Abstract
This study investigated lytic enzyme activities in three indigenous Trichoderma strains namely, Trichoderma asperellum, Trichoderma harzianum and Trichoderma sp. Native Trichoderma strains and a virulent strain of Rhizoctonia solani isolated from infected bean plants were also included in the study. Enzyme activities were determined by measuring sugar reduction by dinitrosalicylic acid (DNS) method using suitable substrates. The antagonists were cultured in minimal salt medium with the following modifications: medium A (1 g of glucose), medium B (0.5 g of glucose + 0.5 g of deactivated R. solani mycelia), medium C (1.0 g of deactivated respective antagonist mycelium) and medium D (1 g of deactivated R. solani mycelia). T asperellum showed presence of higher amounts of chitinases, β-1, 3-glucanases and xylanases in extracellular protein extracts from medium D as compared to medium A. While, the higher activities of glucosidases and endoglucanses were shown in medium D extracts by T. harzianum. β-glucosidase activities were lower compared with other enzymes; however, activities of the extracts of medium D were significantly different. T. asperellum exhibited maximum inhibition (97.7%). On the other hand, Trichoderma sp. did not show any effect on mycelia growth of R. solani on crude extract.
Key words: lytic enzymes, mycoparasitic activity, Rhizoctonia solani, Trichoderma
Introduction
Soil-borne pathogens cause diseases that result in significant loss of quality and yield of the valuable crops around the globe. Fungi, being the most aggressive soil-borne pathogens, have been intensely investigated due to inflicting damage to major crops. The main pathogenic fungal genera involved in such damage are: Phythium, Botrytis, Rhizoctonia and Fusarium (Djonovic et al., 2007). Pesticides have been widely used to control these pathogens (Gerhardson, 2002) but their applications have been increasingly criticized due to environmental and human health concerns (Punja and Utkhede, 2003; Bues et al., 2004).
In Pakistan, being an agricultural country, almost all of the economically important crops are affected by phytopathogenic fungal species such as R. solani, Fusarium moniliforme, and Fusarium solani (Ahmed et al., 1997). R. solani lives in subterranean forms, which makes it the most resistant pathogen. Hence, chemical control is ineffective, unless highly selective fungicides are used. The search for novel bio-control agents, therefore, is a prime target of many plant pathologists (Harman et al., 2004); and Trichoderma species, such as T. harzianum, Trichoderma hamatum, Trichoderma reesei or Trichoderma virens, are recommended by many researchers as preferred choice for controlling phytopathogens (Punja and Utkhede 2003; Steyaert et al., 2003; Harman et al., 2004; Montealegre et al., 2010; Castro et al., 2014).
The genus Trichoderma includes cosmopolitan saprophytic fungi found in soil and have long been known to be effective against plant pathogen, R. solani (Weindling, 1932). Trichoderma acts through direct fungal penetration (Asad et al., 2014) and/or by secreting antifungal compounds, such as hydrolytic enzymes, in order to inhibit the growth of phytopathogens. For instance, T. harzianum releases hydrolytic enzymes against Crinipellis perniciosa, the causative agent of cocoa (Theobroma cacao) disease (De Marco et al., 2003). Due to the significant role of hydrolytic enzymes in the biocontrol activity of Trichoderma species against R. solani, the present study was undertaken to assess the activities of hydrolytic enzymes from three Trichoderma species. Furthermore, the antagonistic potential (antibiosis) of metabolites, obtained from crude extracts of selected isolates, was studied.
Materials and Methods
Microorganisms
Three Trichoderma strains (Trichoderma asperellum, Trichoderma harzianum and Trichoderma sp.) isolated from native agricultural soils were obtained from Fungal Culture Bank of the University of the Punjab, Lahore, Pakistan. A virulent strain of R. solani previously isolated from the infected bean plants was courteously supplied by Agroinnova culture bank, University of Torino, Italy (Minuto et al., 2008; Asad et al., 2014). All microbial cultures were grown and maintained on potato dextrose agar (Difco, Becton, Dickinson, Sparks, MD) at 4 °C. The antagonists were cultured in the minimal salt medium according to the method of Lilly and Barnett (1951) for chitinase, xylanase, (3-1, 3-glucanase, endoglucanase (CMCase) and β-glucosidase activities. Minimal salt medium (Tseng et al., 2008) was used with minor modifications as medium A (1 g of glucose), medium B (0.5 g of glucose + 0.5 g of deactivated R. solani mycelia), medium C (1.0 g of deactivated respective antagonist mycelium) and medium D (1 g of deactivated R. solani mycelia). Fifty milliliters of each of the mentioned media was incubated at 25 °C on a rotary shaker at 150 rpm for 0, 24, 48, 72, 96 and 120 h. Filtrates were collected by centrifugation at 3000 x g for 10 min at 4 °C and the filtrate was used for enzyme activity assays.
Deactivation of mycelium
Mycelia from seven-day old cultures of Trichoderma and R. solani were collected by centrifugation (3000 x g) for 10 min and subsequently washed twice with 50 mL of sterile and deionized water. The collected mycelia were boiled twice for 20 min to obtain the deactivated mycelia and stored at −20 °C until used.
Enzyme activity assays
Enzyme activities of Trichoderma strains were determined by using dinitro-salicylic acid (DNS) reagent (Miller, 1959) and sugar reduction in the respective substrates was measured.
Chitinase activity
Chitinase activity was determined according to Tseng et al. (2008), an artificial substrate containing 10 μL of 0.5% 4-nitrophenyl N, N ‘-diacetyl-β-D-chitobioside (Sigma-Aldrich, USA) and 250 μL of the enzyme samples were mixed in 250 μL of 100 mM acetate buffer having pH 5. After 30 min, 50 μL of 0.4 M Na2CO3 was added to terminate the reaction and turbidity (OD) was measured at 415 nm. One unit of the enzyme activity was defined as the amount of enzyme, required to produce 1 mmol of the product per milligram of the protein per hour.
Beta-1, 3-glucanase activity
Enzyme activity was quantified according to Masih and Paul (2002) by incubating 250 μL of the enzyme samples and 250 μL of 1% laminarin dissolved in 0.2 M acetate buffer (pH 5) at 50 °C for 40 min. The reaction was terminated by 500 μL of DNS reagent and kept for 10 min in a boiling water bath. After boiling, the solution was diluted by adding 4 mL of distilled water and after cooling, the amount of the reducing sugars was measured at 540 nm using D-glucose as benchmark. Specific activity of the enzyme was manifested as mmol of glucose released per milligram of the protein per hour.
Beta-glucosidase activity
Beta-Glucosidase activity was determined by the method of Tokao et al. (1985). The reaction mixtures were prepared by adding 250 μL enzyme sample and 400 μL of 17 mmol L−1 salicin solution dissolved in 0.2 mol L−1 sodium acetate buffer (pH 4.6). 250 μL distilled water was added to the solution and incubated at 50 °C for 40 min. Optical density was recorded at 540 nm using glucose as standard. Specific activity of the enzyme was expressed as mmol of glucose released per milligram of the protein per hour.
Xylanase activity
For xylanase activity, 100 μL enzyme sample was mixed with 500 μL of 1% oat spelt xylan dissolved in 0.1 mol L−1 phosphate buffer (pH 7), and then 400 μL of 0.1 mol L−1 phosphate buffer (pH 7) was added (Bailey et al., 1992). The resultant mixture was incubated at 30 °C for 20 min, cooled, and turbidity was recorded at 540 nm with D-xylose as standard. One unit of enzyme activity was defined as 1 mmol of xylose released per milligram of the protein per hour.
Endoglucanase activity
Endoglucanase activity was determined as described by Ko et al. (2005). Briefly, 250 μL enzyme sample was added to 250 μL of 1% carboxymethyl cellulose dissolved in 0.2 mol L−1 sodium acetate buffer (pH 5). The enzyme containing mixture was incubated at 50 °C for 40 min and terminated by adding 1.5 mL of DNS reagent. Turbidity was recorded at 540 nm using glucose as a standard. Enzyme activity was defined as mmol of xylose released per milligram of the protein per hour.
Total protein concentration in enzyme solution was estimated using bovine serum albumin as standard (Bradford, 1976). The specific activity of the enzyme was expressed as the amount of enzyme that catalyzed formation of 1 mmol of the product per hour under the assay conditions. The experiments were performed twice with three replicates for each treatment.
Effect of crude extract on R. solani mycelial inhibition (Antibiosis)
Three discs of mycelial agar plugs (5 mm diameter) were inoculated in 100 mL potato dextrose broth (PDB). The samples were incubated for 7 days at 25 ± 1 °C on a rotary shaker at 100 rpm and then filtered through a Millipore filter (0.2 μm). The samples were sterilized by passing through a biological membrane filter (0.2 μm). Filtered supernatant (500 μL) from each of the Trichoderma species was spread over the surface of PDA plates and a 5 mm disc of R. solani was inoculated at the center of each plate. The plates were incubated at 25 °C until the colony spread over the surface of PDA plates in the control treatment (Dennis and Webster 1971). Radial growth of the pathogens was recorded on a daily basis. The percent inhibition of the average growth of mycelia in relation to the growth of the controls was calculated by using Equation 1 (Edington et al, 1971).
| (1) |
where C1 = radial mycelial growth of R. solani in the presence of Trichoderma, and C2 = radial mycelial growth of R. solani in control.
Experimental design and statistical analyses
The experiments were repeated twice in a complete randomized block design with four replicates for each treatment. The data were analyzed using SPSS software (version 17.0 Chicago IL, USA). Analysis of variance (ANOVA) was carried out with a significance defined at p < 0.05. Duncan's HSD multiple range test was used as a post-hoc analysis to compare means.
Results
Chitinases
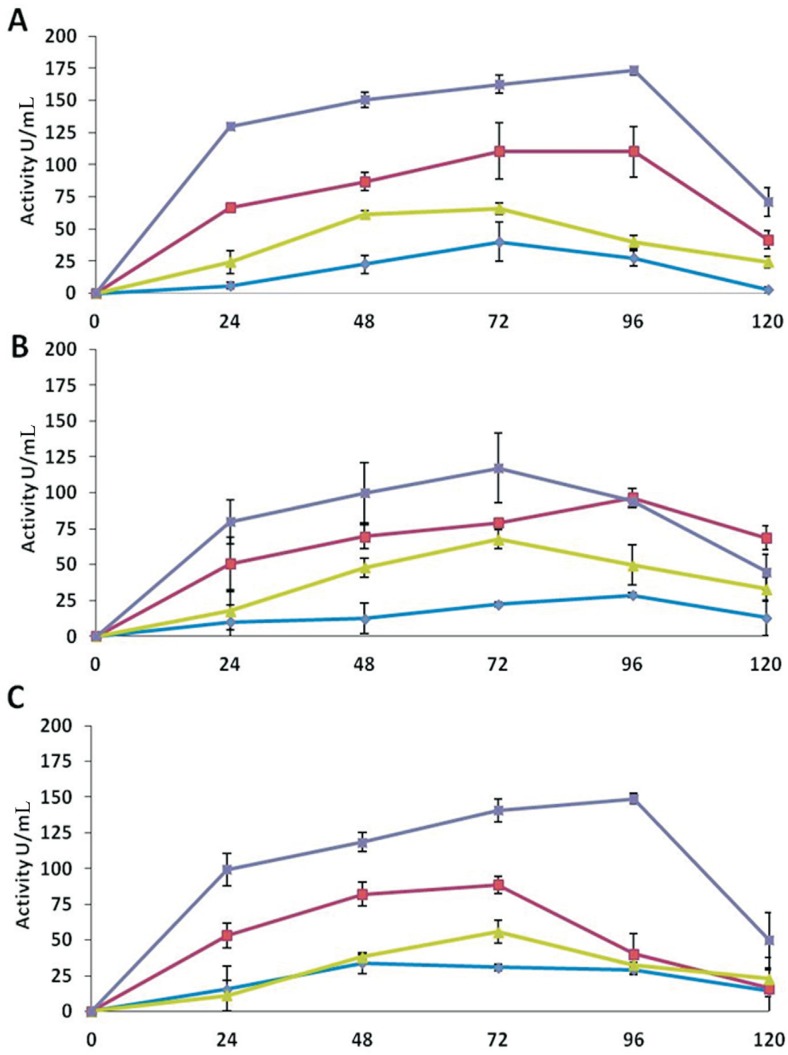
Chitinase activity assays showed that all the antagonists cultured in medium D, (containing deactivated mycelium of R. solani) expressed the highest enzyme activity compared to other three media (Figure 1). The maximum activity (173 U mL−1) was detected by T. asperellum in the extracts of medium D after 96 h of incubation, which was the highest among all the isolates. The medium B, containing glucose and deactivated mycelium of R. solani, also showed high activities (111 U mL−1) as compared to medium A (glucose) and C (deactivated antagonist mycelium).
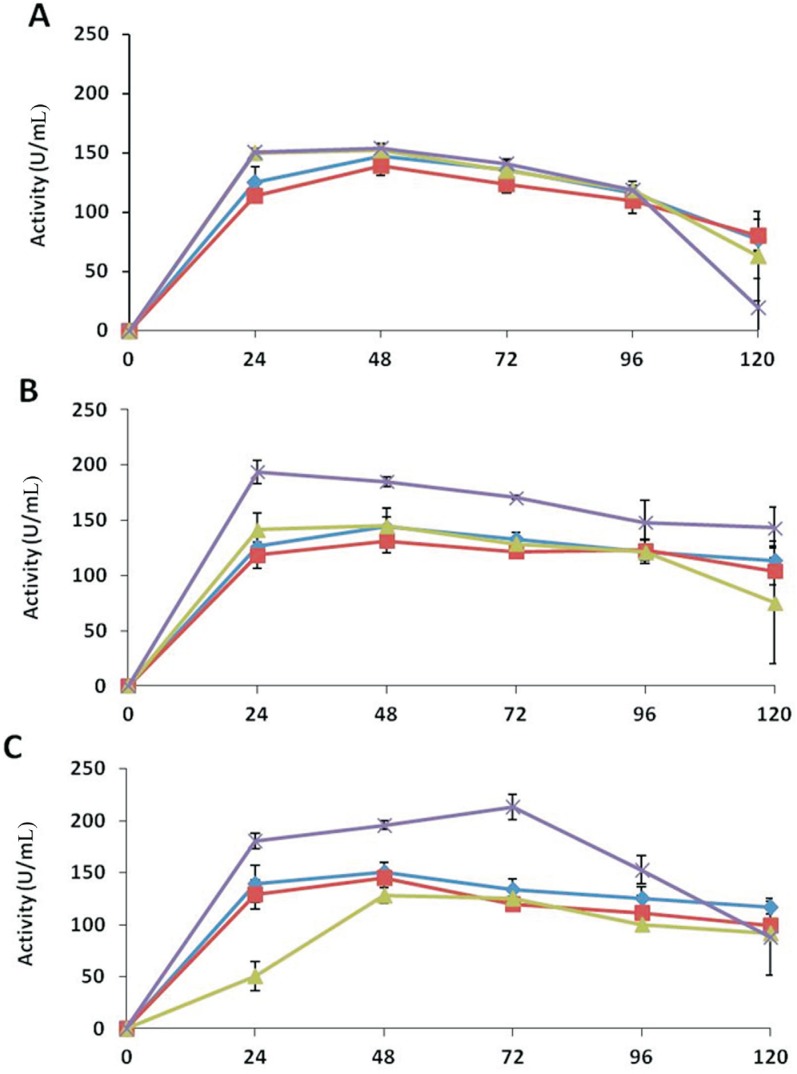
Figure 1. Chitinase activities of the extracellular protein extracts of Trichoderma in different media over an incubation period of 120 h. A: T. asperellum, B: T. harzianum, C: Trichoderma sp. The activities of the enzyme are denoted in different media as: medium A (-♦-), medium B (-■-), medium C (-▲-), and medium D (-x -). Values are means of four replicates, and error bars represent SE.
The maximum chitinase activity by T. harzianum was detected in medium D after 72 h of incubation (117 U mL−1). The medium B showed maximum activity of 97 U mL−1 after 96 h of incubation. While the medium A and C expressed very low chitinases activities (Figure 1).
Trichoderma sp. also showed an increased chitinase activity (149 U mL−1) until 96 h of incubation in the extracts of medium D. While in medium B, the activity increased (89 U mL−1) until 72 h of incubation and then decreased. Significantly low chitinase activities were expressed in medium A and C (Figure 1).
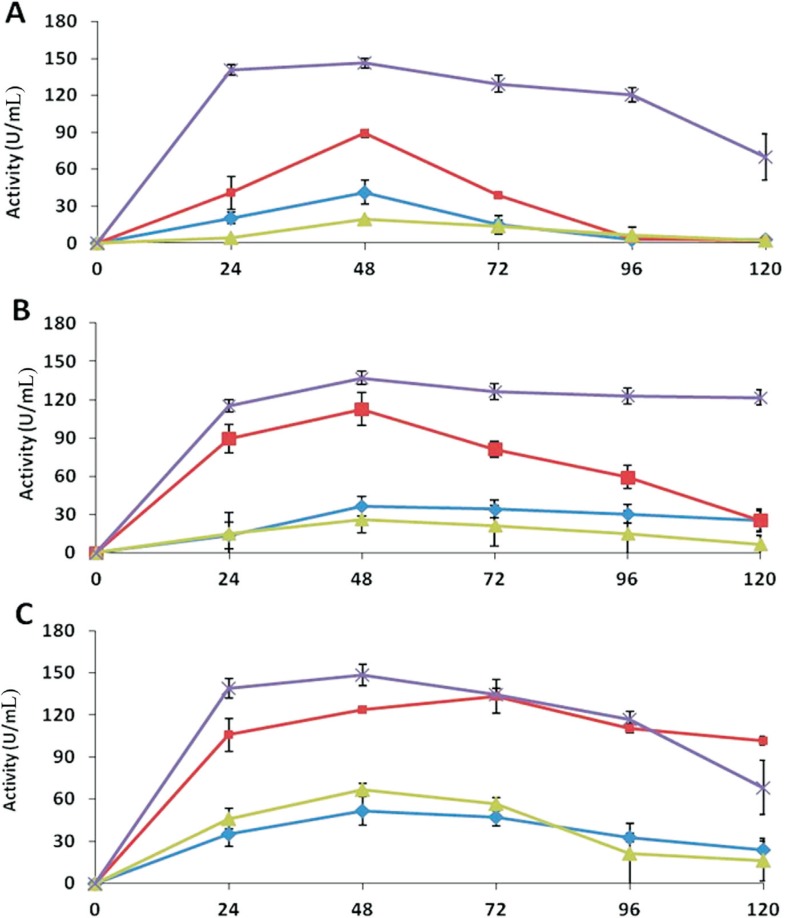
β- 1, 3-glucanase
The assays for β-1,3-glucanase showed that all the antagonists were able to express much higher enzyme activity in extracts of medium D than the extracts of other media. After 48 h of incubation, Trichoderma sp. showed maximum activity of 149 U mL−1, while T. asperellum and T. harzianum exhibited activity of 146 and 137 U mL−1, respectively. T. harzianum maintained maximum activity till 120 h, while the activities decreased in the extracts of T. asperellum and Trichoderma sp. after reaching the maximum level (Figure 2). Very high activities were observed in the extracts of medium B. The activities dropped to minimum after 72 and 96 h of incubation, in the case of T. asperellum and T. harzianum respectively, while for Trichoderma sp., the activity retained at 102 U mL−1 until 120 h of incubation. The results showed that the glucanase activities of all the strains in extracts of medium A and C were non-significant (Figure 2).
Figure 2. β-1,3-glucanase activities of the extracellular extracts of Trichoderma in different media over an incubation period of 120 h. A: T. asperellum, B: T. harzianum, C: Trichoderma sp. The activities of the enzyme are denoted in different media as: medium A (-♦-), medium B (-■-), medium C (-▲-), and medium D (-x -). Values are means of four replicates, and error bars represent SE.
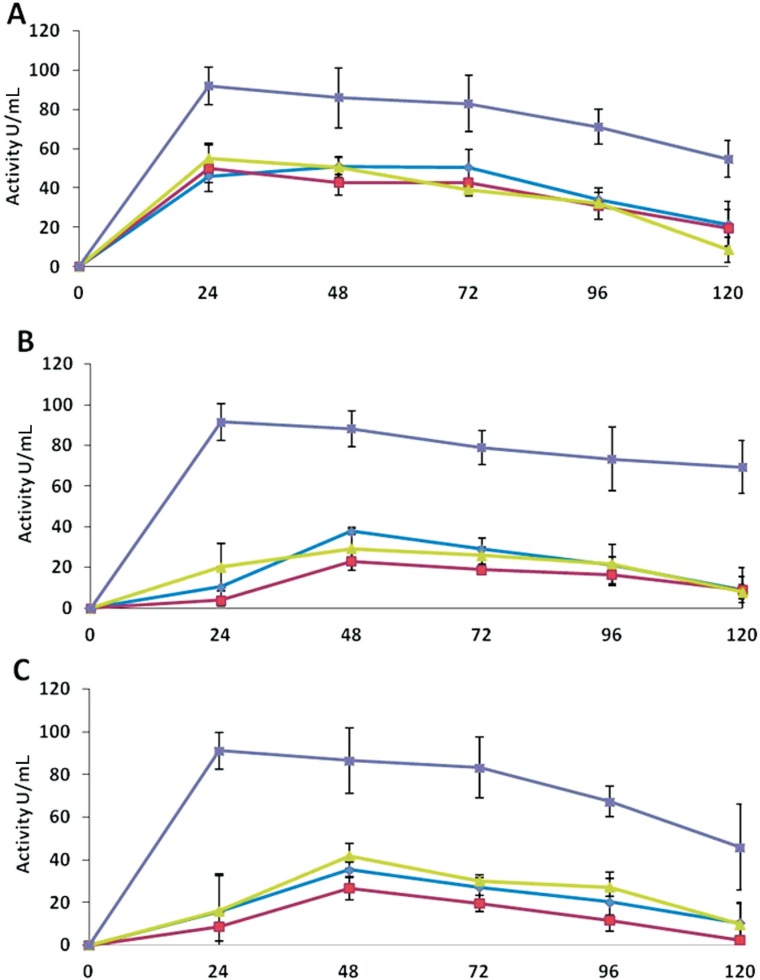
β- glucosidase
Assays indicated that β-glucosidase activities were comparatively lower than that of the chitinase and glucanase. The enzyme activity was significantly higher in the extracts of medium D. The maximum activities were 92 U mL−1 by T. asperellum, and 91 U mL−1 by T. harzianum and Trichoderma sp. (Figure 3). These activities were expressed within first 24 h of the incubation, which decreased immediately thereafter. T. asperellum showed almost similar activities in all the three media, where maximum activities were 51 U mL−1 in medium A after 72 h of incubation and 50 and 55 U mL−1 after 24 h of incubation in medium B and C, respectively. T. harzianum and Trichoderma sp. exhibited very low enzyme activities in the extracts of all the three media. These activities were also lower than the activities expressed by T. asperellum in the respective media (Figure 3).
Figure 3. β- glucosidase activities of the extracellular protein extracts of Trichoderma species in different media over an incubation period of 120 h. A: T. asperellum, B: T. harzianum, C: Trichoderma sp. The activities of the enzyme are denoted in different media as: medium A (-♦-), medium B (-■-), medium C (-▲-), and medium D (-x -). Values are means of four replicates, and error bars represent SE.
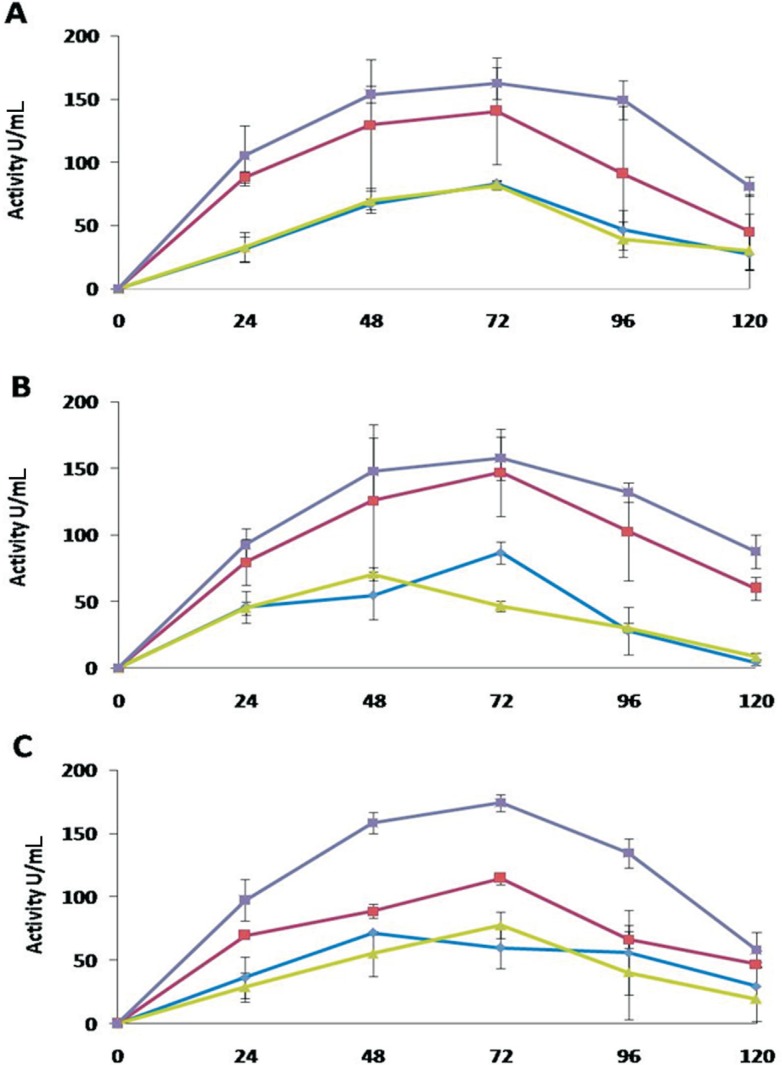
Xylanase
All antagonists showed a higher xylanase activity in medium D compared to other media. T. asperellum exhibited maximum xylanase activity of 162 U mL−1 in the extracts of medium D after 72 h of incubation, which decreased to 81 U mL−1 after 120 h of the incubation (Figure 4). The extracts from medium B exhibited activity of 140 U mL−1 at 72 h of the incubation and then decreased thereafter to 45 U mL−1.
Figure 4. Xylanase activities of the extracellular protein extracts of Trichoderma in different media over an incubation period of 120 h. A: T. asperellum, B: T. harzianum, C: Trichoderma sp. The activities of the enzyme are denoted in different media as: medium A (-♦-), medium B (-■-), medium C (-▲-), and medium D (-x -). Values are means of four replicates, and error bars represent SE.
In the extracts of medium A and C, some activity was shown by T. asperellum after 72 h, which then diminished at the end of the incubation period. The xylanase activities by T. harzianum were also comparatively high in extracts of medium D. The maximum activity was 152 U mL−1 after 72 h of incubation. A significant activity was expressed by the extracts of medium B, while the extracts of medium A and C showed very low activities (Figure 4).
Trichoderma sp. showed highest maximum xylanase activity of 175 U mL−1 in the extracts of medium D after 72 h of the incubation. The medium B also showed significantly higher activities upon induction by deactivated pathogen mycelium, while in the extracts of other media, the enzyme activities were comparable to other isolates (Figure 4).
Endoglucanase
As shown in Figure 5, T. asperellum showed much higher endoglucanase activity than other enzymes in the extracts of all media. There was no effect of the presence of pathogen mycelium in medium D compared with the extracts of medium A, containing only glucose, the same level of enzyme activity was exhibited.
Figure 5. Endoglucanase activities of the extracellular protein extracts of Trichoderma in different media over an incubation period of 120 h. A: T. asperellum, B: T. harzianum, C: Trichoderma sp. The activities of the enzyme are denoted in different media as: medium A (-♦-), medium B (-■-), medium C (-▲-), and medium D (-x -). Values are means of four replicates, and error bars represent SE.
The maximum activities in medium A and B were 147 and 139 U mL−1, respectively; while in media C and D both showed about 153 U mL−1 activity after 48 h of incubation. At the end of the time course, the activities significantly decreased to 77, 80, 63 and 20 U mL−1 in medium A, B, C, and D, respectively (Figure 5).
T. harzianum showed higher activities in extracts of medium D. The maximum enzyme activity (194 U mL−1) was expressed within first 24 h of the incubation and then decreased to 143 U mL−1 after 120 h. In other media, the activities increased after 48 h of incubation to 144, 131 and 145 U mL−1 in medium A, B, and C, respectively; these activities were retained as 114, 104 and 75 U mL−1 for the three media, respectively, after 120 h (Figure 5). Trichoderma sp. also showed high endoglucanase activity (214 U mL−1) in medium D extracts after 72 h of incubation. In medium A, B and C maximum endoglucanase activities were 151, 145 and 128 U mL−1 after 48 h of incubation, which decreased to 116, 99 and 92 U mL−1, respectively, by the end of the time course (Figure 5).
Effect of crude extracts of different species of Trichoderma on the growth inhibition of R. solani
The effect of the metabolites present in the crude extracts of the media was statistically significant for all the strains (p < 0.05). T. asperellum was most effective to inhibit the mycelial growth of R. solani by 97.7%, while T. harzianum was effective with 72.7% of inhibition, and Trichoderma sp. was failed to show any effect on the mycelial growth (Table 1).
Table 1. Effect of crude extracts of different Trichoderma species on growth inhibition of R. solani.
| Isolates | Radial growth of R. solani (cm) | % mycelial inhibition | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| T. asperellum | 0.0 ± 0.0* | 0.0 ± 0.0 | 0.1 ± 0.0 | 97.7a** |
| T. harzianum | 0.9 ± 0.1 | 1.1 +0.1 | 1.2 ± 0.1 | 72.7b |
| Trichoderma sp. | 1.3 ± 0.2 | 2.5 +0.2 | 3.4 ± 0.0 | 22.7c |
| Control (R. solani) | 1.5 ± 0.3 | 3.4 ± 0.2 | 4.4 ± 0.1 | - |
The results are the mean value (± SE) of two independent experiments, each with four replicates.
Values followed by the different letters are statistically different by Duncan's multiple range test (p < 0.05).
Discussion
Apart from other mechanisms, antagonistic ability of Trichoderma also includes production of lytic enzymes that hydrolyze the cell wall of the host fungus (Woo et al., 2006). In most fungi, chitin and glucans are the most abundant microfibrilar components in the cell wall, while proteins and glucans act as cementing matrix (Peberdy, 1990). In the current study, deactivated R. solani mycelium was used rather than cell walls of the pathogen to simulate production of natural metabolites by Trichoderma which resulted in significant activities of chitinase, xylanase and β-1, 3-glucanase. The higher activities of extracellular enzymes in medium D and B as compared to medium A (containing only glucose) illustrate that these enzymes were induced by the presence of deactivated mycelium of R. Solani, this observation concords with the study of Tseng et al. (2008). This behavior can be correlated to the reduction of R. solani mycelium after seven days of incubation, when co-inoculated in vitro. Chitin is the major component of fungal cell walls (Free, 2013). Hence, the chitinases were considered to be involved in the degradation of cell wall of R. solani by T. virens (Baek et al., 1999). Similar observations were recorded by Viterbo et al. (2002), where Chitinase catalyzed cleavage of β-1,4 linkages between N-acetyl-β-D-glucosamine units. Our results demonstrated that T. asperellum produced higher amount of chitinases compared with other species, and T. harzianum produced lowest one. The enzyme activities were observed until 96 h and 72 h of incubation by T. asperellum and T. harzianum, respectively, which decreased significantly after 120 h (Figure 1). De Marco et al. (2003) also reported increase in chitinase activity by Trichoderma isolates within 72 h of incubation. The chitinolytic system of Trichoderma comprises many enzymes, including 1,4-β-acetylglucosaminidase, endochitinases, and exochitinases (Brunner et al., 2003). The chitinases are thought to be the major enzyme reported participating in mycoparasitic interaction. In current study, increased production of the enzymes upon induction by pathogen's deactivated mycelium indicated their possible role in antagonistic activity in dual culture assays. Harighi et al. (2007) purified chitinase-42 (Chi42) from Trichoderma atroviride, which showed a strong potential to lyse R. solani cell wall and inhibit mycelial growth. T. virens mutants over-expressing Chi42 showed an enhanced biocontrol activity against R. solani in cotton seedlings compared to the wild types (Howell, 2003). Haran et al. (1996) detected the presence of GlcNAcases (1,4-β-acetylglucosaminidase), Chit73, and Chit102 in T. harzianum TM and T. asperellum. However, Kullnig et al. (2000) reported that endochitinases were regulated by chit33, chit36 and chit42 genes during stress. Dana et al. (2001) suggested that chit33 is not expressed during overgrowth of R. solani, but expressed only during the contact phase; whereas, chit36Yexpression does not require direct contact with the pathogen. Two proteins, 1,3-β-glucosidase and a 42 kDa endochitinase, were identified by Grinyer et al. (2004) in the culture supernatant of T. Atroviride, grown in the medium containing cell walls of R. solani as the sole carbon source.
The major component of R. solani cell wall has been identified as β-glucan in addition to chitin (Lahsen et al, 2001); therefore, glucanases can play important role in antagonistic activity. β-1,3-glucanases cleave β-1,3 linkages between two molecules of glucose (Viterbo et al., 2002). In the present study, medium D, containing deactivated mycelium of the pathogen, showed highest activity of β-1,3-glucanase for all the three isolates; and, in addition, T. harzianum retained the activity to a maximum level throughout the incubation period (Figure 2). In contrast to this, De Marco et al. (2003) reported increase in glucanase activity by Trichoderma isolates within 72 h of incubation. De La Curz et al. (1995) and Vázquez-Garcidueñas et al. (1998) also showed an increased activity of glucanases by Trichoderma sp. within 48 h of induction, in the presence of different substrates having hydrolytic ability in combination with other enzymes. However, Lorito et al. (1994) purified an endo-β-1,3-glucosidase involved in the inhibition of spore germination of B. cinerea, establishing role of glucanase in mycoparasitism. El-Katatny et al. (2001) also purified a glucanase having a potential to inhibit the growth of S. rolfsii from T harzianum isolate T-24. Therefore, the activities of glucanases by all Trichoderma species can be speculated to be involved in the antagonistic potential in dual culture assays.
In the present study, all tested Trichoderma species showed some activity in medium A containing only glucose until 48 h of incubation and then decreased (Figure 2). However these findings are contrary to Ramot et al. (2000), who concluded that glucose inhibited the secretion of β-1,3-glucanases. Similarly, Tseng et al. (2008) hypothesized that Trichoderma secrets β-1,3-glucanases at a very low level to detect long-chain β-1,3-glucans. The enzymes are secreted for a short period in a glucose-rich medium and degrade if no polysaccharides with β-1,3-linkages are present. However, in a β-1,3-glucan-rich medium, oligosaccharides with β-1,3-linkages are generated that induce β-1,3-glucanases to cleave long chains of β-1,3-glucan producing glucose, which disintegrates cell wall of the pathogen. Xylanase catalyzes hydrolysis of xylan to xylose, and thus xylan is used as a nutrient source but its role in bio-control is not yet established. Trichoderma reesei produces two specific, xylan-inducible xylanases encoded by xyn1 and xyn2 to degrade the β-1,4-D-xylan backbone of hemicelluloses (Zeilinger et al, 1996; Mach et al, 1996). The role of the enzymes in bio-control has not been studied possibly due to the fact that R. solani cell wall lacks xylan. All the tested strains of Trichoderma showed a high activity of xylanase, while grown in a medium containing deactivated pathogen mycelium (Figure 4). Therefore, β-1,3-glucanase activities by Trichoderma species were assumed to exhibit xylanase activity, which was in agreement with Tseng et al. (2008). It might have contributed to the combined antagonistic activities of Trichoderma strains against R. solani in dual culture assays.
Trichoderma species are known to produce cellulases and β-glucosidase which hydrolyze β-1,4-glucans (De Marco et al, 2003). The cre1 gene in the filamentous fungi Trichoderma reesei and T. harzianum regulates cellulase expression (Ilmén et al., 1996). The mycoparasitic interaction relieves binding of the Cre1 carboncatabolite repressor protein to promoter sequences of ech42 (endochitinaseencoding) gene in T. harzianum (Strauss et al., 1995; Lorito et al., 1996; Portnoyet al., 2011; Silva-Rocha et al., 2014). β-1,4-glucans contribute to the cell wall of the R. solani (Lahsen et al., 2001). In the present study, T. harzianum was found to produce β-glucosidase and cellulase enzymes on induction by deactivated mycelium of the pathogen within 24 h of growth, which contributed the antagonistic behavior in dual culture assay. However, T. asperellum did not show any significant effect on induction by deactivated mycelium on the activity of cellulytic enzymes, suggesting that the major enzymes involved in the mycoparasitism by T. asperellum do not include cellulases. De Marco et al. (2003) reported increased activities of cellulytic enzymes within 24 h of incubation in the presence of specific substrates; Trichoderma may be involved in the hydrolysis of cell wall of pathogen, which differs from Tseng et al. (2008), who reported an insignificant cellulose activity by T. harzianum.
Mycoparasitism is associated with the production of cell walldegrading enzymes such as chitinase and glucosidase and their induction as a response to infection by pathogen (Zhang et al., 2010); and these two classes of the hydrolytic enzymes show synergistic activity against several pathogenic fungi (Qin et al., 2003). The cell walldegrading enzymes of Trichoderma are of special importance to induce the defense mechanisms of plants (Jayalakshmi et al., 2009). Abd-El-Khair et al. (2010) also reported the induction of enzymes such as chitinase and peroxidase by Trichoderma, which play an important role in the defense mechanisms of plants against pathogens.
The production of antibiotics as well as secondary metabolites was determined by applying crude cell free extract of Trichoderma indigenous isolates on pathogen growth to establish antagonistic potential. The inhibition of mycelia growth of R. solani by crude extracts produced in liquid medium was significantly different among Trichoderma species. T. asperellum showed maximum inhibition of 97%, while Trichoderma sp. did not show any effect on mycelia growth of the pathogen (Table 1), which indicated that the secondary metabolites/antibiotics production can be related to the combined antagonistic activity by T. asperellum against R. solani. These results are in accordance with Castillo et al. (2011), who observed 100% mycelial growth inhibition of the pathogen by T. asperellum. Similar findings were reported by Etebarian (2006), showing the antifungal effect of metabolites secreted by T. harzianum and T. virens isolates causing 100% inhibition of mycelia growth of Macrophomina phaseolina. However, Vinale et al. (2006) isolated and characterized secondary metabolites from culture filtrates of two commercial T. harzianum isolates (T22 and T39) and reported their production in relation to mycoparasitic interaction with R. solani.
Conclusions
T. asperellum produced higher amounts of chitinases, β-1,3-glucanases and xylanases in a medium containing deactivated R. solani mycelium than the medium containing only glucose. T. asperellum showed maximum inhibition (97.7%) of mycelial growth of R. solani, while Trichoderma sp. did not show any effect. This study established antagonistic potential of Trichoderma using crude protein extract.
Acknowledgments
The authors thank the University of Torino, Italy for partial financial support and Mas-simo Pugliese, Maria Lodovica Gullino for providing the technical assistance in sample analysis. We are also grateful to the anonymous referees who made valuable comments and suggestions. We also thank Professor James Peters from the University of Manitoba, Canada for improving English quality of the manuscript.
References
- Abd-El-Khair H, Khalifa RM, Hagga KE. Effect of Trichoderma Species on damping off diseases incidence, some plant enzymes activity and nutritional status of bean plants. J American Sci. 2010;6:486–497. [Google Scholar]
- Ahmad S, Iqbal SH, Khalid AN. Fungi of Pakistan. Mycological Society of Pakistan. Uni.; Punjab: 1997. 248 pp. [Google Scholar]
- Ait-Lahsen H, Soler A, Rey M, et al. An antifungal exo-β-1,3-glucanase (AGN13.1) from the bio control fungus Trichoderma harzianum . Appl Environ Microbiol. 2001;67:5833–5839. doi: 10.1128/AEM.67.12.5833-5839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asad SA, Ali N, Hameed A, et al. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani . Pol J Microbiol. 2014;63:95–103. [PubMed] [Google Scholar]
- Baek JM, Howell CR, Kenerley CM. The role of extracellular chitinase from Trichoderma virens Gv29-8 in the bio control of Rhizoctonia solani . Curr Genet. 1999;35:41–50. doi: 10.1007/s002940050431. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Bailey P, Poutanen K. Inter laboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:1105–1112. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunner K, Montero M, Mach RL, et al. Expression of the ech42 (endochitinase) gene of Trichoderma atroviride under carbon starvation is antagonized via a BrlA-like cis-acting element. FEMS Microbiol Lett. 2003;218:259–264. doi: 10.1111/j.1574-6968.2003.tb11526.x. [DOI] [PubMed] [Google Scholar]
- Bues R, Bussieres P, Dadomo M, et al. Assessing the environmental impacts of pesticides used on processing tomato crops. Agri Ecosys Environ. 2004;102:155–116. [Google Scholar]
- Castillo FDH, Padilla AMB, Morales GG, et al. In vitro antagonist action of Trichoderma strains against sclerotinia sclerotium and sclerotium cepivorum. Am J Agri Biol Sci. 2011;6:410–417. [Google Scholar]
- Castro Ldos S, Antoniêto ACC, Pedersoli WR, et al. Expression pattern of cellulolytic and xylanolytic genes regulated by transcriptional factors XYR1 and CRE1 are affected by carbon source in Trichoderma reesei . Gene Expr Patterns. 2014;14:88–95. doi: 10.1016/j.gep.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Dana MM, Limón MC, Mejías R. Regulation of chitinase 33 (chit33) gene expressions in Trichoderma harzianum . Curr Genet. 2001;38:335–342. doi: 10.1007/s002940000169. [DOI] [PubMed] [Google Scholar]
- De la Cruz J, Pintor-Toro JA, Benitez T. A novel endo-β-1, 3-glucanase, BGN13.1 involved in the mycoparasitism of Trichoderma harzianum . J Bacteriol. 1995;177:6937–6945. doi: 10.1128/jb.177.23.6937-6945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco JL, Valadares MC, Felix CR. Production of hydrolytic enzymes by Trichoderma isolates with antagonistic activity against Crinipellis perniciosa, the causal agent of witches’ broom of cocoa. Braz J Microbiol. 2003;34:33–38. [Google Scholar]
- Dennis C, Webster J. Antagonistic properties of species groups of Trichoderma I, production of non-volatile antibiotics. Trans Br Mycol Soc. 1971;57:25–39. [Google Scholar]
- Djonovic S, Vargas WA, Kolomiets MV. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007;145:875–889. doi: 10.1104/pp.107.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington LV, Khew KL, Barron GL. Fungi toxic spectrum of benzimidazole compounds. Phytopathol. 1971;61:42–44. [Google Scholar]
- El-Katatny MH, Gudelj M, Robra KH. Characterization of a chitinase and an endo-beta-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii . Appl Microbiol Biotechnol. 2001;56:137–143. doi: 10.1007/s002530100646. [DOI] [PubMed] [Google Scholar]
- Etebarian HR. Evaluation of Trichoderma isolates for biological control of charcoal stem rot in melon caused by Macrophomina phaseolina . J Agri Sci Technol. 2006;8:243–250. [Google Scholar]
- Free SJ. Fungal cell wall organization and biosynthesis. Adv Genet. 2013;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- Gerhardson B. Biological substitutes for pesticides. Trends Biotechnol. 2002;20:338–343. doi: 10.1016/s0167-7799(02)02021-8. [DOI] [PubMed] [Google Scholar]
- Grinyer J, McKay M, Herbert B. Fungal proteomics: mapping the mitochondrial proteins of a Trichoderma harzianum strain applied for biological control. Curr Genet. 2004;45:170–175. doi: 10.1007/s00294-003-0475-3. [DOI] [PubMed] [Google Scholar]
- Haran S, Schickler H, Oppenheim A, Chet I. Differential expression of Trichoderma harzianum chitinases during mycoparasitism. Phytopathol. 1996;86:980–985. [Google Scholar]
- Harman GE, Howell CR, Viterbo A. Trichoderma species-opportunistic, a virulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Harighi MJ, Zamani MR, Motallebi M. Evaluation of antifungal activity of purified chitinase 42 from Trichoderma atroviride PTCC5220. Biotechnol. 2007;6:28–33. [Google Scholar]
- Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant diseases; the history and evolution of current concepts. Plant Dis. 2003;87:4–10. doi: 10.1094/PDIS.2003.87.1.4. [DOI] [PubMed] [Google Scholar]
- Ilmén M, Thrane C, Penttilä M. The glucose repressor gene cre1 of Trichoderma: isolation and expression of a fulllength and a truncated mutant form. Mol Gen Genet. 1996;251:451–460. doi: 10.1007/BF02172374. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi SK, Rajus S, Benagi VI. Trichoderma harzianum L1 as a potential source for lytic enzymes and elicitor of defense responses in chickpea (Cicer arietinum L.) against wilt disease caused by Fusarium oxysporum. sp. ciceri. Aus J Crop Sci. 2009;3:44–52. [Google Scholar]
- Ko HG, Parka SH, Kimb SH. Detection and recovery of hydrolytic enzymes from spent compost of four mushroom species. Foliar Microbiol. 2005;50:103–106. doi: 10.1007/BF02931456. [DOI] [PubMed] [Google Scholar]
- Kullnig C, Mach RL, Lorito M, et al. Enzyme diffusion from Trichoderma atroviride (T. harzianum P1) to Rhizoctonia solani is a prerequisite for triggering of Trichoderma ech42 gene expression before mycoparasitic contact. Appl Environ Microbiol. 2000;66:2232–2234. doi: 10.1128/aem.66.5.2232-2234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly VG, Bernett HL. Physiology of the Fungi. McGraw-Hill; New York: 1951. [Google Scholar]
- Lorito M, Harman CK, Di Pietro A, et al. Purification, characterization and synergistic activity of a glucans 1, 3-beta glucosidase and an N-acetylglucosaminidase from Trichoderma harzianum . Phytopathol. 1994;84:398–405. [Google Scholar]
- Lorito M, Mach RL, Sposato P, et al. Mycoparasitic interaction relieves binding of the Cre1 carbon catabolite repressor protein to promoter sequence of ech-42 (endochitinaseencoding) gene of Trichoderma harzianum . Proc Natl Acad Sci. 1996;93:14868–14872. doi: 10.1073/pnas.93.25.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RL, Strauss J, Zeilinger S, et al. Carbon catabolite repression of xylanase I (xyn1) gene expression in Trichoderma reesei . Mol Microbiol. 1996;21:1273–1281. doi: 10.1046/j.1365-2958.1996.00094.x. [DOI] [PubMed] [Google Scholar]
- Masih EI, Paul B. Secretion of β-1, 3-glucanase by the yeast Pichia membranefaciens and its possible role in the bio control of Botrytis cinerea causing grey mold of grapevine. Curr Microbiol. 2002;44:391–395. doi: 10.1007/s00284-001-0011-y. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- Minuto A, Gaggero L, Gullino ML. Influence of pH, nutrient solution disinfestation and antagonists application in a closed soilless system on severity of Fusarium wilts of gerbera. Phytopara. 2008;36:294–303. [Google Scholar]
- Montealegre J, Valderrama L, Sánchez S. Biological control of Rhizoctonia solani in tomatoes with Trichoderma harzianum mutants. Electron J Biotechnol. 2010;13:1–11. [Google Scholar]
- Peberdy JF. Fungal cell walls. A review. In: Kuhn PJ, Trinci APJ, Jung MJ, et al., editors. Biochemistry of Cell Walls and Membranes in Fungi. Springer-Verlag; London: 1990. pp. 5–30. [Google Scholar]
- Portnoy T, Margeot A, Linke R, et al. The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: a master regulator of carbon assimilation. BMC Genomics. 2011;12:269–269. doi: 10.1186/1471-2164-12-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punja ZK, Utkhede RS. Using fungi and yeasts to manage vegetable crop diseases. Trend Biotechnol. 2003;21:400–407. doi: 10.1016/S0167-7799(03)00193-8. [DOI] [PubMed] [Google Scholar]
- Qin GZ, Tian SP, Xu Y. Enhancement of bio control efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol Mol Plant Pathol. 2003;62:147–154. [Google Scholar]
- Ramot O, Cohen-Kupiec R, Chet I. Regulation of P-1,3-glucanase by carbon starvation in the mycoparasite Trichoderma harzianum . Mycol Res. 2000;104:415–420. [Google Scholar]
- Silva-Rocha R, Castro Ldos S, Antoniêto ACC, et al. Deciphering the Cis-Regulatory Elements for XYR1 and CRE1 Regulators in Trichoderma reesei . PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyaert JM, Ridgway HJ, Elad Y. Genetic basis of mycoparasitism; a mechanism of biological control by species of Trichoderma . New Zea J Crop Hort Sci. 2003;31:281–291. [Google Scholar]
- Strauss J, Mach RL, Zeilinger S, et al. Cre l, the carbon catabolite repressor protein from Trichoderma reesei . FEBS Letters. 1995;376:103–107. doi: 10.1016/0014-5793(95)01255-5. [DOI] [PubMed] [Google Scholar]
- Tokao S, Kamagata Y, Sasaki T. Cellulase production by Penicillium purpurogenum . J Agri Sci. 1985;93:217–222. [Google Scholar]
- Tseng SC, Liu SY, Yang HH. Proteomic Study of Bio control mechanisms of Trichoderma harzianum ETS 323 in response to Rhizoctonia solani . J Agri Food Chem. 2008;56:6914–6922. doi: 10.1021/jf703626j. [DOI] [PubMed] [Google Scholar]
- Vázquez-Garcidueñas S, Leal-Morales C, Herrera-Estrella A. Analysis of the β-1, 3-glucanolytic system of the bio control agent Trichoderma harzianum . Appl Environ Microbiol. 1998;64:1442–1446. doi: 10.1128/aem.64.4.1442-1446.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinale F, Marra R, Scala F, et al. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Letts Appl Microbiol. 2006;43:143–148. doi: 10.1111/j.1472-765X.2006.01939.x. [DOI] [PubMed] [Google Scholar]
- Viterbo A, Ramot O, Chemin LY, et al. Significance of lytic enzymes from Trichoderma spp. in the bio control of fungal plant pathogens. Ant van Leeuw. 2002;81:549–556. doi: 10.1023/a:1020553421740. [DOI] [PubMed] [Google Scholar]
- Weindling R. Trichoderma lignorumas a parasite of other fungi. Phytopathol. 1932;22:837–845. [Google Scholar]
- Woo SL, Scala F, Ruocco M, et al. The molecular biology of the interactions between Trichoderma spp. phytopathogenic fungi and plants. Phytopathol. 2006;96:181–185. doi: 10.1094/PHYTO-96-0181. [DOI] [PubMed] [Google Scholar]
- Zeilinger S, Mach RL, Schindler M, et al. Different inducibility of expression of the two xylanase genes xyn1 and xyn2 in Trichoderma reesei . J Biol Chem. 1996;271:25624–25629. doi: 10.1074/jbc.271.41.25624. [DOI] [PubMed] [Google Scholar]
- Zhang D, Spadaro D, Garibaldi A, et al. Selection and evaluation of new antagonists for their efficacy against postharvest brown rot of peaches. Postharvest Biol Technol. 2010;55:174–181. [Google Scholar]