Abstract
MICs and biofilm inhibitory concentrations (BICs) were measured for 68 cystic fibrosis (CF) Achromobacter isolates for amikacin, aztreonam, colistin, levofloxacin, and tobramycin. With the exception of colistin and levofloxacin, the remaining antibiotics had MIC90s, BICs at which 50% of the isolates were susceptible (BIC50s), and BICs at which 90% of the isolates were susceptible (BIC90s) equal to or above the highest concentrations tested. In a biofilm model, tobramycin was able to significantly increase killing of bacterial cells compared to controls, for intermediate-resistant strains only, at concentrations of 1,000 and 2,000 μg/ml.
TEXT
Achromobacter species, previously known as Alcaligenes species, are multidrug-resistant Gram-negative bacteria that have increasingly been isolated from the sputum from cystic fibrosis (CF) patients worldwide (1). Case-control studies have shown increased decline in lung function following pulmonary infection with Achromobacter xylosoxidans (2, 3). As with Burkholderia cepacia complex and Stenotrophomonas maltophilia, A. xylosoxidans species are intrinsically resistant to several classes of antibiotics (4–7). Currently, there are no recommendations for chronic suppressive aerosolized antimicrobial therapies to treat these infections in CF patients. The objectives of this study were thus to examine the effects of antibiotics available for aerosolization against a range of CF Achromobacter species grown planktonically and as biofilms, which are important in CF pulmonary infections.
Sixty-eight Achromobacter isolates were collected from CF patients at The Hospital for Sick Children, Toronto, Ontario, Canada (n = 15), and the CF Foundation B. cepacia Research Laboratory and Repository, Ann Arbor, MI (n = 53). The collection of Achromobacter CF isolates included five species: A. xylosoxidans (n = 50), A. denitrificans (n = 3), A. dolens (n = 5), A. insolitus (n = 5), and A. ruhlandii (n = 5). Antimicrobial susceptibility testing was performed on isolates grown planktonically and as biofilms for amikacin, aztreonam, colistin, levofloxacin, and tobramycin, as previously described (8, 9). Five A. xylosoxidans CF isolates with intermediate biofilm inhibitory concentrations (BICs) (defined as ≤800 μg/ml tobramycin, representing the mean peak sputum concentration of aerosolized tobramycin [10]) and another 5 isolates from the same species but with high BICs (>800 μg/ml tobramycin) were selected for further study in the biofilm slide chamber model. After 48 h of growth, biofilms were treated with various concentrations of tobramycin (0, 8, 400, 1,000, and 2,000 μg/ml) for 24 h, stained using the FilmTracer LIVE/DEAD biofilm viability kit, and visualized by confocal microscopy (see Methods in the supplemental material).
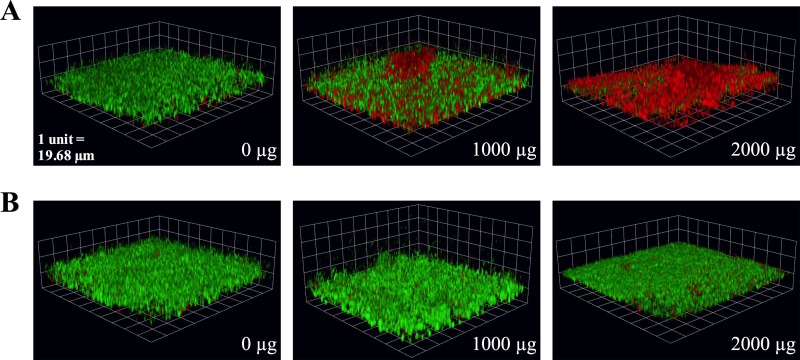
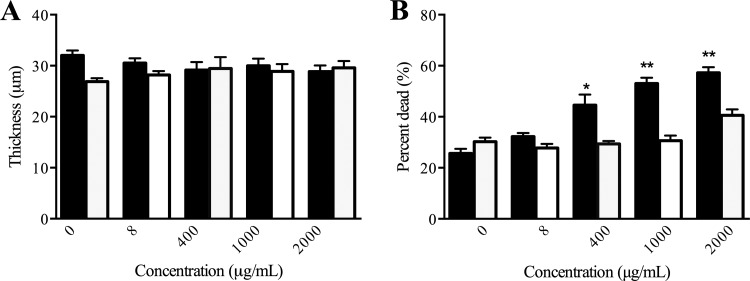
Planktonic and biofilm susceptibility testing were performed on a total of 68 Achromobacter isolates. To describe the overall susceptibility results, the MICs and BICs at which 50% and 90% of the isolates were susceptible are presented in Table 1. For all Achromobacter species, the MIC50 of amikacin (512 μg/ml), aztreonam (256 μg/ml), colistin (4 μg/ml), levofloxacin (20 μg/ml), and tobramycin (200 μg/ml) were determined. With the exception of the MIC90s of colistin (64 μg/ml) and levofloxacin (20 μg/ml), the remaining three antibiotics had MIC90s, BIC50s, and BIC90s that were equal to or above the highest concentrations tested. The susceptibility results were varied among the isolates, and the distributions of the MICs and BICs are presented in Fig. S1 in the supplemental material. From the distributions of the MICs and BICs of all Achromobacter CF isolates, the five antibiotics tested had right-skewed MICs and left-skewed BICs. Correlations between MICs and BICs were calculated for each antibiotic using the Spearman correlation coefficient and were found to be statistically significant for amikacin (r = 0.271, P ≤ 0.05) and tobramycin (r = 0.261, P ≤ 0.05) only. To determine the effect of tobramycin, one of the most commonly used and available inhaled antibiotics in CF, on A. xylosoxidans, the most prevalent Achromobacter species in CF, biofilms grown in a slide chamber model were treated with various concentrations of antibiotic and imaged using confocal microscopy. Upon visualization of the intermediate-resistant strains, there appeared to be an increase in bacterial killing at concentrations of 1,000 and 2,000 μg/ml tobramycin, which was not observed with the highly resistant strains (Fig. 1). In addition, there was a statistically significant increase in the percentage of dead cells at concentrations of 400, 1,000, and 2,000 μg/ml tobramycin that showed a dose-response effect, but no change in thickness was observed in a comparison of intermediate-resistant biofilms to the untreated controls (Fig. 2). With the highly resistant strains, there was no statistically significant change in thickness or killing at any of the concentrations of tobramycin tested.
TABLE 1.
Antibiotic MICs and BICs for Achromobacter CF isolates measured by planktonic and biofilm susceptibility testing
| MIC/BIC by antibiotic | Results for Achromobacter species |
||
|---|---|---|---|
| All species (n = 68) | A. xylosoxidans (n = 50) | Other species (n = 18)a | |
| Amikacin | |||
| MIC50 | 512 | 1,024 | 256 |
| BIC50 | >4,096 | >4,096 | >4,096 |
| MIC90 | >4,096 | >4,096 | >4,096 |
| BIC90 | >4,096 | >4,096 | >4,096 |
| Aztreonam | |||
| MIC50 | 256 | 512 | 512 |
| BIC50 | >2,048 | >2,048 | >2,048 |
| MIC90 | 2,048 | 2,048 | 2,048 |
| BIC90 | >2,048 | >2,048 | >2,048 |
| Colistin | |||
| MIC50 | 4 | 8 | 4 |
| BIC50 | >256 | >256 | >256 |
| MIC90 | 64 | 64 | 64 |
| BIC90 | >256 | >256 | >256 |
| Levofloxacin | |||
| MIC50 | 20 | 20 | 20 |
| BIC50 | 5,120 | 5,120 | 5,120 |
| MIC90 | 20 | 20 | 20 |
| BIC90 | >5,120 | >5,120 | >5,120 |
| Tobramycin | |||
| MIC50 | 200 | 400 | 200 |
| BIC50 | 3,200 | 3,200 | 3,200 |
| MIC90 | >3,200 | >3,200 | >3,200 |
| BIC90 | >3,200 | >3,200 | >3,200 |
“Other species” includes A. denitrificans (n = 3), A. dolens (n = 5), A. insolitus (n = 5), and A. ruhlandii (n = 5).
FIG 1.
Confocal microscopy of live and dead (green and red, respectively) intermediately resistant (A) and highly resistant (B) A. xylosoxidans CF isolates treated with 1,000 and 2,000 μg/ml tobramycin in the biofilm slide chamber model.
FIG 2.
Mean (with standard error of the mean) thickness (A) and percent dead (B) of intermediately (black bars, n = 5) and highly (white bars, n = 5) resistant Achromobacter xylosoxidans CF isolates treated with increasing concentrations of tobramycin in the biofilm slide chamber model. *, P ≤ 0.05; **, P ≤ 0.01 compared to the untreated control.
This study examined the antibiotic susceptibility of a large collection of Achromobacter isolates from CF patients and included the most common Achromobacter species encountered in this population (11, 12). These in vitro results highlight their high degree of resistance to multiple classes of antibiotics in the context of both planktonic and biofilm growth. Several studies have noted that resistance among strains isolated from patients with CF is quite common; however, none have studied antibiotic susceptibility of Achromobacter spp. grown as biofilms (13–15). One of the strengths of this study was the investigation of antimicrobial activity against biofilm structures of Achromobacter using two models of biofilm growth, namely, the Calgary Biofilm Device and a slide chamber model with visualization using confocal microscopy. The results confirmed that when grown as biofilms, these bacteria, like others, exhibited higher tolerance to antibiotics (9, 16). The availability of aerosolized formulations of antibiotics, however, allows for higher pulmonary concentrations to be achieved in patients (10). All of the antibiotics tested in our study are either commercially available or in phase III study for aerosolization in CF patients, and the concentrations tested represent those achievable in the lungs after aerosolization. While the majority of isolates still had BICs above the achievable aerosolized concentration, levofloxacin and tobramycin showed the greatest efficacy against Achromobacter spp. overall, with the highest percentages of isolates with MICs and BICs below the achievable mean sputum drug concentrations (10). As we have previously shown (9), there was a significant correlation between the MICs and BICs for aminoglycosides but not for other antimicrobial classes tested, suggesting potential efficacy against both planktonically grown and biofilm-grown organisms. Data using the biofilm slide chamber model coupled with confocal microscopy confirmed the BIC data in this study. Strains deemed to be intermediate resistant via the Calgary Biofilm Device showed more killing with increasing doses of tobramycin. In contrast, strains with high resistance to tobramycin showed little increase in killing compared to that with the control. Inhaled levofloxacin and tobramycin thus represent the most promising treatment options, with effects that go beyond merely inhibiting Achromobacter growth to actually killing bacterial cells embedded in biofilms. Clinical trials, however, are needed to demonstrate true in vivo efficacy.
Supplementary Material
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02240-15.
REFERENCES
- 1.Emerson J, McNamara S, Buccat AM, Worrell K, Burns JL. 2010. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol 45:363–370. doi: 10.1002/ppul.21198. [DOI] [PubMed] [Google Scholar]
- 2.De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. 2007. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 6:75–78. doi: 10.1016/j.jcf.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Rønne Hansen C, Pressler T, Høiby N, Gormsen M. 2006. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J Cyst Fibros 5:245–251. doi: 10.1016/j.jcf.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Mensah K, Philippon A, Richard C, Nevot P. 1990. Susceptibility of Alcaligenes denitrificans subspecies xylosoxydans to beta-lactam antibiotics. Eur J Clin Microbiol Infect Dis 9:405–409. doi: 10.1007/BF01979470. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsen TH, Hansen MA, Jensen PØ, Hansen L, Riber L, Cockburn A, Kolpen M, Rønne Hansen C, Ridderberg W, Eickhardt S, Hansen M, Kerpedjiev P, Alhede M, Qvortrup K, Burmølle M, Moser C, Kuhl M, Ciofu O, Givskov M, Sørensen SJ, Høiby N, Bjarnsholt T. 2013. Complete genome sequence of the cystic fibrosis pathogen Achromobacter xylosoxidans NH44784-1996 complies with important pathogenic phenotypes. PLoS One 8:e68484. doi: 10.1371/journal.pone.0068484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridderberg W, Nielsen SM, Nørskov-Lauritsen N. 2015. Genetic adaptation of Achromobacter sp. during persistence in the lungs of cystic fibrosis patients. PLoS One 10:e0136790. doi: 10.1371/journal.pone.0136790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bador J, Amoureux L, Blanc E, Neuwirth C. 2013. Innate aminoglycoside resistance of Achromobacter xylosoxidans is due to AxyXY-OprZ, an RND-type multidrug efflux pump. Antimicrob Agents Chemother 57:603–605. doi: 10.1128/AAC.01243-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratories Standards Institute. 2012. Performance standards for 303 antimicrobial susceptibility testing; 22nd informational supplement CLSI document M100-S22. Clinical and Laboratories Standards Institute, Wayne, PA. [Google Scholar]
- 9.Ratjen A, Yau Y, Wettlaufer J, Matukas L, Zlosnik JE, Speert DP, LiPuma JJ, Tullis E, Waters V. 2015. In vitro efficacy of high-dose tobramycin against Burkholderia cepacia complex and Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother 59:711–713. doi: 10.1128/AAC.04123-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmiel JF, Aksamit TR, Chotirmall SH, Dasenbrook EC, Elborn JS, LiPuma JJ, Ranganathan SC, Waters VJ, Ratjen FA. 2014. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, Gram-negative bacteria, and multiple infections. Ann Am Thorac Soc 11:1120–1129. doi: 10.1513/AnnalsATS.201402-050AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spilker T, Vandamme P, Lipuma JJ. 2013. Identification and distribution of Achromobacter species in cystic fibrosis. J Cyst Fibros 12:298–301. doi: 10.1016/j.jcf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Vandamme P, Moore ER, Cnockaert M, Peeters C, Svensson-Stadler L, Houf K, Spilker T, LiPuma JJ. 2013. Classification of Achromobacter genogroups 2, 5, 7 and 14 as Achromobacter insuavis sp. nov., Achromobacter aegrifaciens sp. nov., Achromobacter anxifer sp. nov. and Achromobacter dolens sp. nov., respectively. Syst Appl Microbiol 36:474–482. doi: 10.1016/j.syapm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Biswas S, Dubus JC, Reynaud-Gaubert M, Stremler N, Rolain JM. 2013. Evaluation of colistin susceptibility in multidrug-resistant clinical isolates from cystic fibrosis, France. Eur J Clin Microbiol Infect Dis 32:1461–1464. doi: 10.1007/s10096-013-1898-5. [DOI] [PubMed] [Google Scholar]
- 14.Lambiase A, Catania MR, Del Pezzo M, Rossano F, Terlizzi V, Sepe A, Raia V. 2011. Achromobacter xylosoxidans respiratory tract infection in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis 30:973–980. doi: 10.1007/s10096-011-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trancassini M, Iebba V, Citera N, Tuccio V, Magni A, Varesi P, De Biase RV, Totino V, Santangelo F, Gagliardi A, Schippa S. 2014. Outbreak of Achromobacter xylosoxidans in an Italian cystic fibrosis center: genome variability, biofilm production, antibiotic resistance, and motility in isolated strains. Front Microbiol 5:138. doi: 10.3389/fmicb.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu K, Yau YC, Matukas L, Waters V. 2013. Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother 57:1546–1548. doi: 10.1128/AAC.02215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.