Abstract
Chronic kidney disease is considered an inflammatory state and a high fiber intake is associated with decreased inflammation in the general population. Here, we determined whether fiber intake is associated with decreased inflammation and mortality in chronic kidney disease, and whether kidney disease modifies the associations of fiber intake with inflammation and mortality. To do this, we analyzed data from 14,543 participants in the National Health and Nutrition Examination Survey III. The prevalence of chronic kidney disease (estimated glomerular filtration rate less than 60 ml/min per 1.73 m2) was 5.8%. For each 10-g/day increase in total fiber intake, the odds of elevated serum C-reactive protein levels were decreased by 11% and 38% in those without and with kidney disease, respectively. Dietary total fiber intake was not significantly associated with mortality in those without but was inversely related to mortality in those with kidney disease. The relationship of total fiber with inflammation and mortality differed significantly in those with and without kidney disease. Thus, high dietary total fiber intake is associated with lower risk of inflammation and mortality in kidney disease and these associations are stronger in magnitude in those with kidney disease. Interventional trials are needed to establish the effects of fiber intake on inflammation and mortality in kidney disease.
Keywords: all-cause mortality, chronic kidney disease, inflammation
There are more than 16 million1 US adults with chronic kidney disease (CKD) defined as estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2. CKD is considered an independent risk factor for cardiovascular disease.2–5 Furthermore, CKD is also considered to be an inflammatory milieu,6 and elevated levels of serum C-reactive protein (CRP) is a strong predictor of cardiovascular events and mortality in CKD.7–10 Therefore, it is of great interest to identify potential interventions that could decrease the elevated serum CRP levels that are commonly seen in the CKD population. One such potential, but simple intervention, is increase in dietary fiber intake, as many studies in the general population suggest that high dietary fiber intake is associated with lower serum levels of markers of inflammation11,12 as well as decreased risk of mortality.13,14 However, dietary fiber intake is often low in the CKD population because of decreased intake of fruits and vegetables. To our knowledge, the associations of fiber intake with inflammation and mortality have not been studied in the CKD population. Therefore, we used the National Health and Nutrition Examination Survey (NHANES III) database to examine the associations of dietary fiber (total, soluble, and insoluble) intake with elevated serum CRP (>3 mg/l) and all-cause mortality in the non-CKD and CKD populations. We also examined whether the presence of CKD modifies the associations of fiber intake with serum CRP and mortality.
RESULTS
Baseline characteristics
The study population consisted of 14,533 adult participants aged 20 years or older with eGFR <150 ml/min per 1.73 m2 and non-missing data for dietary fiber intake. The mean (±s.d.) age was 45.0±15.8 years and 48% were men and 10% were African American. The mean intakes of total, soluble, and insoluble dietary fibers were 17.4±9.7, 6.0±3.3, and 11.3±6.8 g/day, respectively.
The prevalence of CKD was 5.8%. On the basis of the median total fiber intake of 14.6 g/day in the entire cohort, both non-CKD and CKD participants were divided into low-and high-fiber intake groups (Table 1). In general, in both non-CKD and CKD populations, male gender was associated with higher fiber intake, whereas African-American race was associated with lower fiber intake. None of the comorbid conditions was associated with fiber intake. Of the lifestyle factors, smoking and physical inactivity were associated with low fiber intake, and alcohol use was associated with high fiber intake. As expected, high total fiber intake was associated with higher intake of both soluble and insoluble fibers as well as calorie and protein intakes (Table 2) in both CKD and non-CKD populations.
Table 1.
Baseline characteristicsa of non-CKD and CKD participants according to dietary total fiber intake
| Non-CKD (≥l60 ml/min per 1.73 m2)
|
P | CKD (<60 ml/min per 1.73 m2)
|
P | |||
|---|---|---|---|---|---|---|
| Low total fiber (<14.5 g/day; n=6523) | High total fiber (≥l14.6 g/day; n=6915) | Low total fiber (<14.5 g/day; n=623) | High total fiber (≥l14.6 g/day; n=482) | |||
| Total dietary fiber (g/day) | 9.5±3.1 | 24.4±8.9 | 9.5±3.7 | 22.7±7.7 | ||
| Demographics | ||||||
| Age (years) | 43±15 | 44±14 | 0.001 | 69±15 | 70±12 | 0.53 |
| Men (%) | 37 (35–39) | 59 (57–60) | <0.001 | 27 (22–32) | 48 (42–55) | <0.001 |
| African Americans (%) | 14 (12–15) | 8 (7–9) | <0.001 | 10 (7–13) | 4 (2–6) | 0.004 |
| Clinical characteristics | ||||||
| Myocardial infarction (%) | 2.5 (2.1–3.1) | 3.0 (2.4–3.8) | 0.29 | 15.9 (11.8–21.2) | 14.9 (10.6–20.5) | 0.76 |
| Stroke (%) | 1.6 (1.2–2.2) | 1.5 (1.1–1.9) | 0.61 | 7.7 (5.8–10.3) | 10.8 (7.5–15.3) | 0.15 |
| Congestive heart failure (%) | 1.6 (1.3–2.0) | 1.5 (1.1–2.0) | 0.75 | 13.5 (10.3–17.4) | 11.1 (7.5–16.2) | 0.43 |
| Malignancy (%) | 3.5 (2.9–4.2) | 3.5 (3.1–4.0) | 0.96 | 12.0 (8.7–16.4) | 9.0 (5.9–13.6) | 0.30 |
| Diabetes mellitus (%) | 6.6 (5.7–7.7) | 6.1 (5.3–7.1) | 0.423 | 21.3 (17.1–26.5) | 19.9 (15.8–24.8) | 0.719 |
| Current smoker (%) | 34 (32–35) | 25 (23–28) | <0.001 | 17 (14–21) | 9 (6–13) | 0.001 |
| Alcohol use (%) | 52 (49–55) | 59 (55–62) | <0.001 | 22 (18–28) | 38 (30–47) | 0.001 |
| Leisure-time physical inactivity (%) | 16 (15–18) | 11 (10–13) | <0.001 | 34 (28–41) | 20 (15–27) | 0.003 |
| Waist circumference of men (inches) | 37.2±4.9 | 37.1±4.4 | 0.77 | 39.5±5.4 | 39.4±4.3 | 0.87 |
| Waist circumference of women (inches) | 34.5±5.5 | 34.3±5.3 | 0.28 | 37.3±5.2 | 37.7±5.4 | 0.48 |
| Systolic blood pressure (mm Hg) | 121±16 | 122±15 | 0.002 | 142±23 | 140±22 | 0.26 |
| Diastolic blood pressure (mm Hg) | 74±9 | 75±9 | <0.001 | 75±13 | 75±12 | 0.94 |
| Laboratory | ||||||
| Serum C-reactive protein >3 mg/l (%) | 28 (25–30) | 22 (20–24) | <0.001 | 48 (42–54) | 40 (34–47) | 0.08 |
| eGFR (ml/min per 1.73 m2) | 95±17 | 93±16 | 0.004 | 49±10 | 50±10 | 0.22 |
| Serum triglycerides (mg/dl) | 135±92 | 144±108 | 0.005 | 199±146 | 195±123 | 0.68 |
| Serum HDL cholesterol (mg/dl) | 51±15 | 51±14 | 0.31 | 49±17 | 49±18 | 0.95 |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
Mean±s.d. or median (25th to 75th percentiles) for continuous variables and proportion (95% confidence interval) for dichotomous variables are presented.
Table 2.
Baseline dietary factorsa of non-CKD and CKD participants according to dietary total fiber intake
| Non-CKD (≥60 ml/min per 1.73 m2)
|
P | CKD (<60 ml/min per 1.73 m2)
|
P | |||
|---|---|---|---|---|---|---|
| Low total fiber (<14.5 g/day; 48.5%) | High total fiber (≥14.6 g/day; 51.5%) | Low total fiber (<14.5 g/day; 56.4%) | High total fiber (≥14.6 g/day; 43.6%) | |||
| Soluble fiber (g/day) | 3.5±1.3 | 8.2±3.1 | <0.001 | 3.6±1.6 | 7.7±2.7 | <0.001 |
| Insoluble fiber (g/day) | 5.9±2.1 | 16.0±6.4 | <0.001 | 5.9±2.5 | 14.9±6.1 | <0.001 |
| Calorie intake (cal/day) | 1721±682 | 2658±1009 | <0.001 | 1329±558 | 1987±792 | <0.001 |
| Protein intake (g/day) | 65±31 | 98±42 | <0.001 | 52±25 | 78±37 | <0.001 |
Abbreviations: CKD, chronic kidney disease; cal, calories.
Mean±s.d. or median (25th to 75th percentiles) for continuous variables and proportion (95% confidence interval) for dichotomous variables are presented.
Dietary fiber and elevated CRP in the entire cohort, and non-CKD, and CKD sub-populations
In the entire population, 25.7% had elevated (>3 mg/l) serum CRP. The CKD sub-population had a higher prevalence of elevated serum CRP compared with the non-CKD sub-population (44.5 vs. 24.5%, P<0.001).
Intake of total, insoluble, and soluble fibers was each inversely associated with elevated serum CRP in unadjusted models (Table 3) in the entire cohort. Adjustment for factors that are potential confounders and unlikely to be in the causal pathway between fiber intake and elevated CRP (model 2 in Table 3) modestly attenuated these associations. Further adjustment for abdominal obesity and diabetes had little impact on the odds ratios (model 3 in Table 3).
Table 3.
Associations of dietary fiber intake with elevated serum CRP (>3 mg/l) in the entire cohort
| Total fiber For each 10-g/day ↑ OR (95% CI) |
Insoluble fiber For each 10-g/day ↑ OR (95% CI) |
Soluble fiber For each 10-g/day ↑ OR (95% CI) |
|
|---|---|---|---|
| Model 1a | 0.79 (0.74, 0.83) | 0.73 (0.68, 0.79) | 0.48 (0.42, 0.57) |
| Model 2b | 0.87 (0.81, 0.94) | 0.84 (0.76, 0.93) | 0.67 (0.54, 0.83) |
| Model 3c | 0.88 (0.81, 0.96) | 0.86 (0.77, 0.96) | 0.69 (0.54, 0.87) |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio.
Unadjusted.
Adjusted for age, gender, race, myocardial infarction, congestive heart failure, stroke, cancer, smoking, alcohol use, leisure-time physical inactivity, systolic blood pressure, diastolic blood pressure, calorie intake, protein intake, serum triglycerides, serum HDL cholesterol, and serum LDL cholesterol.
Adjusted for above+waist circumference and diabetes mellitus.
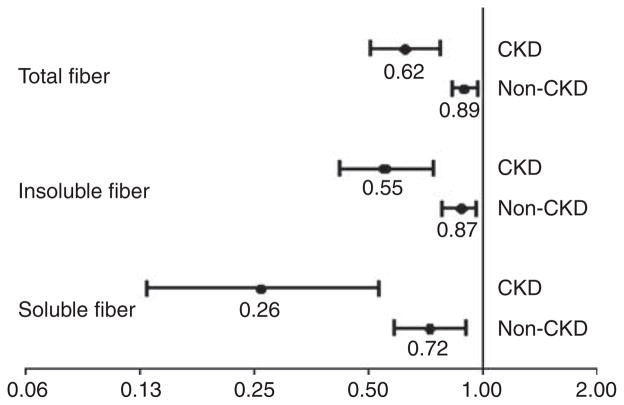
The associations of total, insoluble, and soluble fiber intakes with elevated serum CRP in the non-CKD and CKD sub-populations are summarized in Figure 1. Adjustment for the potential confounders, including age, gender, race, myocardial infarction, congestive heart failure, stroke, cancer, smoking, alcohol use, leisure-time physical inactivity, systolic blood pressure, diastolic blood pressure, calorie and protein intakes, serum triglycerides, serum high-density lipoprotein, serum low-density lipoprotein cholesterol (but not factors on the causal pathways between fiber intake and CRP such as waist circumference and diabetes), and total, soluble, and insoluble fiber intakes, was each inversely associated with the risk of elevated serum CRP in both the CKD and non-CKD populations, but was significantly stronger in those with CKD than those without CKD (interaction P-values were 0.002 for total fiber, 0.008 for soluble, and 0.005 for insoluble fiber).
Figure 1. Associations of dietary fiber with elevated serum C-reactive protein (>3 mg/l) in the non-chronic kidney disease (CKD) and CKD sub-populations.
Odds ratio for every 10-g/day increase in each type of fiber intake in CKD and non-CKD sub-populations. Models adjusted for age, gender, race, myocardial infarction, congestive heart failure, stroke, cancer, smoking, alcohol use, leisure-time physical inactivity, systolic blood pressure, diastolic blood pressure, calorie and protein intakes, serum triglycerides, serum high-density lipoprotein cholesterol, and serum low-density lipoprotein cholesterol.
Dietary fiber and all-cause mortality in entire cohort, and non-CKD and CKD cohort
Out of 14,533 NHANES III participants included in the analysis there were 2141 deaths (14.7%) over an average follow-up of 8.4 years. The non-CKD sub-population consisted of 13,428 participants with 1549 deaths (11.5%) over an average follow-up of 8.6 years. The CKD sub-population of 1,105 participants had 592 deaths (54%) over an average follow-up of 6.5 years. Table 4 summarizes the associations of dietary fiber with all-cause mortality in the entire cohort. In the unadjusted analyses, intake of total, insoluble, and soluble fibers was inversely associated with mortality in the entire cohort. However, after adjusting for demographics, comorbidities, and lifestyle factors this association was nonsignificant.
Table 4.
Associations of dietary fiber intake with all-cause mortality in the entire cohort
| Total fiber For each 10-g/day ↑ HR (95% CI) |
Insoluble fiber For each 10-g/day ↑ HR (95% CI) |
Soluble fiber For each 10-g/day ↑ HR (95% CI) |
|
|---|---|---|---|
| Model 1a | 0.91 (0.85, 0.97) | 0.90 (0.82, 0.99) | 0.72 (0.61, 0.85) |
| Model 2b | 0.98 (0.92, 1.06) | 0.98 (0.89, 1.07) | 0.98 (0.81, 1.18) |
| Model 3c | 1.00 (0.93 1.07) | 1.00 (0.91, 1.09) | 0.98 (0.81, 1.19) |
| Model 4d | 1.00 (0.93, 1.08) | 0.99 (0.90, 1.10) | 1.04 (0.85, 1.26) |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein.
Unadjusted.
Adjusted for age, gender, race, smoking, alcohol, leisure-time physical inactivity, calorie intake, and protein intake.
Model 2+adjusted for myocardial infarction, congestive heart failure, stroke, cancer, diabetes, systolic and diastolic blood pressure, serum triglycerides, serum HDL cholesterol, and serum LDL cholesterol.
Model 3+adjusted for waist circumference and serum CRP.
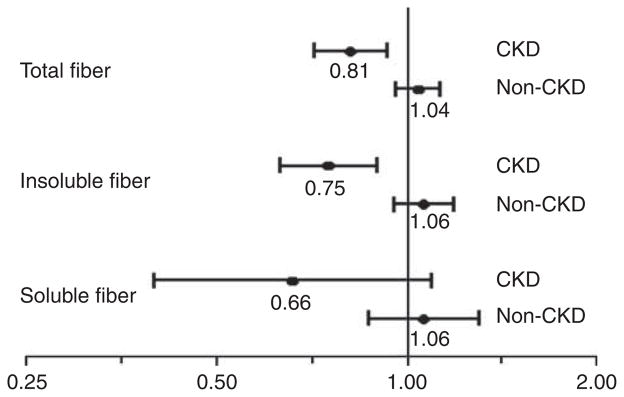
As shown in Figure 2, there was no association of fiber intake with mortality within the non-CKD sub-population adjusted for potential confounders (age, gender, race, smoking, alcohol, physical activity, and calorie and protein intakes). However, within the CKD sub-population, in similar models, intakes of total and insoluble fiber were inversely associated with risk of death, whereas this reached nonsignificance for the soluble fiber (Figure 2). Interaction P-values for CKD on the associations of fiber intake with mortality were 0.006 for total, 0.09 for soluble, and 0.004 for insoluble fibers. In additional sensitivity analyses, after adjusting for factors that are in the potential causal pathway between fiber intake and mortality (myocardial infarction, congestive heart failure, stroke, cancer, hypertension, systolic and diastolic blood pressures, and fasting serum lipids), each 10-g/day increase was associated with hazard ratio (HR) 0.83, 95% confidence interval (CI) 0.73–0.94 for total fiber, HR 0.77, 95% CI 0.65–0.91 for insoluble fiber, and HR 0.67, 95% CI 0.42–1.04 for soluble fiber. With further adjustment for serum CRP levels and waist circumference, the corresponding HR and 95% CI for total, insoluble, and soluble fibers were (HR 1.00, 95% CI 0.93–1.08), (HR 0.99, 95% CI 0.90–1.10), and (HR 1.04, 95% CI 0.85–1.26), respectively.
Figure 2. Associations of dietary fiber with all-cause mortality in the chronic kidney disease (CKD) and non-CKD sub-populations.
Hazard ratio for every 10-g/day increase in each type of fiber intake in CKD and non-CKD sub-populations. Models adjusted for age, gender, race, smoking, alcohol, leisure-time physical inactivity, and calorie intake and protein intakes.
DISCUSSION
The results of this study suggest that in the non-CKD population, higher fiber intake was associated with lower inflammation but not lower mortality. On the other hand, in the CKD population, higher fiber intake not only had strong association with lower inflammation but also was associated with lower mortality. The following discussion interprets these findings in the context of existing literature.
To our knowledge, there are no guidelines for dietary fiber intake in CKD population. In the general population, the current guidelines recommend a total fiber (both soluble and insoluble) intake of 20–35 g/day.15 However, the average American currently consumes one-half this amount.11,12,16 The current national guidelines for fiber intake are based on observational data.
Higher dietary fiber intake was associated with lower serum levels of interleukin-6 and tumor necrosis factor-alpha receptor-2 in postmenopausal women in the Women’s Health Initiative Study.17 Dietary fiber intake was associated with lower serum CRP in cross-sectional studies11,12 as well as in longitudinal analyses18 in the general population. These data raise the question whether dietary fiber per se or the other nutrients that are present in the foods that are high in fiber decrease systemic inflammation. King et al.19 examined the effects of fiber intake on serum CRP levels in a randomized crossover intervention trial of two diets, a high-fiber (30 g/day) Dietary Approaches to Stop Hypertension diet or fiber-supplemented diet (30 g/day). Overall, the mean serum CRP level changed from 4.4 to 3.8 mg/l (−13.7%; P =0.046) in the high-fiber Dietary Approaches to Stop Hypertension diet group and to 3.6 mg/l (−18.1%; P =0.03) in the fiber-supplemented diet group. However, in another trial of 7 g/day or 14 g/day of fiber supplementation, there were no changes in serum levels of markers of inflammation compared with the control group in overweight or obese adults.20 It is unclear whether the differences in these two studies reflect the different doses of fiber supplementation.
Several mechanisms have been proposed for the association of high dietary fiber intake with decreased serum CRP. Dietary fiber may inhibit inflammation by lowering glycemic load of rapidly digestible and absorbable dietary carbohydrates.21,22 High-fiber diet has been associated with higher plasma levels of anti-inflammatory adiponectin.23 Furthermore, substances such as phenols, indoles, and amines produced by colonic bacterial metabolism are absorbed from the gut and might have a role in systemic inflammation.24 These bacterial metabolism end products are normally cleared by the kidney but they accumulate with kidney failure. This might explain the observation that the associations of higher dietary fiber with lower inflammation are stronger in the CKD population than in the non-CKD population, as a high-fiber diet by altering gut bacterial metabolism likely decreases the generation and absorption of these toxins, and thereby, decreases systemic concentrations of these toxins more in the CKD population than in the non-CKD population.
Chronic metabolic acidosis is a complication of CKD and is associated with elevated CRP.25 This is further worsened in the CKD population because of the low intake of fruits and vegetables, which are an important source of alkali.26 Exposure of macrophages to an acidic environment leads to the increased production of tumor necrosis factor-α. In one study, the correction of metabolic acidosis in a small number of patients maintained on chronic ambulatory peritoneal dialysis was associated with a reduction in tumor necrosis factor-α levels.27
In the current study, higher dietary fiber intake was associated with decreased mortality in the CKD but not in the non-CKD population. Furthermore, this association of higher fiber intake with lower mortality in CKD was independent of demographics, cardiovascular disease, other lifestyle factors, blood pressure, serum lipids, and calorie and protein intakes. Further adjustment for serum CRP levels eliminated the association of fiber intake with mortality in CKD. Taken together, these data suggest a stronger role of dietary fiber in lowering inflammation in the CKD population and this is a likely potential mechanism for the association of higher dietary fiber with lower mortality in this population.
Nonetheless, apart from inflammation, there are other potential mechanisms through which dietary fiber could improve survival. Higher intake of whole grain foods or cereal fiber was consistently associated with lower risk of incident diabetes in prospective observational studies.28 Higher cereal fiber intake was associated with slower angiographic progression of coronary artery disease29 and lower risk of incidence of total stroke and ischemic stroke.30 Higher dietary fiber intake was associated with lower risk of coronary heart disease mortality.31 In NHANES I Epidemiological Follow-up Study, dietary fiber in particular soluble fiber was associated with lower risk of subsequent incidence of coronary heart disease and cardiovascular disease events.32 Fruit intake with lower mortality in elderly.33
Although there is mounting evidence from observational studies on the role of dietary fiber in reducing inflammation, diabetes, and cardiovascular disease, caution is needed in interpreting these results. First, whether dietary fiber per se or the other nutrients that are present in the foods that are high in fiber provide the health benefits is difficult to establish in the absence of interventional trials that increase dietary fiber alone. Second, whether the soluble fibers (from sources such as psyllium, fruits, vegetables, oatmeal, beans, and peas) or insoluble fibers (from sources such as wheat bran, whole grains, oats, and cereals) or both result in decrease in inflammation and cardiovascular disease is unclear. Third, even though observational studies might suggest benefit that might not be observed in interventional trials. For instance, among survivors of early-stage breast cancer, adoption of a diet that was very high in vegetables, fruit, and fiber and low in fat did not reduce additional breast cancer events or mortality during a 7.3-year follow-up period.34 Dietary advice to increase cereal intake in men after myocardial infarction was not associated with improved outcomes.35
There are other limitations to the current study; first, it is an observational, retrospective analysis of an existing database, which limits causal inference. Second, residual confounding due to unmeasured confounders cannot be ruled out. This is a major limitation of this type of analyses. Third, the diet was assessed with the use of a single 24-h-diet recall.
In conclusion, this study suggests a stronger inverse association of dietary fiber intake with inflammation and all-cause mortality in the CKD population than in the non-CKD population. Further studies are warranted to determine the mechanisms of these associations. Interventional trials are warranted to establish the causal role of dietary fiber in reducing inflammation and mortality in the CKD population.
MATERIALS AND METHODS
From 1988 to 1994, the National Center for Health Statistics conducted NHANES III, a cross-sectional survey of the US population. A complex, multistage sampling design was used to extrapolate the results to the entire non-institutionalized civilian US population as of the early 1990s.36 Data on age, sex, race, current or past cigarette smoking, and history of comorbid conditions, such as myocardial infarction, stroke, and congestive heart failure, were collected in a structured home interview conducted by trained personnel. A detailed questionnaire on leisure-time physical activity was also administered during the home interview. The home interview was followed by an examination by a physician at a mobile examination center, which included blood pressure measurement, extensive anthropometric, physiological, and laboratory testing.
Serum specimens from collection sites were transported on dry ice to the central laboratories and stored at −70 °C until analysis. Serum CRP was measured by latex-enhanced nephelometry using a Behring Nephelometer Analyzer System and reagents from Behring Diagnostics, Somerville, NJ. The lower limit for detection of this assay was 2.1 mg/dl. Serum creatinine was measured using a kinetic rate Jaffe method in NHANES III. These serum creatinine measurements were recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH) as standard creatinine (=−0.184 +0.960 × NHANES III measured serum creatinine in mg/dl).2 eGFR was calculated as 175 ×(standardized serum creatinine)−1.154 × (age)−0.203 × 0.742 (if the individual is woman) × 1.212 (if the individual is African American) ml/min per 1.73 m2. CKD was defined as eGFR <60 ml/min per 1.73 m2.
Details about dietary assessment methodology have been published elsewhere.37 A computer-based interview system developed by the University of Minnesota’s Nutrition Coordinating Center (Regents of the University of Minnesota) was used to conduct a 24-h dietary recall. The 24-h dietary recall was conducted by trained interviewers. The US Department of Agriculture’s Survey Nutrient DataBase was used to calculate macro- and micronutrient content of the foods consumed during the 24-h recall period for each respondent.
Follow-up data
The National Center for Health Statistics created a NHANES III Linked Mortality File that contains mortality follow-up data from the date of NHANES III survey participation (1988–1994) through 31 December 2000. This information was based upon the results from a probabilistic match between NHANES III and National Death Index death certificate records, the details of which are provided elsewhere.37
Data analyses
Several aspects of the NHANES design must be taken into account in data analysis, including the sampling weights and the complex survey design. We used the svy suite of commands in Stata 11 (Stata 11, College Station, TX) and followed the analytical guidelines for NHANES data proposed by the Centers for Disease Control and Prevention.37 It should be noted that the svy suite of commands in Stata use the complex survey design of NHANES to calculate the expected means and proportions of the entire US non-institutionalized civilian CKD population, and hence, means and proportions are presented with the estimated value and 95% confidence intervals.
Chi-square tests and t-tests (with adjustments for the survey design as implemented by the svy commands in Stata 11) were used as appropriate to compare categorical and continuous baseline factors between the low- and high-fiber groups within the non-CKD and CKD participants.
Associations of fiber intake with elevated serum CRP
Serum CRP >3 mg/l was defined as elevated CRP in this study based upon a consensus statement of the Centers for Disease Control and the American Heart Association.38 The association of total fiber with elevated serum CRP was first examined without covariate adjustment in the entire cohort with a logistic regression model. Next, this model was expanded by adding covariates to adjust for factors that are likely potential confounders and unlikely to be in the causal pathway between fiber and elevated CRP. These variables were age, gender, race, myocardial infarction, congestive heart failure, stroke, cancer, smoking, alcohol use, leisure-time physical inactivity, systolic blood pressure, diastolic blood pressure, calorie and protein intakes, serum triglycerides, serum high-density lipoprotein, and serum low-density lipoprotein cholesterol.
In sensitivity analysis, factors that are potential confounders but also may fall in the causal pathway between fiber and elevated CRP were added to the above covariates. These factors were waist circumference and diabetes.
The above analyses were repeated separately for soluble and insoluble fibers in the entire cohort.
To examine the effect modification by CKD of the associations of fiber intake with elevated CRP, the above analyses were conducted separately in the non-CKD and CKD sub-populations and the regression coefficients of fiber intake with elevated CRP in the non-CKD and CKD sub-populations were compared using a large-sample normal approximation.
Associations of fiber intake with mortality
The association of total fiber with mortality was first examined in the entire cohort with a Cox regression model without covariate adjustment. Next this model was extended by adding covariates that are potential confounders and unlikely to be in the causal pathway between fiber and mortality. These variables were age, gender, race, smoking, alcohol use, leisure-time physical inactivity, calorie and protein intakes.
In sensitivity analysis, factors that are potentially in the causal pathway between fiber and mortality were added to the above covariates. These variables were myocardial infarction, congestive heart failure, stroke, cancer, systolic blood pressure, diastolic blood pressure, serum triglycerides, serum high-density lipoprotein and serum low-density lipoprotein cholesterol, and diabetes. In order to examine the extent to which serum CRP and abdominal obesity potentially mediate the associations of dietary fiber with mortality, serum CRP, and waist circumference were added to the above model.
It is unlikely that the association of dietary fiber with inflammation is mediated by cardiovascular disease, cancer, blood pressure, and serum lipids. On the other hand, these variables could mediate the association of fiber with mortality. Therefore, we chose a different order of variables for the main model and the sensitivity analyses for elevated CRP and mortality.
Similarly, to the analyses of CRP, the Cox regressions for mortality were repeated for soluble and insoluble fibers in the entire cohort, and each models was then fit separately in the non-CKD and CKD sub-populations to evaluate if the relationships of mortality with the fiber intake factors differed between the non-CKD and CKD sub-populations.
The assumption of proportional hazards was examined by comparing the logarithm of the hazard ratio for each predictor variable in the first 36 months of follow-up to the logarithm of the hazard ratio of the predictor variables after 36 months. The factors age, gender, and serum CRP exhibited a significant deviation from proportional hazards for mortality (P<0.05). Hence, the Cox regressions were stratified by each of these factors (using tertiles for the continuous variables age and serum CRP) to allow separate baseline hazard functions within each stratum. Furthermore, within each age stratum, age was adjusted as a continuous variable.
Acknowledgments
This investigation is supported by the following: National Kidney Foundation Research Fellowship grant awarded to VMRK; RO1-DK077298 and RO1-DK078112 awarded to SB; the University of Utah Study Design and Biostatistics Center, with funding in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Beddhu S, Allen-Brady K, Cheung AK, et al. Impact of renal failure on the risk of myocardial infarction and death. Kidney Int. 2002;62:1776–1783. doi: 10.1046/j.1523-1755.2002.00629.x. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 4.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 5.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 6.Ramkumar N, Cheung AK, Pappas LM, et al. Association of obesity with inflammation in chronic kidney disease: a cross-sectional study. J Ren Nutr. 2004;14:201–207. [PubMed] [Google Scholar]
- 7.Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42:44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Yeun JY, Levine RA, Mantadilok V, et al. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 12.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92:1335–1339. doi: 10.1016/j.amjcard.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs DR, Jr, Meyer KA, Kushi LH, et al. Is whole grain intake associated with reduced total and cause-specific death rates in older women? The Iowa Women’s Health Study. Am J Public Health. 1999;89:322–329. doi: 10.2105/ajph.89.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs DR, Pereira MA, Meyer KA, et al. Fiber from whole grains, but not refined grains, is inversely associated with all-cause mortality in older women: the Iowa women’s health study. J Am Coll Nutr. 2000;19:326S–330S. doi: 10.1080/07315724.2000.10718968. [DOI] [PubMed] [Google Scholar]
- 15.Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 17.Ma Y, Hebert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008;24:941–949. doi: 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King DE, Egan BM, Woolson RF, et al. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–506. doi: 10.1001/archinte.167.5.502. [DOI] [PubMed] [Google Scholar]
- 20.King DE, Mainous AG, III, Egan BM, et al. Effect of psyllium fiber supplementation on C-reactive protein: the trial to reduce inflammatory markers (TRIM) Ann Fam Med. 2008;6:100–106. doi: 10.1370/afm.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Manson JE, Buring JE, et al. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 22.Qi L, van Dam RM, Liu S, et al. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–211. doi: 10.2337/diacare.29.02.06.dc05-1903. [DOI] [PubMed] [Google Scholar]
- 23.Qi L, Rimm E, Liu S, et al. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005;28:1022–1028. doi: 10.2337/diacare.28.5.1022. [DOI] [PubMed] [Google Scholar]
- 24.Evenepoel P, Meijers BK, Bammens BR, et al. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;76:S12–S19. doi: 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- 25.Kopple JD, Kalantar-Zadeh K, Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl. 2005;67:S21–S27. doi: 10.1111/j.1523-1755.2005.09503.x. [DOI] [PubMed] [Google Scholar]
- 26.New SA. Intake of fruit and vegetables: implications for bone health. Proc Nutr Soc. 2003;62:889–899. doi: 10.1079/PNS2003310. [DOI] [PubMed] [Google Scholar]
- 27.Pickering WP, Price SR, Bircher G, et al. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. 2002;61:1286–1292. doi: 10.1046/j.1523-1755.2002.00276.x. [DOI] [PubMed] [Google Scholar]
- 28.Priebe MG, van Binsbergen JJ, de Vos R, et al. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008:CD006061. doi: 10.1002/14651858.CD006061.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erkkila AT, Herrington DM, Mozaffarian D, et al. Cereal fiber and whole-grain intake are associated with reduced progression of coronary-artery atherosclerosis in postmenopausal women with coronary artery disease. Am Heart J. 2005;150:94–101. doi: 10.1016/j.ahj.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Kumanyika SK, Lemaitre RN, et al. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA. 2003;289:1659–1666. doi: 10.1001/jama.289.13.1659. [DOI] [PubMed] [Google Scholar]
- 31.Streppel MT, Ocke MC, Boshuizen HC, et al. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: the Zutphen Study. Am J Clin Nutr. 2008;88:1119–1125. doi: 10.1093/ajcn/88.4.1119. [DOI] [PubMed] [Google Scholar]
- 32.Bazzano LA, He J, Ogden LG, et al. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 2003;163:1897–1904. doi: 10.1001/archinte.163.16.1897. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez S, Huerta JM, Fernandez S, et al. Differences in overall mortality in the elderly may be explained by diet. Gerontology. 2008;54:232–237. doi: 10.1159/000135069. [DOI] [PubMed] [Google Scholar]
- 34.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burr ML. Secondary prevention of CHD in UK men: the Diet and Reinfarction Trial and its sequel. Proc Nutr Soc. 2007;66:9–15. doi: 10.1017/S0029665107005241. [DOI] [PubMed] [Google Scholar]
- 36.Statistics NCfH. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Hyattsville, MD: 1995. [Google Scholar]
- 37.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- 38.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]