Abstract
Objective
Myoclonus‐dystonia (M‐D) is a hyperkinetic movement disorder, typically alcohol‐responsive upper body myoclonus and dystonia. The majority of autosomal dominant familial cases are caused by epsilon‐sarcoglycan gene (SGCE) mutations. Previous publications have observed increased rates of psychiatric disorders amongst SGCE mutation‐positive populations. We analyzed the psychiatric data from four international centers, forming the largest cohort to date, to further determine the extent and type of psychiatric disorders in M‐D.
Methods
Psychiatric data from SGCE mutation‐positive M‐D cohorts, collected by movement disorder specialists in the Netherlands, United Kingdom, United States, and Germany, were analyzed. These data were collected using standardized, systematic questionnaires allowing classification of symptoms according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) criteria. Based on motor findings and SGCE mutation analysis, participants were classified into one of three groups: manifesting carriers, nonmanifesting carriers and noncarriers.
Results
Data from 307 participants were evaluated (140 males, 167 females, mean age at examination: 42.5 years). Two‐thirds of motor affected mutation carriers (n = 132) had ≥1 psychiatric diagnosis, specific, and social phobias being most common followed by alcohol dependence and obsessive‐compulsive disorder (OCD). Compared to familial controls, affected mutation carriers had significantly elevated overall rates of psychiatric disorders (P < 0.001). The most significant differences were observed with alcohol dependence (P < 0.001), OCD (P < 0.001), social and specific phobias (P < 0.001).
Interpretation
M‐D due to SGCE mutations is associated with specific psychiatric disorders, most commonly OCD, anxiety‐related disorders, and alcohol dependence. These suggest either a potential pleiotropic function for SGCE within the central nervous system or a secondary effect of the motor disorder.
Introduction
Myoclonus‐dystonia (M‐D) is a rare hyperkinetic movement disorder typically characterized by myoclonus of the trunk and upper limbs in conjunction with dystonia of the neck or hands (writer's cramp).1, 2 Onset of motor symptoms is usually in the first two decades of life and are typically alcohol responsive. A proportion of M‐D cases are caused by mutations in the epsilon‐sarcoglycan gene (SGCE), which encodes the epsilon‐sarcoglycan protein.3 More than 40 different point mutations have now been identified, these resulting in proteosomal degradation and failure of expression of the mutated protein at the cell‐surface membrane.4
Psychiatric symptoms have been reported to be prevalent amongst SGCE mutation‐positive cases.5, 6 Depression, anxiety disorders and obsessive‐compulsive disorder (OCD)7, 8, 9 have been frequently and repeatedly reported whereas other psychiatric diagnoses, for example, psychosis,10 schizophrenia,7 schizoaffective disorder,11 Attention Deficit Hyperactivity Disorder (ADHD)12, and Anorexia Nervosa13 have been described predominantly in single case reports. More recently, larger cohort studies have used systematic and standardized methods to compare results from SGCE mutation‐positive M‐D cohorts to control groups, finding OCD, in particular compulsivity,7 and anxiety‐related disorders to be the most prominent reported neuropsychiatric features.8
This study combines previously collected standardized data from four international centers (Netherlands, United Kingdom, United States, and Germany; see Table 1 for an overview of the clinical centers involved) to form the largest single collection of psychiatric data from SGCE mutation carriers. Given the increasing recognition of nonmotor symptoms as a comorbid feature of dystonic disorders, this large cohort allows further assessment of the type and frequency of psychiatric symptoms in M‐D populations as well as another opportunity to examine whether these symptoms represent a pleiotropic function for the epsilon‐sarcoglycan protein or a secondary consequence of the motor disorder.
Table 1.
Demographic features of the study population
| Demographics | MC (n = 132) | NMC (n = 38) | NC (n = 137) | Total (n = 307) |
|---|---|---|---|---|
| Gender | ||||
| Male (%) | 70 (53) | 22 (58) | 48 (35) | 140 (46) |
| Female (%) | 62 (47) | 16 (42) | 89 (65) | 167 (54) |
| Mean age at examination (SD) | 40.5 years (17.0 years) | 50.4 years (17.1 years) | 41.2 years (14.0 years) | 42.1 years (16.1 years) |
| Age at onset of motor symptoms (n %) | ||||
| <18 years | 65 (49) | – | – | 65 (21) |
| 18–30 years | 4 (3) | – | – | 4 (1.3) |
| 30–40 years | 1 (0.8) | – | – | 1 (0.3) |
| 40–50 years | 1 (0.8) | – | – | 1 (0.3) |
| Unknown | 68 (52) | – | – | 68 (22) |
| Number of cases per center (%) | ||||
| Academic Medical Centre, Amsterdam, the Netherlands8, 14 | 31 (23) | 9 (24) | 33 (24) | 73 (24) |
| Beth Israel Medical Centre, New York, U.S.A.9, 15 | 20 (15) | 9 (24) | 33 (24) | 62 (20) |
| University of Tubingen, Tubingen, Germany | 54 (41) | 10 (26) | 55 (40) | 119 (39) |
| Cardiff University, Cardiff, United Kingdom7 | 27 (20) | 10 (26) | 16 (12) | 53 (17) |
MC, manifesting carriers; NMC, nonmanifesting carriers; NC, noncarrier; SD, standard deviation.
Methods
Movement disorder research groups that had previously published standardized and systematic psychiatric data from SGCE mutation‐positive M‐D cohorts were contacted and asked to provide a summary of their findings for each case. Each center was also asked to provide data from any as yet unpublished cases that could be included in analysis (Table 1; Fig. 1).
Figure 1.
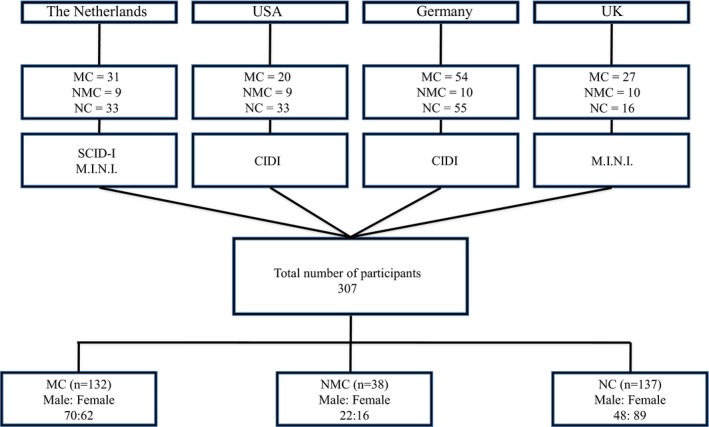
Diagrammatic representation of case recruitment from the four international centers. MC, manifesting carrier; NMC, nonmanifesting carrier; NC, noncarrier; CIDI, Composite International Diagnostic Interview; M.I.N.I., MINI International Neuropsychiatric Interview; SCID‐I, Structured Clinical Interview.
Standard protocol approvals, registrations and patient consents
Ethical approval for data collection was obtained from the respective research and ethics committees in each of the recruiting countries (Netherlands, United Kingdom, United States and Germany). Written informed consent or assent was obtained from each participant or their parent/guardian prior to involvement in the study.
Genetic analysis
As reported previously, DNA from all samples was analyzed by direct sequencing and Multiplex ligation‐dependent probe amplification (MLPA) of SGCE exons 1–12.7, 8, 9, 14, 15
Clinical assessment
All cases underwent a neurological examination by a movement disorders expert at their original medical center and were classified according to their motor and genetic status into three groups: (1) manifesting carriers (MC): SGCE mutation and movement disorder (n = 132), (2) nonmanifesting carriers (NMC): SGCE mutation and no movement disorder (n = 38) and (3) noncarriers (NC): no SGCE mutation and no movement disorder (n = 137) (Table 1).
Psychiatric assessment
All centers used standardized assessment tools to establish lifetime psychiatric diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) criteria in their patient population. These included the Structured Clinical Interview (SCID‐I)16 (Netherlands), MINI International Neuropsychiatric Interview (M.I.N.I.)17 (Netherlands and United Kingdom) and Composite International Diagnostic Interview (CIDI)18 (United States and Germany) (Fig. 1). These included the sections on major affective disorder (MAD), generalized anxiety disorder (GAD), OCD, alcohol dependence, panic disorder, social phobia and specific phobia. Panic disorder, social phobia and specific phobia were not assessed amongst participants recruited in the United States and specific phobia was not assessed in those recruited from the United Kingdom. These cases were therefore excluded from the analysis of these disorders.
Data analysis and statistics
Overall and disorder specific rates of psychiatric diagnoses were analyzed using chi‐squared and Fisher's exact testing where appropriate. Stepwise logistic regression and multilevel logistic regression were used to determine whether additional variables (age at examination, gender, center, questionnaire type) impacted upon the presence of psychiatric symptoms.
Results
Data from 307 cases (140 males and 167 females) with a mean age at examination of 42.5 years (±15.8 years) were analyzed. Subdivided according to genetic and motor criteria 132 MC, 38 NMC and 137 NC were included. There was a near equal sex distribution in MC and NMC subgroups, unlike those with no mutation (NC) where almost two‐thirds of the cases were female. Mean age at assessment was similar across MC and NC cohorts, while those in the NMC sub‐group were, on average, 10 years older. Half of all MC cases had onset of their motor symptoms at <18 years of age, although this data was unknown in a similar number of cases (n = 68). Baseline motor, psychiatric and genetic data from each of the individual centers have been published previously, with the exception of those recruited by the University of Tubingen.7, 8, 9, 14, 15
Sixty five percent of MC cases met DSM‐IV criteria for ≥1 psychiatric disorder, with rates of all disorder subtypes being above population estimates (Table 2). Specific phobias (33%), social phobia (31%) and MADs (30%) were the most common. Overall rates of psychiatric disorders were almost half that of population estimates in both NMC (21%) and NC (27%) cohorts. Symptoms consistent with MAD were the most commonly reported in both groups (NMC: 21%, NC: 27%) while alcohol dependence was the least common with no NMC cases and only 4 (3%) cases amongst nonmutation carriers.
Table 2.
Rate and type of psychiatric disorders in MC, NMC and NC cohorts
| Lifetime DSM‐IV disorder | MC, n (%) (n = 132) | NMC, n (%) (n = 38) | NC, n (%) (n = 137) | Total, n (%) (n = 307) | Population estimates (%) |
|---|---|---|---|---|---|
| Any DSM‐IV disorder | 86 (65) | 8 (21) | 37 (27) | 131 (43) | 4820 |
| Major affective disorder | 40 (30) | 6 (16) | 20 (15) | 66 (21) | 17.120 |
| Generalized anxiety disorder | 23 (17) | 1 (2.6) | 10 (7) | 34 (11) | 5.120 |
| Obsessive‐compulsive disorder | 28 (21) | 1 (2.6) | 5 (4) | 34 (11) | 421 |
| Alcohol dependence | 32 (24) | 0 (0) | 4 (3) | 36 (12) | 14.120 |
| (n = 112) | (n = 29) | (n = 104) | (n = 245) | ||
| Panic disorder | 25 (22) | 1 (3) | 9 (9) | 35 (14) | 3.520 |
| Social phobia | 35 (31) | 1 (3) | 7 (7) | 43 (18) | 13.320 |
| (n = 85) | (n = 19) | (n = 88) | (n = 192) | ||
| Specific phobia | 28 (33) | 1 (5) | 7 (8) | 36 (19) | 8.720 |
MC, manifesting carriers; NMC, non‐manifesting carriers; NC, non‐carrier; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
Comparison of the motor affected MC group with the nonmutation carrying cohort (NC) found a significant difference in the overall rate of psychiatric disorders (OR 5.05, 95% confidence interval [CI] 2.91–8.80, P < 0.001) as well as with each individual diagnosis. Alcohol dependence (OR 10.64, 95% CI 3.44–36.72, P < 0.001) and OCD (OR 7.11, 95% CI 2.50–16.52, P < 0.001) demonstrated the largest differences. Social and specific phobias were also significantly higher amongst the MC cases (P < 0.001) (OR 6.30, 95% CI 2.50, 16.52 and OR 5.68, 95% CI 2.18, 15.44). In an attempt to determine whether SGCE mutations had a direct impact on psychiatric symptoms, NMC cases were compared to both the NC controls and motor affected (MC) cases. No significant difference was observed between NMC and NC patients either overall or with regard to individual diagnoses. However, comparison of MC and NMC participants found a significant difference in overall rates of psychiatric symptoms (OR 7.01, 95% CI 2.79–18.20, P < 0.001) with the largest differences again being seen with alcohol dependence, OCD and social phobia, the latter two diagnoses being 10 and 12 times more likely, respectively, in the MC population. A full summary of analyses can be seen in Table 3. Sensitivity analyses using stepwise and multilevel logistic regression found no significant effect of age at examination, gender, center or questionnaire type upon rate of psychiatric symptoms.
Table 3.
Comparison of rates of psychiatric disorders between MC, NMC and NC cohorts
| Psychiatric diagnosis (DSM‐IV) | MC versus NC | NMC versus NC | MC versus NMC |
|---|---|---|---|
| Any DSM‐IV disorder | <0.001 (5.05; 2.91, 8.80) | 0.46 (0.72; 0.28, 1.83) | <0.001 (7.01; 2.79, 18.20) |
| Major affective disorder | 0.002 (2.54; 1.34, 4.86) | 0.86 (1.10; 0.36, 3.21) | 0.10 (2.32; 0.84, 6.73) |
| Generalized anxiety disorder | 0.011 (2.68; 1.15, 6.34) | 0.29 (0.34; 0.02, 2.76) | 0.02 (7.81; 1.06, 160.59) |
| Obsessive‐compulsive disorder | <0.001 (7.11; 2.50, 21.78) | 0.76 (0.71; 0.03, 6.63) | 0.007 (9.96; 1.37, 203.60) |
| Alcohol dependence | <0.001 (10.64; 3.44, 36.72) | 0.29 (0; 0, 5.65) | 0.001 (∞; 2.38, ∞) |
| Panic disorder | 0.006 (3.03; 1.26, 7.45) | 0.35 (0.38; 0.02, 3.14) | 0.02 (8.04; 1.07, 166.62) |
| Social phobia | <0.001 (6.30; 2.50, 16.52) | 0.51 (0.50; 0.02, 4.32) | 0.002 (12.73; 1.73, 261.28) |
| Specific phobia | <0.001 (5.68; 2.18, 15.44) | 0.68 (0.64; 0.03, 5.85) | 0.015 (8.84; 1.14, 186.62) |
Results analyzed using chi‐squared testing or Fisher's exact testing where appropriate. Results expressed as: P‐value (odds ratio, 95% confidence interval). Statistically significant results (P < 0.05) are highlighted in bold. MC, manifesting carriers; NMC, non‐manifesting carriers; NC, non‐carrier; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
Data relating to age at onset of both motor and psychiatric symptoms were available in 26 patients. Based on recall, none reported onset of psychiatric symptomatology prior to their comorbid motor features and the majority reported that their motor symptoms had begun clearly in advance of any subsequent psychiatric disorder. This was most commonly seen in relation to alcohol dependence (11/31). Twenty‐two cases reported near simultaneous onset of the motor symptoms and psychiatric symptoms, with MAD (7/22) and social phobia (6/22) being the most prevalent.
Discussion
This study represents the largest multicenter cohort of SGCE mutation‐positive M‐D patients investigated using systematic and standardized tools for psychiatric symptomatology. Consistent with previous studies, we have demonstrated an excess of psychiatric symptoms amongst motor affected mutation carriers (MC) compared to intrafamilial controls (NMC and NC), with alcohol dependence, OCD, social and specific phobias demonstrating the largest between group differences.7, 9 Some of the rarer psychiatric diagnoses, for example psychosis and anorexia, were not identified in this cohort suggesting that only a loose association exists between these rarer disorders and SGCE mutations.
The overall rate of psychiatric disorders was almost one and a half times greater in the MC (65%) cohort than population estimates (48%). These latter values were obtained from epidemiological studies in the United States19 and New Zealand20 and considered to be from comparable populations to our study cohort. Interestingly, overall rates of psychiatric diagnoses in the NMC (21%) and NC (27%) groups were only half that of the population controls. There is no clear explanation for this, although the relatively small group sizes, and an awareness of the impact of psychiatric disorders (for example alcohol excess in related MC family members) may in part account for these findings.
Excess alcohol dependence amongst SGCE mutation carriers has been consistently reported in previous studies.7, 8, 15 This was initially felt to be a secondary reactive response to the therapeutic effect of alcohol on motor symptoms.2, 21 More recent studies have suggested that SGCE may exert a gene independent effect in contributing to these symptoms,6, 7 potentially mirroring the mechanisms involved in OCD and compulsivity, also prevalent amongst this population.7 However, this study appears to support the former view of a therapeutic response rather than gene effect with a significant difference in alcohol consumption between MC and NMC groups (P = 0.001) compared to similar levels of intake in NMC and NC patients (P = 0.29). Similarly, in those where age at onset data for motor and psychiatric symptoms was available, a third of those with primary motor symptoms reported subsequent alcohol dependence (Table 4).
Table 4.
Relationship between age at onset of motor and psychiatric symptoms in MC cases
| Psychiatric diagnosis (DSM‐IV) | Motor symptoms as 1st symptom | Psychiatric symptoms as 1st symptom | Onset of motor and psychiatric symptoms at similar time point |
|---|---|---|---|
| Major affective disorder | 5 | 0 | 7 |
| Generalized anxiety disorder | 2 | 0 | 3 |
| Obsessive‐Compulsive disorder | 5 | 0 | 3 |
| Alcohol dependence | 11 | 0 | 1 |
| Panic disorder | 4 | 0 | 1 |
| Social phobia | 2 | 0 | 6 |
| Specific phobia | 2 | 0 | 1 |
| Total (n) | 31 | 0 | 22 |
Data collected from only 26 patients where age relating to both features was known. Ages at onset placed into following categories: <18, 19–30, 31–30, 41–50 and >50 years. Simultaneous onset of symptoms was considered when both sets of symptoms began in the same age bracket. MC, manifesting carriers; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
OCD was more prevalent amongst the MC group. As OCD is not typically considered a secondary response to a chronic, disabling disorder22 its relationship to M‐D and SGCE mutations continues to be of significant interest. However, there remains some conflict in this area with several previous publications reporting a strong association between SGCE mutations and OCD,9 in particular the compulsivity component,7 while others have reported no evidence of this in their cohort.5, 8 Similar results have been seen in mixed dystonia cohorts, some finding an excess of OCD when compared to disability matched and healthy controls23, 24, 25 while others have found no evidence of an association.26 In M‐D this may represent a pleiotropic function of SGCE mutations for the development of both motor and OC symptoms at an age of increased vulnerability to these symptoms.
Anxiety‐related disorders have been widely published in previous M‐D cohorts.8, 13, 27 However, GAD was the least common psychopathology (17%) in this cohort, and although significant, showed the smallest difference when compared to both NMC and NC groups. This may be explained by generalized anxiety and related disorders (panic disorder, social and specific phobias) being analyzed separately in this study, rather than collectively as has been done previously. Although focal dystonia and anxiety‐related symptoms have been postulated to share a common pathophysiological pathway28 debate remains as to whether this is a primary or secondary effect of the movement disorder. Suggesting a primary mechanism, several studies have reported onset of the anxiety symptoms prior to the movement disorder.29, 30 However, both studies involved mixed groups of focal dystonia, limiting interpretation of the results. In our smaller cohort, near equal numbers reported onset of their anxiety‐related disorder either in conjunction with (11 cases) or subsequent (10 cases) to development of their motor symptoms.
In this study, as with previous publications, we attempted to determine whether SGCE mutations exerted a gene independent effect in contributing to psychiatric symptomatology, a finding that could have significant implications to our understanding of underlying pathophysiological mechanisms. No evidence of this was found with exception of the lack of a statistically significant difference in the rate of MAD between MC and NMC groups. However, there was no significant difference between NMC and NC groups either and therefore this is unlikely to represent an independent effect of SGCE mutations. As with almost all previous studies7, 15 our attempts to identify a pleiotropic role for SGCE were limited by the small size of the NMC cohort. However, it is also important to consider that manifestation of psychiatric symptoms, as is seen with the motor features, may be similarly silenced due to the imprinting mechanism in the presence of pathogenic mutations.
The use of different psychiatric assessments both between and within individual centere represents a potential limitation of this study. Although all allow classification of symptoms according to DSM‐IV criteria, the manner in which this is achieved differs between interviews. There is, however, evidence to suggest strong concordance between SCID‐I and MINI questionnaires,17 while the CIDI is designed for epidemiological studies and demonstrates greater sensitivity. These interviews were also administered by a number of different assessors with differing training backgrounds. However, the standardized and systematic nature of all three questionnaires limits the potential impact of personal bias and represents the most pragmatic means of assessing a large population across several countries. Finally, although much of the standardized data collection referred to lifetime and present diagnosis, age at onset for example was retrospectively collected and therefore may be limited by recall bias. A systematic prospective data collection in families of affected probands, starting at an early age, remains the only means to overcome this potential bias and given the rarity of this disorder a multicenter international collaboration would provide an opportunity to investigate this further.
Conclusion
We have demonstrated a higher incidence of psychiatric disorders in the motor affected SGCE mutation carriers compared to nonmutation carrying familial controls in the largest published cohort to date. Alcohol dependence, OCD, social and specific phobias were the most strongly associated disorders, consistent with the findings from previous smaller studies. Although no gene independent role for SGCE was demonstrated in this cohort, the consistent finding of comorbid psychiatric disorders within M‐D cohorts suggests that in its active mutated form, SGCE contributes to both motor and psychiatric symptoms. Further prospective work following affected probands and family members from an early age is required to clarify the temporal pattern of onset of these symptoms, how they relate to one another, and what implications this may have on our understanding of the pathogenesis of this disorder.
Author Contribution
Drs Peall, Dijk and Professor Tijssen were involved in the study concept and design, acquisition of data, analysis and interpretation and critical revision of the manuscript. Drs Saunders‐Pullman, Dreissen, van Loon, Cath, Kurian, Foncke and Professors Owen, Morris, Gasser, Bressman and Asmus were involved in the acquisition of data and critical revision of the manuscript.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank the patients and their relatives for participation in this study. Peall is funded by the Welsh Clinical Academic Track (WCAT) training program, The Ipsen Fund and a COST Dystonia Europe Fellowship. J. M. Dijk is funded by Prinses Beatrix Fonds. R. Saunders‐Pullman is funded by National Institutes of Health K02 NS073836. M. A. Kurian is funded by the Wellcome Trust and receives funding from Great Ormond Street Hospital Children's Charity. F Asmus, has no financial interest to disclose related to this work. He is an employee of Bayer Pharma AG, Berlin. M.A.J. Tijssen is funded by STW Technology Society – NeuroSIPE, Netherlands Organization for Scientific Research – NWO Medium, Fonds Nuts‐Ohra, Prinses Beatrix Fonds, Gossweiler Foundation, Stichting wetenschapsfonds dystonie vereniging and educational grants from Ipsen, Allergan and Medtronic. Y. E. M. Dreissen, I. van Loon, D. Cath, M. J. Owen, E. M. J. Foncke, H. R. Morris, T. Gasser and S. Bressman have no financial disclosures.
References
- 1. Asmus F, Zimprich A, Tezenas Du Montcel S, et al. Myoclonus‐dystonia syndrome: epsilon‐sarcoglycan mutations and phenotype. Ann Neurol 2002;52:489–492. [DOI] [PubMed] [Google Scholar]
- 2. Kinugawa K, Vidailhet M, Clot F, Apartis E, Grabli D, Roze E. Myoclonus‐dystonia: an update. Mov Disord 2009;24:479–489. [DOI] [PubMed] [Google Scholar]
- 3. Zimprich A, Grabowski M, Asmus F, et al. Mutations in the gene encoding epsilon‐sarcoglycan cause myoclonus‐dystonia syndrome. Nat Genet 2001;29:66–69. [DOI] [PubMed] [Google Scholar]
- 4. Esapa CT, Waite A, Locke M, et al. SGCE missense mutations that cause myoclonus‐dystonia syndrome impair epsilon‐sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet 2007;16:327–342. [DOI] [PubMed] [Google Scholar]
- 5. Weissbach A, Kasten M, Grunewald A, et al. Prominent psychiatric comorbidity in the dominantly inherited movement disorder myoclonus‐dystonia. Parkinsonism Relat Disord 2013;19:422–425. [DOI] [PubMed] [Google Scholar]
- 6. Peall KJ, Waite AJ, Blake DJ, Owen MJ, Morris HR. Psychiatric disorders, myoclonus dystonia, and the epsilon‐sarcoglycan gene: a systematic review. Mov Disord 2011;26:1939–1942. [DOI] [PubMed] [Google Scholar]
- 7. Peall KJ, Smith DJ, Kurian MA, et al. SGCE mutations cause psychiatric disorders: clinical and genetic characterization. Brain 2013;136:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Tricht MJ, Dreissen YE, Cath D, et al. Cognition and psychopathology in myoclonus‐dystonia. J Neurol Neurosurg Psychiatry 2012;83:814–820. [DOI] [PubMed] [Google Scholar]
- 9. Hess CW, Raymond D, de Aguiar PC, et al. Myoclonus‐dystonia, obsessive‐compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology 2007;68:522–524. [DOI] [PubMed] [Google Scholar]
- 10. Dale RC, Nasti JJ, Peters GB. Familial 7q21.3 microdeletion involving epsilon‐sarcoglycan causing myoclonus dystonia, cognitive impairment, and psychosis. Mov Disord 2011;26:1774–1775. [DOI] [PubMed] [Google Scholar]
- 11. Wong SH, Steiger MJ, Larner AJ, Fletcher NA. Hereditary myoclonus dystonia (DYT11): a novel SGCE gene mutation with intrafamilial phenotypic heterogeneity. Mov Disord 2010;25:956–957. [DOI] [PubMed] [Google Scholar]
- 12. Doheny D, Danisi F, Smith C, et al. Clinical findings of a myoclonus‐dystonia family with two distinct mutations. Neurology 2002;59:1244–1246. [DOI] [PubMed] [Google Scholar]
- 13. Nardocci N, Zorzi G, Barzaghi C, et al. Myoclonus‐dystonia syndrome: clinical presentation, disease course, and genetic features in 11 families. Mov Disord 2008;23:28–34. [DOI] [PubMed] [Google Scholar]
- 14. Ritz K, Gerrits MC, Foncke EM, et al. Myoclonus‐dystonia: clinical and genetic evaluation of a large cohort. J Neurol Neurosurg Psychiatry 2009;80:653–658. [DOI] [PubMed] [Google Scholar]
- 15. Saunders‐Pullman R, Shriberg J, Heiman G, et al. Myoclonus dystonia: possible association with obsessive‐compulsive disorder and alcohol dependence. Neurology 2002;58:242–245. [DOI] [PubMed] [Google Scholar]
- 16. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM‐IV Axis I Disorders—Non‐Patient Edition (SCID‐I/NP), version 2.0. New York: New York State Psychiatric Institute, Biometrics Research, 1996. [Google Scholar]
- 17. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 1998;59(Suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 18. Andrews G. Case ascertainment: the Composite International Diagnostic Interview. Aust N Z J Psychiatry 2000;34(Suppl):S161–163. [DOI] [PubMed] [Google Scholar]
- 19. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12‐month prevalence of DSM‐III‐R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51:8–19. [DOI] [PubMed] [Google Scholar]
- 20. Douglass HM, Moffitt TE, Dar R, McGee R, Silva P. Obsessive‐compulsive disorder in a birth cohort of 18‐year‐olds: prevalence and predictors. J Am Acad Child Adolesc Psychiatry 1995;34:1424–1431. [DOI] [PubMed] [Google Scholar]
- 21. DeBerardinis RJ, Conforto D, Russell K, et al. Myoclonus in a patient with a deletion of the epsilon‐sarcoglycan locus on chromosome 7q21. Am J Med Genet A 2003;121A:31–36. [DOI] [PubMed] [Google Scholar]
- 22. Fullana MA, Mataix‐Cols D, Caspi A, et al. Obsessions and compulsions in the community: prevalence, interference, help‐seeking, developmental stability, and co‐occurring psychiatric conditions. Am J Psychiatry 2009;166:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Broocks A, Thiel A, Angerstein D, Dressler D. Higher prevalence of obsessive‐compulsive symptoms in patients with blepharospasm than in patients with hemifacial spasm. Am J Psychiatry 1998;155:555–557. [DOI] [PubMed] [Google Scholar]
- 24. Barahona‐Correa B, Bugalho P, Guimaraes J, Xavier M. Obsessive‐compulsive symptoms in primary focal dystonia: a controlled study. Mov Disord 2011;26:2274–2278. [DOI] [PubMed] [Google Scholar]
- 25. Cavallaro R, Galardi G, Cavallini MC, et al. Obsessive compulsive disorder among idiopathic focal dystonia patients: an epidemiological and family study. Biol Psychiatry 2002;52:356–361. [DOI] [PubMed] [Google Scholar]
- 26. Fabbrini G, Berardelli I, Moretti G, et al. Psychiatric disorders in adult‐onset focal dystonia: a case‐control study. Mov Disord 2010;25:459–465. [DOI] [PubMed] [Google Scholar]
- 27. de Carvalho Aguiar P, Fazzari M, Jankovic J, Ozelius LJ. Examination of the SGCE gene in Tourette syndrome patients with obsessive‐compulsive disorder. Mov Disord 2004;19:1237–1238. [DOI] [PubMed] [Google Scholar]
- 28. Ron MA. Primary focal dystonia – a disease of brain and mind: motor and psychiatric manifestations have a common neurobiological basis. J Neurol Neurosurg Psychiatry 2009;80:1059. [DOI] [PubMed] [Google Scholar]
- 29. Lencer R, Steinlechner S, Stahlberg J, et al. Primary focal dystonia: evidence for distinct neuropsychiatric and personality profiles. J Neurol Neurosurg Psychiatry 2009;80:1176–1179. [DOI] [PubMed] [Google Scholar]
- 30. Wenzel T, Schnider P, Wimmer A, Steinhoff N, Moraru E, Auff E. Psychiatric comorbidity in patients with spasmodic torticollis. J Psychosom Res 1998;44:687–690. [DOI] [PubMed] [Google Scholar]


