Abstract
Sex differences in learning and memory suggest differences between men and women in mechanisms of neural plasticity. Such differences have been reported in a variety of explicit memory tasks, but implicit memory has not been studied in this context. We investigated differences between men and women in offline consolidation of perceptual learning (PL) of motion direction discrimination. Initially, discrimination thresholds were measured for two opposite directions of motion, followed by approximately forty minutes of training on one of the directions. During a post-training consolidation period, subjects either took a nap or remained awake. Thresholds were then reassessed for both directions of motion. We found that rapid eye movement (REM) sleep facilitates consolidation of PL but that the pattern of specificity in the REM condition differed between men and women. PL for men whose naps contained REM sleep was highly specific to the trained direction of motion, whereas REM sleep in women resulted in generalized learning to the untrained direction as well as to a novel direction that was not previously tested. Moreover, for subjects in the REM condition, men exhibited greater PL than women for the trained direction. Our findings provide the first evidence of sex differences in the magnitude and specificity of PL and in the role of REM sleep in implicit learning. Our results have important implications for optimization of educational and training strategies designed for males and females.
Keywords: Perceptual learning, REM sleep, napping, memory consolidation, specificity, generalization
1. Introduction
Cognitive performance is influenced by a variety of psychological and biological factors, including sex. In the domain of episodic memory, there are systematic differences between men and women in performance of hippocampal-dependent tasks (reviewed in Herlitz, Airaksinen, & Nordstrom, 1999). In particular, women outperform men on episodic memory tasks, including word recall, word recognition, story recall, name recognition, face recognition, and concrete picture recall and recognition (Lewin, Wolgers, & Herlitz, 2001). Women also have better memory for emotional stimuli than men (Canli et al., 2002). In contrast, men excel on visuospatial episodic memory tasks (Herlitz, Airaksinen, & Nordstrom, 1999; Lewin et al., 2001). Although complete mechanistic explanations of sex differences in cognition are still lacking, there are many biological dimorphisms that could account for these differences, such as dimorphisms in brain structure, sex hormones and neurotransmitters, and differing responses to stress hormones (reviewed in Cahill, 2006). In particular, no studies have determined whether sex differences exist for implicit learning and whether such differences interact with the documented effects of sleep on implicit learning. In the present study, we directly measure sex differences in sleep-dependent implicit learning of a visual perceptual skill.
Perceptual learning (PL) is the long-term improvement of performance on a sensory task. One of the hallmarks of PL is that it is specific to the physical features of the trained stimulus. That is, the performance improvement does not fully generalize to stimuli that are not used during training. In the visual system, specificity of PL has been demonstrated for spatial location (Ball & Sekuler, 1987; Nishina, Kawato, & Watanabe, 2009), orientation (Ahissar & Hochstein, 1997), spatial frequency (Fiorentini & Berardi, 1980), and ocularity, when training is monocular (Fahle, Edelman, & Poggio, 1995; Karni & Sagi, 1991), suggesting that the mechanism of training effects is a change in encoding in early stages of visual processing and/or decoding of activity in these early stages by higher-order areas involved in perceptual decisions. In particular, visual PL of motion direction discrimination is specific to the direction of motion and visual field location used for training (Ball & Sekuler, 1987; Rokem & Silver, 2010). In the present study, we assessed sex differences in the magnitude and specificity of PL of motion direction discrimination following sleep-dependent consolidation.
Offline consolidation during sleep has substantial effects on the magnitude and specificity of PL (Mednick et al., 2002; Mednick, Nakayama, & Stickgold, 2003). For example, post-training improvement of texture discrimination is dependent on both slow wave sleep and rapid eye movement (REM) sleep (Karni et al., 1994; Stickgold et al., 2000). A recent study reported sex differences in motor and verbal learning following a nap and found that sleep-dependent learning effects in women were mediated by the phase of the menstrual cycle (Genzel et al., 2012). However, PL was not examined in this study.
In the current study, we examined the effects of sleep during the consolidation period on the magnitude and specificity of PL of motion direction discrimination. We also assessed sex differences in these sleep effects. We utilized a nap paradigm that controls for circadian confounds and daytime interference. Our nap paradigm also allows for exquisite control of sleep stages (i.e., naps with and without REM sleep) and can produce the same magnitude of PL as a full night of sleep (Mednick et al., 2002, 2003). The experimental design includes a group of subjects that rested quietly during the consolidation period but were electroencephalographically monitored to insure they did not fall asleep (quiet wake) and a group that conducted their normal daily activities (without sleep or rest) during consolidation (active wake). Our results reveal a novel interaction between sex and sleep that affects both the magnitude and specificity of PL. This interaction demonstrates differences in the mechanisms of offline consolidation of PL between men and women.
2. Methods
2.1 Subjects
150 healthy non-smoking adults between the ages of 18 and 35 gave informed consent to participate in the study. All experimental procedures were approved by the University of California, San Diego Human Research Protections Program. Exclusion criteria included: a) irregular sleep-wake schedule; b) sleep disorder; c) significant psychopathology in immediate family; d) current use of any psychotropic medications; e) history of head injury and/or seizures; f) history of substance dependence; g) any other major medical condition. These exclusion criteria were evaluated based on subject self-report.
Subjects were asked to maintain their usual sleep-wake schedule during the week prior to the experiment and to refrain from consumption of caffeine, alcohol, and all stimulants for 24 hours prior to the beginning of the experiment as well as throughout the study day. Heavy caffeine users were not enrolled to exclude the possibility of significant withdrawal symptoms during the experiment. Subjects completed sleep diaries during the entire week prior to the experiment and wore actigraph wristwatches (Actiwatch-64, Respironics) the night before the experiment to provide subjective and objective measures of sleep-wake activity, respectively. We also assessed trait daytime sleepiness with the Epworth Sleepiness Scale (Johns, 1991) and evaluated circadian phase preference for morningness or eveningness with the Horne-Ostberg Morningness-Eveningness Questionnaire (Horne & Ostberg, 1976).
2.2 Stimulus and task
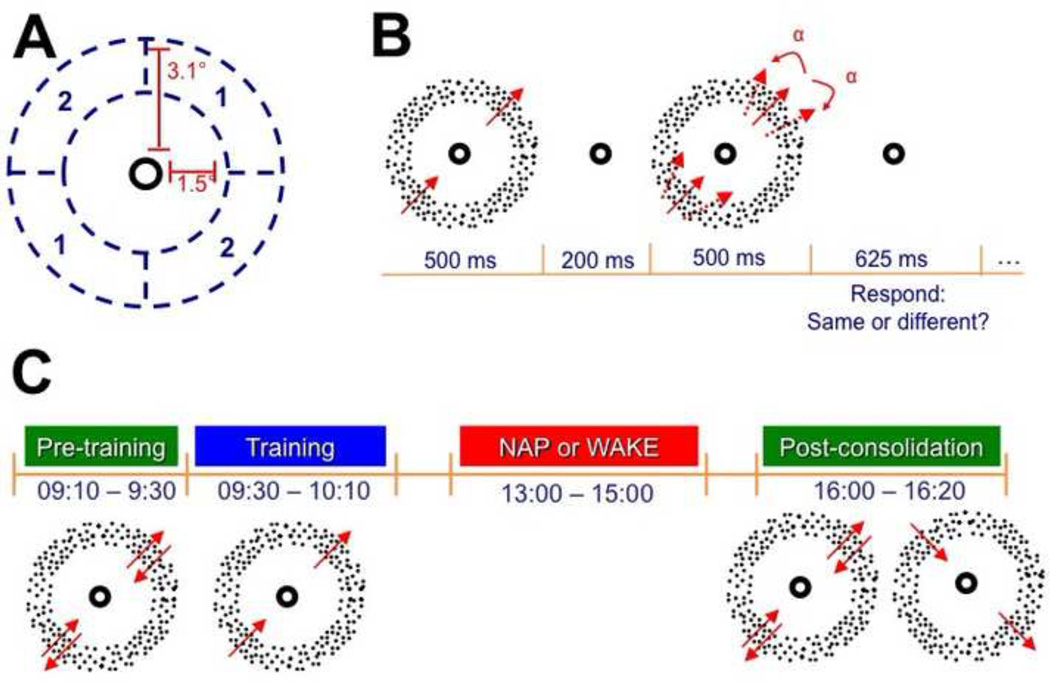
Visual stimuli for the motion direction discrimination (MDD) task have been previously described (Rokem & Silver, 2010) and were created using the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). Random dot kinetograms were presented within an annulus subtending 1.5–3.1 degrees of visual angle and centered at the fixation point (Figure 1A). The radius of each dot was 0.03 deg, and the dot density was 17 dots/deg2. The dots were moving at a speed of 8 deg/sec, and each dot moved continuously for two monitor frames (approximately 27 msec at the 75 Hz refresh rate used) before being reassigned to another random location within the annulus. The dots were displayed at full luminance (158.9 cd/m2). Two quadrants of the annulus, located on opposite sides of the fixation point, contained 100% coherent dot motion, and the remaining quadrants contained 0% coherent motion (Figure 1A).
Figure 1.
Experimental procedures. A) Stimulus configuration. Coherent motion was presented in one of two pairs of spatial locations (1 or 2), and the other pair of spatial locations contained dots with 0% motion coherence. B) Motion direction discrimination task. In each trial, two fields of dots with 100% coherent motion were sequentially presented. The two stimuli contained either the same or slightly different directions of motion. Direction of motion is indicated by the arrows, and angular difference in motion direction is denoted here by α. C) Experimental timeline. At 9:10, pre-training thresholds were obtained for two oblique directions of motion (in this example, 45° and 225° in location 1). One of these directions (here, 45° in location 1) was then randomly chosen to be the trained direction, and subjects performed the task with this direction/location combination for 1000 trials. Subjects then either napped or remained awake from 13:00 to 15:00. At 16:00, post-consolidation thresholds were obtained for the two directions of motion used in the pre-training measurements as well as a novel direction/location combination (in this example, 135° in location 2).
In each trial, subjects reported whether the dots in two sequentially-presented stimuli were moving in the same or in different directions within the two quadrants containing coherent dot motion (Figure 1B) (Ball & Sekuler, 1987; Rokem & Silver, 2009, 2010, 2013). Presentation of task-relevant information in locations on opposing sides of the fixation point encouraged subjects to maintain central fixation throughout the trial, and subjects were also explicitly instructed to maintain fixation. The angular difference between the sequentially-presented stimuli was adjusted according to a Quest psychophysical staircase, converging on 70% correct performance, and each threshold was estimated from all trials in the staircase (Watson & Pelli, 1983). In addition, the Quest algorithm was used to calculate a 95% confidence interval for each threshold measurement. The stimulus presentation software can be downloaded from: http://github.com/arokem/motion_th.
2.3 Protocol
The full experimental timeline is displayed in Figure 1C. At 09:00, subjects practiced MDD for several minutes until they could reliably perform the task (95% confidence interval of discrimination threshold less than 30 degrees). Next, pre-training thresholds were measured for each of two opposite oblique directions and one of the two possible visual field locations within the stimulus annulus, randomly chosen for each subject (Rokem & Silver, 2010, 2013). Each pre-training threshold was the average of thresholds from two assessments of 50 trials each. At 09:30, one of the pre-training directions was randomly chosen to be the trained direction (the opposite direction was the untrained direction), and subjects performed 1000 trials of the MDD task for this direction/location combination.
At 11:00, subjects were randomly assigned to one of three groups. Subjects took a nap recorded with polysomnography (PSG), sat in a recliner listening to classical music with PSG monitoring (quiet wake (QW), n=23), or carried out their normal daily activities but were instructed to abstain from caffeine, alcohol, and napping (active wake (AW), n=30). Within the nap group, sleep stage scoring was used to assign subjects to either the REM (n=41, naps contained one or more minutes of REM sleep) or non-REM (NREM, n=36) groups after completion of the experiment. Subjects in the nap group were randomly assigned to either a 60-minute or 90-minute nap condition. Given that shorter naps tend to have less REM sleep than longer naps, the use of these two durations increased the likelihood of having a significant number of subjects in both the REM and NREM groups. Wakefulness in the AW group was monitored using actigraph wristwatches.
At 16:00, MDD thresholds were again obtained for both the trained and untrained directions of motion. For each direction of motion, the threshold was based on an average of four 50-trial assessments. A subset of subjects (n= 81) was also tested on a completely novel direction of motion in a novel location. Over the course of the experimental day, we assessed momentary state levels of subjective sleepiness/alertness with the Karolinska Sleepiness Scale (KSS; Akerstedt & Gillberg, 1990) at 09:00, 11:00, 16:00, and 18:00.
2.4 Polysomnography
PSG data were collected using Astro-Med Grass Heritage Model 15 amplifiers and Grass Gamma software. Scalp electroencephalogram and electrooculogram electrodes were referenced to unlinked contralateral mastoids (C3/A2, C4/A1, O1/A2, LOC/A2 and ROC/A1), and muscle tone electromyogram electrodes were attached under the chin. PSG data were digitized at 256 Hz and visually scored in 30-second epochs according to the sleep staging criteria of Rechtschaffen and Kales (1968). A given subject’s data were excluded if he or she had less than fifteen minutes of total sleep time in the nap group (3 subjects), if sleep efficiency (defined as the ratio of total sleep time to time spent in bed) was less than 30% (2 subjects), or if the PSG data indicated that he or she had slept despite being assigned to the QW group (5 subjects).
2.5 Statistical Analyses
A given subject’s data were excluded if he or she had a 95% confidence interval of larger than 30 degrees for all pre-training, training, or post-training MDD thresholds (2 subjects) or if the absolute value of the difference between pre-training thresholds for the two directions of motion was more than three standard deviations from the mean of these thresholds, indicating unreliable pre-training measurements (4 subjects). Individual subject data were also excluded due to experimenter error (4 subjects). Data from a total of 130 remaining subjects are presented here.
Sleep variables were examined using one-way analysis of variance (ANOVA), with sex as a between-subject factor. The relationship between specific sleep variables and behavioral performance was examined by computing bivariate Pearson correlations. Because there is substantial between-subject variability in MDD thresholds, we computed a measure of learning (percent improvement) that is normalized to each subject’s individual pre-training performance. This measure was calculated by comparing thresholds in the pre-training and post-consolidation sessions:
Specificity of learning was calculated for each subject by subtracting percent improvement for the untrained direction of motion from percent improvement for the trained direction. For each participant, percent improvement for the novel condition was calculated relative to the average of pre-training thresholds from the trained and untrained directions of motion. To assess effects on PL magnitude, a three-way ANOVA was performed, with direction (trained/untrained) as a within-subject factor and sex and nap condition (AW/QW/NREM/REM) as between-subject factors. Similarly, specificity of PL was examined with a two-way ANOVA, with sex and condition as between-subject factors. All correlations and post-hoc pairwise comparisons were family-wise corrected for multiple comparisons. For all statistical tests, we report effect size in the form of R2 for t-tests and partial eta squared (ηp2) for ANOVA.
3. Results
3.1 Experimental nap parameters and other sleep variables
Consistent with previous studies of nocturnal sleep architecture in young adults (Dijk, Beersma, & Bloem, 1989; Voderholzer et al., 2003), we found no significant sex differences in sleep architecture for either NREM or REM experimental naps. In particular, there were no significant differences between men and women in total sleep time, sleep latency, wake after sleep onset, and sleep efficiency. There were also no detectable sex differences in minutes or percent of Stage 1, Stage 2, SWS, or REM sleep. Table 1 contains a summary of values of experimental nap sleep variables.
Table 1.
Experimental nap sleep variables as measured with polysomnography
| Non-REM Naps | REM Naps | |||
|---|---|---|---|---|
| Men (n=17) |
Women (n=19) |
Men (n=15) |
Women (n=26) |
|
| Total Sleep Time (min) | 56.0 (4.3) | 52.2 (4.3) | 76.9 (5.3) | 80.6 (2.8) |
| Sleep Latency (min) | 10.4 (3.4) | 10.4 (1.1) | 7.1 (1.6) | 7.3 (0.7) |
| WASO (min) | 15.5 (4.0) | 19.2 (4.1) | 12.7 (3.8) | 7.8 (1.6) |
| Sleep Efficiency | 70.8 (4.7) | 62.4 (4.1) | 80.2 (3.6) | 84.3 (1.5) |
| Stage 1 Sleep (min) | 5.5 (1.4) | 6.9 (1.3) | 6.7 (1.5) | 4.2 (0.5) |
| Stage 2 Sleep (min) | 35.4 (3.1) | 36.8 (4.4) | 47.4 (4.3) | 45.5 (2.7) |
| Slow Wave Sleep (min) | 15.0 (3.4) | 8.5 (2.3) | 10.6 (2.7) | 15.7 (2.8) |
| REM Sleep (min) | 0 | 0 | 12.2 (2.2) | 15.1 (1.7) |
Note:
Mean (SEM). WASO, wake after sleep onset; REM, rapid eye movement.
There were also no significant differences between men and women on any of the other sleep variables examined in this study: 1) prior nocturnal sleep; 2) trait or state subjective sleepiness; 3) morningness versus eveningness preference; or 4) nap habits (as assessed by the sleep diary).
3.2 Pre-training thresholds
There were no significant sex differences in pre-training thresholds (t(128) = 1.58, p > .05, R2 = .02), and all subsequent analyses employ a percent improvement measure that is normalized to each subject’s pre-training performance (see section 2.5). All pre-training and post-training thresholds are reported in Table 2.
Table 2.
Motion direction discrimination pre- and post-training thresholds
| Direction of Motion |
MEN | WOMEN | ||||||
|---|---|---|---|---|---|---|---|---|
| AW | QW | NREM | REM | AW | QW | NREM | REM | |
| Trained (pre) | 19.2 (1.5) | 19.1 (2.0) | 16.0 (1.4) | 18.8 (1.6) | 19.7 (1.7) | 17.6 (1.6) | 19.6 (1.2) | 18.2 (0.9) |
| Trained (post) | 17.2 (1.9) | 17.3 (1.5) | 13.9 (1.6) | 12.4 (1.3) | 16.2 (1.1) | 15.1 (0.8) | 17.1 (1.3) | 14.8 (0.8) |
| Untrained (pre) | 16.8 (1.3) | 20.8 (2.2) | 16.5 (1.4) | 15.2 (1.7) | 17.4 (1.2) | 18.6 (1.7) | 20.8 (1. 2) | 19.7 (1.0) |
| Untrained (post) | 17.7 (1.2) | 15.5 (1.5) | 14.2 (1.5) | 13.7 (1.6) | 15.9 (1.5) | 15.2 (1.5) | 17.3 (1.4) | 14.0 (0.8) |
| Novel | 20.7 (2.2) | 20.3 (3.3) | 15.2 (1.9) | 16.7 (1.4) | 16.9 (1.6) | 15.9 (1.0) | 14.9 (1.1) | 14.0 (1.8) |
Note: Mean (SEM) in degrees.
AW, active wake; QW, quiet wake; NREM, non-rapid eye movement naps; REM, rapid eye movement naps.
3.3 Sleep affects motion PL
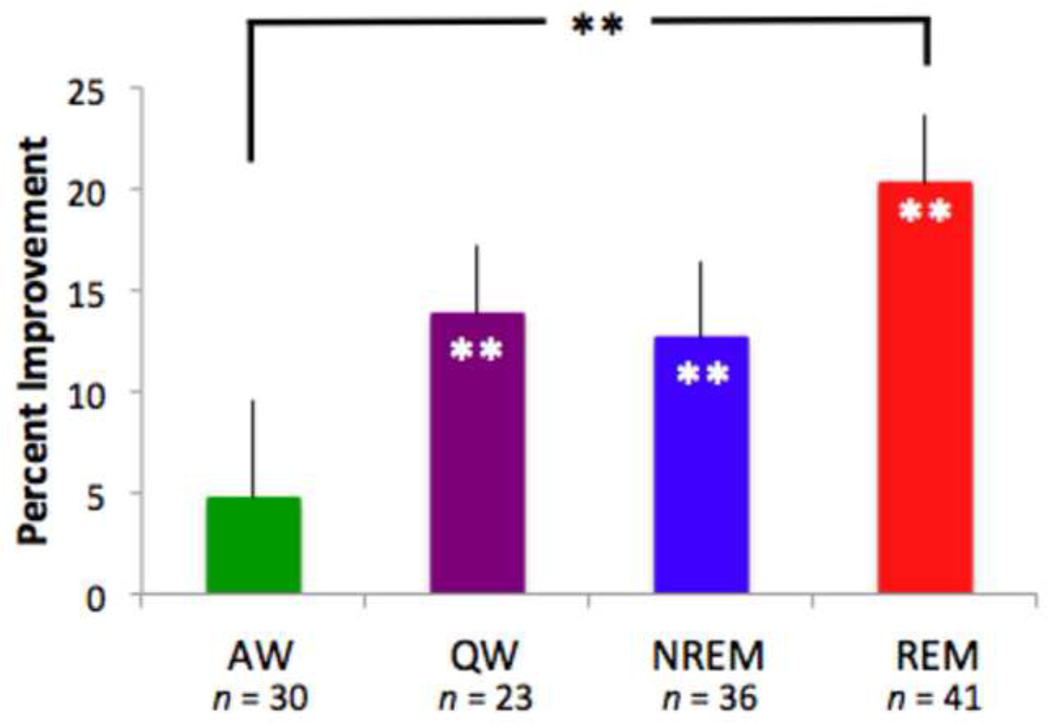
We assessed the effects of sex and sleep on magnitude of motion PL with a 2 × 2 × 4 ANOVA, with direction of motion (trained or untrained) as a within-subject factor and sex and nap condition (AW, QW, NREM, REM) as between-subject factors. There were no significant main effects of either motion direction or sex, but there was a significant main effect of nap condition (F(3,122) = 2.81, p < .05, ηp2 = .07), indicating a role of sleep in consolidation of motion PL. Post-hoc t-tests demonstrated significant learning in the QW (t(22) = 4.00, p = .001, R2 = .42), NREM (t(35) = 3.33, p < .01, R2 = .24), and REM (t(40) = 5.91, p < .001, R2 = .46) conditions. However, percent improvement was not significantly different from zero in the AW group (t(29) = 0.97, p > .05, R2 = .03). We directly compared percent improvement in the QW, REM and NREM groups to the AW control group and found that only the REM group had significantly more learning than the AW group (t(69) = 2.70, p < .01, R2 = .10) (Figure 2). These results reveal a substantial facilitation of motion PL consolidation by REM sleep.
Figure 2.
REM sleep after training increases motion perceptual learning. Quiet wake (QW), non-REM (NREM), and REM nap conditions all showed significantly enhanced motion direction discrimination, but only the REM group had significantly more percent improvement compared to active wake (AW). Error bars denote SEM. **indicates p < .01
There were no significant correlations between basic sleep variables and percent improvement (all p values >.05): time in Stage 1 (NREM naps: r = −.08, REM naps: r = −.36), time in Stage 2 (NREM naps: r = .02; REM naps: r = −.14), slow wave sleep time (NREM naps: r = .12; REM naps: r = −.10), REM sleep time (r = .01), and total sleep time (NREM naps: r = .08; REM naps: r = −.28).
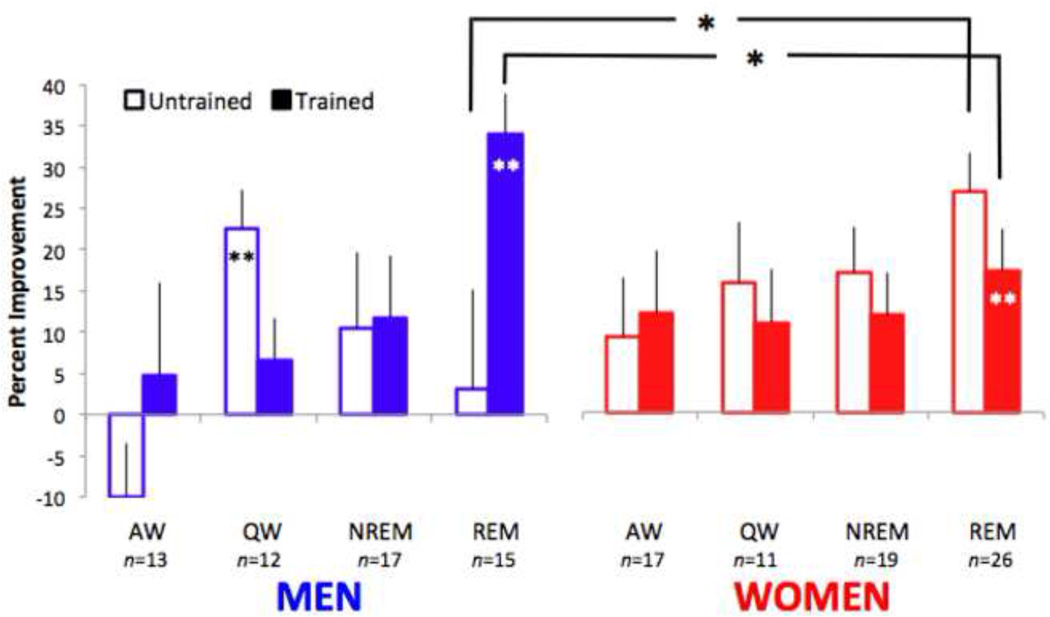
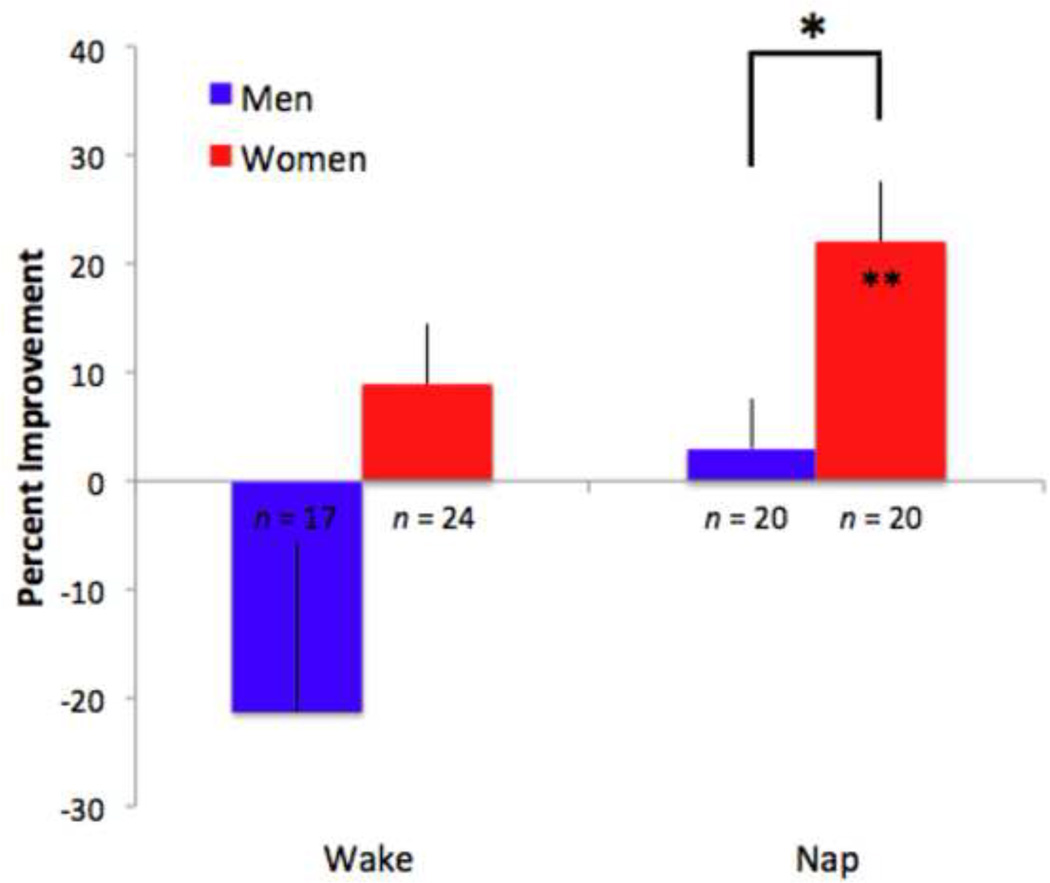
3.4 The effects of naps on magnitude of PL are sex-specific
In addition to the main effect of nap condition on percent improvement, we found a significant three-way interaction among nap condition, trained versus untrained directions of motion, and sex (F(3,122) = 3.27, p < .05, ηp2 = .07) (Figure 3). We further explored this interaction with post-hoc t-tests. In the REM nap condition, both men and women exhibited significant learning for the trained direction of motion (men: t(14) = 6.97, p < .001, R2 = .77; women: t(25) = 3.36, p < .01, R2 = .31). Additionally, women in the REM nap condition had significant learning for the untrained direction (t(25) = 5.58, p < .001, R2= .56), while men did not (t(14) = 0.25, p > .05, R2 = .005).
Figure 3.
Specificity of motion perceptual learning differs in men and women. Following a nap with REM sleep, men showed significant improvement for the trained (filled bars) direction of motion, whereas women showed improvement for both trained and untrained (open bars) directions of motion. Error bars denote SEM. *indicates p < .05 and **indicates p < .01
Within the REM nap group, men showed more learning than women for the trained direction of motion (t(39) = 2.34, p < .05, R2 = .12), whereas women showed significantly more learning than men for the untrained direction (t(39) = 2.08, p < .05, R2 = .10). These differential effects of sex on learning for the trained and untrained directions in the REM nap group suggest that there are differences between men and women in the specificity of PL following REM sleep, a possibility that we explicitly test below. Finally, there was learning for the untrained direction for men in QW (t(11) = 4.79, p = .001, R2 = .67).
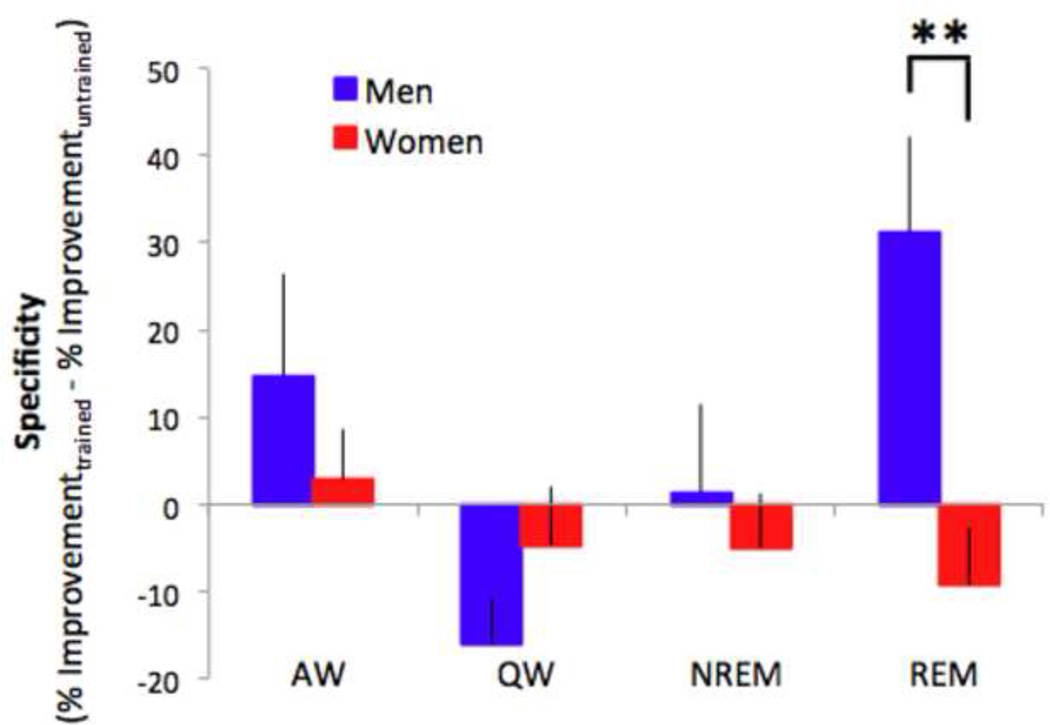
3.5 REM sleep enhances specificity of PL in men but not women
To directly assess the effects of sex on specificity of PL, we calculated the difference in percent improvement for the trained and untrained directions of motion for each subject (Figure 4). There was a significant sex-by-nap condition interaction for this measure of direction specificity of PL (F(3,122) = 3.27, p < .05, ηp2 = .07). For men, there was a main effect of nap condition on specificity (F(3,53) = 3.62, p < .05, ηp2 = .17), with the greatest specificity occurring in the REM group (significantly greater specificity for REM than for QW: t(25) = 3.52, p < .01, R2 = .33). In contrast, there was no detectable effect of nap condition on specificity of PL for women (F(3,69) = .62, p = .61, ηp2 = .03), with women showing generalization of PL to the untrained direction in all nap conditions. Within the REM nap condition, men showed significantly more direction specificity of PL than women (t(39) = 3.33, p < .01, R2 = .22).
Figure 4.
REM sleep enhances direction specificity of motion perceptual learning more for men than women. Following REM sleep, men showed greater specificity of PL than women. Error bars denote SEM. **indicates p < .01
3.6 Women generalize learning to a novel direction and location
It is possible that exposure to the untrained direction during pre-training measurements could have influenced percent improvement for the untrained direction (Zhang et al., 2010). Therefore, we also examined transfer of PL to a completely novel direction of motion and visual field location in a subset of subjects (nmen = 37, nwomen = 44). Results from a three-way ANOVA, with motion direction (trained, untrained, or novel) as a within-subject factor and sex and nap condition as between-subject factors, revealed a significant interaction between motion direction and sex (F(2,146) = 4.22, p < .05, ηp2 = .06). There is insufficient power (due to small sample size in some groups) to fully assess the effects of nap condition for the novel stimulus. Therefore, to increase power for this analysis, we combined nap groups (NREM + REM) and wake groups (AW + QW). In general, women showed greater percent improvement for the novel stimulus than men (t(79) = 2.79, p < .05, R2 = .09), and this pattern of results was obtained following both wake and sleep (Figure 5). However, percent improvement was only significantly greater in women compared to men following a nap (t(38) = 2.63, p < .05, R2 = .15), and the magnitude of improvement was only significantly different from zero in women following a nap (t(19) = 3.90, p < .01, R2 = .44). These data demonstrate generalization of PL to a completely novel stimulus in women but not men and that this effect was enhanced following a nap, consistent with our finding of significant learning of the untrained direction in the REM nap group in women but not men.
Figure 5.
Women generalize learning to a novel motion direction and location, but men do not. Following a nap, women showed greater percent improvement for the novel stimulus compared to men. Error bars denote SEM. *indicates p < .05 and **indicates p < .01
4. Discussion
This is the first study of sleep-dependent PL to 1) measure the effects of sleep during offline consolidation of motion PL, and 2) demonstrate sex differences in consolidation of PL. We have found that the critical stage of sleep for these consolidation processes is REM sleep and that the magnitude and specificity of learning of the trained stimulus depends on sex.
4.1 REM sleep facilitates motion PL
We defined motion perceptual learning (PL) as the percent improvement in motion direction discrimination after an offline consolidation period during which subjects either took a nap (with or without REM sleep) or remained awake (quiet or active wake). We found that while quiet wake, NREM and REM groups all showed significant motion PL, only the REM group had greater learning than the active wake group. Importantly, total sleep time was not correlated with motion PL, consistent with other studies reporting that the quality of sleep is more important than the quantity (Mednick et al., 2003). Our finding of a unique role of REM sleep in consolidation of motion PL is consistent with prior studies showing that REM sleep, either from nighttime sleep (Stickgold et al., 2000) or a daytime nap (Mednick et al., 2003), is necessary for PL of texture discrimination. Motion PL has previously been examined across multiple days of training (Ball & Sekuler, 1987; Rokem & Silver, 2010, 2013), with nocturnal sleep occurring between training sessions. However, sleep was not monitored in those studies.
In the present study, we interpret the effects of sex and sleep on PL as effects on consolidation, as our design treated all subjects identically during encoding and retrieval, while the nap groups differed in the consolidation portion of the experiment. The sex differences we observed interacted with nap condition, providing further evidence that the effects we report are on the process of consolidation of PL. However, our methods, like those used in any behavioral study of PL, employ performance at retrieval to measure the consequences of consolidation. It is therefore possible that the effects of different types of nap during consolidation actually manifest as differences in post-nap retrieval. Similarly, the sex differences we found could reflect retrieval processes (although these sex differences in retrieval would have to depend on the type of sleep in the prior consolidation period). Thus, while the most straightforward explanation of our findings involves effects of sleep and sex on consolidation of PL, we cannot exclude effects on retrieval of PL that are modulated by experience during the consolidation period. It is possible that consolidation and retrieval could be dissociated in future studies employing physiological measures of the effectiveness of consolidation and/or pharmacological manipulations that separately target the consolidation and retrieval phases of PL.
4.2 Sex differences in motion PL
Within the REM group, men showed greater learning of the trained direction and also more specificity of PL for the trained motion direction, whereas women showed generalized learning across motion directions and visual field locations. Across nap conditions, these sex differences in specificity of PL extended to a novel direction and location, with women showing greater learning than men for a stimulus configuration that they had never seen before. Our findings suggest that while REM sleep facilitates specific PL in men, generalization of learning to untrained and novel stimuli occurs for women, regardless of nap condition.
Using an experimental nap paradigm, we identified REM sleep as the sleep stage involved in sex-dependent specificity of PL. The sex differences reported here were not due to other sleep-related factors that we studied: there were no significant differences between men and women in any other measured sleep variable: nap architecture, prior nocturnal sleep, nap habits, trait or state daytime sleepiness, and morningness-eveningness preference. Thus, the sex differences in sleep-dependent learning we report here were not due to sex differences in any of these factors.
There were also no significant pre-training differences in task performance between men and women for displays with 100% motion coherence, generally consistent with the finding that young men and women do not differ in their sensitivity in detecting low coherence motion signals, although older women have lower sensitivity than older men (Atchley & Andersen, 1998). Thus, the sex differences we report are not due to overall differences in motion direction discrimination ability between the men and women studied here but instead are specific to consolidation of PL during REM sleep.
Sex differences have recently been reported for fast task-irrelevant PL (TIPL) in younger adults (Leclercq & Seitz, 2012). Specifically, fast TIPL depended on whether subjects were instructed to explicitly memorize or to simply attend to the information presented with the target. Men exhibited fast TIPL in both instruction conditions, whereas women showed fast TIPL only in the explicit memorization condition. However, fast TIPL is used to study learning within a single training session, not across a post-training consolidation period, suggesting that the results of Leclercq and Seitz (2012) stem from a different underlying mechanism than the sex differences in REM sleep-dependent consolidation we report here.
One unexpected result from our study was the magnitude of learning of the untrained motion direction for men in the quiet wake condition. One previous study has shown similar profiles of learning in quiet wake and sleep conditions but found no learning in an active wake condition (Mednick et al., 2009). This result should be examined in future studies on sex differences in PL in the context of active wake, quiet wake, and sleep.
4.3 Cognitive and biological factors contributing to PL specificity
Many factors modulate specificity of PL. During encoding of training stimuli, high-level mechanisms such as attention (Ahissar & Hochstein, 1993) and decision-making (Law & Gold, 2009) influence specificity of learning, as do training methods (Hussain, Bennett, & Sekuler, 2012; Liu, 1999; Wang et al., 2012), task difficulty (Ahissar & Hochstein, 1997), exposure to different stimulus features (e.g., Xiao et al., 2008), and sensory adaptation (Harris et al., 2012).
The neurotransmitter acetylcholine (ACh) modulates both the magnitude and specificity of PL. Cholinergic enhancement with the cholinesterase inhibitor donepezil augments the magnitude and specificity of motion PL (Rokem & Silver, 2010; 2013), and administration of chewing tobacco containing nicotine, an agonist of nicotinic ACh receptors, during PL consolidation increased the magnitude and specificity of PL of texture discrimination (Beer, Vartak, & Greenlee, 2013). In general, high levels of cholinergic signaling have been proposed to set the appropriate neural dynamics for consolidation during REM sleep (Buzsáki, 1989; Hasselmo, 1999). Additionally, ACh is crucial for the induction and maintenance of long-term potentiation of synaptic transmission (Hasselmo & Bower, 1993; Matsukawa et al., 1997), a likely mechanism of synaptic plasticity in PL (Sale et al., 2011). Therefore, ACh modulation may be one candidate mechanism for understanding the differences between men and women in PL following REM sleep.
Indeed, animal studies have reported that male rats have more hippocampal ACh release than female rats (Mitsushima, 2011; Mitsushima, Masuda, & Kimura, 2003), particularly during the dark phase of the daily cycle (Masuda et al., 2005). If similar sex differences are present in humans, they could contribute to the lower specificity of PL in women, compared with men, that we found following a nap with REM sleep. One way to further investigate this hypothesis is to examine different perceptual learning tasks. For example, the fact that cholinergic enhancement increases both the magnitude and specificity of PL of motion (Rokem and Silver, 2010) and texture (Beer et al., 2012) discrimination provides evidence for a general facilitatory role of ACh in PL as opposed to a task-specific mechanism. Therefore, if ACh mediates the sex-dependent differences in motion PL we report here, these sex differences may also generalize to other forms of sleep-dependent PL. Further research is needed to determine the relationships among ACh, sex, and PL.
Another possible source of sex differences in the magnitude and specificity of PL are menstrual cycle effects mediated by sex hormones. Mitsushima et al. (2009) found that sex steroids – testosterone in males and estradiol (estrogen) in females – increase ACh release in the hippocampus of rats. Indeed, in humans, fluctuations in estradiol levels across the menstrual cycle are associated with changes in learning and memory (Genzel et al., 2012; Maki, Rich, & Rosenbaum, 2002), and a recent study reported sex differences in verbal and motor learning following a nap (Genzel et al., 2012). Specifically, women in their luteal phase (when estrogen levels are higher) and men showed significant improvement on both tasks following a nap, compared to a wake group, but women in the follicular phase (the first week of the menstrual cycle) did not. Our study included a relatively large sample of women participants (n=26 in the REM group alone), so it is unlikely that there was a significant bias towards a particular phase of the menstrual cycle in our sample. Nevertheless, we did not determine menstrual phase of the female subjects in our study and could therefore not assess possible effects of menstrual cycle on sleep-dependent PL.
Recent work suggests that effects on the magnitude and specificity of PL may be dissociated. Changing the state of local adaptation during texture discrimination PL did not affect the magnitude of learning but did increase generalization (Harris et al., 2012). Moreover, specificity and magnitude of learning are reflected in different components of visual event-related potentials, suggesting that they are based on different neural mechanisms (Zhang et al. 2013). Thus, the two main sex-dependent results we report, that men learn more in the trained condition, and that women generalize more to other conditions, may arise from separate underlying biological causes.
4.4 Implications of our results for design of training procedures
Our finding that sex is an important factor in the sleep-dependent consolidation of PL has several significant practical implications. First, consolidation differences between men and women should be taken into account when designing courses of training, especially those having an element of PL, such as training in the detection of the occurrence of rare visual signals (satellite and radar imagery; video from security cameras). This could lead to refined learning strategies and even differential assignment of individuals to tasks that place high demands on visual processing. In most real-life learning situations, generalization of learning to novel and untrained stimuli is desirable. We have shown that REM sleep facilitates the consolidation of motion PL in both men and women but that generalization to untrained and novel stimuli is only apparent in women, suggesting that women may be better suited for tasks requiring generalization of perceptual skill learning. Finally, PL studies have typically used small sample sizes and have therefore been underpowered to detect sex differences (e.g., for motion PL: Ball & Sekuler, 1987; Liu, 1999; Rokem & Silver, 2010). Our findings indicate that future studies would benefit from having sample sizes large enough to adequately test for individual differences such as sex, as this may help inform our understanding of underlying biological mechanisms and determine the generalizability of results.
4.5 Clinical implications
PL is used as a treatment for a variety of visual disorders, including amblyopia (Levi & Li, 2009). Importantly, in patients with amblyopia, learning on a variety of PL tasks transfers to improvements in Snellen acuity (Levi & Polat, 1996, Zhou et al., 2006), stereoacuity (Li, Provost, & Levi, 2007), and visual counting (Li & Levi, 2004). PL-driven improvements in these fundamental aspects of vision in patients with amblyopia should therefore enhance natural scene processing and quality of life. However, the efficacy of these treatments may be increased by individualized training procedures. Our findings suggest that sex and sleep should be taken into account when designing therapeutic interventions involving PL.
-
➤
We studied sex differences in consolidation of perceptual learning.
-
➤
We used napping to study the effects of REM and non-REM sleep on consolidation.
-
➤
REM sleep enhanced consolidation of learning of motion direction discrimination.
-
➤
Men whose naps contained REM sleep had highly specific perceptual learning.
-
➤
Women whose naps had REM sleep generalized learning to untrained and novel stimuli.
Acknowledgments
We thank Ryan Wong, Rocco Ragano, and Brooke Kettering for their help in collecting the data. Research was supported by the following NIH awards: K01 MH080992 (S.M.), R21 EY19992 (M.A.S.), and F31 AG032209 (A.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth A. McDevitt, Email: mcdevitt@ucr.edu.
Ariel Rokem, Email: arokem@gmail.com.
Michael A. Silver, Email: masilver@berkeley.edu.
Sara C. Mednick, Email: smednick@ucr.edu.
References
- Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(12):5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387(6631):401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. International Journal of Neuroscience. 1990;52(1–2):29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and Aging. 1998;13(2):297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Research. 1987;27(6):953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Beer AL, Vartak D, Greenlee MW. Nicotine facilitates memory consolidation in perceptual learning. Neuropharmacology. 2013;64(1):443–451. doi: 10.1016/j.neuropharm.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: A role for "noisy" brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep: Journal of Sleep Research & Sleep Medicine. 1989;12(6):500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S, Poggio T. Fast perceptual learning in hyperacuity. Vision Research. 1995;35(21):3003–3013. doi: 10.1016/0042-6989(95)00044-z. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287(5777):43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Genzel L, Kiefer T, Renner L, Wehrle R, Kluge M, Grozinger M, et al. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology. 2012;37(7):987–998. doi: 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Harris H, Gliksberg M, Sagi D. Generalized perceptual learning in the absence of sensory adaptation. Current Biology. 2012;22(19):1813–1817. doi: 10.1016/j.cub.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends in Cognitive Sciences. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Acetylcholine and memory. Trends in Neuroscience. 1993;16(6):218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Airaksinen E, Nordstrom E. Sex differences in episodic memory: the impact of verbal and visuospatial ability. Neuropsychology. 1999;13(4):590–597. doi: 10.1037//0894-4105.13.4.590. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Hussain Z, Bennett PJ, Sekuler AB. Versatile perceptual learning of textures after variable exposures. Vision Research. 2012;61:89–94. doi: 10.1016/j.visres.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Science of the United States of America. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265(5172):679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Law CT, Gold JI. Reinforcement learning can account for associative and perceptual learning on a visual-decision task. Nature Neuroscience. 2009;12(5):655–663. doi: 10.1038/nn.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq V, Seitz AR. Fast-TIPL occurs for salient images without a memorization requirement in men but not in women. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research. 2009;49(21):2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology. 2001;15(2):165–173. doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. Journal of Vision. 2004;4(6):476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Investigative Ophthalmology and Visual Science. 2007;48(11):5046–5051. doi: 10.1167/iovs.07-0324. [DOI] [PubMed] [Google Scholar]
- Liu Z. Perceptual learning in motion discrimination that generalizes across motion directions. Proceedings of the National Academy of Sciences USA. 1999;96(24):14085–14087. doi: 10.1073/pnas.96.24.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40(5):518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Masuda J, Mitsushima D, Funabashi T, Kimura F. Sex and housing conditions affect the 24-h acetylcholine release profile in the hippocampus in rats. Neuroscience. 2005;132(2):537–542. doi: 10.1016/j.neuroscience.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Matsukawa M, Ogawa M, Nakadate K, Maeshima T, Ichitani Y, Kawai N, et al. Serotonin and acetylcholine are crucial to maintain hippocampal synapses and memory acquisition in rats. Neuroscience Letters. 1997;230(1):13–16. doi: 10.1016/s0304-3940(97)00460-6. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Makovski T, Cai D, Jiang Y. Sleep and rest facilitate implicit memory in a visual search task. Vision Research. 2009;49(21):2557–2565. doi: 10.1016/j.visres.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, et al. The restorative effect of naps on perceptual deterioration. Nature Neuroscience. 2002;5(7):677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mitsushima D. Sex differences in the septo-hippocampal cholinergic system in rats: behavioral consequences. Current Topics in Behavioral Neurosciences. 2011;8:57–71. doi: 10.1007/7854_2010_95. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Masuda J, Kimura F. Sex differences in the stress-induced release of acetylcholine in the hippocampus and corticosterone from the adrenal cortex in rats. Neuroendocrinology. 2003;78(4):234–240. doi: 10.1159/000073707. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Takase K, Takahashi T, Kimura F. Activational and organisational effects of gonadal steroids on sex-specific acetylcholine release in the dorsal hippocampus. Journal of Neuroendocrinology. 2009;21(4):400–405. doi: 10.1111/j.1365-2826.2009.01848.x. [DOI] [PubMed] [Google Scholar]
- Nishina S, Kawato M, Watanabe T. Perceptual learning of global pattern motion occurs on the basis of local motion. Journal of Vision. 2009;9(9) doi: 10.1167/9.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service, University of California; 1968. [Google Scholar]
- Rokem A, Silver MA. A model of encoding and decoding in V1 and MT accounts for motion perception anisotropies in the human visual system. Brain Research. 2009;1299:3–16. doi: 10.1016/j.brainres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Rokem A, Silver MA. Cholinergic enhancement augments magnitude and specificity of visual perceptual learning in healthy humans. Current Biology. 2010;20(19):1723–1728. doi: 10.1016/j.cub.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, Silver MA. The benefits of cholinergic enhancement during perceptual learning are long-lasting. Frontiers in Computational Neuroscience. 2013;7:66. doi: 10.3389/fncom.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, De Pasquale R, Bonaccorsi J, Pietra G, Olivieri D, Berardi N, et al. Visual perceptual learning induces long-term potentiation in the visual cortex. Neuroscience. 2011;172:219–225. doi: 10.1016/j.neuroscience.2010.10.078. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. Journal of Cognitive Neuroscience. 2000;12(2):246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depression and Anxiety. 2003;17(3):162–172. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang JY, Klein SA, Levi DM, Yu C. Task relevancy and demand modulate double-training enabled transfer of perceptual learning. Vision Research. 2012;61:33–38. doi: 10.1016/j.visres.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Xiao L-Q, Zhang J-Y, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Current Biology. 2008;18(24):1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xiao LQ, Klein SA, Levi DM, Yu C. Decoupling location specificity from perceptual learning of orientation discrimination. Vision Research. 2010;50(4):368–374. doi: 10.1016/j.visres.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Zhang G-L, Cong L-J, Song Y, Yu C. ERP P1-N1 changes associated with Vernier perceptual learning and its location specificity and transfer. Journal of Vision. 2013;13:1–13. doi: 10.1167/13.4.19. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, Lu Z-L. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Research. 2006;46(5):739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]