Abstract
Differentiation of oligodendroglial precursor cells (OPCs), a crucial prerequisite for central nervous system (CNS) remyelination in diseases such as Multiple Sclerosis (MS), is modulated by a multitude of extrinsic and intrinsic factors. In a previous study we revealed that the chemokine CXCL12 stimulates rodent OPC differentiation via activation of its receptor CXCR7. We could now demonstrate that CXCR7 is also expressed on NogoA- and Nkx2.2-positive oligodendroglial cells in human MS brains and that stimulation of cultured primary fetal human OPCs with CXCL12 promotes their differentiation as measured by surface marker expression and morphologic complexity. Pharmacological inhibition of CXCR7 effectively blocks these CXCL12-dependent effects. Our findings therefore suggest that a specific activation of CXCR7 could provide a means to promote oligodendroglial differentiation facilitating endogenous remyelination activities.
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory demyelinating disease of the human central nervous system (CNS), leading to gradual degeneration and loss of myelin sheaths and oligodendrocytes. As a consequence, axonal function is impaired and axons are severely damaged [1]. Although repair activities are limited within the adult CNS, remyelination can be observed as a result of resident oligodendroglial precursor cell (OPC) activation particularly in early disease stages. These cells can be recruited into MS lesions where they differentiate into functional myelinating cells [2]. However, due to a blockade of oligodendroglial differentiation remyelination efficiency remains overall poor [3–6]. Neutralization of inhibitory cues or activation of stimulatory pathways could therefore be a viable strategy to enhance CNS remyelination. Chemokines are highly conserved among mammalian species and regulate a plethora of different physiological processes, such as, for instance the modulation of cell-cell interactions, immune cell chemotaxis and developmental processes in a variety of tissues including the brain [7, 8]. Chemokines bind mainly to G-protein coupled receptors and exert strong effects in neuroinflammatory diseases [9, 10]. In the past the impact of chemokines on the survival and behavior of OPCs has been under closer investigation [11, 12]. Particularly CXCL12 (stromal derived growth factor 1; SDF-1) has been described as a relevant factor for the behavior of oligodendroglial precursors [13] and the regulation of blood-brain-barrier integrity in neuroinflammation [14]. In a previous study we have identified the CXCL12 receptor CXCR7 as a potent mediator of OPC differentiation in the inflamed rodent brain [15]. We could now translate our results to the human paradigm confirming that signaling through CXCR7 leads to increased human oligodendroglial precursor cell differentiation suggesting that specific activation of this receptor could be a novel therapeutic approach to promote endogenous remyelination activities.
Material and Methods
Isolation, culture and immunocytochemistry of fetal hOPCs
Human fetal CNS tissue obtained from 15 to 18 gestational week embryos was provided by the Human Fetal Tissue Repository (Albert Einstein College of Medicine, Bronx, NY). This developmental stage precedes CNS myelination. Human fetal OPCs (hOPCs) as A2B5 expressing cells were isolated immuno-magnetically as previously described [16]. The purified cells were plated on poly-L-lysine-coated plastic coverslips (Nunc, Rochester, NY). The cultures were grown in Dulbecco’s modified essential medium (DMEM)-F12 supplemented with N1 (Sigma, Oakville, ON), 0.01% bovine serum albumin (BSA), 1% penicillin-streptomycin, B27 supplement (Invitrogen, Burlington, ON), thyroid hormone (T3, 2 ng/ml, Sigma, Oakville, ON), platelet-derived growth factor AA (PDGF-AA, 10 ng/ml, Sigma, Oakville, ON) and basic fibroblast growth factor (FGF2, 10 ng/ml, Sigma, Oakville, ON). All tissue samples were obtained under protocols approved by the McGill University institutional review boards. Fetal material was obtained from the Albert Einstein School of Medicine fetal repository program with written consents obtained at that site. All experiments were conducted in accordance with the Helsinki Declaration. Additional studies were performed using human fetal OPCs and respective media purchased from 3H Biomedical (Uppsala, Sweden) and cultured according to the manufacturer’s protocol.
To assess the effects of CXCL12 stimulation on hOPCs (myelin marker expression, morphological maturation) fetal hOPCs were cultured for up to 12 days in defined media supplemented with recombinant human CXCL12 (100ng/ml in PBS buffer supplemented with 0.1% bovine serum albumin; R&D Systems, Minneapolis, MN) and growth factors (10ng/ml BDNF, Calbiochem, San Diego, CA and 10ng/ml IGF, MJS BioLynx, Brackville, ON) or growth factors alone. Medium was changed on days 3, 6 and 9. CCX771 (ChemoCentryx, Mountain View, CA) was reconstituted in DMSO according to the manufacturer’s instructions and used at a concentration of 10 nM in a 30 min pre-treatment step on hOPC cultures prior to the addition of CXCL12 at the above-described concentration. Immunocytofluorescent staining was performed using the following primary antibodies: hybridoma anti-O4 IgM antibody (1:50; Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada and [17]), hybridoma anti-GalC IgG3 antibody (1:50; Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada and [18]) and mouse anti-2‘,3‘-cyclic nucleotide 3‘-phosphodiesterase antibody (CNPase; 1:1000; Sternberger Monoclonals, Lutherville, MD). For visualization IgM-FITC or TxR (1:100; Jackson ImmunoResearch, Westgrove, PA), IgG3-FITC or TxR (1:100; Biosource, Camarillo, CA) and IgG1 Alexa Fluor 488 (1/500; Molecular Probes, Leiden, the Netherlands) were used. Data are presented as mean +/- standard error of the mean (SEM) and significance was assessed by either two-sided Student’s t-test (unpaired comparison for means) or one-way ANOVA (GraphPad Prism). Experimental groups were considered significantly different at *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
Immunohistochemistry
We retrospectively investigated 12 brain biopsy tissue samples from 12 MS patients (9 women, 3 men; age 21 to 74 years, mean age 44 +/- 16 years) and six control cases (3 women, 3 men; age 25 to 72, mean age 56 +/- 8 years; see Table 1). All lesions fulfilled the generally accepted criteria for the diagnosis of MS. The tissue samples showed the characteristics of active lesions with loss of myelin, infiltration by numerous phagocytes and fewer lymphocytes as well as gliosis. We classified the de- and remyelinating activity as described earlier [19]. Actively demyelinating lesion areas (AD; n = 7) were located at the plaque border, these areas were partially demyelinated and infiltrated by numerous macrophages containing myelin degradation products, such as myelin basic protein (MBP) or CNPase within their cytoplasm. Demyelinated lesion areas (DM) were infiltrated by macrophages and T cells, but macrophages did not contain myelin degradation products (biopsies; n = 2). In remyelinating areas (RM; biopsies; n = 3), thin, irregularly formed myelin sheaths were seen. Periplaque white matter (PPWM; biopsies; n = 7) showed no signs of demyelination. Additionally, tissue samples from six control patients without CNS pathology besides mild microglia activation and reactive gliosis were analyzed. Tissue specimens were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue samples were cut in 4 μm thick sections and stained with hematoxylin/eosin (Merck, Darmstadt, Germany). Immunohistochemical staining was performed using a biotin-streptavidin peroxidase protocol (Dako, Glostrup, Denmark). After deparaffinization intrinsic peroxidase activity was blocked by incubation with 5% H2O2 in PBS for 20 min. Non-specific antibody binding was inhibited with 10% FCS in PBS for 25 min. Sections were pre-treated in a steamer with citrate (pH 6.0) prior to incubation with the primary antibody. 3,3'-diaminobenzidine (DAB) was used as a chromogen and sections were counterstained using hematoxylin. As primary antibodies we used rabbit anti-CMKOR1 (1:200; Proteintech, Chicago, USA), rabbit anti-CXCR7 (1:100; Millipore), rabbit anti-Nogo-A (1:750; Chemicon International, Temecula, CA), mouse anti-Nkx2.2 (1:100; Developmental Studies Hybridoma Bank, University of Iowa, Iowa) and mouse anti-Nogo-A (1:15.000; 11c7, a generous gift from M.E. Schwab, Brain Research Institute, University of Zürich and Department of Biology, Swiss Federal Institute of Technology Zürich, Switzerland). General tissue characterization (data not shown) was carried out using Luxol-fast blue (Sigma), mouse anti-KiM1P (1:5000; H.-J. Radzun, Department of Pathology, University of Göttingen, Germany), mouse anti-CD3 (1:25; Dako, Glostrup, Denmark), rabbit anti-Olig2 (1:300; IBL, Spring Lake Park, Minnesota), rabbit anti-GFAP (1:2000; Dako, Glostrup, Denmark), rabbit anti-MBP (1:1000; Boehringer Mannheim, Mannheim, Germany) and mouse anti-GFAP (1:50; Dako, Glostrup, Denmark). Double immunofluorescent staining was performed using rabbit anti-CXCR7 (see above), and mouse anti-NogoA (see above) or mouse anti-Nkx2.2 antibodies followed by Cy3 (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA) or Alexa488 (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA) conjugated antibodies and counterstained with 4´,6-diamidino-2-phenylindole (DAPI; 1:5000, Invitrogen, Burlington, ON). Numbers of CXCR7-positive cells were determined in at least 10 standardized microscopic fields of 10.000 μm2 each defined by an ocular morphometric grid as indicated in the text and figures as the mean number of cells/μm2 ± SEM. For statistical analysis, a Bonferroni-corrected one-way ANOVA tests was performed. The test was classified as significant if the p-value was <0.05 (GraphPad PRISM). All images were taken on an Olympus fluorescent microscope. None of the study authors was involved in decision-making with respect to biopsy. The study was approved by the Ethics Committee of the University of Münster.
Table 1. Human brain tissues used for immunohistochemical analysis.
| Diagnosis of biopsy | Sampling location | Age/Sex | |
|---|---|---|---|
| 1 | Brain tissue with reactive changes | right frontal | 72/m |
| 2 | Brain tissue with reactive changes | right paraventricular | 66/f |
| 3 | Brain tissue with reactive changes | right parieto-occipital | 25/f |
| 4 | Brain tissue with reactive changes | unknown | 51/m |
| 5 | Brain tissue with reactive changes | right parietal | 72/m |
| 6 | Brain tissue with reactive changes | unknown | 47/f |
| 7 | Inflammatory demyelinating lesion consitent with MS | right frontal | 44/f |
| 8 | Inflammatory demyelinating lesion consitent with MS | left frontal | 74/f |
| 9 | Inflammatory demyelinating lesion consitent with MS | supraventricular | 50/f |
| 10 | Inflammatory demyelinating lesion consitent with MS | subcortical | 41/f |
| 11 | Inflammatory demyelinating lesion consitent with MS | right occipital | 26/f |
| 12 | Inflammatory demyelinating lesion consitent with MS | left temporal | 21/f |
| 13 | Inflammatory demyelinating lesion consitent with MS | left parieto-occipital | 63/f |
| 14 | Inflammatory demyelinating lesion consitent with MS | cerebellar | 25/f |
| 15 | Inflammatory demyelinating lesion consitent with MS | leukocortical | 34/f |
| 16 | Inflammatory demyelinating lesion consitent with MS | cerebellar | 48/m |
| 17 | Inflammatory demyelinating lesion consitent with MS | semioval center | 45/m |
| 18 | Inflammatory demyelinating lesion consitent with MS | left parieto-occipital | 61/f |
Results
CXCR7 is expressed in mature NogoA positive oligodendrocytes and Nkx2.2 positive OPCs in remyelinating MS lesions
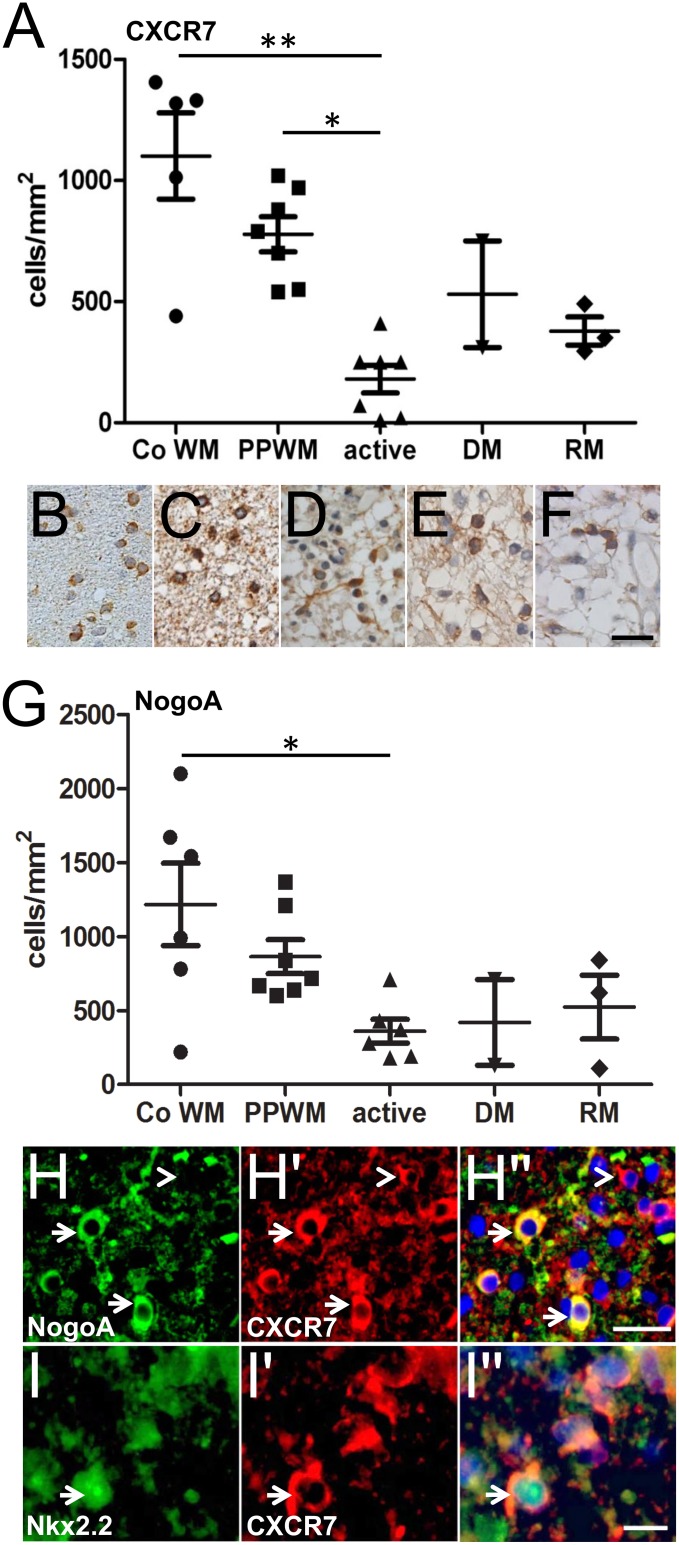
Immunohistochemistry of human MS tissue revealed that CXCR7-expressing cells are present in control brains and different areas of the MS brain (Fig 1A–1F). While in the periplaque white matter (PPWM) an average of 750 CXCR7-expressing cells per mm2 could be detected this number was significantly decreased to approximately 200 cells/mm2 in actively demyelinating lesion areas (active) at the plaque border. As these areas were infiltrated by numerous macrophages containing myelin degradation products this drop in numbers probably reflects ongoing oligodendroglial cells death—a common observation in MS pathology. The number of CXCR7-expressing cells was also lower in demyelinated (DM) areas featuring macrophages and T cells not containing myelin degradation products (approx. 500 cells/mm2) and remyelinating (RM) areas characterized by newly formed thin and irregular myelin sheaths (approx. 400 cells/mm2). In order to clarify the identity of the CXCR7-expressing cells in the above-described regions of the MS brain we then performed stainings of NogoA, a reliable marker for mature oligodendrocytes [20], of the same regions in parallel sections (Fig 1G). This revealed that the distribution of NogoA largely mirrored that of CXCR7 with an overall correlation coefficient of 0,783. Double immunofluorescent labeling of CXCR7 in combination with Nogo-A (see arrows in Fig 1H–1H'') and Nkx2.2, a transcription factor strongly expressed in the nucleus of OPCs (see arrow in Fig 1I–1I''; [5]) then confirmed that both mature and precursor cells in remyelinating MS lesions express CXCR7. Of note—and as expected since expression of CXCR7 has been described in several CNS cell types such as astrocytes [21, 22]—not all of the detected CXCR7-expressing cells belonged to the oligodendroglial lineage (see arrowhead in Fig 1H–1H'').
Fig 1. CXCR7 detection in MS tissue.
(A-F) CXCR7 staining revealed receptor expression on cells in control brains (B) and different regions of the MS brain including demyelinated (E; DM) and remyelinating (F; RM) lesions as well as active white matter lesions (D). Most CXCR7-positive cells in the MS brain were detected in the periplaque white matter (C; PPWM) while their number was significantly decreased in actively demyelinating lesion areas (D; active) at the plaque border probably reflecting ongoing oligodendroglial cell death (one-way ANOVA, **p < 0,05). (G) NogoA staining of control and MS brains demonstrated a similar distribution as CXCR7 (A) with a correlation coefficient of 0,783 between stainings. Double immunostaining then confirmed that mature NogoA positive oligodendrocytes (H-H''; see arrows) and Nkx2.2 positive oligodendroglial precursor cells (I-I''; arrows) express CXCR7. Not all CXCR7-positive cells were of oligodendroglial origin which is in line with studies describing this receptor in other CNS cell types (see arrowhead in H-H''). Scale bars: 25μm (F), 25μm (H-H''), 10μm (I-I'').
CXCL12 stimulation promotes the expression of oligodendroglial differentiation markers
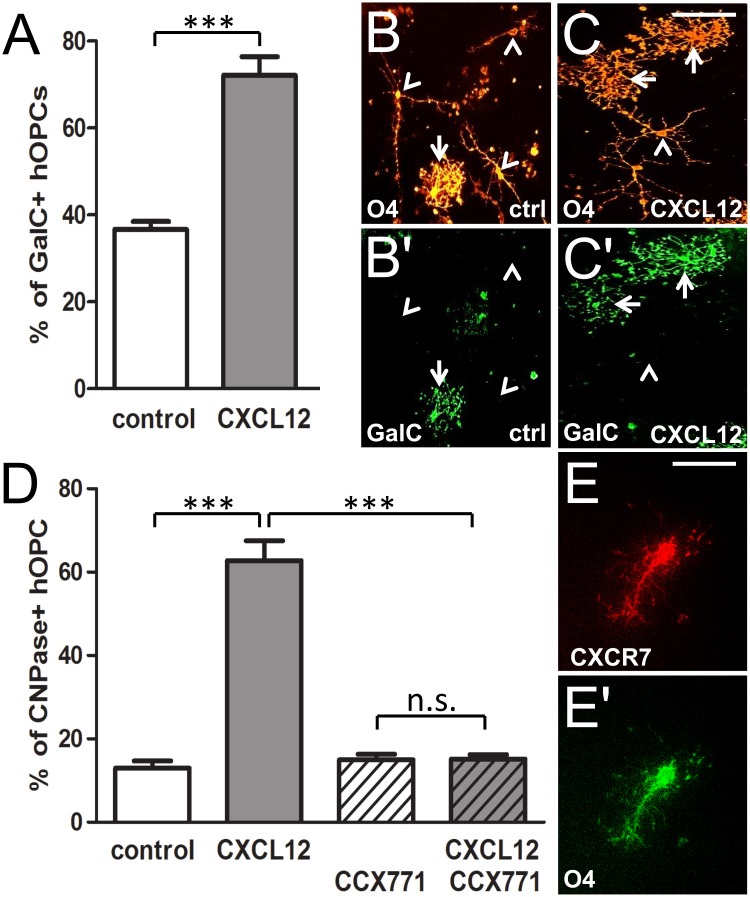
For a functional analysis of CXCL12-dependent effects on human OPCs, we exposed A2B5 antibody selected fetal human precursor cells to this chemokine. As oligodendroglial maturation is reflected by the induction of specific lineage markers, we determined whether expression kinetics was altered upon CXCL12 stimulation (Fig 2). To this end, fetal progenitors from the Human Fetal Tissue Repository were stimulated with 100ng/ml recombinant CXCL12 for 7 days, fixed, and subjected to immunofluorescent staining with antibodies directed against O4 and GalC. The assessment of OPC differentiation based on double positivity for O4 and GalC is widely used in the literature as a maturation read-out [23, 24] and revealed here that after 7 days of CXCL12 stimulation, the percentage of O4 cells that expressed GalC was increased more than 2-fold, compared to controls (Fig 2A–2C'). In parallel to our previous study investigating the effects of CXCL12 on rodent OPCs [15] most CXCL12-stimulated hOPCs featured more complex morphologies (arrows) as compared to cells in control cultures (arrowheads). Of note, with regard to potential effects of CXCL12 on hOPC proliferation and survival, DAPI counts of cells at different time points revealed no significant difference in cell numbers between stimulated or non-stimulated cells (data not shown). To determine whether the observed CXCL12-dependent differentiation effects were mediated through CXCR7 and in order to reproduce and corroborate the previous results in hOPCs from a different source, we then conducted specific blocking experiments with commercially available hOPCs. Using the expression of CNPase as a readout, we applied the specific CXCR7 antagonist CCX771. When applied alone (dashed white bar), CCX771 did not affect the degree of CNPase positive hOPCs (Fig 2D), whereas CXCL12 application (grey bar) boosted CNPase expression 3-fold similar to our previous observations using GalC-positivity of hOPCs from the Human Fetal Tissue Repository. Of note, this induction was completely abolished in CXCL12-stimulated OPCs that had been pre-treated with CCX771 (dashed grey bar), suggesting a crucial role for CXCR7 in the transmission of oligodendrocyte differentiation signals. In order to rule out potential DMSO-associated effects we also stimulated hOPCs with DMSO alone (white bar). In addition, CXCL12 stimulation was also carried out in the presence of DMSO to provide an appropriate control for CCX771 inhibition. Furthermore, double immunostaining confirmed that O4 positive hOPCs expressed CXCR7 (Fig 2E and 2E’).
Fig 2. CXCL12-mediated promotion of differentiation marker expression in cultured human oligodendroglial precursor cells.
(A) The percentage of O4 positive cells expressing GalC was significantly increased after 7 days of CXCL12 stimulation as compared to control cells. (B-C') Representative immunostainings of GalC/O4 positive OPCs stimulated with CXCL12 and control cells. Note that in CXCL12-stimulated cultures complex cell morphologies (arrows) were more common than in control cultures (arrowheads indicating cells with fewer cellular processes). (D) Determination of the percentage of CNPase positive hOPCs under CXCL12 stimulation after 7 days. This demonstrated that the CXCL12-dependent promotion of myelin induction (white vs. gray bars) was completely abolished in the presence of CCX771 (dashed gray bar). CCX771 alone did not affect myelin expression (compare white to dashed white bar). (E-E') Double immunostaining revealed that O4 positive precursor cell express CXCR7. Data are shown as mean values +/- SEM derived from 3 independent experiments. (t-test, ***p <0,001 and ANOVA, **p < 0,01). Scale bars: 70 μm (B-C'), 30 μm (E-E') μm.
CXCL12 stimulation leads to enhanced morphological maturation of CXCR7-positive human OPCs
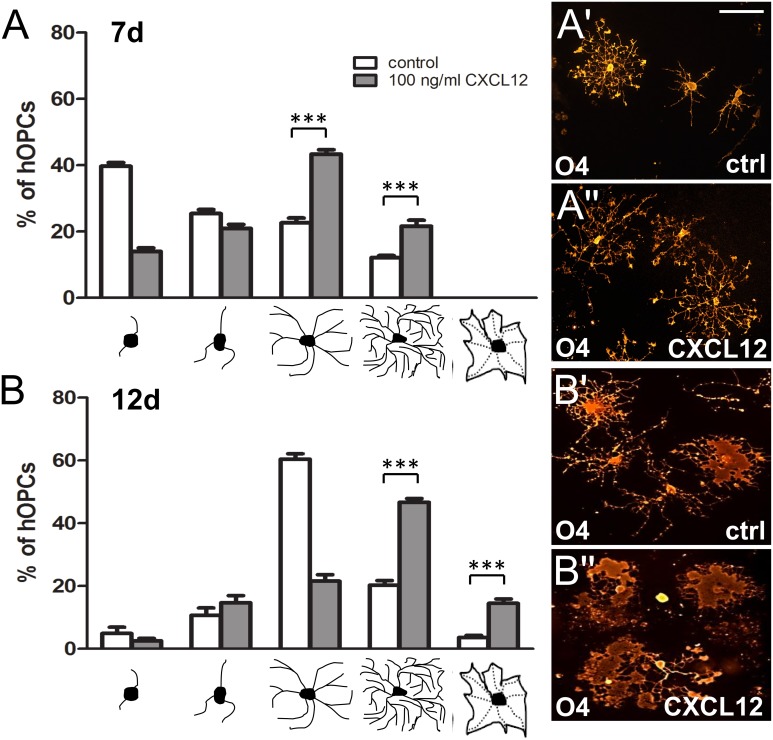
As oligodendrocyte lineage marker staining of control versus CXCL12-stimulated hOPCs suggested that cell shape and size are also influenced by this chemokine, we determined quantitatively whether hOPC morphological maturation is promoted as well (Fig 3). For visualization of single cells, we stained hOPCs from the Human Fetal Tissue Repository with the previously used anti-O4 antibody. Differentiation of cultured hOPCs is not a synchronized process and heterogeneous cell populations are generally observed featuring various degrees of morphological maturation with increasing numbers of processes and branches. In order to classify cell morphology we therefore used a well-established grading system that was already introduced in several previous studies [25–27] which distinguishes between five different morphologies ranging from a very low number of processes in precursor cells to multiple process-bearing cells and mature cells with a very high degree of arborization or membrane sheet appearance (see below graphs in Fig 3). We initiated oligodendroglial differentiation and applied recombinant CXCL12 or buffer as control condition, and fixed cells after 7 (Fig 3A–3A'') and 12 days (Fig 3B–3B''), respectively. This clearly demonstrated that application of CXCL12 resulted in a significant shift towards more complex hOPC morphologies as compared to control (buffer stimulated) cells. At 7 days, the majority of control cells featured low and medium morphologies, whereas the majority of cells stimulated with 100ng/ml CXCL12 were already at a highly differentiated stage. At 12 days CXCL12 stimulation resulted in an additional shift, with the majority of cells now displaying membrane sheet structures (see Fig 3B'').
Fig 3. CXCL12 promotes morphological maturation of cultured human oligodendroglial precursor cells.
Five different morphologies were distinguished ranging from a very low number of processes in precursor cells to multiple process-bearing cells (low, medium, high) to mature cells with a very high degree of arborization or flattened appearance (membrane sheet). (A,B) Analysis of hOPC morphology distribution revealed a CXCL12-dependent shift towards more mature cells (white bars: buffer treated cells; gray bars: CXCL12-stimulated cells) after 7 and 12 days in culture. Data are shown as mean values +/- SEM derived from 3 independent experiments (ANOVA, **p < 0,01). Representative O4-expressing hOPCs stimulated with either buffer or CXCL12 at 7d (A',A'') and 12d (B',B''). Scale bars: 50μm.
Discussion
Inefficient CNS remyelination observed in neuroinflammatory demyelinating diseases such as MS is tightly linked to the limited ability of resident OPCs to differentiate properly—a prerequisite for subsequent remyelination [5, 28]. While previous studies have demonstrated CXCR7 expression in human adult neurons [29], brain tumors [30] and human brain microvascular endothelial cells (HBMECs, [31]), we here demonstrate that CXCR7 is expressed on oligodendroglial cells in MS brain lesions and that its activation by CXCL12 leads to an acceleration of human glial maturation in culture as demonstrated by expression of oligodendrocyte lineage markers and enhanced morphological maturation. Regarding the relevance of CXCL12 in vivo several groups have already demonstrated that in the MS brain CXCL12 is expressed by reactive astrocytes particularly near the lesion edge [32] and that the proinflammatory cytokine interleukin-1β and myelin debris in the form of MBP can induce astrocytic CXCL12 [33]. With respect to the underlying mechanisms of CXCR7 signaling our previous studies have demonstrated that CXCR7 activation on oligodendroglial cells by CXCL12 leads to ERK1/2 phosphorylation [15] which is in line with several other studies indicating that factors involved in oligodendroglial differentiation and myelination exert their effects through the ERK1/2 signaling cascade [34–36]. Nevertheless, the mechanisms that promote oligodendrocyte differentiation upon ERK phosphorylation are still largely elusive even though a recent study revealed that both ERK1 and -2 promote MBP gene expression in oligodendroglial cells via phosphorylation of the transcription factor Sp1 [37]. On the other hand, other groups have clearly shown that G protein-coupled receptor kinase 2 (Grk2) is essential for CXCR7 signaling in CNS cells [21]. In how far Grks also play a role in oligodendroglial CXCR7 signaling is currently unclear and needs to be further elucidated in future studies on human tissue.
Of note, related studies have linked CXCL12-mediated OPC maturation to signaling through its second well-described receptor CXCR4. However, the experiments in these studies were performed in the non-inflammatory toxic cuprizone animal model [38] which makes a potential translation to MS with its strong inflammatory component difficult. In contrast the data presented here were generated in the human paradigm and demonstrate for the first time that CXCR7 is present on OPCs and oligodendrocytes in the MS brain. In addition, Williams and colleagues used the controversial substance AMD3100, a compound initially developed as a specific CXCR4 inhibitor. Pharmacological studies have, however, demonstrated that this molecule cannot be used as a reliable and specific blocking reagent as it was shown to act as a weak partial CXCR4 agonist leading, for instance, to an intracellular calcium levels increase similar to CXCL12 [39].
Nonetheless, CXCR4 might still play a role in CXCR7-mediated OPC maturation as several studies demonstrated receptor heterodimerization [40, 41] being relevant for CXCL12-mediated signal transmission. Of note, the goal of this study was not to demonstrate an exclusive responsibility of CXCR7 for OPC maturation but rather aimed at proving that CXCR7 is critically involved in this process thus providing evidence that this receptor could by a target for therapeutic repair. Yet, the exact downstream signaling of such heterodimers is still controversially discussed since some studies indicated, for instance, that coexpression enhances Ca2+ mobilization or chemotaxis [41, 42] while others demonstrated compromised CXCR4 signaling [40]. We and others support the idea that CXCR7 signals independently from CXCR4 and the results of our specific blocking experiments presented here have clearly demonstrated that CXCR7 is highly relevant for human OPC differentiation. This is of even greater interest since recently two small-molecules, VUF11207 and VUF11403, were identified as highly potent and selective ligands for CXCR7 that induce recruitment of β-arrestin [43]. These new compounds might represent promising lead substances for the development of specific CXCR7-based remyelination strategies with the benefit of avoiding CXCR4-mediated effects. However, a prerequisite for such therapies is a sufficient presence of CXCR7 on target cells and as we found that CXCR7 positivity undergoes an overall decrease in MS tissue in comparison to controls this is a critical aspect with potential therapeutic implications. In general, it is conceivable that the overall decrease of CXCR7-positive cells may partly reflect oligodendroglial cell death. However, CXCR7 positivity increases again in remyelinating lesions possibly based on migration of recruited OPCs. During this phase of lesion repair a window of opportunity might open up allowing for specific CXCR7-based OPC stimulation. However, lesion repair is overall insufficient which is thought to be partly due to the expression of chemorepellent molecules in MS lesions such as, for instance, Semaphorin 3A. Thereby, instead of entering MS lesions OPCs could be arrested in the periplaque white matter where, accordingly, we found significantly more CXCR7-positive cells in comparison to lesions themselves. This suggests that while CXCR7 activation could be an overall beneficial cue for remyelination in MS the key prerequisite for such an effect is successful entry of OPCs into MS lesions. We therefore conclude that besides and beyond a potential exogenous CXCR7 receptor upregulation to facilitate repair future combinatorial therapies are required that stimulate both OPC migration and differentiation to ultimately induce lesion repair.
Data Availability
All relevant data are within the paper.
Funding Statement
The MS Center at the Department of Neurology is supported in part by the Walter and Ilse Rose Foundation and the James and Elisabeth Cloppenburg, Peek & Cloppenburg Düsseldorf Stiftung.
References
- 1.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–85. [DOI] [PubMed] [Google Scholar]
- 2.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(17):6404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremer D, Aktas O, Hartung HP, Kury P. The complex world of oligodendroglial differentiation inhibitors. Ann Neurol. 2011;69(4):602–18. 10.1002/ana.22415 [DOI] [PubMed] [Google Scholar]
- 4.Kremer D, Schichel T, Forster M, Tzekova N, Bernard C, van der Valk P, et al. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol. 2013;74(5):721–32. 10.1002/ana.23970 [DOI] [PubMed] [Google Scholar]
- 5.Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain: a journal of neurology. 2008;131(Pt 7):1749–58. [DOI] [PubMed] [Google Scholar]
- 6.Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain: a journal of neurology. 1998;121 (Pt 12):2221–8. [DOI] [PubMed] [Google Scholar]
- 7.Adler MW, Rogers TJ. Are chemokines the third major system in the brain? J Leukoc Biol. 2005;78(6):1204–9. [DOI] [PubMed] [Google Scholar]
- 8.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4(6):444–55. [DOI] [PubMed] [Google Scholar]
- 9.Mennicken F, Maki R, de Souza EB, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20(2):73–8. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739 10.1155/2013/480739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadi L, Selvaraju R, de Lys P, Proudfoot AE, Wells TN, Boschert U. Differential effects of chemokines on oligodendrocyte precursor proliferation and myelin formation in vitro. Journal of neuroimmunology. 2006;174(1–2):133–46. [DOI] [PubMed] [Google Scholar]
- 12.Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain: a journal of neurology. 2005;128(Pt 5):1003–15. [DOI] [PubMed] [Google Scholar]
- 13.Maysami S, Nguyen D, Zobel F, Pitz C, Heine S, Hopfner M, et al. Modulation of rat oligodendrocyte precursor cells by the chemokine CXCL12. Neuroreport. 2006;17(11):1187–90. [DOI] [PubMed] [Google Scholar]
- 14.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, et al. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172(3):799–808. 10.2353/ajpath.2008.070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Göttle P, Kremer D, Jander S, Ödemis V, Engele J, Hartung HP, et al. Activation of CXCR7 receptor promotes oligodendroglial cell maturation. Ann Neurol. 2010;68(6):915–24. 10.1002/ana.22214 [DOI] [PubMed] [Google Scholar]
- 16.Cui QL, Fragoso G, Miron VE, Darlington PJ, Mushynski WE, Antel J, et al. Response of human oligodendrocyte progenitors to growth factors and axon signals. Journal of neuropathology and experimental neurology. 2010;69(9):930–44. Epub 2010/08/20. 10.1097/NEN.0b013e3181ef3be4 [DOI] [PubMed] [Google Scholar]
- 17.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83(2):311–27. [DOI] [PubMed] [Google Scholar]
- 18.Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(8):2709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38(5):788–96. [DOI] [PubMed] [Google Scholar]
- 20.Kuhlmann T, Remington L, Maruschak B, Owens T, Bruck W. Nogo-A is a reliable oligodendroglial marker in adult human and mouse CNS and in demyelinated lesions. Journal of neuropathology and experimental neurology. 2007;66(3):238–46. [DOI] [PubMed] [Google Scholar]
- 21.Lipfert J, Odemis V, Engele J. Grk2 is an essential regulator of CXCR7 signalling in astrocytes. Cell Mol Neurobiol. 2013;33(1):111–8. 10.1007/s10571-012-9876-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odemis V, Boosmann K, Heinen A, Kury P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123(Pt 7):1081–8. 10.1242/jcs.062810 [DOI] [PubMed] [Google Scholar]
- 23.Warrington AE, Pfeiffer SE. Proliferation and differentiation of O4+ oligodendrocytes in postnatal rat cerebellum: analysis in unfixed tissue slices using anti-glycolipid antibodies. Journal of neuroscience research. 1992;33(2):338–53. Epub 1992/10/01. [DOI] [PubMed] [Google Scholar]
- 24.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain: a journal of neurology. 2002;125(Pt 2):338–49. Epub 2002/02/15. [DOI] [PubMed] [Google Scholar]
- 25.Göttle P, Sabo JK, Heinen A, Venables G, Torres K, Tzekova N, et al. Oligodendroglial maturation is dependent on intracellular protein shuttling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(3):906–19. Epub 2015/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jadasz JJ, Kremer D, Gottle P, Tzekova N, Domke J, Rivera FJ, et al. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PloS one. 2013;8(8):e71814 Epub 2013/08/21. 10.1371/journal.pone.0071814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremer D, Heinen A, Jadasz J, Gottle P, Zimmermann K, Zickler P, et al. p57kip2 is dynamically regulated in experimental autoimmune encephalomyelitis and interferes with oligodendroglial maturation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):9087–92. Epub 2009/05/22. 10.1073/pnas.0900204106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–73. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Brown M, Sengupta R, Penfold ME, Meucci O. CXCR7 protein expression in human adult brain and differentiated neurons. PloS one. 2011;6(5):e20680 10.1371/journal.pone.0020680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang T, Xia QJ, Chen JB, Xi MR, Lei D. Expression of the CXCL12/SDF-1 chemokine receptor CXCR7 in human brain tumours. Asian Pac J Cancer Prev. 2012;13(10):5281–6. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Carson-Walter E, Walter KA. Chemokine receptor CXCR7 is a functional receptor for CXCL12 in brain endothelial cells. PloS one. 2014;9(8):e103938 10.1371/journal.pone.0103938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, et al. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. Journal of neuropathology and experimental neurology. 2005;64(8):706–15. Epub 2005/08/18. [DOI] [PubMed] [Google Scholar]
- 33.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, et al. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. Journal of neuroimmunology. 2006;177(1–2):27–39. Epub 2006/06/20. [DOI] [PubMed] [Google Scholar]
- 34.Du Y, Lercher LD, Zhou R, Dreyfus CF. Mitogen-activated protein kinase pathway mediates effects of brain-derived neurotrophic factor on differentiation of basal forebrain oligodendrocytes. Journal of neuroscience research. 2006;84(8):1692–702. [DOI] [PubMed] [Google Scholar]
- 35.Galabova-Kovacs G, Catalanotti F, Matzen D, Reyes GX, Zezula J, Herbst R, et al. Essential role of B-Raf in oligodendrocyte maturation and myelination during postnatal central nervous system development. J Cell Biol. 2008;180(5):947–55. 10.1083/jcb.200709069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao J, Ferner AH, Wong AW, Denham M, Kilpatrick TJ, Murray SS. Extracellular signal-regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro. J Neurochem. 2012;122(6):1167–80. 10.1111/j.1471-4159.2012.07871.x [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Eviatar-Ribak T, Miskimins R. Sp1 phosphorylation is involved in myelin basic protein gene transcription. Journal of neuroscience research. 2010;88(15):3233–42. 10.1002/jnr.22486 [DOI] [PubMed] [Google Scholar]
- 38.Williams JL, Patel JR, Daniels BP, Klein RS. Targeting CXCR7/ACKR3 as a therapeutic strategy to promote remyelination in the adult central nervous system. J Exp Med. 2014;211(5):791–9. 10.1084/jem.20131224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem. 2002;277(27):24515–21. [DOI] [PubMed] [Google Scholar]
- 40.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–93. 10.1182/blood-2008-12-196618 [DOI] [PubMed] [Google Scholar]
- 41.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188–97. 10.1074/jbc.M111.277038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wijtmans M, Maussang D, Sirci F, Scholten DJ, Canals M, Mujic-Delic A, et al. Synthesis, modeling and functional activity of substituted styrene-amides as small-molecule CXCR7 agonists. Eur J Med Chem. 2012;51:184–92. 10.1016/j.ejmech.2012.02.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.