Abstract
Epidemiological studies have shown that dietary sugar intake has a significant impact on the development of breast cancer. One proposed mechanism for how sugar impacts cancer development involves inflammation. In the current study, we investigated the impact of dietary sugar on mammary gland tumor development in multiple mouse models, along with mechanisms that may be involved. We found that sucrose intake in mice comparable to levels of Western diets led to increased tumor growth and metastasis, when compared to a non-sugar starch diet. This effect was ascribed in part to increased expression of 12-lipoxygenase 12-LOX) and its arachidonate metabolite 12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid 12-HETE). We determined that fructose derived from the sucrose was responsible for facilitating lung metastasis and 12-HETE production in breast tumors. Overall, our data suggested that dietary sugar induces 12-LOX signaling to increase risks of breast cancer development and metastasis.
Introduction
Identifying the risk factors for development of breast cancer is a continuing public health priority. In various epidemiological studies, the risk of breast cancer may be increased by diets with high intake of added sugar, defined by either glycemic index or glycemic load that physiologically measures the ability of food to increase postprandial glucose levels (1–3). The per capita consumption of sugar in the United States has surged to 70 lb per year (4), and an increase in consumption of sugar-sweetened beverages has been identified as an important contributor to the epidemic of obesity, heart disease and cancer worldwide (5). The glucose, insulin and subsequent Warburg effect have been the major focuses of investigations into the role of sugar, especially glucose, in cancer development; however, the inflammatory cascade may be an alternative route of studying sugar-driven carcinogenesis that warrants further investigation. No prior studies have investigated the direct effect of sugar consumption on development of breast cancer using breast cancer animal models or examining the purported mechanisms.
Different carbohydrates may have distinct effects on tumorigenesis. In 1979, Hoehn et al. demonstrated that after injection of 7,12-dimethylbenz(α)-anthracene (DMBA), rats that consumed sucrose/dextrose diets developed more mammary tumors than rats fed with starch (6). However using the same rat model, Klurfeld and colleagues observed opposite results that consumption of a corn starch diet was associated with the most palpable and largest tumors than rats fed with sucrose or lactose diets (7). Therefore, the effects of dietary sucrose versus starch on breast tumor growth are still inconclusive. Further, in these studies, the authors did not report the effects of carbohydrate diets on breast tumor metastasis, which could be more susceptible to dietary factors.
Dysregulation of bioactive lipids, especially cyclooxygenase and lipoxygenase metabolites of arachidonate, collectively called eicosanoids, or their pathway constituents is implicated in many degenerative diseases, including inflammation and cancers (8). These bioactive lipids are critical components of the cell membrane that are involved in cell membrane function and cell signaling. Among various lipoxygenases, 12-LOX and 12-HETE are known to play a role in tumorigenesis in animal models and humans. Overexpression of 12-LOX protein was detected in multiple types of cancers (9–11). Interestingly, 12-HETE has been shown to increase the proliferation and invasion of breast cancer cells by induction of collagenase secretion (12). Tissue 12-LOX protein expression was significantly correlated with tumor–node–metastasis (TNM) staging suggesting that 12-LOX can be a prognosis marker for breast cancer (13). Therefore, we hypothesized that dietary sugar induces breast tumor development by altering 12-LOX signaling. To test this hypothesis, we performed four dietary intervention studies in the three animal models including a mouse mammary gland tumor model that carries a MMTV/unactivated neu transgene, a human triple-negative breast cancer cell (MDA-MB–231) orthotopic mouse model, and a breast cancer lung metastasis mouse model (injected with 4T1 mouse breast cancer cells).
Materials and Methods
Mice
Mice of strain FVB/N-Tg(MMTVneu)202Mul/J were purchased from the Jackson Laboratory (Bar Harbor, Maine), bred and housed at the MD Anderson Cancer Center animal facility. For the MDA-MB-231 orthotopic mouse model, 6- to 8-week-old mice were supplied from MD Anderson’s Department of Experimental Radiation Oncology and were allowed to acclimatize for 3 days prior to study initiation. Mice were fed an AIN-76 diet (Harlan Laboratories, Livermore, CA) and water ad libitum. MDA-MB-231 cells (1.5 × 106) were injected into the fourth mammary fat pad (groin mammary fat). For the 4T1 orthotopic model, BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) at 5 weeks old. They were acclimated for 1 week before being injected with 4T1 mouse breast cancer cells (10,000 cells per mouse) into their fourth mammary fat pad.
Diets
Mice in the four studies were randomized to different diet groups and fed one of four diets (Supplementary Table S1). Both starch control and sucrose-enriched diets were made by Research Diet, Inc. (New Brunswick, NJ) and kept in the refrigerator upon arrival. They were composed of 15% fat by weight, which equates to 30% of energy per day for humans. The amount of sucrose was increased from 0 g/kg (control) to 62.5 g/kg, 125 g/kg, 250 g/kg and 500 g/kg.
Eicosanoid analyses
Frozen mammary tumor tissue (20–25 mg) was analyzed for eicosanoid levels using a modification of a previously described method (14).
Study Approval
All animal experiments were approved by The University of Texas MD Anderson Cancer Center Animal Care and Use Committee.
Statistical analysis
GraphPad Prism was used to perform statistical tests (t-test or ANOVA). P-values less than 0.05 were considered statistically significant. All data are presented as mean ± SEM. * p<0.05; ** p<0.001 compared to control.
Results and Discussion
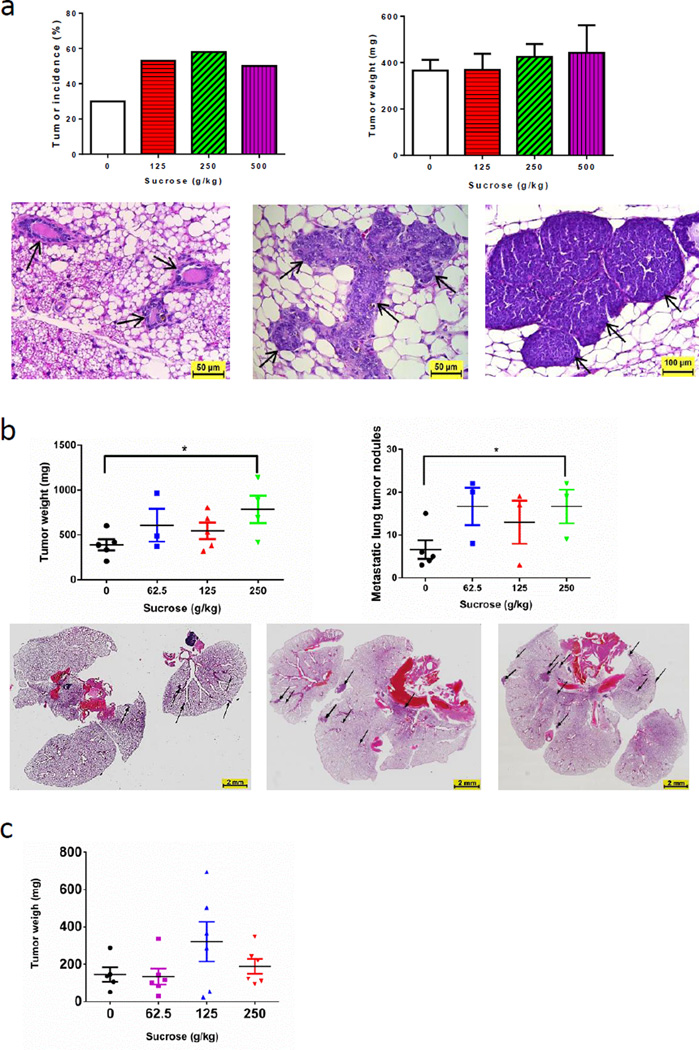
At 6 months of age, 30% of MMTV/neu mice on the starch control diet had measurable tumors, whereas 50%, 58% and 50% of mice on the sucrose-enriched diet mice (125, 250 and 500-g/kg, respectively) had developed mammary tumors. The average tumor weight from the 250-g/kg sucrose diet-fed mice was 50 mg higher than that of the starch control group (Fig. 1a), indicating that sucrose not only shortened the onset of mammary tumors but also increased the proliferation of mammary carcinoma cells. Histological examination revealed that the starch control diet fed mice had minor hyperplasia in their mammary glands, while mice that were on the 500-g/kg sucrose diet had adenomas in their mammary glands as early as 3 months of age. Interestingly, dietary sucrose had no statistically significant effect on body weight (Supplementary Fig. S1) after 7 months of treatment. This is consistent with reports in the literature that sucrose does not affect body weight in rodents (15,16).
Figure 1. Dietary sucrose consistently increased mammary gland tumor growth in three mouse models.
a. Tumor development in MMVT/neu mouse model. Top left: Tumor incidence at 6 months of age (n ≥12). Top right: Tumor weight. Bottom: H&E staining of normal mammary gland (left) and hyperplasia (middle) from control diet-fed mice and of adenomas from mice fed a 500-g/kg sucrose diet (right) at 3 months of age. b. Sucrose increased primary mammary gland tumor growth and metastasis of tumor in 4T1 cell-bearing mice (n≥3). Top left: Tumor weight. Top right: Lung tumor metastasis (micro and macro metastases combined). Bottom: Lung nodules in control (left), 62.5 g/kg sucrose-treated (middle) and 125 g/kg sucrose-treated (right) mice, showing that the tumor size was larger in the lungs from sugar treated mice compared that to the control mice. c. Tumor weight in MDA-MB-231 tumor-bearing mice (n≥5). Data are represented as mean ± s.e.m. *p<0.05.
We further investigated the effect of the sucrose-enriched diet on the growth of primary tumors and metastasis potential using mouse orthotopic models injected with mouse mammary carcinoma 4T1 cells or human MDA-MB-231 cells. In the 4T1 cell-bearing mice, both the average weight of primary tumors and numbers of lung metastases were statistically significantly higher in the 250-g/kg sucrose-diet group than in the starch control-diet group (Fig. 1b). The average tumor weights were 784.0±153.1 mg in the 250-g/kg sucrose group versus 389.4±64.3 mg in the starch control group (p<0.05). Histopathological examination showed that there were an average of 6.6±2.2 lung nodules due to mammary tumor metastases in the starch control group but 16.7±4.4 nodules in the 250-g/kg sucrose group (p<0.05). Similarly, compared to the starch control diet, dietary sucrose at a dose of 125 g/kg increased tumor growth in an MDA-MB-231 cell mouse orthotopic model (Fig. 1c). Again, in both models the sucrose-enriched diet did not lead to changes in body weight relative to the control mice (Supplementary Fig. S1). The data from the three breast carcinoma models consistently showed that sucrose-enriched diets compared to starch based control diet, not only shortened the onset and increased proliferation of mammary gland tumors but also notably increased the lung metastatic potential of mammary carcinoma. Sugar has been linked to increased risk of cancer (17,18); however, such a link in breast cancer is still controversial. Our animal data supports the tumor promoting effect of sugar in breast cancer development and metastasis.
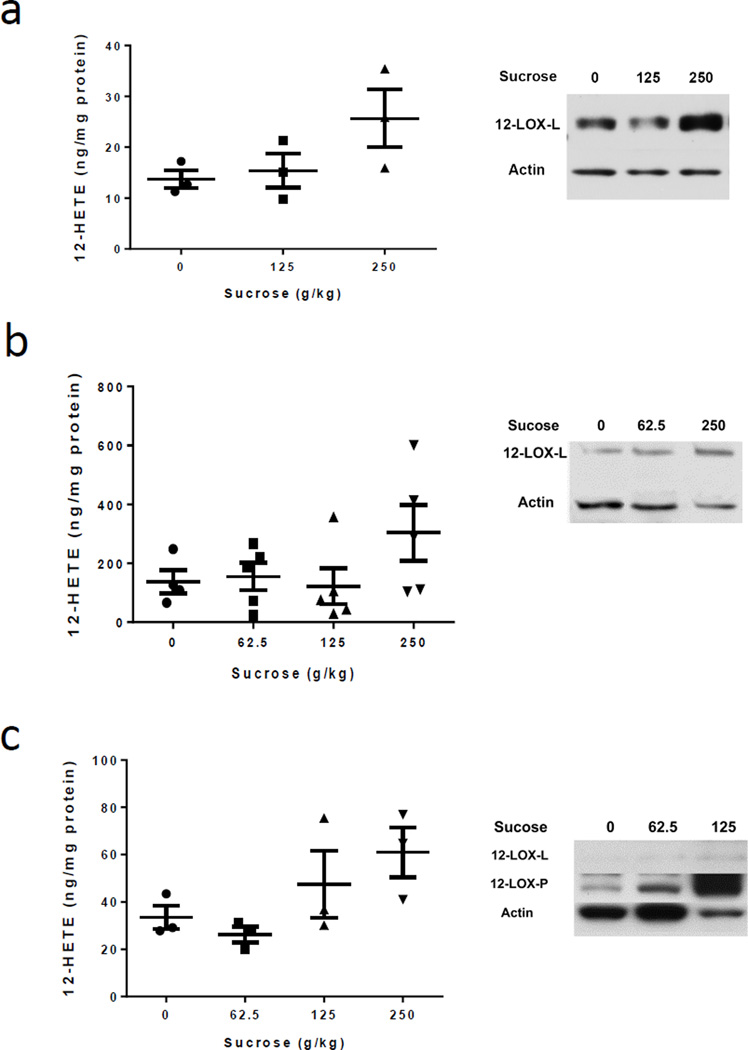
As high-glycemic-index food has been shown to increase the risk of breast cancer (20) and may result in modulation of inflammation (21), we determined the effects of sugar-enriched diets on levels of arachidonic acid metabolites, known to be important in inflammation, in MMTV/neu mouse mammary tumors. Among various prostaglandins (cyclooxygenase-related metabolites) and HETEs and HODE (LOX metabolites), 12-HETE levels were elevated about 2.6-fold in the sucrose-diet group, especially in the 250-g/kg group, compared to that of the starch control-diet group (Fig. 2a). Similarly, the 250 g/kg sucrose-enriched diet increased 12-HETE levels by 1.8-fold and 2.2-fold in the mammary tumors of 4T1 and MDA-MB-231 models, respectively (Fig. 2b and 2c). Other HETE levels in the tumor tissues are presented in Supplementary Table S2; with 5-HETE consistently being the lowest and similar levels of 13-HODE and 15-HETE. We also noticed that breast tumor 5-HETE was increased by sugar consumption in 4T1 and MDA-MB-231 cell-bearing mice compared to that in the starch control fed mice. We then examined 12-LOX protein levels in the tumor by western blotting. There are three isoforms of the 12-LOX enzyme abundance: platelet-type 12-LOX 12-LOX-P), leukocyte-type 12-LOX 12-LOX-L) and epidermal-type 12-LOX 12-LOX-E, only in mouse) (22). We observed a detectable level of 12-LOX-L protein but not 12-LOX-P protein in the tumors generated from mammary glands of MMTV/neu mice (Fig. 2a) and 4T1 cell-bearing mice (Fig. 2b). The mammary gland tumors that developed from MDA-MB-231 cells, which originated from human breast cancer, expressed both 12-LOX-L and 12-LOX-P protein (Fig. 2c). Intriguingly, although different isoforms of 12-LOX were detected in different mammary gland carcinoma tissues, we found consistently increased abundance of 12-LOX protein in the tumor tissue from mice that consumed high levels of dietary sucrose compared to control mice. These data imply that sucrose-enriched diets are associated with increased 12-LOX protein expression and 12-HETE levels in mouse mammary gland carcinoma and human breast tumor tissues, suggesting potential mechanisms through which dietary sucrose induces tumor growth.
Figure 2. Dietary sucrose altered 12-LOX protein expression and 12-HETE production in mouse tumors.
a. MMTV/neu mouse tumors; b. 4T1 tumor-bearing mice; c. MDA-MB-231 tumor-bearing mice. Data are represented as mean ± s.e.m.
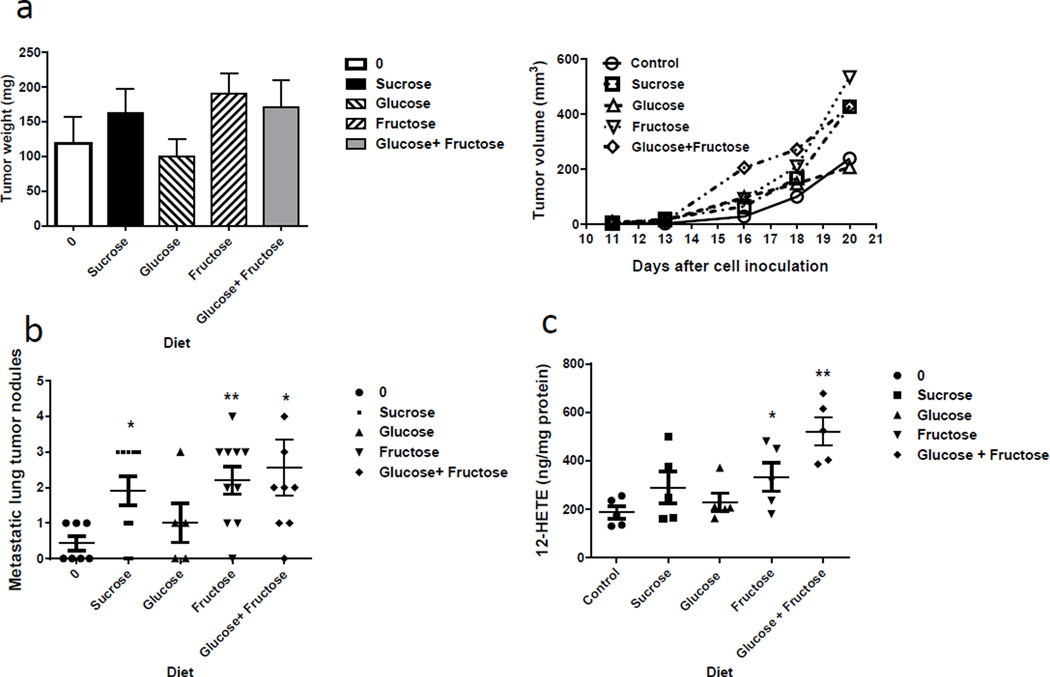
A human study reported that dietary sucrose/fructose/glucose but not starch is associated with increased risk of breast cancer (19). This is possibly due to the fact that simple sugars such as sucrose leads to greater fluctuations in blood sugar (23), which in turn could cause differences in hormonal patterns and other metabolisms (17). Since sucrose contains half glucose and half fructose, it is not clear whether glucose or fructose is the active component of sucrose that contributes to sucrose induced tumorigenesis. To tease out the effect of glucose or fructose, we compared the tumorigenic effect of sucrose (250g/kg) to glucose (125g/kg), fructose (125g/kg) and glucose plus fructose diets in 4T1 cell-bearing mice (Fig. 3). We found that in addition to the sucrose enriched diet, the fructose enriched diet and the fructose plus glucose diets led to larger tumors than that in the control mice (Fig. 3a). More importantly, the sucrose, fructose and fructose plus glucose diets all resulted in significantly more lung metastasis compared to control diets (1.9±0.4 per mouse, 2.2±0.4 per mouse, 2.6±0.8 per mouse vs. 0.4±0.2 per mouse, respectively), suggesting fructose may be a major dietary carbohydrate that contributed to sucrose induced mammary gland tumorigenesis and metastasis (Fig. 3b). This is consistent with literature that described that fructose is associated with more aggressive/metastatic phenotype of breast cancer cells (24) and its transporters (GLUT2 and GLUT5) are linked to numerous diseases and syndromes including breast cancer (25). Strikingly, while sucrose diet continually increased the breast tumor 12-HETE production (289.3±65.6 ng/mg protein), average tumor 12-HETE concentration was significantly higher in tumors from mice fed with fructose (333.1±58.6 ng/mg protein) and with fructose plus glucose diets (521.3±57.3 ng/mg protein) compared to the 12-HETE levels in control diet fed mice (186.2±25.5 ng/mg protein) (Fig. 3c). These data suggest that the fructose component in the diet is potentially responsible for promoting the primary tumor growth and tumor metastasis to the lungs of the mice. Literatures suggest that dietary fructose is taken up by the liver and converted into lactate and glucose which are subsequently released into the circulation or converted into hepatic glycogen or fat (26), however, it is unclear how fructose alters 12-LOX abundance and 12-HETE production.
Figure 3. The sucrose diet promoted lung metastasis potentially due to the fructose.
Dietary sucrose, fructose and glucose plus fructose diets all induced tumor weight and volume (a), metastasis (b) (macro metastasis only) and 12-HETE production (c) in breast tumors from the mice after treatment. Data are represented as mean ± s.e.m. *p<0.05; **p < 0.001.
The molecular mechanisms of the effects of sugar/carbohydrate-enriched on breast cancer are incompletely understood. The current study provides a promising mechanism that for the first time directly links dietary sugar (sucrose/fructose) to breast cancer development and metastasis, which is worthy of further investigation. Although the 12-LOX/12-HETE signaling has been studied in cancer for some time, its involvement in tumor development still remains inconclusive. For example, Dilly et al, showed that overexpression of 12-LOX increases invasion and angiogenesis of prostate cancer (27), but Gondek et al reported that the plasma concentration of 12-LOX was significantly higher in the patient with BPH compared to those with prostate cancer (28). In comparison, in breast cancer, Liu and colleagues transfected MCF-7 breast cancer cells with 12-LOX and found that transfection led to a loss of estrogen dependency and an increased tumor growth (12). Singh et al observed that serum 12-LOX concentration was significantly higher in breast cancer patients with lymph node involvement comparing to that of age-match controls (29). Higher levels of 12-LOX were also observed in tumors from patients who died of breast cancer (30). Furthermore, a non-synonymous polymorphism of A12LOX (mRNA, A535G; gln261Arg) has been associated with increased risk of breast cancer, especially the Arg/Gln and Arg/Arg variants (31). In line with this, a recent preclinical study further documented that 12-HETE serves as a key mediator of tumor cell invasion into lymphatic vessels and formation of lymph node metastasis in ductal mammary carcinomas (32). All this evidence indicates that the 12-LOX/12-HETE axis is a useful prognostic marker for progression and metastasis of breast cancer. Our findings suggested that dietary sucrose/fructose induced 12-LOX/12-HETE production in breast tumor cells in vivo is a possible signaling pathway responsible for sugar-promoted tumor growth in mice. How dietary sucrose/fructose induces 12-HETE and whether it is a direct or an indirect effect remains in question. Given that fructose consumption in the U.S. has surged from 0.5 lb/year/person in 1970 to 62.4 lb/year/person in 1997 (33) and studies have suggested that fructose may play a role in metabolic syndromes (34,35), the mechanism by which dietary fructose or fructose containing sugar (sucrose) affects breast tumorigenesis and metastasis, especially through the 12-lipoxygenase pathways, warrants further investigation.
Supplementary Material
Acknowledgments
This project is supported by Mr. Leighton Steward and EOG Resource Inc., Houston, Texas; core resources supported by NIH/NCI P30CA016672.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Romieu I, Ferrari P, Rinaldi S, Slimani N, Jenab M, Olsen A, et al. Dietary glycemic index and glycemic load and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Am J Clin Nutr. 2012;96:345–355. doi: 10.3945/ajcn.111.026724. [DOI] [PubMed] [Google Scholar]
- 2.Dong JY, Qin LQ. Dietary glycemic index, glycemic load, and risk of breast cancer: meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;126:287–294. doi: 10.1007/s10549-011-1343-3. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Bergkvist L, Wolk A. Glycemic load, glycemic index and breast cancer risk in a prospective cohort of Swedish women. Int J Cancer. 2009;125:153–157. doi: 10.1002/ijc.24310. [DOI] [PubMed] [Google Scholar]
- 4.USDA's Economic Research Service. http://www.ers.usda.gov/data-products/food-availability-(per-capita)-data-system.aspx. [Google Scholar]
- 5.Miller PE, McKinnon RA, Krebs-Smith SM, Subar AF, Chriqui J, Kahle L, et al. Sugar-sweetened beverage consumption in the U.S.: novel assessment methodology. Am J Prev Med. 2013;45:416–421. doi: 10.1016/j.amepre.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Hoehn SK, Carroll KK. Effects of dietary carbohydrate on the incidence of mammary tumors induced in rats by 7, 12-dimethylbenz[α] anthracene. Nutr Cancer. 1979;1:27–30. [Google Scholar]
- 7.Klurfeld DM, Weber MM, Kritchevsky D. Comparison of dietary carbohydrates for promotion of DMBA-induced mammary tumorigenesis in rats. Carcinogenesis. 1984;5:423–425. doi: 10.1093/carcin/5.3.423. [DOI] [PubMed] [Google Scholar]
- 8.Honn KV, Marnett LJ, Nigam S, Jones RL, Wong PYK. Eicosanoids and other bioactive lipids in cancer, inflammation, and radiation injury. Cancer Metastasis Rev. 1997;407:365–396. [Google Scholar]
- 9.Yoshimura R, Inoue K, Kawahito Y, Mitsuhashi M, Tsuchida K, Matsuyama M, et al. Expression of 12-lipoxygenase in human renal cell carcinoma and growth prevention by its inhibitor. Int J Mol Med. 2004;13:41–46. [PubMed] [Google Scholar]
- 10.Gao X, Grignon DJ, Chbihi T, Zacharek A, Chen YQ, Sakr W, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 11.Pidgeon G. The role of cyclooxygenases and lipoxygenases in the regulation of tumor angiogenesis. Tumor Angiogenesis Regulators. 2013;239:246. [Google Scholar]
- 12.Liu XH, Connolly JM, Rose DP. Eicosanoids as mediators of linoleic acid-stimulated invasion and type IV collagenase production by a metastatic human breast cancer cell line. Clin Exp Metastasis. 1996;14:145–152. doi: 10.1007/BF00121211. [DOI] [PubMed] [Google Scholar]
- 13.Mohammad AM, Abdel HA, Abdel W, Ahmed AM, Wael T, Eiman G. Expression of cyclooxygenase-2 and 12-lipoxygenase in human breast cancer and their relationship with HER-2/neu and hormonal receptors: impact on prognosis and therapy. Indian J Cancer. 2006;43:163–168. doi: 10.4103/0019-509x.29421. [DOI] [PubMed] [Google Scholar]
- 14.Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. 2010;97:101–106. doi: 10.1016/j.pbb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potischman N, Coates RJ, Swanson CA, Carroll RJ, Daling JR, Brogan DR, et al. Increased risk of early-stage breast cancer related to consumption of sweet foods among women less than age 45 in the United States. Cancer Causes Control. 2002;13:937–946. doi: 10.1023/a:1021919416101. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Uchida K, Ohnaka K, Morita M, Toyomura K, Kono S, et al. Sugars, sucrose and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol. 2014;49:581–588. doi: 10.3109/00365521.2013.822091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romieu I, Lazcano-Ponce E, Sanchez-Zamorano LM, Willett W, Hernandez-Avila M. Carbohydrates and the risk of breast cancer among Mexican women. Cancer Epidemiol Biomarkers Prev. 2004;13:1283–1289. [PubMed] [Google Scholar]
- 20.Choi Y, Giovannucci E, Lee JE. Glycaemic index and glycaemic load in relation to risk of diabetes-related cancers: a meta-analysis. Br J Nutr. 2012;108:1934–1947. doi: 10.1017/S0007114512003984. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 22.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balzer BWR, Atkinson FS, Bell KJ, Steinbeck KS. Low glycemic index dietary interventions in cystic fibrosis. In: Watson RR, editor. Diet and exercise in cystic fibrosis. UK: Elsevier Inc.; 2014. p. 210. [Google Scholar]
- 24.Monzavi-Karbassi B, Hine RJ, Stanley JS, Ramani VP, Carcel-Trullols J, Whitehead TL, et al. Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int J Oncol. 2010;37:615–622. doi: 10.3892/ijo_00000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol. 2013;591:401–414. doi: 10.1113/jphysiol.2011.215731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 27.Dilly AK, Ekambaram P, Guo Y, Cai Y, Tucker SC, Fridman R, et al. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-kappaB. Int J Cancer. 2013;133:1784–1791. doi: 10.1002/ijc.28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gondek T, Szajewski M, Szefel J, Aleksandrowicz-Wrona E, Skrzypczak-Jankun E, Jankun J, et al. Evaluation of 12-lipoxygenase (12-LOX) and plasminogen activator inhibitor 1 (PAI-1) as prognostic markers in prostate cancer. BioMed research international. 2014;2014:102478. doi: 10.1155/2014/102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AK, Kant S, Parshad R, Banerjee N, Dey S. Evaluation of human LOX-12 as a serum marker for breast cancer. Biochem Biophys Res Commun. 2011;414:304–308. doi: 10.1016/j.bbrc.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 30.Jiang WG, Douglas-Jones A, Mansel RE. Levels of expression of lipoxygenases and cyclooxygenase-2 in human breast cancer. Prostaglandins Leukot Essent Fatty Acids. 2003;69:275–281. doi: 10.1016/s0952-3278(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 31.Prasad VV, Kolli P, Moganti D. Association of a functional polymorphism (Gln261Arg) in 12-lipoxygenase with breast cancer. Exp Ther Med. 2011;2:317–323. doi: 10.3892/etm.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bray GA, Popkin BM. Calorie-sweetened beverages and fructose: what have we learned 10 years later. Pediatr Obes. 2013;8:242–248. doi: 10.1111/j.2047-6310.2013.00171.x. [DOI] [PubMed] [Google Scholar]
- 35.Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24:198–206. doi: 10.1097/MOL.0b013e3283613bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.