In this study, an animal model of moderate-severe traumatic brain injury (TBI) was treated with an acute dose of propranolol followed by a delayed dose of human mesenchymal stem cells (MSCs), resulting in improved short- and long-term measurements. These results reinforce the inevitable clinical trial of MSCs to treat TBI by demonstrating, among other benefits, a notable decrease in chronic neuroinflammation and demonstrating that MSCs and propranolol are compatible treatments that improve overall outcome.
Keywords: Traumatic brain injury, Propranolol, Mesenchymal stromal cell, β-Blocker
Abstract
More than 6.5 million patients are burdened by the physical, cognitive, and psychosocial deficits associated with traumatic brain injury (TBI) in the U.S. Despite extensive efforts to develop neuroprotective therapies for this devastating disorder, there have been no successful outcomes in human clinical trials to date. Retrospective studies have shown that β-adrenergic receptor blockers, specifically propranolol, significantly decrease mortality of TBI through mechanisms not yet fully elucidated but are thought to counterbalance a hyperadrenergic state resulting from a TBI. Conversely, cellular therapies have been shown to improve long-term behavior following TBI, likely by reducing inflammation. Given the nonredundancy in their therapeutic mechanisms, we hypothesized that a combination of acute propranolol followed by mesenchymal stem cells (MSCs) isolated from human bone marrow would have additive effects in treating a rodent model of TBI. We have found that the treatments are well-tolerated individually and in combination with no adverse events. MSCs decrease BBB permeability at 96 hours after injury, inhibit a significant accumulation of activated microglia/macrophage in the thalamic region of the brain both short and long term, and enhance neurogenesis short term. Propranolol decreases edema and reduces the number of fully activated microglia at 7 days and the number of semiactivated microglia at 120 days. Combinatory treatment improved cognitive and memory functions 120 days following TBI. Therefore, the results here suggest a new, efficacious sequential treatment for TBI may be achieved using the β-blocker propranolol followed by MSC treatment.
Significance
Despite continuous efforts, traumatic brain injury (TBI) remains the leading cause of death and disability worldwide in patients under the age of 44. In this study, an animal model of moderate-severe TBI was treated with an acute dose of propranolol followed by a delayed dose of human mesenchymal stem cells (MSCs), resulting in improved short- and long-term measurements. These results have direct translational application. They reinforce the inevitable clinical trial of MSCs to treat TBI by demonstrating, among other benefits, a notable decrease in chronic neuroinflammation. More importantly, these results demonstrate that MSCs and propranolol, which is increasingly being used clinically for TBI, are compatible treatments that improve overall outcome.
Introduction
In the U.S., traumatic brain injury (TBI) constitutes the leading cause of mortality and morbidity in individuals younger than 44 years and is predicted to be the third leading cause of death and disability worldwide by 2020 [1]. TBI occurs in approximately 2.5 million people annually, contributing to a third all of injury-related deaths in the U.S. [2], with an estimated cost of $76.5 billion in 2010 [3]. TBI consists of an acute mechanical injury that shears neurons, ruptures blood vessels, and causes broad tissue damage [4]. Unfortunately, not all neurological damage occurs during the primary injury at impact; instead, increasing tissue injury develops hours and days later in a highly complex set of secondary injuries [4]. Secondary or subacute brain injury is the leading cause of in-hospital deaths after traumatic brain injury [5] and is thought to account for the development of many of the neurological deficits observed after TBI [6]. The delayed onset of secondary injuries permits therapeutic intervention to prevent progressive tissue damage and improve functional recovery after injury.
Currently, treatment of TBI is limited to controlling intracranial pressure and optimizing cerebral perfusion pressure acutely to prevent further physical injury, whereas chronic treatment centers on motor, cognitive, and behavioral rehabilitation [7]. There is a growing interest in combinatorial treatments using complementary or multipotent agents that modulate multiple injury mechanisms [8, 9].
β-Adrenergic receptor antagonists, often propranolol, have been recently proposed as a possible treatment for TBI. Retrospective studies of propranolol treatments in TBI have indicated significantly improved survival rates attributed to decreased cerebral metabolism, edema, and blood-brain barrier (BBB) permeability, along with increased cerebral perfusion [10–17]. In another recent retrospective study, propranolol has been shown to be the best β-blocker to limit secondary injury and decrease mortality in patients with traumatic brain injury when compared with others like atenolol, carvedilol, esmolol, labetalol, metoprolol, and sotalol [18]. Although the mechanisms behind the therapeutic benefits have not been fully explained, it is thought that propranolol could counteract a hyperadrenergic state following a catecholamine surge after TBI, which may last a variable time period [17]. Systemic increases in epinephrine and norepinephrine negatively correlate with a prognosis with high levels associated with coma and neurological dysfunction [19].
In recent years, mesenchymal stem/progenitor/stromal cells (MSCs) and other cell therapies have been explored for the treatment of TBI. MSCs were originally explored as a tissue or cell replacement strategy that ultimately failed to meet expectations but demonstrated that MSCs are remarkably safe clinically, even when genetically modified [20]. As the field of MSCs progresses, more studies are focusing on the ability of MSCs to modulate immune responses [21, 22]. Neuroinflammation plays a key role in TBI pathology. Following TBI, resident microglia activation perpetuates the neuroinflammatory response, leading to a chronic neurodegenerative process that last months to years in TBI patients [23–25]. Recently, MSCs isolated from rat bone marrow have been shown to improve TBI outcome by inhibiting the inflammatory cascade following TBI [26]. However, it is known that the anti-inflammatory potential of MSC can vary upon many factors, including species [27], tissue [28–31] of origin, and even gender [32]. In addition, studies involving MSCs or MSC-like cells use acute infusion protocols following brain trauma, within 24 hours of injury [26, 33], despite the chronic nature of the neuroinflammatory response following TBI.
Hence, based on the positive outcomes observed when propranolol is used on TBI models and the anti-inflammatory properties of MSCs, we hypothesize that combining the use of propranolol acutely, with an infusion of human MSCs from the bone marrow 72 hours postinjury, targeting subacute neuroinflammation, will result in superior therapeutic benefits in our experimental model of TBI. To our knowledge, there are no studies investigating a sequential propranolol-MSC treatment. We used cerebral edema, vascular permeability, microglia infiltration, neurogenesis, and cognitive functions as parameters to assess the therapeutic potential of the combinatorial treatment.
Materials and Methods
All protocols involving the use of animals were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee (AWC-13-065).
Cell Culture
We isolated MSCs from commercially available fresh human bone marrow aspirates of a 34-year-old male (AllCells, Emeryville, CA, http://www.allcells.com) according to the protocols previously established using density centrifugation and plastic adherence [34]. MSCs were isolated based on plastic adherence and screened for typical MSC spindle-like morphology; growth kinetics; positive expression of MSC markers CD90, CD73, and CD44; and negative expression of CD45. MSC were expanded by thawing a frozen vial of 106 passage 2 cells, and plating at 200 cells per cm2 in 2,528 cm2 in Cell Factory Systems (Nunc, Rochester, NY, http://www.nuncbrand.com) with complete culture medium that consisted of α-minimal essential medium (Life Technologies, Grand Island, NY, http://www.lifetech.com), 17% fetal bovine serum (lot-selected for rapid growth of MSC; Atlanta Biologicals, Norcross, GA, http://www.atlantabio.com), 100 units/ml penicillin (Life Technologies), 100 μg/ml streptomycin (Life Technologies), and 2 mM l-glutamine (Life Technologies). MSC were incubated with medium replaced every 2 days until approximately 70% confluent. The medium was discarded, the cultures were washed with phosphate-buffered saline (PBS; Life Technologies), and the adherent cells were harvested with 0.25% trypsin and 1 mM EDTA (Life Technologies) for 5 min at 37°C and frozen at 106 cells per milliliter for subsequent experiments as passage 3 cells. Frozen vials containing passage 3 cells were thawed, resuspended at 3 × 106 cells per milliliter in PBS, and used for injection.
Controlled Cortical Injury
Controlled cortical impacts were performed as previously described [35]. Rats were divided into 5 groups: controlled cortical impact injury (CCI) alone, CCI with propranolol (propranolol given i.p. at 10 mg/kg 1 hour after CCI), CCI with MSC (106 MSCs per kg given i.v. 72 hours after CCI), CCI with propranolol and MSC (propranolol i.p. at 1 hour and MSCs i.v. at 72 hours after injury), and a sham group who received an incision only. A CCI device (Impact One; Leica Biosystems, Buffalo Grove, IL, http://www.leicabiosystems.com) was used to administer a unilateral brain injury. Male Sprague-Dawley rats weighing 250–275 g were anesthetized with 4% isoflurane. The head was mounted in a stereotactic frame and held in a horizontal plane. A midline incision was made for exposure, and a 6-7-mm diameter craniectomy was performed on the right cranial vault. The center of the craniectomy was placed at the midpoint between bregma and λ, 3 mm lateral to the midline, overlying the temporoparietal cortex. Animals received a single impact of 3.1-mm depth of deformation with an impact velocity of 5.8 m/s and a dwell time of 150 ms (moderate-severe injury) to the parietal association cortex orthogonal to the surface and at an angle of 10° from the vertical plane. A 6-mm-diameter flat impactor tip was used. Sham injuries were performed by anesthetizing the animals, making the midline incision, and separating the skin, connective tissue, and aponeurosis from the cranium. Incisions in both the sham and TBI groups were closed using skin clips.
Edema
Brain tissue water content was determined in 24 animals (8 sham rats, 8 CCI alone, and 8 CCI + propranolol). The rats were euthanized 24 hours after CCI and exsanguinated, brains were quickly removed, and coronal sections (6 mm) from the ipsilateral side containing the injury and the contralateral side were placed separately on preweighed aluminum foils and weighed to yield wet weight. Brain samples were dried in a desiccation oven at 60°C for 48 hours and reweighed to determine the water content in each section. Water content was calculated as follows: percentage of water = wet weight − dry weight/wet weight × 100.
Serum Norepinephrine Measurement
Systemic levels of norepinephrine were measured in 26 animals (3 shams, 6 CCI, 6 CCI + propranolol, 5 CCI + MSCs, and 6 CCI + propranolol + MSCs). The rats were anesthetized, and blood was collected from the tail vein 4 days after injury and centrifuged at 12,000g for 15 min for isolation of serum. Serum norepinephrine was then assessed using a norepinephrine enzyme-linked immunosorbent assay kit (Abnova, Taipei, Taiwan, http://www.abnova.com) following the manufacturer’s protocol. One sample from a MSC animal was discarded because of technical difficulties during serum collection.
BBB Permeability Measurement
Permeability was measured in 71 animals (13 shams, 16 CCI alone, 14 CCI + propranolol, 14 CCI + MSCs, and 14 CCI + propranolol + MSCs) using a technique recently published by our group [36]. At 4 days after CCI and 30 minutes prior to euthanasia, rats were injected with 0.5 ml of 1 mg/ml Alexa Fluor 680 (Life Technologies) solution, a far red dye bioconjugated to a 10-kDa dextran molecule. Following exsanguination and 4% paraformaldehyde perfusion, the brains were harvested, sliced in 2-mm coronal sections, and scanned for Alexa Fluor 680 using a Odissey IR laser scanner (Li-Cor Lincoln, NE, http://www.licor.com/). The amount of dye accumulated within the whole brain was calculated relative to sham animals.
Immunohistochemistry
Rats were anesthetized, and brain was harvested following perfusion with PBS and paraformaldehyde, 7 days or 150 days after the injury. Coronal sections at 30 μm were cut in a vibrating blade microtome (VT1000 S; Leica, Heerbrugg, Switzerland, http://www.leica.com) and stained in suspension using standard staining protocol. Briefly, the sections were first rinsed with PBS with 0.01% Triton X-100 (PBST; T-9284, Sigma Aldrich) twice for a minute each followed by permeabilizing step for 20 minutes with PBS with 0.2% Triton X-100. Then they were blocked for 30 minutes in PBST containing 3% goat serum (005-000-121; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com). The primary and secondary antibodies were both prepared in PBST with 2% bovine serum albumin (A2153; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and 1% goat serum incubated overnight at 4°C and 2 hours at room temperature, respectively. The sections were washed thrice with PBST for 10 minutes each before and after incubation with secondary antibodies. Lastly, the sections mounted onto slides and were let dry at room temperature before they were coverslipped with DAPI Fluoromount-G (0100; Southern Biotech, Birmingham, AL, https://www.southernbiotech.com). The primary antibodies were as follows: microglia (Iba-1, 1:300, 019-19741; Wako Chemical, Richmond, VA, http://www.wako-chem.co.jp/english) and regenerating neurons (doublecortin, AB2253, 1:1,000; EMD Millipore, Billerica, MA, http://www.emdmillipore.com). The secondary antibodies were as follows: 1:1,000, green/488:A11073 and 1:1,000, red/568:A11011 (Life Technologies).
Microglia Distribution in Thalamus
For cell quantification and characterization, we used a total of 25 rats for the short-term group (7 days after CCI; 3 shams, 6 CCI alone, 5 CCI + propranolol, 6 CCI + MSCs, and 5 CCI + propranolol + MSCs; 7 days postinjury) and 28 rats for the long-term group (150 days after CCI; 6 shams, 6 CCI only, 5 CCI + propranolol, 6 CCI + MSCs, and 5 CCI + propranolol + MSCs; 150 days postinjury). The difference in number per group reflects brains damaged during tissue processing that were discarded. All brain slices used were within sections bregma −2.85 mm to −3.25 mm, and three brain slices per animal, at least 30 μm apart, were counted. We used a Nikon TE2000U fluorescent microscopy with a Nikon PlanFluor 10× objective (Nikon, Tokyo, Japan, http://www.nikon.com) to determine the number of Iba-1+ cells in three random regions within the thalamic nuclei, adjacent to the hippocampus. For representative pictures of each treatment group, inactivated, semiactivated, and fully activated microglia/macrophage, we used a 20× objective. To determine the number and distribution of microglia in the brain following injury, images from both contralateral and ipsilateral sides of injured and sham rats were randomized, and Iba-1+ cells were counted by a blinded researcher.
Neurogenesis
Neurogenesis was determined using the same brain slices used for microglia/macrophage characterization but assessing the number of doublecortin-positive cells (DCX+) in the subgranular zone of the dentate gyrus of animals. Cell quantification was performed using a TE2000U fluorescent microscope (Nikon) with a PlanFluor 20× objective (Nikon). Representative pictures were performed using a Nikon PlanFluor 10×. Three slices per brain, at least 30 μm apart, were analyzed for the total number of DCX+ cells present in the subgranular zone of the dentate gyrus from randomized and blinded image files. For the short-term experiment, the number of DCX+ cells was normalized by the total length of the subgranular zone of the dentate gyrus on each brain slice analyzed.
Morris Water Maze
We assessed cognitive function in a group of 30 rats (6 for each condition tested) using the Morris water maze (MWM) at 120 days postinjury to assess spatial memory and spatial learning in rats as previously described [37]. Standard protocol was followed where a blinded investigator tested all the groups in a hidden platform MWM setup by performing 4 trials per day with 4 minutes between each trial for 6 days for each rat. The latency to platform was measured as the time to find the platform. Probe trials (removal of platform) were done on day 7, 24 hours after the conclusion of platform testing. The EtoVision XT 8.0 tool from Noldus Information Technology (Leesburg, VA, http://www.noldus.com) used to record and analyze the probe trials.
Statistical Analysis
All data are represented as means ± SD. Comparisons between means of each group were made with the use of one-way analysis of variance (ANOVA) followed by Tukey’s test, except when measuring latency to platform, which was compared using two way ANOVA and differences in DCX+ cells between ipsilateral and contralateral, which were compared using unpaired t test. p < .05 was considered significant.
Results
In our study, a total of 207 rats were subjected to CCI treated with vehicle, propranolol, human bone marrow-derived MSCs, or a combination of propranolol and MSCs (Fig. 1A). Of those, three animals died immediately following CCI because of the severity of the injury received before treatment and were therefore discarded from this study. No apparent adverse effects caused by treatment were observed.
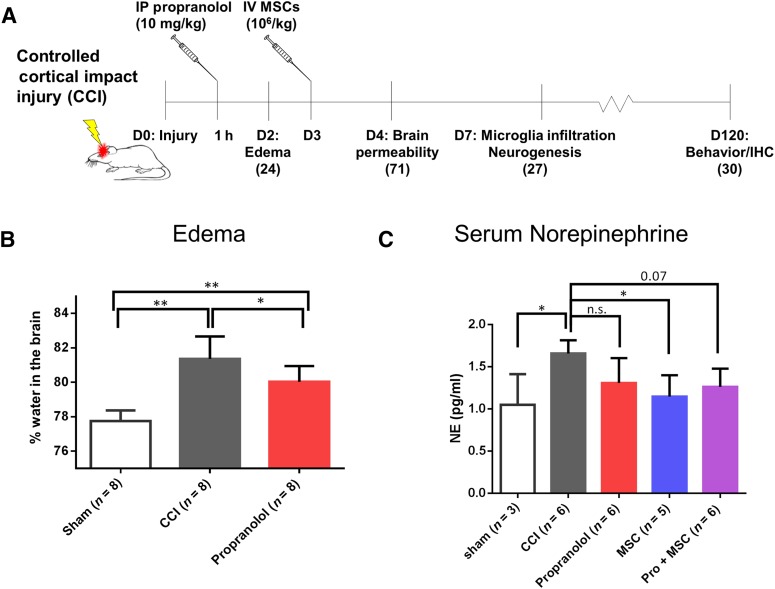
Figure 1.
Propranolol inhibits cerebral edema and MSC decrease systemic norepinephrine following CCI. (A): Experimental design and timeline. The total number of animals used for each time point is noted in parentheses. (B): Water accumulation in the brain of sham-operated rats, rats subjected to CCI, and CCI followed by an acute IP propranolol infusion at 1 hour postinjury. (C): Serum levels of norepinephrine of sham-operated rats, rats subjected to CCI, CCI followed by IP propranolol infusion (10 mg/kg) 1 hour postinjury, CCI followed by IV MSCs infusion (107 cells per kilogram) 72 hours postinjury, and CCI followed by the propranolol and MSC treatment, all measured at 4 days postinjury. ∗, p < .05; ∗∗, p < .01 by one-way analysis of variance followed by Tukey’s test. Abbreviations: CCI, controlled cortical impact injury; D, days; IP, intraperitoneal; IV, intravenous; MSC, mesenchymal stromal cell; n.s., not significant; Pro, propranolol.
Propranolol Decreases Cerebral Edema
In previous studies in mice subjected to TBI, propranolol treatment has been shown to improve TBI outcome. Acute propranolol infusion (4 mg/kg) at 15 or 60 min following TBI improves cerebral perfusion in mice [15, 38]. In addition, 2.5 mg/kg of propranolol given immediately after TBI has shown to decrease brain edema [39] following TBI. In order to develop a TBI treatment closely related to a clinical setting, in here we assessed the effect of our treatment regimens 60 min after CCI on cerebral edema following our rat model of TBI. Our results show that acute propranolol (10 mg/kg) treatment diminished but could not prevent brain edema in the ipsilateral side of the brain following CCI 24 hours after injury (Fig. 1B).
MSC Treatment Decreases Systemic Norepinephrine Levels Following TBI
It is known that following TBI, systemic norepinephrine levels rapidly increase [17, 19]. After this initial surge, a hyperadrenergic state persists a variable time period after the initial trauma [17]. In here, we sought to determine the effect of our treatment regimen on levels of norepinephrine in the serum of rats subjected to TBI. Our results show that 96 hours after CCI, systemic norepinephrine levels of injured rats were significantly higher than sham-operated rats (Fig. 1C). More importantly, MSC treatment significantly reduced norepinephrine, and the sequential treatment also showed a reduction but just failed to achieve statistical significance (p = .07).
MSC and Propranolol + MSC Treatment Improve/Restore BBB Permeability
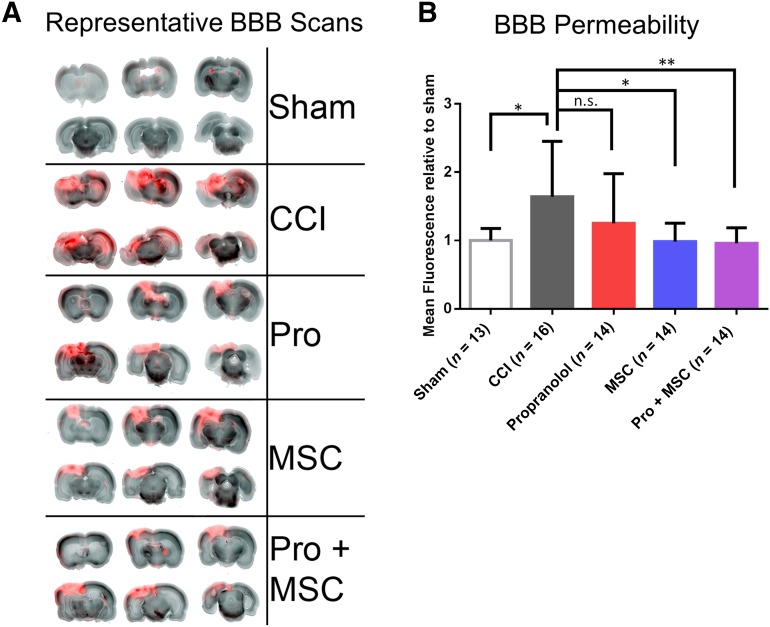
Animal studies and clinical data both suggest that BBB breakdown following head trauma can last from several days to weeks, extending to even years after the acute event [40]. In here, we assessed the extent to which our treatment regimen would affect BBB integrity and consequent brain permeability. Our results showed that the BBB breakdown and consequent brain permeability are significantly higher in rats subjected to CCI compared with sham animals at 96 hours (Fig. 2A, 2B). Overall, propranolol-treated animals had lower dye accumulation when compared with CCI alone, but not significantly so because of variability (Fig. 2B). However, animals treated with MSC alone or the combinatory treatment (Pro + MSCs) displayed significantly lower brain permeability when compared with CCI-only animals (Fig. 2B).
Figure 2.
MSC and sequential treatment decrease brain permeability in rats following CCI. (A): Representative pictures of 2-mm brain slices from rats perfused with Alexa Fluor far red dye 30 minutes before euthanasia. (B): Quantification of BBB permeability by accumulation of dye in the brain of sham-operated rats, rats subjected to CCI, CCI followed by intraperitoneal propranolol infusion (10 mg/kg) 1 hour postinjury, CCI followed by intravenous MSC infusion (107 cells per kilogram) 72 hours postinjury, and CCI followed by the propranolol and MSC treatment, all measured 96 hours postinjury. ∗, p < .05; ∗∗, p < .01 by one-way analysis of variance followed by Tukey’s test. Abbreviations: BBB, blood-brain barrier; CCI, controlled cortical impact injury; MSC, mesenchymal stromal cell; n.s., not significant; Pro, propranolol.
MSC, Propranolol, and Sequential Treatment Reduce Number and Activation of Microglia/Macrophages in the Brain
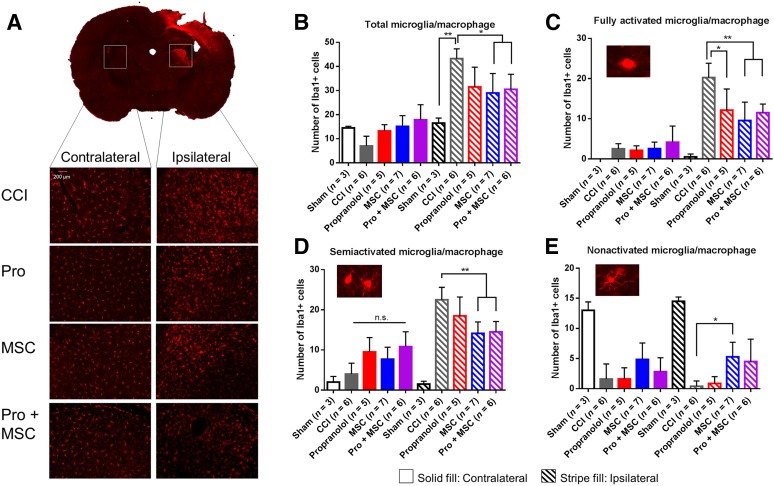
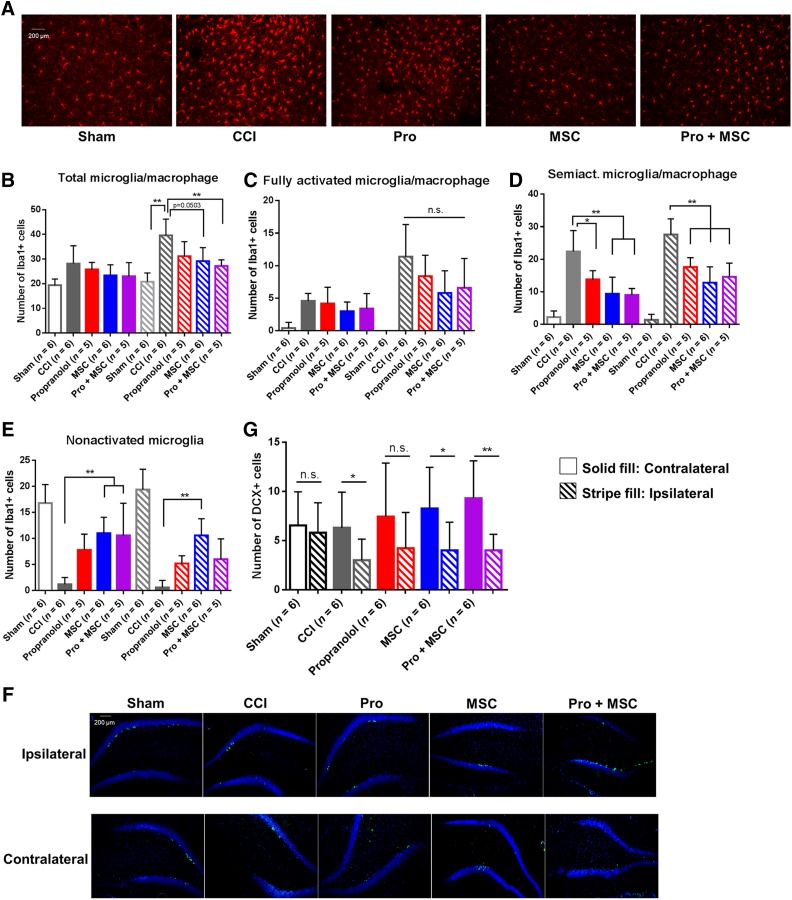
Activation of microglia following TBI is one of the central inflammatory responses to brain injury [41]. In humans and rodents, microglia activation and accumulation in the cortex is apparent 72 hours postinjury [42, 43], and chronic microglial activation may persist for years in humans [23]. Hence, we sought to determine the impact of our treatment regimen on the number and distribution of microglia in the brain following TBI focusing on the extended subacute or secondary stage of the injury at 7 days. Because of the severity of our model, microglia quantification in the cortex was problematic because of significant loss of cortical tissue at the impact site. However, we observed a dramatic accumulation of microglia/macrophages 7 days postinjury, particularly in the ipsilateral thalamic region of injured rats, more specifically in the lateral dorsal nucleus thalamus, adjacent to the hippocampus (Fig. 3A). This microglia/macrophage accumulation was three times higher when compared with sham animals, and MSC and Pro + MSCs significantly reduced the number of microglia/macrophage in the ipsilateral side compared with CCI-treated animals (Fig. 3B). In addition, we analyzed the morphology of the accumulated microglia/macrophages in the brain of treated animals (Fig. 3C–3E). We observed a significant decrease in fully activated (ameboid) microglia when treated with propranolol (p < .05), MSCs, and Pro + MSCs (p < .01) (Fig. 3C). Only the treatments containing MSCs decreased semiactivated microglia (Fig. 3D), and MSCs alone increased the number of ipsilateral nonactivated microglia (Fig. 3E). Overall, propranolol demonstrates a smaller effect on microglial number and activation compared with MSC treatment.
Figure 3.
MSC and combinatory treatment inhibit microglia/macrophage accumulation in the brain after injury. (A): Representative image of injured brain stained for Iba-1 depicting regions for the detection of microglia/macrophage cells. (B–E): Quantification of microglia/macrophage in different activation phenotypes in the thalamus of sham-operated rats, rats subjected to CCI, CCI followed by intraperitoneal propranolol infusion (10 mg/kg) 1 hour postinjury, CCI followed by intravenous MSCs infusion (107 cells per kilogram) 72 hours postinjury, and CCI followed by the propranolol and MSC treatment (Pro + MSC), all measured 7 days postinjury. ∗, p < .05; ∗∗, p < .01 by one-way analysis of variance followed by Tukey’s test. Abbreviations: CCI, controlled cortical impact injury; MSC, mesenchymal stromal cell; n.s., not significant; Pro, propranolol.
MSC Treatment Increase Short-Term Neurogenesis
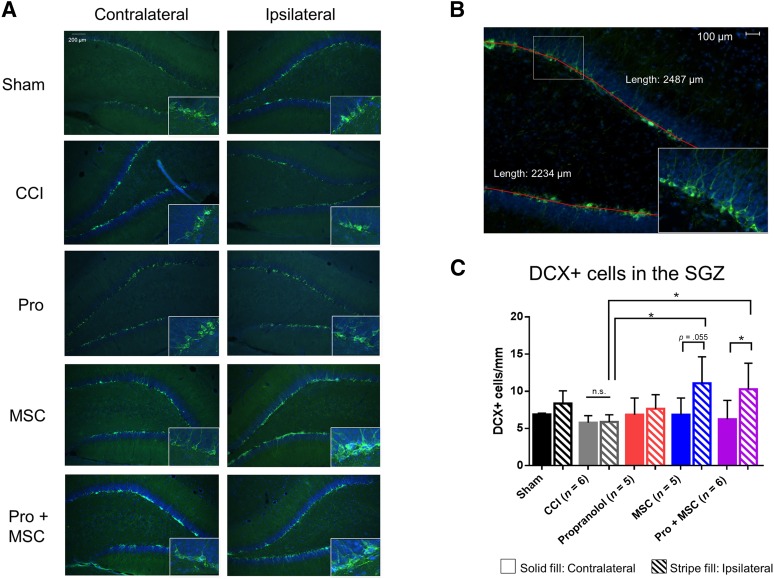
It is known that the mammalian nervous system can replace populations of damaged neurons by triggering the proliferation of neural stem cells (NSCs) [44, 45]. NSCs have been shown to exist in the subgranular zone at the dentate gyrus/hilus interface [46]. Doublecortin (DCX), a microtubule-associated protein specifically expressed in all migrating neuronal precursors of the developing brain and therefore expressed in newly formed immature neurons [47], was used to determine the effects of our treatment regimen on neurogenesis. In this study, treatment with MSC alone and the combinatory treatment (Pro + MSCs) increased neurogenesis in the ipsilateral but not in the contralateral side of the brain 7 days postinjury when compared with CCI alone (Fig. 4A), as evidenced by the increase in DCX-positive cells. When we normalized the number of DCX+ cells by the length of the dentate gyrus region (Fig. 4B), we observed that MSC and Pro + MSC treatments increase the number of DCX+ cells in the ipsilateral side. In contrast, there was no increase neurogenesis following CCI or CCI following propranolol when assessed 7 days postinjury in both ipsilateral and contralateral sides (Fig. 4A, 4B), suggesting that MSC treatment is the major contributor for the augment neurogenesis following CCI.
Figure 4.
MSC and combinatory treatments augment neurogenesis after injury. (A): Representative picture of the dentate gyrus (DG) depicting DCX+ cells in the SGZ. (B): Representative picture illustrating length used to normalize the number of DCX+ cells. (C): Quantification of DCX+ cells in the DG of sham-operated rats, rats subjected to CCI, CCI followed by intraperitoneal propranolol infusion (10 mg/kg) 1 hour postinjury, CCI followed by intravenous MSCs infusion (107 cells per kilogram) 72 hours postinjury, and CCI followed by the propranolol and MSC treatment, all measured 7 days postinjury. ∗, p < .05; ∗∗, p < .01. One-way analysis of variance was followed by Tukey’s test for differences between groups and unpaired t test for differences between ipsilateral and contralateral. Abbreviations: CCI, controlled cortical impact injury; DCX+, doublecortin-positive; MSC, mesenchymal stromal cell; n.s., not significant; Pro, propranolol; SGZ, subgranular zone.
Combinatory Treatment Improves Spatial Learning and Memory
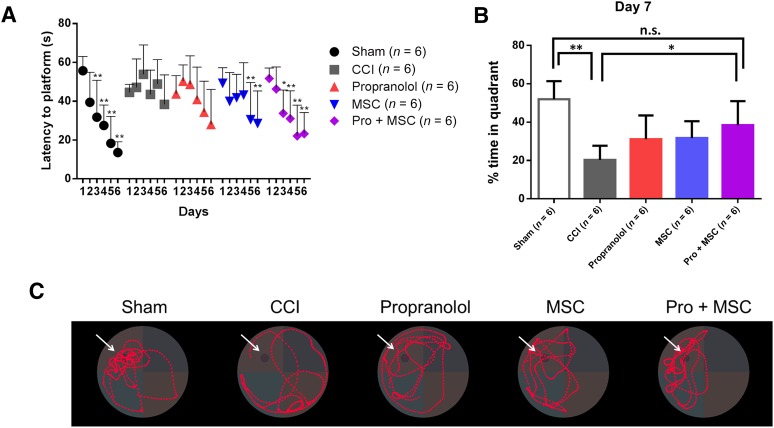
Finally, following the amelioration of multiple secondary complications following CCI, we hypothesized that the combinatory treatment would improve long-term, cognitive functions of rats subjected to TBI. We used the MWM 120 days after injury to assess spatial memory and spatial learning. Sham animals displayed significant improvement in latency time to the hidden platform by the third day of training. Following CCI, injured rats displayed a significant learning deficit, with no significant changes in latency time (Fig. 5A). In contrast, MSC- and Pro + MSC-treated rats showed significant reduced latency to platform by the fifth and third day, respectively (Fig. 5A). With the withdraw of the platform at 7 days after learning, Pro + MSC-treated rats showed a significant improvement in memory when compared with CCI animals, spending more time in the platform quadrant where the platform had been previously located (Fig. 5B, 5C).
Figure 5.
Combinatory treatment improves spatial learning and memory 120 days after injury. (A): Spatial learning in the Morris water maze. (B, C): Seventh-day probe trial. (B): Percentage of time spent in the platform quadrant. (C): Representative pictures showing path traveled during probe trials. Arrows indicate previous locations of the platform. The images are for sham-operated rats, rats subjected to CCI, CCI followed by intraperitoneal propranolol infusion (10 mg/kg) 1 hour postinjury, CCI followed by intravenous MSCs infusion (107 cells per kilogram) 72 hours postinjury, and CCI followed by the propranolol and MSC treatment. ∗, p < .05; ∗∗, p < .01. For differences in latency to platform, a two-way analysis of variance was used with a day as the repeated measure. For differences in percentage time in quadrant, one-way analysis of variance followed by Tukey’s test was used. Abbreviations: CCI, controlled cortical impact injury; MSC, mesenchymal stromal cell; n.s., not significant; Pro, propranolol.
MSC and Combinatory Treatment Impact Long-Term Cellular Changes
Based on the reports of chronic accumulation of microglia following TBI [23], we searched for microglia in the brain of injured rats following our long-term experiments. Similar to our previous observations at 7 days, there were more microglia/macrophages in the ipsilateral side (Fig. 6A). However, this accumulation was less pronounced, and differences between sham and CCI were less than two times higher (Fig. 6B). When we morphologically analyzed the microglia/macrophage, we observed differences between the numbers of total, semiactivated, and nonactivated microglia/macrophages. MSCs and Pro + MSCs decreased the total number of microglia/macrophage (Fig. 6B). Propranolol, MSC, and Pro + MSC treatments led to significantly lesser accumulation of semiactivated cells (Fig. 6D). Interestingly, MSC and Pro + MSC increased the number of contralateral, nonactivated microglia/macrophage, but only MSCs increased such number in the ipsilateral side when the compared animals were subjected to CCI only (Fig. 6E). Finally, we noticed that the number of DCX+ cells was greatly reduced when compared with our short time assessment (Fig. 6F). In sham animals, the number of DCX+ cells did not differ between the ipsilateral and contralateral sides, whereas both CCI and treatment groups displayed a decrease in DCX+ cells in the ipsilateral side of the injured brain (Fig. 6G), suggesting that neurogenesis plays a significant role in the early, but not in the late phase following our treatments.
Figure 6.
MSC and combinatory treatment decreases long-term microglia/macrophage activation and augments neurogenesis. (A): Representative picture of the ipsilateral thalamus stained for Iba-1+ cells. (B–E): Quantification of microglia/macrophage cells in the thalamus 150 days after injury. (F): Representative picture of the dentate gyrus (DG) stained for DCX+ cells. (G): Quantification of total DCX+ cells located in the DG of injured rats. The images are for sham-operated rats, rats subjected to CCI, CCI followed by intraperitoneal propranolol infusion (10 mg/kg) 1 hour postinjury, and CCI followed by intravenous MSCs infusion (107 cells per kilogram) 72 hours postinjury. ∗, p < .05. One-way analysis of variance followed by Tukey’s test was used for differences between groups. Unpaired t test was used for differences between ipsilateral and contralateral for neurogenesis. ∗, p < .05; ∗∗, p < .01. Abbreviations: CCI, controlled cortical impact injury; DCX+, doublecortin-positive; MSC, mesenchymal stromal cell; n.s., not significant; Pro, propranolol; Semiact., semiactivated.
Discussion
Our data demonstrate that an acute treatment of propranolol and/or a delayed MSC infusion can significant ameliorate key pathogenic outcomes of TBI in our experimental model. More specifically, an acute, single dose of propranolol can decrease cerebral edema and accumulation of activated microglia/macrophage following injury in the brain. In addition, a single dose of intravenous MSCs 72 hours after injury is sufficient to reduce serum levels of epinephrine, decrease brain permeability, inhibit the accumulation of activated microglia/macrophage in the brain, and augment neurogenesis after TBI. The combinatory treatment produced a significant improvement in long-term spatial learning and memory following TBI when compared with the propranolol-only or MSC-only treatment. This improvement in cognitive functions associates with a reduction in the chronic accumulation of total and activated microglia/macrophage. In all of our assays, the combination of propranolol and MSC treatments did not diminish the beneficial effects observed from the individual components alone, indicating that propranolol and MSC have potential to additively improve outcome following TBI.
Because of the complexity and variability of TBI-associated secondary injuries, a number of TBI research efforts have shifted away from single neuroprotective strategies and focus on multimodality treatments that can modulate multiple secondary injury pathways. Consistent evidence suggest that exposure to “β-blockers,” such as propranolol, following TBI conveys a 4%–23% improvement in absolute mortality advantage, a value that can be even larger if the data are stratified by early physiological measures of sympathetic excess, such as decreased heart rate variability [17]. The mechanisms responsible for the improved survival observed with β-blockers remain highly speculative. Given its physiological properties, β-blockers may lower myocardial stroke work and oxygen demand, therefore lowering the risk of myocardial infarctions, a common complication associated with TBI in humans [17], but an unknown complication in our model. In addition, propranolol can reduce a catecholamine-induced catabolic state, as observed in burn victims [48], and could reverse the hypermetabolism associated with brain injury [17]. In our model, there was no significant difference in weight gain between sham, injured, and treated rats (data not shown). Our results, however, show that a single acute dose of propranolol 1 hour after injury was sufficient to reduce cerebral edema caused by CCI. Unfortunately, the effects of MSCs or the combinatory treatment (MSCs + propranolol) on edema could not be determined because brain water content did not differ between sham and CCI at 96 hours (data not shown). This is in accordance with a study using a fluid percussion model, which has shown that brain edema subsides by day 5 following injury in rats [49]. Brain edema directly affects TBI mortality and morbidity as it increases intracranial pressure, impairs cerebral perfusion and oxygenation, and contributes to additional ischemic injuries [50]. Although we did not measure blood pressure in our animals, the ability of propranolol to block epinephrine and norepinephrine binding to β-adrenergic receptors, preventing vasoconstriction and reducing heart rate, systolic pressure, cardiac contractility, and output [51], could decrease mean arterial pressure and consequent dampen hydrostatic pressure and edema in the brain. In this regard, our result is in accordance with previous studies investigating the therapeutic effects of propranolol in mice brains injured by weight drop [15, 38, 39], which suggested propranolol treatment in mice reduced edema by improving cerebral perfusion and decreasing cerebral hypoxia.
MSCs and Pro + MSCs decreased systemic norepinephrine in injured rats, although only MSC reached statistical significance, whereas propranolol did not demonstrate a significant difference. This result suggests that early administration of MSCs may share some mechanism of efficacy with propranolol by decreasing circulating norepinephrine as opposed to blocking β-adrenoreceptors. Previous studies have suggested that MSCs express monoamine oxidase, an enzyme that catalyzes the oxidation of monoamines, including norepinephrine [52]. In addition, studies have proposed that MSC may serve as a sink for norepinephrine, because MSCs express α1B-, α2A-, and β2-adrenoreceptors, whose activation in MSCs leads to a robust release of intracellular calcium [53].
The breakdown of the BBB following TBI has been known for more than 20 years in both animal and clinical studies [54]. Following the initial trauma, the endothelia of small blood vessels in the brain often become subjected to a concomitant shear injury, which leads to impairments in the regulation of the BBB and increased brain permeability [55]. By using a technique previously described by our group to measure BBB integrity [36], we observed that MSCs alone could significantly restore BBB integrity. Previous studies in a mouse model of TBI have indicated that MSCs can ameliorate endothelial dysfunction through secretion of tissue inhibitor of matrix metalloproteinase-3 (TIMP-3) [56]. The authors reason that TIMP-3-derived MSCs could modulate endothelial barrier function by inhibiting binding of vascular endothelial growth factor (VEGF)-A to VEGF receptor 2, known to induce BBB permeability both in vitro and in vivo after TBI or by inhibiting matrix metalloproteinases (MMPs) or blocking inflammatory cascades. Another proposed mechanism through which MSCs could preserve BBB integrity comes from a study involving MSCs in a rat model of intracerebral hemorrhage [57]. In this study, the authors suggest that MSCs could confer BBB protection by secreting TSG-6, known to inhibit MMP activation [58] and the inflammatory NF-κB signaling pathway [59].
TBI is a neuroinflammatory condition of the central nervous system (CNS). Trauma to the brain results in loss of BBB integrity, leading to accumulation of leukocytes from the systemic circulation, release of proinflammatory signals, and activation of native glia, including microglia cells [60]. Although a microglial response is necessary to remove necrotic tissue and induce myelin repair, exacerbated and prolonged microglial activation can be detrimental for neurons and astrocytes [37]. Activation of microglia constitutes one of the central inflammatory responses to brain injury because activated microglia proliferates and moves to the site of injury, where they secrete proinflammatory cytokines that contribute to neuronal dysfunction and cell death [41]. In contrast to data from human TBI patients [60], we could not detect significant levels of proinflammatory cytokines (TNF-α and IFN-γ) in the circulation of injured rats at days 1, 3, 4, or 7 (data not shown), suggesting that the inflammatory response after TBI in rats is localized within the CNS, as previously indicated [26]. It is unclear whether the accumulation of microglia derives from resident or circulating monocytes, because microglia become morphologically and immunologically indistinguishable from the infiltrating macrophages of the periphery [60]. We can, however, distinguish fully activated, semiactivated, and nonactivated microglia/macrophage based on their morphology [61]. Treated animals displayed not only a reduced number of total microglia but also decreased number of activated microglia/macrophages. Interestingly, there seems to be an increase in the number of nonactivated microglia in the contralateral hemisphere because of MSC treatment at 7 days that becomes significant at 120 days. Previous studies suggest that nonactivated microglia play a constructive role in the brain, promoting oligodendrocyte development and instructing differentiation of stem cells toward postnatal neurogenesis [62, 63]. Therefore, the increase of nonactivated microglia/macrophage could constitute a therapeutic benefit following MSC treatment in addition to the reduction in activated microglia.
As mentioned, MSCs from rat bone marrow have been shown to reduce inflammatory cytokine levels in the brains of rats subjected to TBI [26], possibly through enhanced expression of TSG-6, a known anti-inflammatory secreted by MSC [58]. In fact, TSG-6 expression may be able to predict the anti-inflammatory potential of MSC in models of cornea injury, peritonitis and, to lesser extent, lung injury [32]. However, the same study indicates that after adjusting for height and weight, sex coefficient is the common predictive item for TSG-6 levels in MSC [32]. Remarkably, therapeutic improvement in the cornea and TSG-6 expression positively correlated with female but not male donor, which was used in our study. Nevertheless, MSCs have been shown to directly inhibit microglia activation [64, 65], which was confirmed using our male-derived MSCs (supplemental online Fig. 1). In particular, murine MSCs were also shown to inhibit microglia activation through TSG-6 [66]. However, there are always concerns when using MSCs from other species. For instance, the expression of immunomodulatory factors such as NO and IL-10, although observed in rodent MSC, are not detected in human MSCs, whose species-specific immunomodulatory factor appears to be IDO [27]. Nevertheless, it is likely that the anti-inflammatory properties of MSC may explain the therapeutic effects of our treatment regimen on the accumulation of microglia.
A number of investigations have targeted neurogenesis to improve functional recovery after adult brain injury [67]. Doublecortin immunostaining has been reliably used to identify neurogenesis, by staining immature, migrating neurons precursors in the dentate gyrus [68, 69]. The subgranular layer of the dentate gyrus is one of two primary areas where adult neural progenitors reside [70]. In our study, MSC-only and Pro + MSC treatment increased the number of DCX+ cells in the dentate gyrus of injured animals, indicating that MSC are solely responsible for this effect in our study. MSCs have been shown to secrete neurotropic factors such as brain-derived neurotrophic factor, fibroblast growth factor-2, glial cell-derived neurotrophic factor, insulin-like growth factor 1, hepatocyte growth factor, VEGF, and transforming growth factor-β [71–74] and promote genesis of neurons and oligodendrocytes from neural stem cells [75]. There are also reports of increased neurogenesis associated with release of the chemokine (C-C motif) ligand 2 from MSCs [76]. Recent studies indicate that some anti-inflammatory treatments improve recovery by promoting neurogenesis [77]. A decrease in body temperature, known to decrease inflammatory responses, resulted in an increase in cellular proliferation frequency of dentate gyrus DCX-immunoreactive cells 7 days post-trauma [67]. Given the immunomodulatory potential of MSC in TBI [26, 33], it is conceivable that the anti-inflammatory effects of MSCs contribute to neurogenesis in our model.
Finally, our study demonstrates that the combinatory treatment led to superior cognitive functions in our model 120 days following TBI compared with untreated rats as measured by MWM performance. Treated rats had an accelerated learning curve, measured as time to platform, as well as increased time in the same quadrant during the probe trial on the 7th day. The improvement in our study was associated with a reduction of subacute and chronic activated microglia/macrophage accumulation in the thalamus of the brain at both 7 days and over 4 months after injury. We believe this finding because it has been previously reported in head-injured patients [78]. In such studies, by using brains from control, moderately disabled, severely disabled, and vegetative patients, the authors observed that the number of activated microglia, astrocytes, and macrophages in the thalamic nuclei increased according to the severity of the condition. In addition, MRI studies have correlated damage to neural fibers projecting to and from the thalamus to chronic impairments in both cognition and behavior. Given the function of the thalamus as a relay center for the majority of cortical fiber projections, a thalamic hypothesis as a central mechanism of injury in TBI has been proposed [79, 80]. Stimulation of the thalamic region years after TBI improves arousal level, motor control, and behavioral responses [81], supporting preservation of the thalamus as a new and promising target to improve TBI outcome. Lastly, the reduction in neuroinflammatory responses holds promise in other neurodegenerative diseases besides TBI [26, 33, 37], such as Alzheimer’s disease [82] and multiple sclerosis [83].
Conclusion
Our study demonstrates that a safe, efficacious therapy can be achieved by combining the β-blocker propranolol with MSCs. A single dose of propranolol treatment given acutely following CCI reduced edema and altered microglial activation, whereas MSCs given at a later time point improved a number of other biomarkers, decreasing norepinephrine; improving BBB; altering subacute and chronic microglial number, distribution, and activation; and increasing neurogenesis. This result suggests the feasibility of a delayed MSC treatment for TBI patients suffering from subacute and chronic neuroinflammation. Notably, only the combination treatment of propranolol with MSCs achieved significance to improve long-term cognitive function, and throughout the study there was no evidence of the treatments interacting adversely. Future research is needed to further elucidate the mechanism of the therapeutic effects of propranolol following TBI and any potential interactions between propranolol and MSC when used in conjunction. The increasing use of propranolol for severe TBI patients [16] and the inevitable use of MSC to treat TBI based upon preclinical models and the safety of MSC clinically strongly support the necessity of evaluating the sequential treatment proposed here.
Supplementary Material
Acknowledgments
This study was performed with funding support from The Institute for Rehabilitation and Research (TIRR) Foundation Mission Connect Award 13-110 and the generous support of the Clare A. Glassell Family Pediatric Stem Cell Research Fund.
Author Contributions
D.J.K.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; K.S.P.: collection and/or assembly of data, data analysis and interpretation; A.J.v.B. and H.X.: collection and/or assembly of data; S.B.: technical support; B.D.: provision of study material; C.S.C.: data analysis and interpretation, manuscript writing; S.D.O.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors have no direct competing financial interests. C.S.C. is on the scientific advisory board of CBR, Inc. and has sponsored research with CBR, Inc., Athersys Inc., and Celgene Cellular Therapeutics, Inc. S.D.O. has sponsored research with Genzyme, Inc. D.J.K. has sponsored research from CBR, Inc., Athersys, Inc., and Genzyme, Inc. S.B. has compensated research funding. The other authors indicated no potential conflicts of interest.
References
- 1.Gean AD, Fischbein NJ. Head trauma. Neuroimaging Clin N Am. 2010;20:527–556. doi: 10.1016/j.nic.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 3.Finkelstein ECP, Miller T. The Incidence and Economic Burden of Injuries in the United States. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 4.Moppett IK. Traumatic brain injury: Assessment, resuscitation and early management. Br J Anaesth. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh TK, Smith DH, Meaney DF, et al. Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315–342. [PubMed] [Google Scholar]
- 7.Cox CS, Jr, Baumgartner JE, Harting MT, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68:588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 8.Margulies S, Hicks R. Combination therapies for traumatic brain injury: Prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoica B, Byrnes K, Faden AI. Multifunctional drug treatment in neurotrauma. Neurotherapeutics. 2009;6:14–27. doi: 10.1016/j.nurt.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotton BA, Snodgrass KB, Fleming SB, et al. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62:26–35. doi: 10.1097/TA.0b013e31802d02d0. [DOI] [PubMed] [Google Scholar]
- 11.Foley N, Marshall S, Pikul J, et al. Hypermetabolism following moderate to severe traumatic acute brain injury: A systematic review. J Neurotrauma. 2008;25:1415–1431. doi: 10.1089/neu.2008.0628. [DOI] [PubMed] [Google Scholar]
- 12.Heffernan DS, Inaba K, Arbabi S, et al. Sympathetic hyperactivity after traumatic brain injury and the role of beta-blocker therapy. J Trauma. 2010;69:1602–1609. doi: 10.1097/TA.0b013e3181f2d3e8. [DOI] [PubMed] [Google Scholar]
- 13.Ker K, Blackhall K. Beta-2 receptor antagonists for acute traumatic brain injury. Cochrane Database Syst Rev. 2008:CD006686. doi: 10.1002/14651858.CD006686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley EJ, Clond MA, Bukur M, et al. β-Adrenergic receptor inhibition affects cerebral glucose metabolism, motor performance, and inflammatory response after traumatic brain injury. J Trauma Acute Care Surg. 2012;73:33–40. doi: 10.1097/TA.0b013e31825a769b. [DOI] [PubMed] [Google Scholar]
- 15.Ley EJ, Scehnet J, Park R, et al. The in vivo effect of propranolol on cerebral perfusion and hypoxia after traumatic brain injury. J Trauma. 2009;66:154–161. doi: 10.1097/TA.0b013e31819388be. [DOI] [PubMed] [Google Scholar]
- 16.Riordan WP, Jr, Cotton BA, Norris PR, et al. Beta-blocker exposure in patients with severe traumatic brain injury (TBI) and cardiac uncoupling. J Trauma. 2007;63:503–511. doi: 10.1097/TA.0b013e3181271c34. [DOI] [PubMed] [Google Scholar]
- 17.Tran TY, Dunne IE, German JW. Beta blockers exposure and traumatic brain injury: A literature review. Neurosurg Focus. 2008;25:E8. doi: 10.3171/FOC.2008.25.10.E8. [DOI] [PubMed] [Google Scholar]
- 18.Schroeppel TJ, Sharpe JP, Magnotti LJ, et al. Traumatic brain injury and β-blockers: Not all drugs are created equal. J Trauma Acute Care Surg. 2014;76:504–509. doi: 10.1097/TA.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 19.Patel MB, McKenna JW, Alvarez JM, et al. Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): Study protocol for a randomized controlled trial. Trials. 2012;13:177. doi: 10.1186/1745-6215-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer G, Dao MA, Case SS, et al. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther. 2008;16:1308–1315. doi: 10.1038/mt.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 23.Gentleman SM, Leclercq PD, Moyes L, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Johnson VE, Stewart JE, Begbie FD, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Liu Y, Yan K, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Jung J, Lee HJ, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219–224. doi: 10.1016/j.intimp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4:125. doi: 10.1186/scrt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–156. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Lee RH, Yu JM, Foskett AM, et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci USA. 2014;111:16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedi SS, Hetz R, Thomas C, et al. Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem Cells Translational Medicine. 2013;2:953–960. doi: 10.5966/sctm.2013-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekiya I, Larson BL, Smith JR, et al. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 35.Walker PA, Bedi SS, Shah SK, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: Modulation of the resident microglia population. J Neuroinflammation. 2012;9:228. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao GP, Olson SD, Kota DJ, et al. Far-red tracer analysis of traumatic cerebrovascular permeability. J Surg Res. 2014;190:628–633. doi: 10.1016/j.jss.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedi SS, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. J Trauma Acute Care Surg. 2013;75:410–416. doi: 10.1097/TA.0b013e31829617c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley EJ, Park R, Dagliyan G, et al. In vivo effect of propranolol dose and timing on cerebral perfusion after traumatic brain injury. J Trauma. 2010;68:353–356. doi: 10.1097/TA.0b013e3181c8269a. [DOI] [PubMed] [Google Scholar]
- 39.Liu MY. Protective effects of propranolol on experimentally head-injured mouse brains. J Formos Med Assoc. 1995;94:386–390. [PubMed] [Google Scholar]
- 40.Shlosberg D, Benifla M, Kaufer D, et al. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csuka E, Hans VH, Ammann E, et al. Cell activation and inflammatory response following traumatic axonal injury in the rat. Neuroreport. 2000;11:2587–2590. doi: 10.1097/00001756-200008030-00047. [DOI] [PubMed] [Google Scholar]
- 43.Engel S, Schluesener H, Mittelbronn M, et al. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol. 2000;100:313–322. doi: 10.1007/s004019900172. [DOI] [PubMed] [Google Scholar]
- 44.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 45.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 46.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 48.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 49.Bareyre F, Wahl F, McIntosh TK, et al. Time course of cerebral edema after traumatic brain injury in rats: Effects of riluzole and mannitol. J Neurotrauma. 1997;14:839–849. doi: 10.1089/neu.1997.14.839. [DOI] [PubMed] [Google Scholar]
- 50.Unterberg AW, Stover J, Kress B, et al. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 51.Hörtnagl H, Hammerle AF, Hackl JM, et al. The activity of the sympathetic nervous system following severe head injury. Intensive Care Med. 1980;6:169–177. doi: 10.1007/BF01757299. [DOI] [PubMed] [Google Scholar]
- 52.Trouche E, Mias C, Seguelas MH, et al. Characterization of monoamine oxidases in mesenchymal stem cells: Role in hydrogen peroxide generation and serotonin-dependent apoptosis. Stem Cells Dev. 2010;19:1571–1578. doi: 10.1089/scd.2009.0353. [DOI] [PubMed] [Google Scholar]
- 53.Kotova PD, Sysoeva VY, Rogachevskaja OA, et al. Functional expression of adrenoreceptors in mesenchymal stromal cells derived from the human adipose tissue. Biochim Biophys Acta. 2014;1843:1899–1908. doi: 10.1016/j.bbamcr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez-Baeza A, Reina-de la Torre F, Poca A, et al. Morphological features in human cortical brain microvessels after head injury: A three-dimensional and immunocytochemical study. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:583–593. doi: 10.1002/ar.a.10069. [DOI] [PubMed] [Google Scholar]
- 56.Menge T, Zhao Y, Zhao J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. 2012;4:161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M, Li X, Zhang X, et al. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: Contribution of TSG-6. J Neuroinflammation. 2015;12:61. doi: 10.1186/s12974-015-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi H, Lee RH, Bazhanov N, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morganti-Kossmann MC, Satgunaseelan L, Bye N, et al. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 62.Nicholas RS, Wing MG, Compston A. Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-kappa B. Eur J Neurosci. 2001;13:959–967. doi: 10.1046/j.0953-816x.2001.01470.x. [DOI] [PubMed] [Google Scholar]
- 63.Walton NM, Sutter BM, Laywell ED, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 64.Ooi YY, Ramasamy R, Rahmat Z, et al. Bone marrow-derived mesenchymal stem cells modulate BV2 microglia responses to lipopolysaccharide. Int Immunopharmacol. 2010;10:1532–1540. doi: 10.1016/j.intimp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Yan K, Zhang R, Sun C, et al. Bone marrow-derived mesenchymal stem cells maintain the resting phenotype of microglia and inhibit microglial activation. PLoS One. 2013;8:e84116. doi: 10.1371/journal.pone.0084116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, Zhang R, Yan K, et al. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation. 2014;11:135. doi: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bregy A, Nixon R, Lotocki G, et al. Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp Neurol. 2012;233:821–828. doi: 10.1016/j.expneurol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acosta SA, Diamond DM, Wolfe S, et al. Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS One. 2013;8:e81585. doi: 10.1371/journal.pone.0081585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu TS, Zhang G, Liebl DJ, et al. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 71.Calzarossa C, Bossolasco P, Besana A, et al. Neurorescue effects and stem properties of chorionic villi and amniotic progenitor cells. Neuroscience. 2013;234:158–172. doi: 10.1016/j.neuroscience.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 72.Nakano N, Nakai Y, Seo TB, et al. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett. 2010;483:57–61. doi: 10.1016/j.neulet.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 73.Teixeira FG, Carvalho MM, Sousa N, et al. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res (Amst) 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Bai L, Caplan A, Lennon D, et al. Human mesenchymal stem cells signals regulate neural stem cell fate. Neurochem Res. 2007;32:353–362. doi: 10.1007/s11064-006-9212-x. [DOI] [PubMed] [Google Scholar]
- 76.Lee H, Kang JE, Lee JK, et al. Bone-marrow-derived mesenchymal stem cells promote proliferation and neuronal differentiation of Niemann-Pick type C mouse neural stem cells by upregulation and secretion of CCL2. Hum Gene Ther. 2013;24:655–669. doi: 10.1089/hum.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitney NP, Eidem TM, Peng H, et al. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maxwell WL, MacKinnon MA, Smith DH, et al. Thalamic nuclei after human blunt head injury. J Neuropathol Exp Neurol. 2006;65:478–488. doi: 10.1097/01.jnen.0000229241.28619.75. [DOI] [PubMed] [Google Scholar]
- 79.Grossman EJ, Ge Y, Jensen JH, et al. Thalamus and cognitive impairment in mild traumatic brain injury: A diffusional kurtosis imaging study. J Neurotrauma. 2012;29:2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Little DM, Kraus MF, Joseph J, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 82.Belarbi K, Jopson T, Tweedie D, et al. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Darlington PJ, Boivin MN, Bar-Or A. Harnessing the therapeutic potential of mesenchymal stem cells in multiple sclerosis. Expert Rev Neurother. 2011;11:1295–1303. doi: 10.1586/ern.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.