The role of keratinocyte growth factor (KGF) in human umbilical cord-derived mesenchymal stem cell (hUC-MSC) differentiation remains unknown. Building on previous work, the authors found KGF expression in sweat gland-like cells (SGCs) and determined that recombinant human KGF could induce hUC-MSC differentiation into SGCs. These differentiated SGCs were applied to a mouse burn model and sweat glands were regenerated. These cells may have potential therapeutic application for regeneration of destroyed sweat glands and injured skin.
Keywords: Burns, Mesenchymal stem cell, Sweat gland, Tissue regeneration, Transdifferentiation, Umbilical cord, Wharton’s jelly
Abstract
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) have higher proliferation potency and lower immune resistance than human bone marrow MSCs and can differentiate into various functional cells. Many regulatory factors, including keratinocyte growth factor (KGF), are involved in the development of skin and cutaneous appendages. Although KGF is important in wound healing, the role of KGF in hUC-MSC differentiation remains unknown. In our previous work, we found the mixing medium (nine parts of basic sweat-gland [SG] medium plus one part of conditioned heat-shock SG medium) could induce hUC-MSC differentiation to sweat gland-like cells (SGCs). In this study, we further improved the inducing medium and determined the effects of KGF in hUC-MSC differentiation. We found KGF expression in the SGCs and that recombinant human KGF could induce hUC-MSC differentiation into SGCs, suggesting KGF plays a pivotal role in promoting hUC-MSC differentiation to SGCs. Furthermore, the SGCs differentiated from hUC-MSCs were applied to severely burned skin of the paw of an in vivo severe combined immunodeficiency mouse burn model. Burned paws treated with SGCs could regenerate functional sparse SGs 21 days after treatment; the untreated control paws could not. Collectively, these results demonstrated that KGF is a critical growth factor for SGC differentiation from hUC-MSCs and the differentiated SGCs from hUC-MSCs may have a potential therapeutic application for regeneration of destroyed SGs and injured skin.
Significance
There is growing evidence demonstrating a potential therapeutic application of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in injured skin. In the current study, conditioned media and chemically defined media with recombinant human keratinocyte growth factor (KGF) could induce hUC-MSC differentiation into sweat gland-like cells (SGCs). Moreover, the differentiated SGCs from hUC-MSCs could regenerate functional sparse sweat glands in a mouse burn model, which provides further insight into the mechanisms of the role of KGF and a potential therapeutic application of differentiated SGCs for regeneration of destroyed sweat glands and injured skin.
Introduction
As an essential function of skin, sweating is manipulated by sweat glands (SGs) embedded in the deep dermal layer [1, 2]. When the original structure and function of whole skin is destroyed in seriously burned patients, the skin loses its self-regenerative/self-repairing ability [3, 4]. In past decades, the major objective of surgeons and researchers of burns was to accelerate closure of burn wounds through the application of an autograft of a patient’s own skin or allograft transplants of other kinds of skin substitutes, like tissue-engineered skin [5–9]. However, the original structure and functions of seriously injured skin, especially for perspiration (sweating), could not be well re-established during rehabilitation.
Mesenchymal stem cells derived from human bone marrow (BM-MSCs) and human umbilical cord Wharton’s jelly (hUC-MSCs) have been used as novel and effective sources of stem cell therapy and regenerative medicine to repair injured cutaneous structures and appendages [10–14]. As a prominent cell resource, BM-MSCs have been widely used for treating various kinds of diseases and injured tissues, like burns and other wounds [11, 15–17]. As shown by previous studies, BM-MSCs can be induced to differentiate into sweat gland-like cells (SGCs) in vitro, and patients’ own differentiated SGCs from BM-MSCs can be autografted for regenerating injured SGs [10, 13, 18, 19]. Compared with BM-MSCs, hUC-MSCs have higher proliferation potency [20–22], multiple differentiation potentials, and lower immune resistance [22–24]. Additionally, human umbilical cord tissues can be stored at −80°C for the preparation of hUC-MSCs [23]. In our previous study, we induced hUC-MSC differentiation to SGCs in a conditioned induction medium consisting of nine parts basic SG medium and one part sterile supernatants from conditioned heat-shock SG medium [12]. However, the pivotal inducting factors in the SGC differentiation have not been identified. In this study, we further improved the inducing medium by mixing eight parts basic SG medium and two parts of sterile supernatants from conditioned heat-shock SG medium, referred to as induction medium-mix, and we identified the key regulatory factors of hUC-MSC differentiation to SGCs.
Several growth factors were found to be involved in the development of skin and SGs: keratinocyte growth factor (KGF; one subtype of fibroblast growth factor [FGFs], also called FGF-7) [25–28], epidermal growth factor (EGF) [28], and anhidrotic ectodermal dysplasias (EDAs; one subtype of the tumor necrosis factor family) [27]. It has been demonstrated that a deficiency of EDA during embryo development could result in dysfunctional sweating, missing teeth, and sparse hair [27, 29]. EGF can induce several types of mesenchymal stem cell (MSC) differentiation into epidermal lineage cells, maintain proliferation potency of epithelial cells, and accelerate skin wound healing. Recently, KGF was found to play an important role in wound healing and maintenance of cutaneous homeostasis [25, 30–33]. However, the role of KGF in SGC differentiation is unknown.
In the present study, we hypothesized that KGF is an essential factor in hUC-MSC differentiation to SGCs, and could be applied for reconstruction of destroyed sweat glands and skin wounds. The aim of this study was to clarify the potential of recombinant human KGF (rhKGF) during the differentiation of SGCs from hUC-MSCs, and then to ascertain the reconstruction effect of differentiated SGCs in a severe damaged skin model in vivo.
Materials and Methods
Mice
Severe combined immunodeficient (SCID) mice (male, 8–12 weeks old) were purchased from Zhejiang Academy of Medical Sciences. Mice were maintained under pathogen-free conditions in the Department of Laboratory Animal Resources of Zhejiang University. All protocols related to animal experiments were approved by the institutional animal care and use committee of Second Affiliated Hospital, Zhejiang University School of Medicine (SAHZU). Experiments were performed according to the National Institutes of Health guidelines and Animal Research: Reporting of In Vivo Experiments guidelines on the use of laboratory animals.
hUC-MSCs Isolated From Human Umbilical Cord Tissues
Human umbilical cords were obtained with informed consent from healthy donors who finished their routine deliveries at Women’s Hospital Zhejiang University School of Medicine. All the procedures related to harvesting human tissues in this study were approved by the ethical committee of SAHZU.
The umbilical cords were kept at 4°C and transported to the laboratory. They were then repeatedly rinsed by sterile phosphate-buffered saline (PBS). After removing all the vessels, the remaining tissues were minced into 1-mm3 cubes and digested with type II collagenase (2 mg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) at 37°C for 6–8 hours. The samples were centrifuged at 3,000g for 5 minutes at room temperature. The sediments were resuspended and cultured in basic hUC-MSC medium (Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% fetal bovine serum [FBS] [Gibco/Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com]; 100 U/ml penicillin (Sigma-Aldrich), 100 mg/ml streptomycin (Sigma-Aldrich), and 2 mM l-glutamine (Sigma-Aldrich) in a cell culture incubator at 37°C in a humidified atmosphere containing 5% CO2 (Hera Cell; Thermo Fisher Scientific). hUC-MSCs were routinely examined under a phase-contrast inverted microscope (Leica, Wetzlar, Germany, http://www.leica.com). Cells were subcultured when cells reached 80% confluence in the plates, and then cells were used for the subsequent study after 3–5 passages [12].
Construction of SGC Differentiation Medium
Normal human skin was collected from five female plastic-surgery patients who had small skin grafts harvested from the inside of their upper arms. Skin tissue (0.5–1 cm2) was minced into 1-mm3 skin particles after removal of subcutaneous fat, and then digested with type II collagenase (2 mg/ml; Sigma-Aldrich) at 37°C for 3–4 hours. Mature SGs were cultured in basic SG medium used as a positive control. Proliferated SGs were heat shocked and then recultured with regular culture processes. The supernatants of conditioned medium for heat-shock SGs were collected, filtered through a 0.22-µm diameter filter to eliminate potential bacteria, and stored at −80°C. The induction medium-mix consists of 80% basic SG medium and 20% supernatants of conditioned heat-shocked SG medium. Additionally, induction medium-KGF medium was prepared by adding rhKGF (10–100 ng/ml) into basic SG medium. One pilot experiment indicated that the optimal concentration of rhKGF in the induction medium-KGF was 40 ng/ml, so we chose this concentration of rhKGF for subsequent experiments.
Inducing hUC-MSC Differentiation to SGCs
To induce hUC-MSC differentiation to SGCs, hUC-MSCs were cultured in 2 types of inducing media, induction medium-mix and induction medium-KGF, for 3 weeks as described previously [12]. The differentiated SGCs were then used for various analyses in this study.
Human SGs Isolated From Normal Skin Tissues
Approximately 0.5–1 cm2 of normal skin was collected from 6 healthy donors with their signed consent after clinical surgery. After removing the fat and blood on the skin, the skin was rinsed three times with PBS. The skin tissues were minced into 0.5- to 1.0-mm3 fragments and digested with type II collagenase (2 mg/ml; Sigma-Aldrich) at 37°C for 4–6 hours. When SGs were released from skin tissues, they were collected with a fine needle and transferred to culture plates containing basic SG medium containing DMEM supplemented with 10% FBS (Gibco/Thermo Fisher Scientific), 100 U/ml penicillin (Sigma-Aldrich), 100 mg/ml streptomycin (Sigma-Aldrich), 2 mM l-glutamine (Sigma-Aldrich), insulin-transferrin-sodium selenite solution (1 ml/100 ml; Sigma-Aldrich), 2 nM/ml triiodothyronine (T3; Sigma-Aldrich), 0.4 mg/ml hemisuccinate hydrocortisone (Sigma-Aldrich), and 10 ng/ml human recombinant EGF (Invitrogen/Thermo Fisher Scientific) [10, 12]. The SGs from skin tissues were cultured for approximately 1–2 weeks, and the medium was changed every 2–3 days. The SGs were maintained at a density of 1 × 104 cells/cm2 as positive controls in this study [10, 12].
Characterization of hUC-MSCs
Flow cytometry analysis was used to identify phenotypes of hUC-MSCs. hUC-MSCs at their third to fifth passage were trypsinized and collected from culture dishes; 4.5 × 105 cells were labeled with the following biomarkers: fluorescein isothiocyanate (Sigma-Aldrich)-conjugated anti-CD29, anti-CD34, anti-CD45, and phycoerythrin (BD-Pharmingen, Franklin Lakes, NJ, http://www.bdbiosciences.com)-conjugated (anti-90, anti-105) antibodies, as well as antibodies including anti-Oct-4 (Abcam, San Francisco, CA, http://www.abcam.com). All primary antibodies were diluted at a ratio of 1:50–100. As a negative control, mouse isotype-matched antibodies (Sigma-Aldrich) were used. Experiments were performed with a FAC Scan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, http://www.bd.com) [12].
Multipotent Differentiation of hUC-MSCs
For osteogenic differentiation, cells were incubated in osteogenic medium (DMEM supplemented with 10% FBS [Hyclone; GE Healthcare Life Sciences, Atlanta, GA, http://www.gelifesciences.com], 0.1 µM dexamethasone [Sigma-Aldrich], 0.5 mM ascorbic acid 2-phosphate [Sigma-Aldrich], and 10 mM b-glycerophosphate [Sigma-Aldrich]). After 21 days of induction, cells displayed calcium-like aggregates of matrix mineralization that were examined for calcium [12]. For adipogenesis [16], cells were cultured in the specific induction medium (DMEM supplemented with 10% FBS, 50 mg/ml ascorbate 1-phosphate, 0.1 µM dexamethasone, and 50 mg/ml indomethacin [Sigma-Aldrich]). Adipogenic differentiation was analyzed for the formation of the lipid droplet, which was detected by Oil Red O staining (Sigma-Aldrich) after induction had been ongoing for 3 weeks [12]. For chondrogenic differentiation, cells were cultured in induction medium (alpha modification of Eagle’s medium supplemented with 3.5 g/ml glucose [Sigma-Aldrich], 1% vol/vol ITS Plus [Sigma-Aldrich], 2 mM l-glutamine [Invitrogen/Thermo Fisher Scientific], 100 g/ml sodium pyruvate [Invitrogen/Thermo Fisher Scientific], 0.2 mM ascorbic acid 2-phosphate [Sigma-Aldrich], 0.1 µM dexamethasone [Sigma-Aldrich], and 10 ng/ml transforming growth factor-β3 [R&D Systems, Minneapolis, MN, https://www.rndsystems.com]) for 3 weeks. The expression of chondrogenic lineage was analyzed by expression of antigen anti-collagen type II (Sigma-Aldrich) [12].
Enzyme-Linked Immunosorbent Assay
The media from mature SGs, uninduced hUC-MSCs, and transdifferentiated SGC cultures were collected from all subcultures every 3 days and for final culture in this study. The concentrations of EGF and KGF in the conditioned media from cell cultures were determined using enzyme-linked immunosorbent assay (ELISA) with an EGF kit (Abcam) and KGF kit (R&D Systems), according to manufacturer’s instructions. The absorbance was measured at 450 nm.
Cell Proliferation Assay
The proliferation potency of hUC-MSCs cultured under different types of media was tested by the Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich), according to the manufacturer’s instructions. In brief, the hUC-MSCs that had been passaged 3–5 times were prepared, and the cell suspension (100 µl) was subcultured in a 96-well plate at a density of 5,000 cells per well and cultured for 24 hours. Then, 10 μl of CCK-8 solution was added to each well and the plate was incubated for 1–4 hours at 37°C with 5% CO2. Cell proliferation was assessed by measuring absorbance at 450 nm using a microplate reader (Spectra MR; Dynex Technologies, Chantilly, VA, http://www.dynextechnologies.com).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde and stained with the following SG markers: primary mouse polyclonal anti-carcinoembryonic antigen (CEA), anti-CK14 [15, 34], and rabbit polyclonal anti-CK19 at 1:500 dilutions (Abcam) [12, 35]. The cells were washed with PBS and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse or goat anti-rabbit antibodies (Santa Cruz Biotechnology, Dallas, TX, http://www.scbt.com) for 4 hours at room temperature. Cells were washed 3 times with PBS and then stained with 3,3′-diaminobenzidine (DAB) according to the manufacturer’s instructions.
Polymerase Chain Reaction Assay
To examine expression of KGF, EDA, and EGF, total RNA was isolated from mature SGs, differentiated SGCs, and hUC-MSCs (TaKaRa Bio, Shiga, Japan, http://www.clontech.com/takara) [12]. Total RNA was used for reverse transcription, and transcript levels were determined using reverse-transcription polymerase chain reaction with the following primers: KGF: sense 5′-CTTGGTGTCTTCCGTCCC-3′, antisense 5′- GGCAACAACTC CGATTTCTACTG-3′; EDA: sense 5′-GACAGTCCGCAGTTGTAGCAG-3′, antisense 5′-GGATGGGTGAAAGAACATAAAGG-3′; EGF: sense 5′-TTTGGG AGTTGATGACCTTTG-3′, antisense 5′-CGGAACTTTGGGCGACTATCT-3′; β-actin: sense 5′-CACACTGTG CCCATCTACGA-3′, antisense 5′-TACAGGT CTTTGC GG ATGTC-3′.
Western Blot Analyses
hUC-MSCs, natural SGs, and differentiated SGCs were collected and total proteins were extracted from the cells with ice-cold radioimmunoprecipitation assay buffer containing phenylmethylsulfonyl fluoride (Sigma-Aldrich). The samples were centrifuged at 12,000g for 10 minutes. The supernatant was collected for further analysis. Protein concentrations were measured using the micro-Bradford method (Bio-Rad, Hercules, CA, http://www.bio-rad.com). Each sample (40 µg per lane) was denatured and separated using a 12% Tris-glycine acrylamide gel (Invitrogen/Thermo Fisher Scientific), and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, http://www.emdmillipore.com), and then the blotting membrane was incubated with primary antibody against KGF, EDA, or EGF (Abcam). The membrane was incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit antibodies (Santa Cruz Biotechnology). The immune-reactive bands were then visualized using an enhanced chemiluminescence kit (Amersham/GE Healthcare Life Sciences, Little Chalfont, U.K., http://www.gelifesciences.com) [12].
SCID Mouse Burn Model and SGC Treatment for Injured Paws
The burn injury model was performed by putting a mouse paw on a heated steel pad for 5 seconds at 65°C and then dipping the paw into ice water for 3 seconds to deprive retained heat. For the SGC-treated group, burned paws were transplanted with SGCs differentiated from hUC-MSCs at a concentration of 1 × 105 per 0.2 ml per paw, while in the control (untreated) group, the paw was treated with 0.2 ml of PBS. After the burn wounds were healed, mouse paws were collected for immunohistochemical analysis [10, 36, 37].
Histological Analysis
After collection, paw tissues were fixed in formalin, decalcified in nitrate, and embedded in paraffin. Sections of approximately 5 μm from 6 mice from each group were stained with primary antibodies of SG markers. The slides were washed with PBS and incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit antibodies for 4 hours at room temperature. The slides were washed with PBS and stained with DAB. The histopathology of paw injury was examined blindly by two experienced investigators [38].
Statistical Analysis
The data were analyzed for statistical significance using SPSS version 12.0 software (IBM, Armonk, NY, http://www.ibm.com). Data are presented as mean values. Statistical analyses were performed by a Student unpaired t test or one-way analysis of variance, with p < .05 considered to be statistically significant.
Results
Characteristics of hUC-MSCs
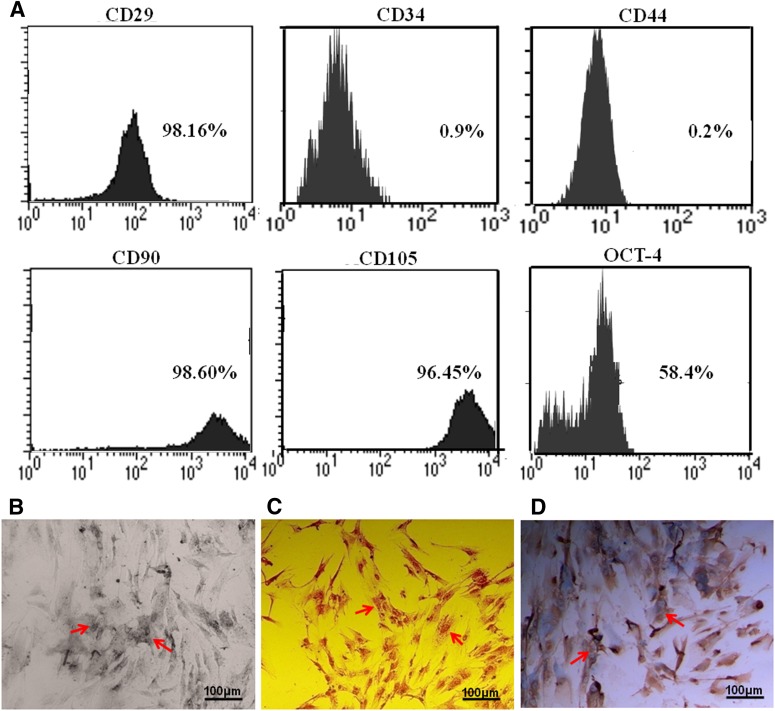
hUC-MSCs were isolated from human umbilical cord Wharton’s jelly through the digestion of type II collagenase and then cultured for 3–5 passages as described in the Materials and Methods. To characterize phenotypes of the hUC-MSCs, we examined expression of several cell markers by flow cytometry analysis. The results showed that the hUC-MSCs were positive for the mesenchymal markers CD29, CD90, and CD105, and the embryonic marker OCT-4, but were negative for the hematological markers CD34 and CD44 (Fig. 1A), suggesting that the hUC-MSCs prepared in this study were an appropriate MSC population for further experiments. Furthermore, we examined the differentiation capacity of the hUC-MSCs, using several standard MSC differentiation protocols. After multipotent induction for 21 days, hUC-MSCs could differentiate to osteogenic, adipogenic, or chondrogenic cells (Fig. 1B). These indicated hUC-MSCs used in this study possessed the differentiation capacity to form functional cells.
Figure 1.
Characteristics of mesenchymal stem cells derived from human umbilical cord Wharton’s jelly (hUC-MSCs). hUC-MSCs isolated from human umbilical cords were cultured for three to five passages. The expressions of MSC biomarkers (CD29, CD90, CD105), an embryonic marker (OCT-4), and hematological markers (CD34, CD44) were examined by flow cytometry analysis. (A): hUC-MSCs were positive for all three MSC biomarkers and the embryonic marker, but negative for hematological markers. (B–D): Multipotent differentiation capacity of hUC-MSCs. hUC-MSCs were cultured in osteogenic, adipogenic, or chondrogenic induction medium for 3 weeks. The differentiated cells were positive by specific methods: alkaline phosphatase for osteogenic cells (B), Oil Red O for adipogenic cells (C), and collagen type II for chondrogenic cells (D). Scale bars = 100 μm.
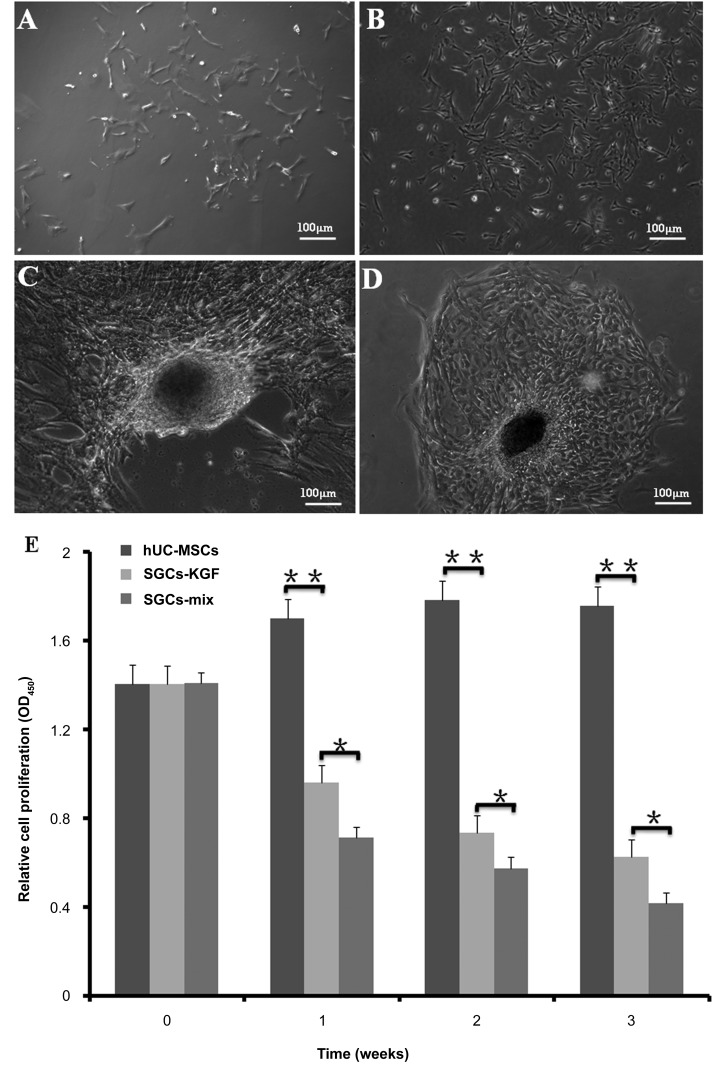
Outgrowth and Morphology of hUC-MSCs and Induced Differentiation to SGCs
hUC-MSCs adhered to plastic plates and exhibited good proliferation potential after three to five subcultures (Fig. 2A, 2B). When hUC-MSCs were cultured in SG-inducing media (i.e., induction medium-mix or induction medium-KGF for 3 weeks), SGCs lost the appearance of primary hUC-MSCs but formed a hypertrophic appearance. The differentiated SGCs from hUC-MSCs had a gathering-growth capacity and formed a “paving stones” structure as natural SGs (Fig. 2C, 2D).
Figure 2.
Morphology and proliferation potency of hUC-MSCs, differentiated SGCs, and natural sweat gland cells. (A): Primary hUC-MSCs exhibit fibroblast-like morphology. (B): The hUC-MSC cells after three to five passages in culture showed spindle-like shapes. (C, D): The differentiated SGCs from hUC-MSCs after 3 weeks in culture in SGC-induction media formed a “gathering” structure, like natural “paving stone” sweat glands. (E): Cell proliferation potencies of hUC-MSCs in three types of media were analyzed using cell counting kit-8. hUC-MSCs were cultured in basic medium, induction medium-KGF, and induction medium-mix for 1–3 weeks. The results showed lower proliferation rates in the induction medium-KGF and induction medium-mix compared with hUC-MSC basic medium. n = 5 per group. Scale bars = 100 μm. ∗, p < .05; ∗∗, p < .01. Abbreviations: hUC-MSC, mesenchymal stem cell derived from human umbilical cord Wharton’s jelly; KGF, keratinocyte growth factor; SGC, sweat gland-like cell.
We examined hUC-MSC proliferation potency in two types of inducing media (induction medium-KGF and induction medium-mix) and basic hUC-MSC medium (control) using CCK-8 analysis. Cells showed decreased proliferation in the induction medium-KGF and induction medium-mix, compared with basic hUC-MSC medium (p < .01) (Fig. 2E).
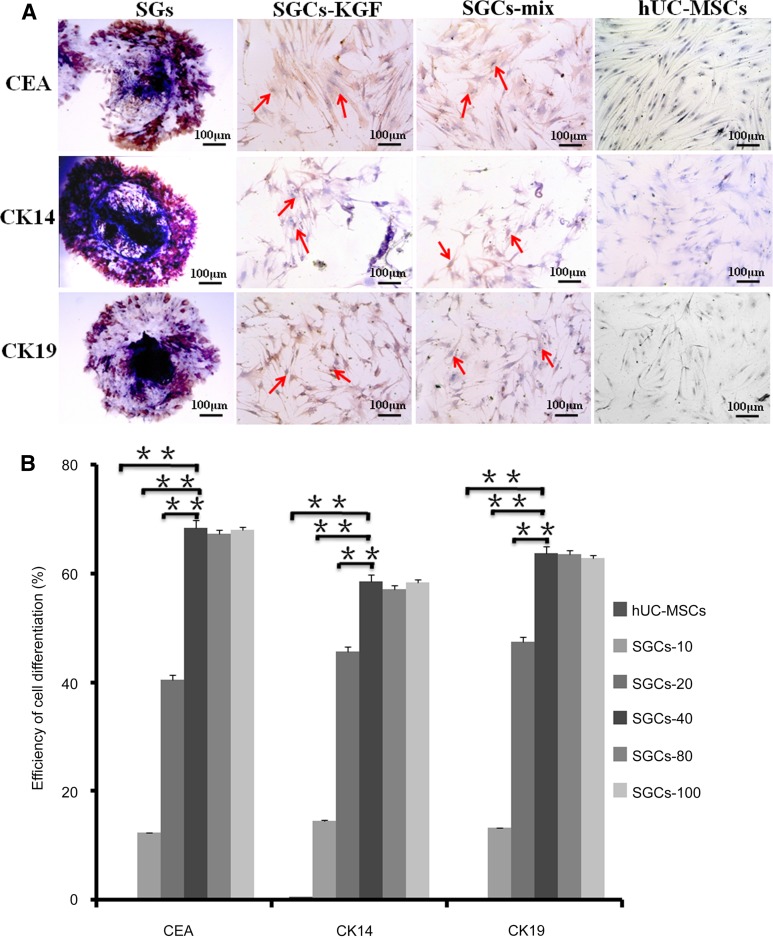
Phenotypes of Natural SGs, Differentiated SGCs, and hUC-MSCs
In order to evaluate the differentiation efficiency of hUC-MSC, natural SGs, differentiated SGCs (SGCs-KGF and SGCs-MIX) and hUC-MSCs (control) were analyzed by immunocytochemical analysis with SG-biomarkers CEA, CK14, CK19, respectively. As expected, natural SGs strongly expressed three SG markers (CEA, CK14, CK29); SGCs in the induction medium-KGF (SGC-KGF) and those in induction medium-mix (SGC-mix) were positive for all three SG biomarkers. Undifferentiated hUC-MSCs, however, were negative for them (Fig. 3A). We also determined the efficiency of hUC-MSC differentiation in the induction medium-KGF with a range of concentrations (10–100 ng/ml) of rhKGF. Approximately 60% of the cells were positive for SG biomarkers when the concentration of rKGF was at 40 ng/ml or higher. Therefore, the optimal concentration of rhKGF was 40 ng/ml to induce hUC-MSC differentiation (Fig. 3B).
Figure 3.
Phenotypes of natural sweat glands (SGs), differentiated SGCs, and hUC-MSCs. Three SG biomarkers—CEA, CK14, and CK19—were examined in natural SGs (positive control), differentiated SGCs (SGCs-KGF and SGCs-mix) and hUC-MSCs (negative control), using immunocytochemical analysis. Natural SGs highly expressed all three biomarkers. Differentiated SGCs (SGCs-KGF differentiated in the induction medium-KGF and SGCs-mix differentiated in the induction medium-mix) were positive for all three SG biomarkers. (A): As expected, hUC-MSCs were negative for all three SG biomarkers. To evaluate the efficiency of cell differentiation, hUC-MScs were cultured for 3 weeks in the induction medium-KGF with a range of recombinant human KGF (rhKGF) concentrations (0, 10, 20, 40, 80, 100 ng/ml) and were examined by flow cytometry analysis with the 3 SG biomarkers. Undifferentiated hUC-MSCs were used as negative control. (B): The results indicated induction medium-KGF containing a concentration of 40 ng/ml of rhKGF or higher could induce approximately 60% hUC-MSC differentiation to SGCs. Differentiation efficiencies in the induction medium-KGF containing a concentration of 40 ng/ml rhKGF or higher were significantly higher than other groups (∗∗, p < .01). Therefore, the optimal concentration of rhKGF in the induction medium-KGF was 40 ng/ml of rhKGF for hUC-MSC differentiation. n = 3 per group. Scale bars = 100 μm. Abbreviations: CEA, carcinoembryonic antigen; hUC-MSC, mesenchymal stem cell derived from human umbilical cord Wharton’s jelly; KGF, keratinocyte growth factor; SGC, sweat gland-like cell; SGCs-10 (20, 40, 80, 100), sweat gland-like cells, 10 ng/ml.
Analyses of KGF and EGF Concentrations in Medium
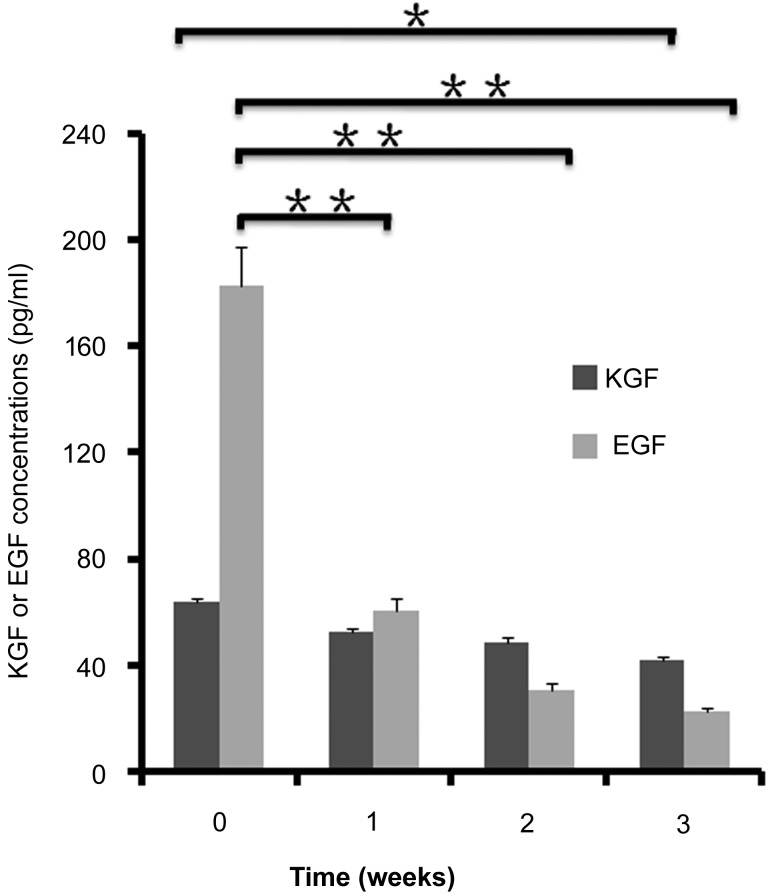
The levels of KGF and EGF were determined by ELISA. The levels of KGF and EGF in the induction medium-mix were 63.5 ng/ml and 182.6 ng/ml, respectively, indicating that KGF and EGF in the medium came from the 20% sterile supernatants from conditioned heat-shock SG medium. The levels of KGF and EGF decreased in the medium after 1–2 weeks in cell culture (Fig. 4).
Figure 4.
Determination of KGF or EGF levels in the media by enzyme-linked immunosorbent assay (ELISA). The levels of KGF and EGF were determined in the fresh induction medium-mix and conditioned media collected from differentiation cultures of mesenchymal stem cells derived from human umbilical cord Wharton’s jelly for 1–3 weeks by ELISA. The results showed that the levels of KGF and EGF in the fresh induction medium-mix were 63.5 ng/ml and 182.6 ng/ml, respectively. The EGF levels decreased significantly in the conditioned media after 1–3 weeks in culture, but the levels of KGF did not change significantly. n = 3 per group. Abbreviations: EGF, epidermal growth factor; KGF, keratinocyte growth factor.
Expression of SG Development-Related Genes
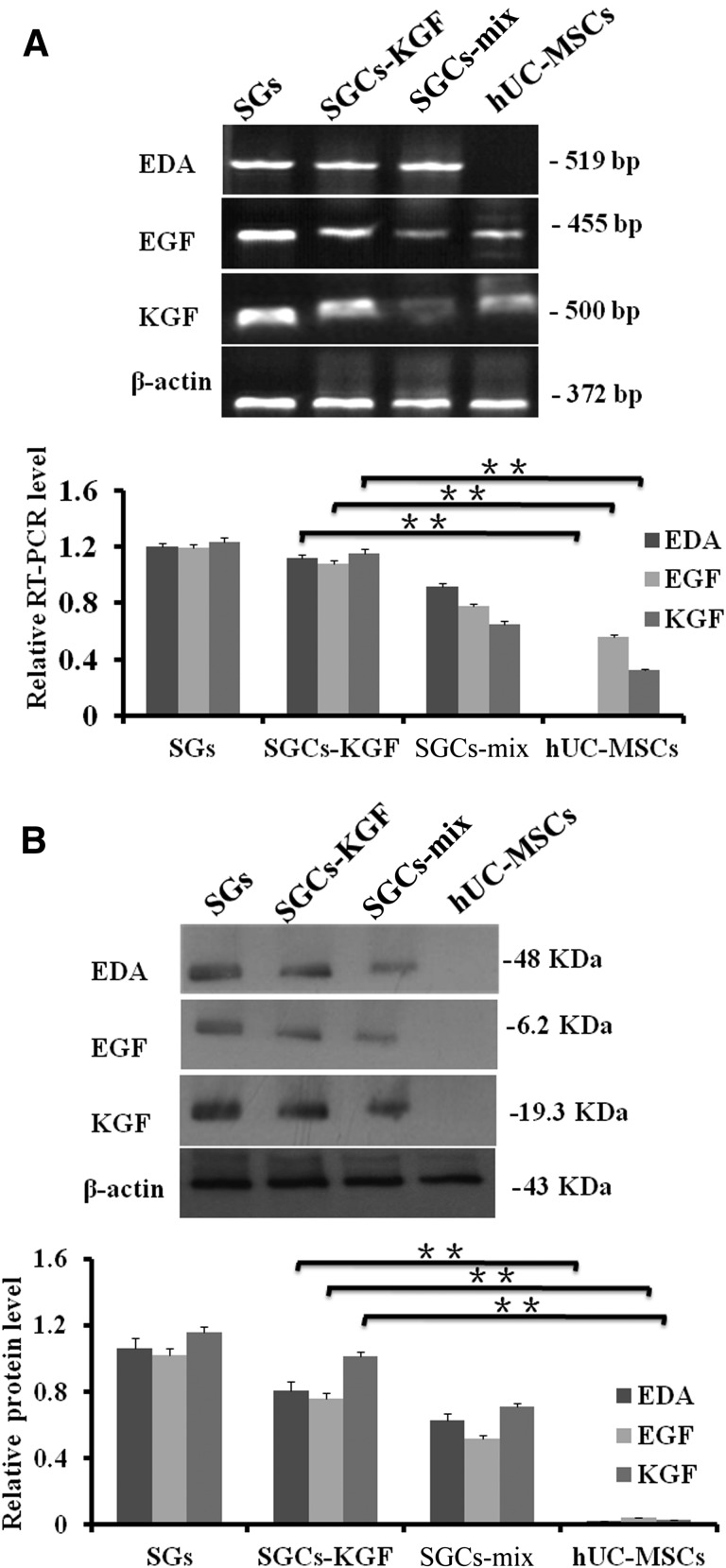
The expression of SG development-related genes, including KGF, EGF, and EDA were examined in natural SGs (positive control), differentiated SGCs (SGCs-KGF and SGCs-MIX), hUC-MSCs (negative control). The results showed that KGF, EDA, and EGF mRNA and proteins could be expressed in differentiated SGCs (SGCs-KGF and SGCs-MIX) (Fig. 5A). As expected, natural SGs strongly expressed KGF, EDA, and EGF mRNA and proteins. However, hUC-MSCs did not express any proteins of the three SG development-related genes (Fig. 5B).
Figure 5.
The expression of SG development-related genes (i.e., EDA, EGF, and KGF) were examined in natural SGs, differentiated SGCs (SGCs-mix and SGCs-KGF), and hUC-MSCs using RT-PCR and Western blotting analysis. (A): mRNA levels of EDA, EGF, and KGF expression. Differentiated SGCs (SGCs-KGF and SGCs-mix) from hUC-MSCs expressed EDA, EGF, and KGF mRNA, but hUC-MSCs expressed lower levels of mRNA of EGF and KGF. The expression of β-actin was used as a control. (B): Protein expressions of EDA, EGF, and KGF. Natural SGs and differentiated SGCs (SGCs-KGF and SGCs-mix) from hUC-MSCs expressed proteins of EDA, EGF, and KGF but did not in hUC-MSCs in Western blot analysis. Abbreviations: bp, base pair; hUC-MSC, mesenchymal stem cell derived from human umbilical cord Wharton’s jelly; KGF, keratinocyte growth factor; RT-PCR, reverse-transcriptase polymerase chain reaction; SG, sweat gland; SGC, SGC, sweat gland-like cell.
SG-Injured Mouse Model and the Outcome After Treatment With SGCs
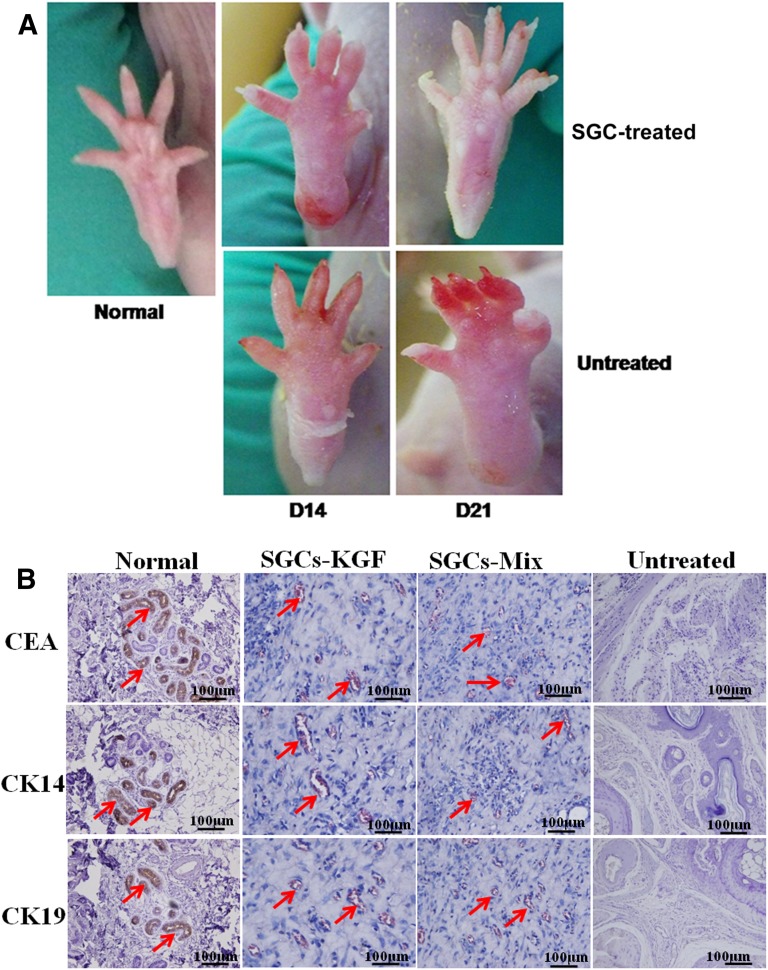
We used a SG-injured mouse model to examine the effect of SGCs on repair and regeneration of injured tissue (Fig. 6). The injured paw skins were obviously swollen with some tiny bubbles, compared with normal paw skin. At 14 days after treatment with SGCs, SGC-treated paws showed reduced swollen tissues compared with untreated paws. At 21 days after treatment with SGCs, the SGC-treated paws could re-establish the destroyed SG structure, unlike untreated paws (Fig. 7A).
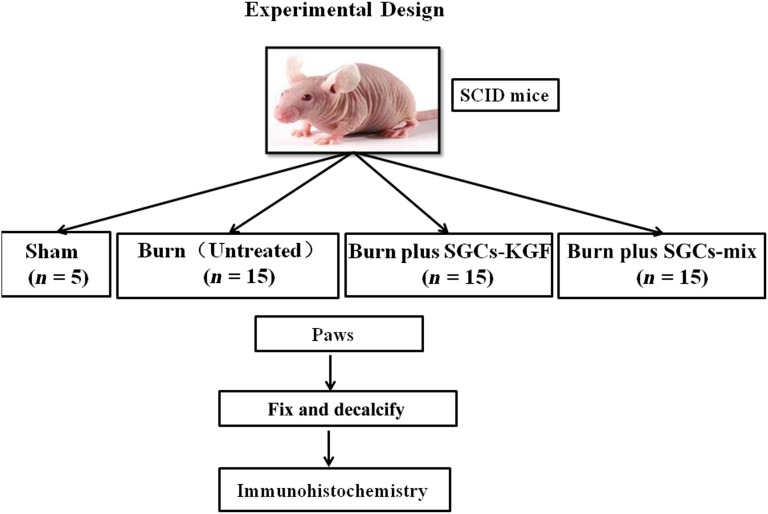
Figure 6.
A diagram of a mouse burn model. SCID mice were used for the in vivo animal burn model. SCID mice were randomly distributed to 4 groups: sham (n = 5), burned mice without SGC treatment (untreated; n = 15), burned mice treated with SGCs-KGF (n = 15), burned mice treated with SGCs-mix (n = 15). All mice were monitored for burn wound healing for 21 days, after which the animals were sacrificed, and the tissues of the paws were harvested and fixed for immunohistochemical analysis. Abbreviations: SGCs-KGF, sweat gland-like cells differentiated from induction medium-KGF; SGCs-mix, sweat gland-like cells differentiated from induction medium-mix; SCID, severe combined immunodeficiency.
Figure 7.
Effect of SGCs treatment on burned paws of a severe combined immunodeficiency (SCID) mouse model. The paws of SCID mice were injured by thermal hazards and then treated with SGCs differentiated from mesenchymal stem cells derived from human umbilical cord Wharton’s jelly at a concentration of 1 × 105 cells per 0.2 ml per paw or 0.2 ml of PBS (control). At 14 days after treatment, the paw skin in the control group was seriously peeled away from tissues, but not in the SGC-treated group. The paw skin in the SGC-treated group could accelerate the velocity of healing compared with untreated group. At 21 days after treatment, the SGC-treated group had regenerated new skin in the paws, whereas the untreated control had not. The sweat gland (SG) formation was examined by immunohistochemical (IHC) analysis with the SG markers carcinoembryonic antigen (CEA), CK14, and CK19. The results showed that the SGC-treated group (SGC-KGF, SGC-mix) formed SG-like structures with positive staining for the three SG markers, like natural paw shin. In the untreated group, no SG regeneration was observed and IHC staining results were negative with all three SG markers. Scale bars =100 μm. Abbreviation: SGC, sweat gland-like cell.
We also examined SG-like formation in the paw-injured mouse model by histological analysis. By 21 days, the skin tissues of the paws treated with SGCs differentiated from both induction medium-KGF and induction medium-mix formed sparse SG structures with the expression of SG biomarkers CEA, CK14, and CK19 (Fig. 7). As expected, the skin tissues of the untreated paws showed damaged structures without the expression of SG biomarkers (Fig. 7B).
Discussion
The repair and regeneration of cutaneous structure and function after severe burn injury are tremendous challenges for clinical therapy. Major objectives are simultaneously to complete acceleration of wound closure, replenish sweating function, and regain cosmetic appearance of regenerated skin [17, 39]. Although enhancing efficacy of burn wound closure could be achieved through transplantation of stem cells, skin substitutes, and application of growth factors, one critical problem is the difficulty of reestablishing the functional structure of severely burned skin (such as sweating) [39]. Stem cell therapy has emerged as a new tool to improve the therapeutic quality of burn wounds, and may not only repair destroyed skin structure and injured sweat glands but also allow return of sweating function [12, 13].
With good proliferation and differentiation potency and lower immune resistance, hUC-MSCs are considered to be an alternative source of stem cells for cutaneous regeneration in patients with severe burn wounds [21, 23, 40]. This study has provided direct evidence that hUC-MSCs could transdifferentiate into SGCs after being cultured in induction medium-mix and induction medium-KGF. Previous studies have shown that SG development needed several growth factors/receptors like KGF and its receptor (FGFR2). The results from this and previous studies demonstrate that basic SG medium with KGF could induce hUC-MSC differentiation to SGCs [12]. Furthermore, a remarkable level of KGF expression in the conditioned heat-shock SG medium as well as differentiated SGCs was observed in this study, which is consistent with previous works [25, 32, 41].
The phenotypes of differentiated SGCs from hUC-MSCs in the induction medium-mix and induction medium-KGF are similar to natural SGs, suggesting that rhKGF plays a critical role in SGC differentiation. However, the detailed mechanism of how KGF induces SGC differentiation is unknown. The findings and the protocol in this study may be a useful tool for basic research on SGC differentiation from hUC-MSCs and, potentially, clinical application. It is especially convenient for studying the role of KGF or other growth factors for SGC differentiation in a chemically defined medium like the induction medium-KGF. Additionally, the induction medium-KGF may become a commercial product for application of SGC differentiation.
Compared with SGC differentiation from BM-MSCs, SGCs from hUC-MSCs showed a higher transdifferentiation efficiency [10]. To our knowledge, SGCs could be induced from BM-MSC differentiation through coculturing with heat-shocked SGs or transfecting with the EDA gene; the differentiated SGCs from BM-MSCs were transplanted in the patients who had damaged SGs [10, 13, 42]. BM-MSC therapy in severe burn patients has to use patients’ own MSCs for autograft transplantation [10, 13, 42]. One major disadvantage of the therapy is that the operation to collect BM-MSCs may cause additional complications and minor injuries to patients or donors. Although tissue-engineered skin provides an effective method for patients with severe burns to regenerate SGs, it also needs to obtain functional BM-MSCs or natural SG cells from patients [43, 44]. Although hair-follicle stem cells possess the differentiation potential of epithelial cells [45] and eccrine SGs can repair and regenerate injured skin [46], hair-follicle stem cells and eccrine SGs are easily destroyed by severe burn injury, thus losing their reconstructive potential for injured skin. Therefore, differentiated SGCs from hUC-MSCs may provide an alternative therapeutic method for SG repair and regeneration because of their good proliferation, higher differentiation efficiency, and lower immune resistance, and tissue sources are plentiful.
The SGCs differentiated from hUC-MSCs in two types of inducing media could repair and regenerate damaged sweat glands in the SCID mouse burn model in this study. SCID mice are used in the regeneration of injured SGs because they lack an immune response to xenografts. For in vivo transplantation application, hUC-MSCs have been found with consistently lower human leukocyte antigen-DR (HLA-DR) during the differentiation process [47, 48]. Because we did not examine the expression of HLA-DR in the SGCs differentiated from hUC-MSCs, we chose to use SCID mice to examine the effect of differentiated SGCs in this study. We have observed significantly reduced inflammation in the injured paws in the SGC-treated mice compared with non-SGC-treated mice. Inflammation is a very important step in the wound healing process and tissue repair and regeneration. An overactive inflammatory response induced tissue damage and influenced formation of such functional structures as sweat gland.
Although we observed formation of new sweat gland-like structures after treatment with differentiated SGCs from hUC-MSCs (but not in the control) in this in vivo model, it is unknown whether SGCs from hUC-MSCs directly or indirectly contributed to the SG formation in vivo. It will be determined using human-specific biomarker(s) in our further study. We will focus on the translational application of differentiated SGCs from hUC-MSCs for repair/regeneration of injury SGs and skins in the future to evaluate the changes of immune characteristics between SGCs and hUC-MSCs and to improve the survival of transplanted SGCs in situ, as well as to test the application of human SGC transplantation as a preclinical study in different animal models like the red Duroc pig or monkey burn models.
In completing the safety and efficiency study in animal models, we will perform one pilot clinical study to repair destroyed SGs with the differentiated SGCs in a few burned patients. Based on previous studies of SGC transplantation, SGCs in situ to the injured skin might be influenced by a small fraction of cell rejection. It is unknown whether, because of their lower immune resistance potential, the differentiated SGCs from hUC-MSCs could overcome this problem. Therefore, differentiated SGCs from hUC-MSCs with this protocol may highlight a potential new application for repair and regeneration of severely injured SGs and skin.
Conclusion
We have established a novel and useful SGC-differentiation method from hUC-MSCs and we found that KGF is an essential factor in SGC differentiation. SGCs differentiated from hUC-MSCs may be a useful tool to repair and regenerate destroyed sweat glands in the skin.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China (Grants 81201478, 81372079, and 81571916), the Specialized Research Fund of Doctoral Program of Higher Education of China (Grant 20120101120035), Medicine and Health Projects of Science and Technology of Zhejiang Province of China (Grants 2013KYA098 and 2011R50018-18), and NIH Grant HL096007.
Author Contributions
Y.X.: conception and design, collection and/or assembly of data, manuscript writing, final approval of manuscript; Y.H., M.X., K.M., and X.F.: conception and design, data analysis and interpretation, final approval of manuscript; M.Z.: conception and design, manuscript writing, final approval of manuscript; G.W.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Cheshire WP, Freeman R. Disorders of sweating. Semin Neurol. 2003;23:399–406. doi: 10.1055/s-2004-817724. [DOI] [PubMed] [Google Scholar]
- 2.Wilke K, Martin A, Terstegen L, et al. A short history of sweat gland biology. Int J Cosmet Sci. 2007;29:169–179. doi: 10.1111/j.1467-2494.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez R. [Chemical and electrical burns] Rev Prat. 2002;52:2234–2239. [PubMed] [Google Scholar]
- 4.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedrick MH, Daniels EJ. The use of adult stem cells in regenerative medicine. Clin Plast Surg. 2003;30:499–505. doi: 10.1016/s0094-1298(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 6.Oshima H, Inoue H, Matsuzaki K, et al. Permanent restoration of human skin treated with cultured epithelium grafting--wound healing by stem cell based tissue engineering. Hum Cell. 2002;15:118–128. doi: 10.1111/j.1749-0774.2002.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 7.Snyder RJ, Doyle H, Delbridge T. Applying split-thickness skin grafts: A step-by-step clinical guide and nursing implications. Ostomy Wound Manage. 2001;47:20–26. [PubMed] [Google Scholar]
- 8.Weissman IL. Translating stem and progenitor cell biology to the clinic: Barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 9.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 10.Sheng Z, Fu X, Cai S, et al. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009;17:427–435. doi: 10.1111/j.1524-475X.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 11.Fu X, Han B, Cai S, et al. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen. 2009;17:185–191. doi: 10.1111/j.1524-475X.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Huang S, Ma K, et al. Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton’s jelly: Sweat gland cell-like differentiative capacity. J Tissue Eng Regen Med. 2012;6:645–654. doi: 10.1002/term.468. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Huang S, Fu X. Autologous transplantation of bone marrow-derived mesenchymal stem cells: A promising therapeutic strategy for prevention of skin-graft contraction. Clin Exp Dermatol. 2012;37:497–500. doi: 10.1111/j.1365-2230.2011.04260.x. [DOI] [PubMed] [Google Scholar]
- 14.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 15.Braiman-Wiksman L, Solomonik I, Spira R, et al. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35:767–779. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- 16.Chung JK, Park TK, Ohn YH, et al. Modulation of retinal wound healing by systemically administered bone marrow-derived mesenchymal stem cells. Korean J Ophthalmol. 2011;25:268–274. doi: 10.3341/kjo.2011.25.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu X, Fang L, Li X, et al. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325–335. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Guo WY, Wang GJ, Wang P, et al. Acceleration of diabetic wound healing by low-dose radiation is associated with peripheral mobilization of bone marrow stem cells. Radiat Res. 2010;174:467–479. doi: 10.1667/RR1980.1. [DOI] [PubMed] [Google Scholar]
- 20.Badraiq H, Devito L, Ilic D. Isolation and expansion of mesenchymal stromal/stem cells from umbilical cord under chemically defined conditions. Methods Mol Biol. 2015;1283:65–71. doi: 10.1007/7651_2014_116. [DOI] [PubMed] [Google Scholar]
- 21.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 22.Batsali AK, Kastrinaki MC, Papadaki HA, et al. Mesenchymal stem cells derived from Wharton’s jelly of the umbilical cord: Biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8:144–155. doi: 10.2174/1574888x11308020005. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Wang D, Du WT, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Childs AJ, Saunders PT, Anderson RA. Modelling germ cell development in vitro. Mol Hum Reprod. 2008;14:501–511. doi: 10.1093/molehr/gan042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner S. Keratinocyte growth factor: A unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 26.Werner M, Hatt H, Gottmann K. Afferent innervation influences HVA Ca2+ current expression in cultured neocortical neurones. Neuroreport. 1998;9:1255–1260. doi: 10.1097/00001756-199804200-00054. [DOI] [PubMed] [Google Scholar]
- 27.Kunisada M, Cui CY, Piao Y, et al. Requirement for Shh and Fox family genes at different stages in sweat gland development. Hum Mol Genet. 2009;18:1769–1778. doi: 10.1093/hmg/ddp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Kuramoto T, Yokoe M, Hashimoto R, et al. A rat model of hypohidrotic ectodermal dysplasia carries a missense mutation in the Edaradd gene. BMC Genet. 2011;12:91. doi: 10.1186/1471-2156-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heng BC, Cao T, Liu H, et al. Directing stem cells into the keratinocyte lineage in vitro. Exp Dermatol. 2005;14:1–16. doi: 10.1111/j.0906-6705.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 31.Ornitz DM, Xu J, Colvin JS, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 32.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 33.Werner S, Peters KG, Longaker MT, et al. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA. 1992;89:6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun TT, Shih C, Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci USA. 1979;76:2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DK, Bubier JA, Silva KA, et al. Development, structure, and keratin expression in C57BL/6J mouse eccrine glands. Vet Pathol. 2012;49:146–154. doi: 10.1177/0300985811430511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard WJ, Choudhry M, Schwacha MG, et al. Cecal ligation and puncture. Shock. 2005;24(suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 37.Tang Z, Ni L, Javidiparsijani S, et al. Enhanced liver autophagic activity improves survival of septic mice lacking surfactant proteins A and D. Tohoku J Exp Med. 2013;231:127–138. doi: 10.1620/tjem.231.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurman JM, Lucia MS, Ljubanovic D, et al. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67:524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 39.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 40.Rebelatto CK, Aguiar AM, Moretão MP, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 41.Yun YR, Won JE, Jeon E, et al. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai S, Pan Y, Han B, et al. Transplantation of human bone marrow-derived mesenchymal stem cells transfected with ectodysplasin for regeneration of sweat glands. Chin Med J (Engl) 2011;124:2260–2268. [PubMed] [Google Scholar]
- 43.Huang S, Lu G, Wu Y, et al. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci. 2012;66:29–36. doi: 10.1016/j.jdermsci.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Xu Y, Wu C, et al. In vitro constitution and in vivo implantation of engineered skin constructs with sweat glands. Biomaterials. 2010;31:5520–5525. doi: 10.1016/j.biomaterials.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Liu ZY, Zhao Q, et al. Future application of hair follicle stem cells: Capable in differentiation into sweat gland cells. Chin Med J (Engl) 2013;126:3545–3552. [PubMed] [Google Scholar]
- 46.Biedermann T, Pontiggia L, Böttcher-Haberzeth S, et al. Human eccrine sweat gland cells can reconstitute a stratified epidermis. J Invest Dermatol. 2010;130:1996–2009. doi: 10.1038/jid.2010.83. [DOI] [PubMed] [Google Scholar]
- 47.Yan M, Sun M, Zhou Y, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopamine neurons mediated by the Lmx1a and neurturin in vitro: Potential therapeutic application for Parkinson’s disease in a rhesus monkey model. PLoS One. 2013;8:e64000. doi: 10.1371/journal.pone.0064000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Chen G, Yue A, Ruan Z, et al. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One. 2014;9:e98565. doi: 10.1371/journal.pone.0098565. [DOI] [PMC free article] [PubMed] [Google Scholar]