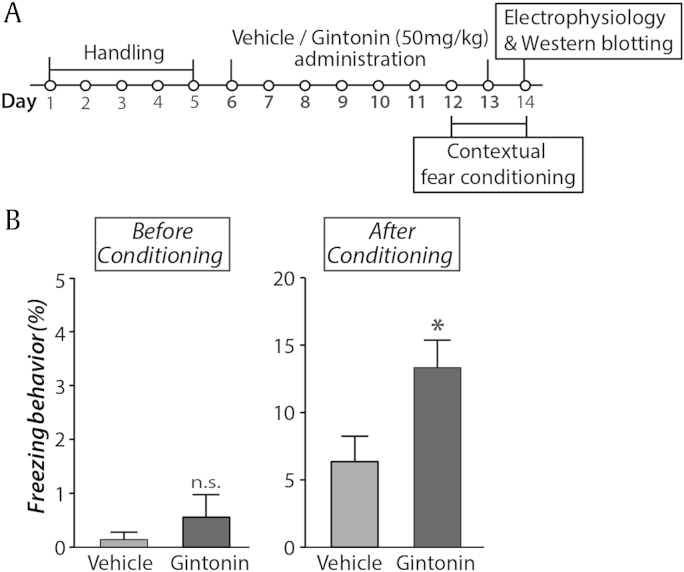
Fig. 1.
Gintonin enhances contextual fear memory. (A) Experimental scheme. (B) On the testing day of fear conditioning task, freezing levels in vehicle or gintonin administered mice were quantified. The gintonin-administered mice (n = 8) showed an increased fear memory retention when compared with vehicle-administered mice (n = 7, *p < 0.05) whereas there is no difference in basal anxiety (p > 0.4). Error bars represent standard error of the mean. n.s., not significant.
2.4. Slice preparation
Seven-wk-old mice were sacrificed after daily oral administration of either vehicle or gintonin for electrophysiology recordings, 1 d after the final treatment. Researchers were blind to the experimental group of mice. Brain slices (350 μm) were prepared with a microtome (VT 1000S, Leica, Nussloch, Germany) in ice-cold sucrose cutting buffer (212mM sucrose, 3mM KCl, 26mM NaHCO3, 1.25mM NaHPO4, 7mM MgCl2, and 10mM glucose) bubbled with 95% O2/5% CO2 mixed gas. Brain slices are placed in a chamber containing artificial cerebrospinal fluid (aCSF: 1mM NaH2PO4, 26.2mM NaHCO3, 118mM NaCl, and 2.5mM KCl, and freshly added 11mM glucose, 2mM CaCl2, and 1mM MgCl2) and recovered in a 35°C water bath for 1 h, after which they were maintained at room temperature. Temperature was maintained between 31°C and 33°C during recording. For acute gintonin experiments, brain slices were incubated with 3 μg/mL concentration of gintonin, which we have shown to induce rapid, LPAR-dependent synaptic enhancement.
2.5. Electrophysiology
Field excitatory postsynaptic potentials (fEPSPs) were recorded in CA1 dendrites using glass pipettes (0.5–1.5 MΩ) filled with aCSF with two bipolar stimulating electrodes being placed in the stratium radiatum. fEPSPs were alternately evoked through each bipolar electrode with 30-s intervals. Theta burst stimulation (TBS, 20 bursts of 4 pulses at 100 Hz) was delivered at one pathway to induce LTP while the other pathway remained unstimulated during the TBS stimulation.
2.6. Behavioral apparatus
The contextual fear-conditioning task was carried out in square chambers (17.78 cm W × 17.78 cm D × 30.48 cm H, Coulbourn Instruments, Allentown, PA, USA). The chamber was equipped with a grid floor that transmits a foot shock during training. The chamber was surrounded with soundproofed shells and a ventilation fan provided background noise. The foot shock was generated via a Coulbourn programmable tone generator (model #A69-20; Coulbourn Instruments). The stimulus was controlled by Coulbourn Graphic State software (Coulbourn Instruments). The chamber was cleaned with 70% alcohol before and after each trial.
2.7. Contextual fear-conditioning procedure
The learning procedures consisted of three stages: (1) preexposure, which allowed the mice to adapt the training chamber, (2) training, during which the mice were exposed to foot shocks, and (3) testing, in which mice responded to the context and freezing behaviors were measured. Each phase was conducted at 24-h intervals. On the first day of contextual fear conditioning (preexposure), mice were placed into the chamber for 12 min. On the training day, mice were placed into the same chamber, and allowed to adapt for 148 s. Following this, the mice received a 2-s, 0.75 mA shock for three trials, with an intertrial interval of 30 s. On the testing day, mice were placed in the training chamber and allowed to move freely for 3 min. Fear memory was assessed by measuring the freezing behavior of the mice. Freezing behavior was defined as the absence of any movement, except for breathing. The percentage of time spent freezing was observed every 2 s by a trained observer [11].
2.8. Western blot analysis
Thirty min after testing on the contextual fear-conditioning test, all mice were sacrificed; the hippocampus was dissected and snap-frozen in dry ice, and stored at −80°C before processing for analysis by Western blot. The hippocampus was homogenized in ice-cold buffer containing 20mM Tris (pH 7.5), 5% glycerol, 1.5mM EDTA, 40mM KCl, 0.5mM dithiothreitol, and protease inhibitors (539131, Calbiochem). Homogenates were centrifuged at 14,000 g for 1 h at 4°C. The supernatant was collected and protein concentration was determined using the Bradford assay [12]. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked for 1 h in 5% skimmed milk in Tris-buffered saline containing Tween-20 and then incubated with primary antibodies against cyclic adenosine monophosphate-response element binding protein (CREB; 1:1,000, Cell Signaling Technology, Danvers, MA, USA), phospho-CREB (1:2,000, Millipore, Darmstadt, Germany), brain-derived neurotrophic factor (BDNF) (1:500, Santa Cruz biotechnology, Santa Cruz, CA, USA), and β-Actin (1:5,000, Sigma, St. Louis, MO, USA). Following this, membranes were incubated with the antirabbit immunoglobulin G (IgG) horseradish-peroxidase-conjugated secondary antibodies (1:2,000, Cell Signaling Technology, Danvers, MA, USA) or antimouse IgG antibody (1:5,000, Millipore, Darmstadt, Germany). The protein bands were immunodetected using an enhanced chemiluminescence kit (GE Healthcare, St. Giles, UK) and detected on an X-ray film. The bands were quantified by densitometry scanning using the Image Gauge program (Fuji Film, Tokyo, Japan).
2.9. Statistical analysis
All data are presented as the mean ± standard deviation. Two-tailed unpaired t tests were used for statistical comparisons using built-in data analysis tool pack in Microsoft Office Excel 2007. A p value < 0.05 was considered significant.
3. Results
3.1. DBA/2 mice show better retention of fear memory after oral administration of gintonin
To investigate whether LPAR activation via systemic gintonin delivery could contribute to hippocampal memory formation, we assessed contextual fear conditioning, a well-known hippocampal-dependent learning procedure in DBA/2 mice after daily oral administration of vehicle or gintonin for 1 wk (50 mg/kg, Fig. 1A) [13]. Animals were exposed to an unfamiliar context (CS) with foot shocks (US) during training on Day 2. Fear memory was tested 24 h later by observing freezing behavior for 3 min when animals are exposed to CS only. The overall freezing of gintonin-administered mice was significantly higher than the vehicle-administered mice (Fig. 1B, right, p < 0.05), suggesting that daily administration of gintonin enhances hippocampal dependent contextual fear memory. The long-term administration of gintonin at a given dose seems to have minimal effect on general health of animals. We observed no difference in weight changes with chronic gintonin treatment compared to vehicle treatment (saline group: 22.29 ± 1.70 g; gintonin group: 22.50 ± 1.69 g, p > 0.8). In addition, there was no difference in freezing between the two groups during preexposure (Fig. 1B, left, p > 0.4), indicating that gintonin did not affect the alertness of the mice and that the gintonin itself was not anxiogenic at the concentration we administered.
3.2. Gintonin administration enhances long-lasting synaptic potentiation in the Schaffer collateral pathway
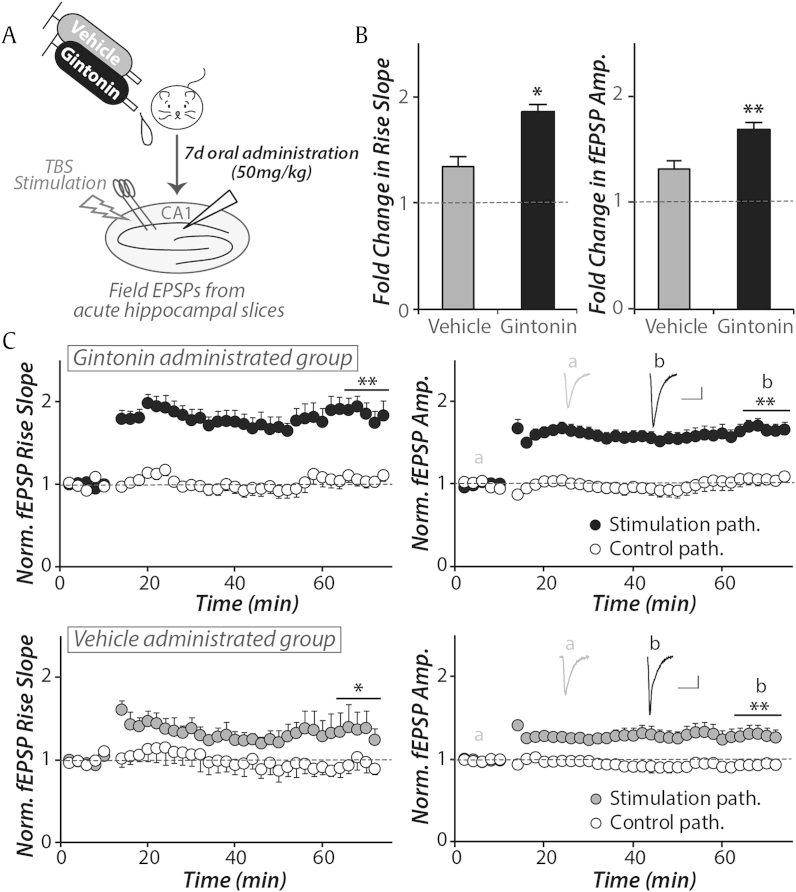
Next, we examined the effect of chronic oral administration of gintonin on synaptic plasticity in the hippocampus. Acute hippocampal slices were prepared 24 h after 7 d administration of vehicle or gintonin (50 mg/kg, Fig. 2A). The experimenter was blind to the different drug groups. fEPSPs were measured in the CA1 dendritic region, while stimulating the stratium radiatum region of CA1 (Fig. 2A). Theta-burst stimulation (TBS, 20 bursts of 4 pulses at 100 Hz, each 0.2 ms) was used to induce LTP. The rise slope and amplitude of the fEPSPs was significantly more potentiated by approximately 2.5-fold in the gintonin-administered group when compared with the vehicle-administered group (Figs. 2B, 2C, n = 10–12, p < 0.05). The control pathway remained unchanged between groups (Figs. 2B, 2C, p > 0.5). These results indicated that gintonin could contribute to enhance memory formation at the cellular level, in agreement with our behavioral observations.
Fig. 2.
Oral administration of gintonin enhances long-term synaptic potentiation in the Shaffer collateral pathway. (A) Theta burst stimulations (TBS, 20 bursts of 4 pulses at 100 Hz) were given at CA3-CA1 synapses from acutely prepared hippocampal slices after daily oral administration of vehicle or gintonin for 7 d (50 mg/kg). (B) In the gintonin-administered group, both the rise slope (10–90%) and amplitude of the stimulated pathway were significantly more potentiated than the vehicle-administered group (34% vs. 86% for rise slope, *p < 0.05; 30% vs. 67% for amplitude, **p < 0.01, vehicle vs. gintonin group, respectively, n = 7–17). The bar graph showed the fold change in rise slope and amplitude of 60 min after LTP induction compared to baseline. (C) Stimulated pathways were significantly potentiated after gintonin administration compared to control pathway (gintonin: rise slope, **p < 0.01, amplitude, **p < 0.01, vehicle: rise slope, **p < 0.01, amplitude, *p < 0.05). The gray trace represents an averaged field excitatory postsynaptic potentials (fEPSP) during baseline (a) and the black trace represents an averaged fEPSP response 50–60 min after TBS stimulation (b) (scale bar: 20 ms and 0.2 mV). Error bars represent standard error of the mean.
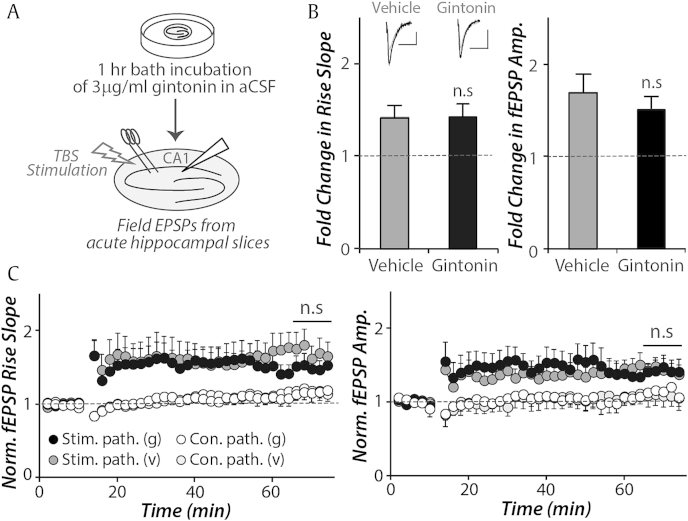
Interestingly, when brain slices was incubated in gintonin (3 μg/mL in aCSF) for 1 h prior to recording fEPSPs, TBS induced only a comparable amount of synaptic potentiation to vehicle incubation (Fig. 3, p > 0.4 for amplitude; p > 0.9 for rise slope). This observation suggests that prolonged and acute LPAR activation via gintonin bath application may have distinctive effects on synaptic plasticity.
Fig. 3.
Acute gintonin bath application has no effect on synaptic plasticity in the CA3-CA1 synapses. (A) Theta burst stimulations (TBS, 20 bursts of 4 pulses at 100 Hz, repeated twice in 10 s) were given at Shaffer collateral pathway 1 h after gintonin or vehicle incubation (3 μg/mL). (B, C) Upon 1 h gintonin incubation, TBS-induced fold changes in both rise slope (10–90%) and amplitude of the stimulated pathway (stim. path.) remained comparable with vehicle group (42% vs. 42% for rise slope, p > 0.9; 70% vs. 50%, for amplitude, p > 0.4, vehicle (v) vs. gintonin (g) group, respectively, con.path.: control pathway, n = 5–7). The gray trace represents an averaged field excitatory postsynaptic potentials (fEPSP) during baseline and the overlaid black trace represents an averaged fEPSP response 50–60 min after TBS stimulation (scale bar: 20 ms and 0.2 mV). Error bars represent standard error of the mean.
3.3. Gintonin increases the expression of memory-related proteins in the hippocampus
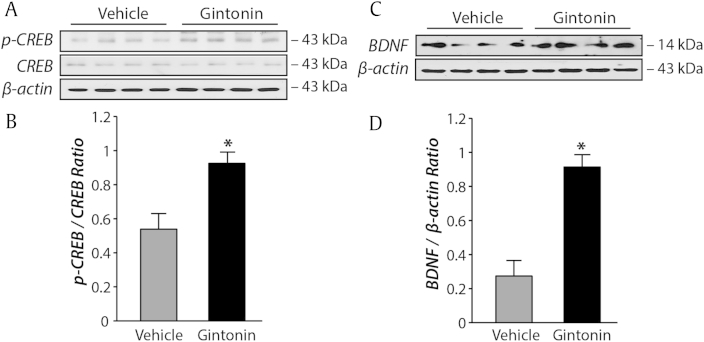
Given that the chronic gintonin administration but not acute gintonin incubation enhances LTP, gintonin-induced enhancement of synaptic potentiation is anticipated to accompany changes in gene expression levels. We examined whether prolonged gintonin oral administration would alter the expression of CREB, a well-characterized transcription factor, which can modulate the expression of a number of learning and memory-related genes [14]. It is well known that phosphorylation of site by various protein kinases initiates gene transcription, thereby leads to consolidation of the memory [15], [16]. Therefore, elevated phosphorylated-CREB (p-CREB) is considered as a molecular marker of late LTP in the hippocampus after learning [17]. We found that the p-CREB was significantly increased following fear learning in the gintonin-administered mice when compared with vehicle-administered mice (Fig. 4, p < 0.05). There was no difference in levels of CREB between vehicle- and gintonin-administered groups (Fig. 4, p > 0.9).
Fig. 4.
Gintonin administration increases hippocampal cyclic adenosine monophosphate-response element binding (CREB) phosphorylation and brain-derived neurotrophic factor (BDNF) expression. (A) Representative immunoblots of hippocampal CREB and phosphorylated CREB (p-CREB). (B) CREB and p-CREB levels were quantified 30 min after testing in the contextual fear conditioning paradigm. Data are expressed as the ratio between p-CREB/CREB. There was a significant increase in p-CREB in gintonin-administered mice when compared with vehicle-administered mice (n = 7–8, *p < 0.05). (C) Representative immunoblots of hippocampal BDNF. (D) Data are expressed as the ratio between BDNF/β-Actin. Overall hippocampal BDNF expression was significantly higher in gintonin-administered mice when compared with vehicle-administered mice (n = 7–8, *p < 0.05). Error bars represent standard error of the mean.
Next, we investigated the expression level of BDNF after vehicle or gintonin administration to examine whether increased p-CREB contributed to an increase in its downstream substrates. BDNF is a primary target of CREB and learning increases BDNF expression [18]. A decrease in BDNF was reported to disrupt learning and memory formation [19]. Our results showed that the BDNF expression was significantly increased in gintonin-administered mice when compared with vehicle-administered mice (Fig. 4, p < 0.05), suggesting that prolonged systemic gintonin administration caused an increase in the phosphorylation of CREB and BDNF expression, which may contribute to enhance hippocampal-dependent fear memory formation.
4. Discussion
In the current study, we have explored the effect of gintonin-mediated LPAR activation on synaptic plasticity and hippocampal-dependent memory task in adult mice. Chronic administration of gintonin significantly strengthened hippocampal-dependent contextual fear memory retention and augmented long-term synaptic potentiation in the Schaffer collateral pathway of the hippocampus. We found increased expression of synaptic plasticity-related molecules after gintonin administration. Our findings suggest that the prolonged oral delivery of gintonin could contribute to improve contextual memory formation.
Previous studies using LPAR1-deficient mice have shown that a lack of LPAR1 causes impaired cognitive performances. For instance, LPAR1 deficiency causes impairments in hippocampus-dependent spatial memory test [4]. Genetic LPAR1 deficiency or a blockade of LPAR1 with antagonists results in impaired fear memory extinction [20]. Previous studies have shown that gintonin activates LPARs in vitro and ex vivo preparations [5], [6], [8]. Therefore, gintonin-mediated activation of LPAR was anticipated to improve cognition. In line with this, when we examined the fear memory retention using the contextual fear conditioning test, we found that freezing behavior was remarkably increased upon prolonged gintonin administration in DBA/2 mouse. Among strains of mice available, DBA/2 mouse strain has been previously reported to perform poorly in the contextual fear learning paradigm [10]. Previously, gintonin administration was shown to decrease amyloid beta protein 1-42 release in the hippocampus in an animal model of Alzheimer's disease [13]. Our current observation provides further support that gintonin's memory-enhancing effect is not limited to neurodegenerative conditions, thus allowing the extended application of gintonin for healthy individuals with declined cognition.
Previously, we have shown that LPAR activation via gintonin potentiates evoked synaptic transmission in the hippocampus of juvenile animals [5]. However, the effect of gintonin on synaptic efficacy in the adult hippocampus has not been investigated so far. Here, we assessed LTP in Schaffer collateral pathway, which expresses N-methyl-D-aspartate receptor (NMDAR)-dependent postsynaptic LTP [21]. Gintonin has been shown to work through NMDAR activation [6]; it activates phospholipase C (PLC)/protein kinase C (PKC) pathway, which is known to mediate LTP priming and maintenance in hippocampal neurons [22]. In addition, it has been suggested that the fear learning is strongly correlated with the synaptic potentiation of the Schaffer collateral pathway [23]. Therefore, it is anticipated that gintonin-mediated chronic LPAR activation would enhance synaptic potentiation of the CA1-CA3 synaptic circuitry.
When we examined how LPAR activation modulated the synaptic plasticity in the Schaffer collateral pathway, a remarkable enhancement in the potentiation of synaptic activity (∼2.5-fold of vehicle group) following 1 wk of daily gintonin administration was observed. This observation suggests that chronic gintonin administration augmented LTP in the adult hippocampus. It has been shown that a direct bilateral infusion of LPAs into rodent hippocampi activates Rho-dependent signaling pathways but not ERK-dependent pathways, which results in improved performance in the Morris water maze test [4]. The activation of Rho-dependent signaling pathway contributes to LTP initiation, consolidation and dendritic plasticity [24]. Gintonin is a strong LPAR-binding component and we observed a significant enhancement in LTP following gintonin administration; therefore, it is possible that gintonin-mediated LPAR activation might lead to Rho pathway activation and thereby contribute to enhance LTP [24], although this speculation needs further examination.
Interestingly, hippocampal LTP was enhanced only following chronic systemic administration but not acute incubation of gintonin, strongly suggesting that gintonin-mediated LPAR activation could have two distinct mechanisms of action depending on duration of activation. Finally, we explored molecular changes that accompanied the improved fear memory and enhanced LTP. BDNF is a neurotrophic factor that acts as key regulator of protein synthesis required for LTP induction [25], [26], [27]. The interaction between BDNF and tyrosine kinase receptor B (TrkB) triggers signalling cascades that induce both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and NMDAR trafficking in synapses [28]. In addition, BDNF increases postsynaptic density protein 95 (PSD95), which is important for dendritic spine formation [29]. When BDNF is released from vesicles in an activity-dependent manner, one of the primary targets of action is CREB and associated kinases [30]. CREB is one of the well-studied transcriptional factors that govern the expression of a series of synaptic proteins in an activity-dependent manner [31], [32]. We observed that increased BDNF expression and CREB phosphorylation were accompanied by prolonged systemic LPAR activation. It still remains to be investigated whether the activation of LPARs may directly stimulate the release of BDNF or the phosphorylation of CREB in the hippocampus.
Our study has analyzed synaptic and molecular changes occurring in the hippocampus; however, prolonged LPAR activation may affect other brain structures. LPAR signaling cascades have been implicated in different aspects of cognition beyond hippocampus-dependent memory. For example, LPAR1-deficient mice have significant deficits in prepulse inhibition, which is found in patients with schizophrenia [33]. In addition, the amygdala of LPAR1-deficient mice was reported to express higher c-fos upon exposure to acute stressors [20]. These studies suggest that the action of gintonin is not limited to the hippocampus, implying that the chronic activation of LPARs may modulate other behaviors, including emotional behaviors. Further investigations may reveal additional sites of action for gintonin and gintonin-dependent behavioral modulations.
In summary, our study suggests that systemic gintonin administration enhances the capacity of synaptic plasticity in the mature hippocampus and significantly improves fear memory formation. We have shown that enhanced LTP and improved cognitive performances were accompanied by an increase in BDNF expression and phosphorylation of CREB protein. Therefore, the activation of LPARs could be a useful target for alleviating memory impairments and cognitive deficits in patients with neurodegenerative diseases in addition to healthy aged people.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by Konkuk University in 2014 (C.C.).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Lee Y.S., Silva A.J. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi J.W., Herr D.R., Noguchi K., Yung Y.C., Lee C.W., Mutoh T., Lin M.E., Teo S.T., Park K.E., Mosley A.N. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 3.Pilpel Y., Segal M. The role of LPA1 in formation of synapses among cultured hippocampal neurons. J Neurochem. 2006;97:1379–1392. doi: 10.1111/j.1471-4159.2006.03825.x. [DOI] [PubMed] [Google Scholar]
- 4.Dash P.K., Orsi S.A., Moody M., Moore A.N. A role for hippocampal Rho-ROCK pathway in long-term spatial memory. Biochem Biophys Res Commun. 2004;322:893–898. doi: 10.1016/j.bbrc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Park H., Kim S., Rhee J., Kim H.J., Han J.S., Nah S.Y., Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113:1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 6.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyo M.K., Choi S.H., Shin T.J., Hwang S.H., Lee B.H., Kang J., Kim H.J., Lee S.H., Nah S.Y. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J Ginseng Res. 2011;35:209–218. doi: 10.5142/jgr.2011.35.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nah S.Y. Gintonin: a novel ginseng-derived ligand that targets G protein- coupled lysophosphatidic acid receptors. Curr Drug Targets. 2012;13:1659–1664. doi: 10.2174/138945012803529947. [DOI] [PubMed] [Google Scholar]
- 9.Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Lee S.M., Kim H.C., Rhim H.W., Nah S.Y. Molecular mechanisms of large-conductance ca (2+) -activated potassium channel activation by ginseng gintonin. Evid Based Complement Alternat Med. 2013;2013:323709. doi: 10.1155/2013/323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen P.V., Abel T., Kandel E.R., Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weeber E.J., Atkins C.M., Selcher J.C., Varga A.W., Mirnikjoo B., Paylor R., Leitges M., Sweatt J.D. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 13.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 14.Kandel E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bito H., Deisseroth K., Tsien R.W. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez G.A., Montminy M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 17.Barco A., Alarcon J.M., Kandel E.R. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 18.Hall J., Thomas K.L., Everitt B.J. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 19.Liu I.Y., Lyons W.E., Mamounas L.A., Thompson R.F. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedraza C., Sanchez-Lopez J., Castilla-Ortega E., Rosell-Valle C., Zambrana-Infantes E., Garcia-Fernandez M., Rodriguez de Fonseca F., Chun J., Santin L.J., Estivill-Torrus G. Fear extinction and acute stress reactivity reveal a role of LPA receptor in regulating emotional-like behaviors. Brain Struct Funct. 2014;219:1659–1672. doi: 10.1007/s00429-013-0592-9. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y.Z., Chen R.S., Rothwell J.C., Wen H.Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Cohen A.S., Raymond C.R., Abraham W.C. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus. 1998;8:160–170. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Song C., Detert J.A., Sehgal M., Moyer J.R., Jr. Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neurophysiol. 2012;107:3397–3408. doi: 10.1152/jn.00692.2011. [DOI] [PubMed] [Google Scholar]
- 24.Rex C.S., Chen L.Y., Sharma A., Liu J., Babayan A.H., Gall C.M., Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y., Christian K., Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson S.L., Abel T., Deuel T.A., Martin K.C., Rose J.C., Kandel E.R. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 27.Gruart A., Sciarretta C., Valenzuela-Harrington M., Delgado-Garcia J.M., Minichiello L. Mutation at the TrkB PLCgamma-docking site affects hippocampal LTP and associative learning in conscious mice. Learn Mem. 2007;14:54–62. doi: 10.1101/lm.428307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 29.Yoshii A., Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno M., Yamada K., Maekawa N., Saito K., Seishima M., Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002;133:135–141. doi: 10.1016/s0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 31.Mayr B., Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 32.Gruart A., Benito E., Delgado-Garcia J.M., Barco A. Enhanced cAMP response element-binding protein activity increases neuronal excitability, hippocampal long-term potentiation, and classical eyeblink conditioning in alert behaving mice. J Neurosci. 2012;32:17431–17441. doi: 10.1523/JNEUROSCI.4339-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison S.M., Reavill C., Brown G., Brown J.T., Cluderay J.E., Crook B., Davies C.H., Dawson L.A., Grau E., Heidbreder C. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol Cell Neurosci. 2003;24:1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]