Abstract
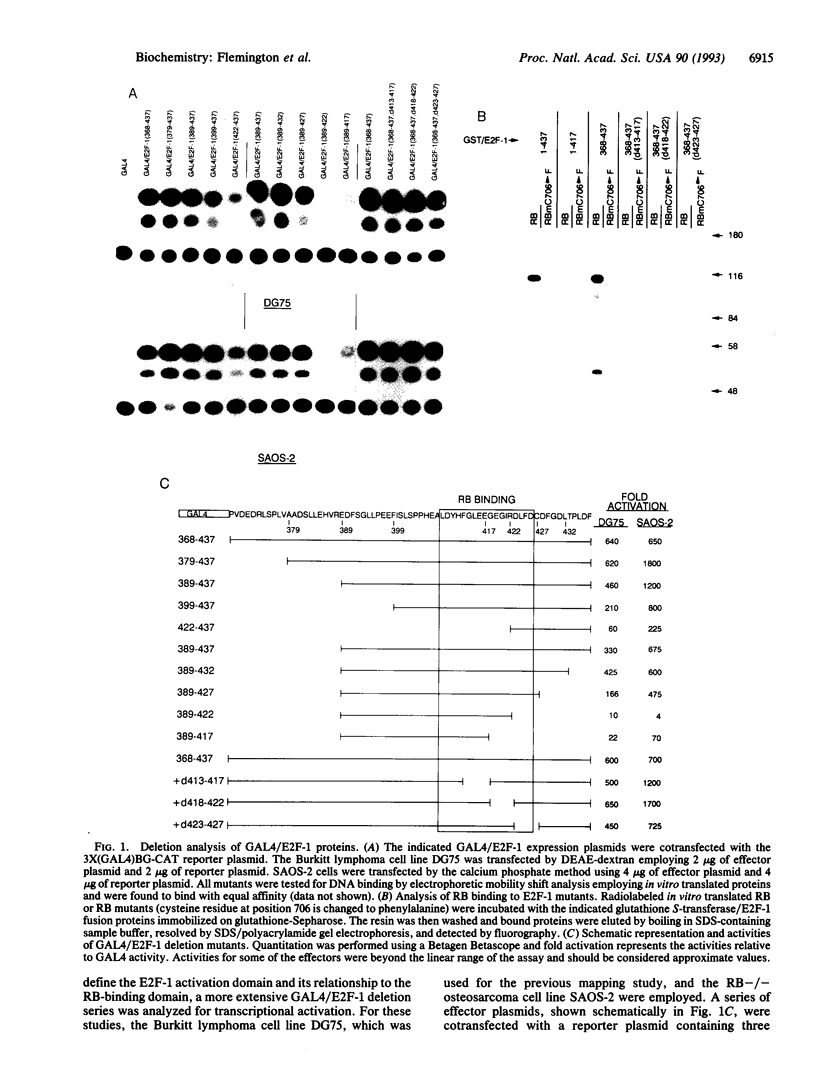
Previous studies have shown that the carboxyl-terminal region of E2F-1 (residues 368-437) can support transcriptional activation when linked to the DNA-binding domain of the yeast transcription factor GAL4. This region also contains an 18-residue retinoblastoma (RB)-binding sequence, raising the possibility that RB binding might inhibit the ability of E2F-1 to form protein-protein contacts required for activation. Here we report a further analysis of the E2F-1 activation domain. In addition, we show that overexpression of RB, but not the RB mutant, RBd22, can inhibit GAL4/E2F-1 activity in vivo. Moreover, expression of the simian virus 40 large tumor antigen (T antigen), but not the RB-binding defective T antigen point mutant, K1, can overcome this repression. Three different GAL4/E2F-1 mutants that activate transcription, but fail to bind to RB, are not significantly affected by overexpression of RB. These findings support a model wherein RB suppresses E2F-1-mediated transcriptional activation through direct physical association.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo M., Raychaudhuri P. Retinoblastoma-repression of E2F-dependent transcription depends on the ability of the retinoblastoma protein to interact with E2F and is abrogated by the adenovirus E1A oncoprotein. Nucleic Acids Res. 1992 Nov 25;20(22):5947–5954. doi: 10.1093/nar/20.22.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chellappan S., Kraus V. B., Kroger B., Munger K., Howley P. M., Phelps W. C., Nevins J. R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Flemington E. K., Borras A. M., Lytle J. P., Speck S. H. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J Virol. 1992 Feb;66(2):922–929. doi: 10.1128/jvi.66.2.922-929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss K., McClain W. H. Rapid site-specific mutagenesis in plasmids. Gene. 1987;59(2-3):285–290. doi: 10.1016/0378-1119(87)90336-2. [DOI] [PubMed] [Google Scholar]
- Girling R., Partridge J. F., Bandara L. R., Burden N., Totty N. F., Hsuan J. J., La Thangue N. B. A new component of the transcription factor DRTF1/E2F. Nature. 1993 Mar 4;362(6415):83–87. doi: 10.1038/362083a0. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Wang N. P., Qian Y. W., Lee E. Y., Lee W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991 Oct 18;67(2):293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin K., Lees J. A., Vidal M., Dyson N., Harlow E., Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992 Jul 24;70(2):337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Huang S., Lee W. H., Lee E. Y. A cellular protein that competes with SV40 T antigen for binding to the retinoblastoma gene product. Nature. 1991 Mar 14;350(6314):160–162. doi: 10.1038/350160a0. [DOI] [PubMed] [Google Scholar]
- Huang S., Wang N. P., Tseng B. Y., Lee W. H., Lee E. H. Two distinct and frequently mutated regions of retinoblastoma protein are required for binding to SV40 T antigen. EMBO J. 1990 Jun;9(6):1815–1822. doi: 10.1002/j.1460-2075.1990.tb08306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Ewen M. E., Livingston D. M. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol. 1990 Jul;10(7):3761–3769. doi: 10.1128/mcb.10.7.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Kaye F. J., Kratzke R. A., Gerster J. L., Horowitz J. M. A single amino acid substitution results in a retinoblastoma protein defective in phosphorylation and oncoprotein binding. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6922–6926. doi: 10.1073/pnas.87.17.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudryj M., Hiebert S. W., Nevins J. R. A role for the adenovirus inducible E2F transcription factor in a proliferation dependent signal transduction pathway. EMBO J. 1990 Jul;9(7):2179–2184. doi: 10.1002/j.1460-2075.1990.tb07387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Transcriptional activation by viral regulatory proteins. Trends Biochem Sci. 1991 Nov;16(11):435–439. doi: 10.1016/0968-0004(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Qian Y., Luckey C., Horton L., Esser M., Templeton D. J. Biological function of the retinoblastoma protein requires distinct domains for hyperphosphorylation and transcription factor binding. Mol Cell Biol. 1992 Dec;12(12):5363–5372. doi: 10.1128/mcb.12.12.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. Q., Chittenden T., Livingston D. M., Kaelin W. G., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992 Jun;6(6):953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- Shan B., Zhu X., Chen P. L., Durfee T., Yang Y., Sharp D., Lee W. H. Molecular cloning of cellular genes encoding retinoblastoma-associated proteins: identification of a gene with properties of the transcription factor E2F. Mol Cell Biol. 1992 Dec;12(12):5620–5631. doi: 10.1128/mcb.12.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S. J., Prater C. A., Dean D. C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992 Jul 16;358(6383):259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- Zamanian M., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from repressing the activity of a cellular transcription factor. EMBO J. 1992 Jul;11(7):2603–2610. doi: 10.1002/j.1460-2075.1992.tb05325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]