Abstract
Increasing atmospheric carbon dioxide (CO2) impacts plant growth and metabolism. Indirectly, the performance and feeding of insects is affected by plant nutritional quality and resistance traits. Life history and feeding behaviour of Myzus persicae were studied on pepper plants under ambient (aCO2, 400 ppm) or elevated CO2 (eCO2, 650 ppm), as well as the direct impact on plant growth and leaf chemistry. Plant parameters were significantly altered by eCO2 with a negative impact on aphid’s life history. Their pre-reproductive period was 11% longer and fecundity decreased by 37%. Peppers fixed significantly less nitrogen, which explains the poor aphid performance. Plants were taller and had higher biomass and canopy temperature. There was decreased aphid salivation into sieve elements, but no differences in phloem ingestion, indicating that the diminished fitness could be due to poorer tissue quality and unfavourable C:N balance, and that eCO2 was not a factor impeding feeding. Aphid ability to transmit Cucumber mosaic virus (CMV) was studied by exposing source and receptor plants to ambient (427 ppm) or elevated (612 ppm) CO2 before or after virus inoculation. A two-fold decrease on transmission was observed when receptor plants were exposed to eCO2 before aphid inoculation when compared to aCO2.
Ambient carbon dioxide (aCO2) concentration has exceeded 400 ppm and future estimations predict an increase up to 550 ppm within a few decades1,2. Apart from the consequences that increasing CO2 is the main driver behind climate change, it plays a mayor role in plant growth, physiology and metabolism as it is the direct substrate for photosynthesis3. Among the observed effects on plants, typical responses to elevated CO2 (eCO2) include increased plant growth and biomass, canopy size, reduction in stomatal conductance and transpiration, improved water-use efficiency and higher photosynthetic rates4,5,6,7,8,9,10,11,12,13. At the same time, increasing CO2 alters the chemical composition of plant tissue, with the accumulation of non-structural carbohydrates such as soluble sugars and starch14,15,16. Elevated CO2 also has an impact on the nitrogen cycle that translates into a decrease in protein content and higher C:N ratio7,15,17,18. The reduction in stomatal conductance may lead to a decrease in micronutrients such as calcium, magnesium or phosphorus due to the lower water uptake from the soil17.
Indirectly, the performance and feeding behaviour of herbivorous insects is greatly affected by plant nutritional quality and resistance, which are likely to be altered by increasing CO26,10,11,15,19. Also, changes in plant chemistry and physiology alter natural enemies9,20,21,22,23, as well as the incidence of plant viruses24,25,26,27. Conversely to plant growth, there is no general agreement on the effects of elevated CO2 on aphid-plant interactions as aphids are among the sap-feeding insects that have responded either positively or negatively to CO2-induced changes in plants5,6,28. Aphid abundance under eCO2 was higher in Aphis gossypii Glover, Sitobion avenae Fabricius or Acyrthosiphon pisum Harris on barrel medic29,30,31,32. Increasing CO2 was also beneficial for Rhopalosiphum padi L. growth rates and weight on wheat11,15,33.
On the contrary, a negative response was found in A. pisum on broad bean and R. padi on tall fescue6,18. Brevicoryne brassicae L. reduced Brussels sprout colonization after long-term exposure to eCO213. Other effects on life history parameters included an increased proportion of Rhopalosiphum maidis Fitch alates32. Lastly, several aphid species were reported to show a neutral response, such as Macrosiphum euphorbiae Thomas and Aulacorthum solani Kaltenbach6,34,35. In addition, responses seem to be host- and even genotype-specific, e.g. A. pisum on lucerne and broad bean6,35,36,37.
Myzus persicae Sulzer, the aphid species studied here, is a cosmopolitan, polyphagous pest of greenhouses and field crops. More importantly, it is a highly efficient vector of more than 100 plant viruses; therefore consecutive insecticide applications to lower vector density have constituted the traditional control strategy in the past, causing environmental and energetic costs38. Recent findings suggest that eCO2 is detrimental for its progeny, growth rates and adult weight on Brassicaceae10,15,21,33, although opposite results have been found on Solanum dulcamara L. and Arabidopsis thaliana L.6,39.
Feeding behaviour of insect pests can be monitored by the Electrical Penetration Graph (EPG) technique, which provides a live visualization and recording of plant penetration by insect mouthparts40,41. Few studies have considered the feeding behaviour of aphids under rising CO2, but decreased aphid salivation into sieve elements, increased phloem sap ingestion and shorter non-pathway phase are among the effects observed for A. pisum on barrel medic31,42.
There is limited information about the consequences of increasing CO2 on viral dynamics. Although it has been proven that high doses provoke resistance against infection with Tobacco mosaic virus (TMV, Tobamovirus) in tomato plants43, and Potato virus Y (PVY, Potyvirus)44 and Cucumber mosaic virus (CMV, Cucumovirus) in tobacco, due to the fact that plant defences appear to deviate from viruses to aphids under increasing CO245. On the other hand, Barley yellow dwarf virus (BYDV, Luteovirus) incidence has been predicted to increase in wheat under elevated CO227. This would imply that the larger amount of biomass could constitute a reservoir of infected material with subsequent higher risk of virus transmission by insect vectors24. Whilst, symptomatology of vegetable viruses can also be affected by rising CO2, enhancing earlier or more pronounced phenotypic differences between healthy and infected plants and making infected hosts more attractive to vectors26,27.
Investigating plant-insect interactions under increasing CO2 is crucial to evaluate their consequences on environment and ecosystems, and to develop new crop management strategies under future climate change scenarios. Therefore, the objective of our work was to study the life history parameters and feeding behaviour of M. persicae on pepper plants under aCO2 (400 ppm) and eCO2 (650 ppm). We also analysed the direct impact of increasing CO2 on plant growth and leaf chemistry. Links between plant tissue quality and aphid responses are discussed. Lastly, the cuticula-borne, non-persistent virus CMV was included in this investigation. We studied CMV transmission by M. persicae on ambient (427 ppm) and elevated CO2 (612 ppm) conditions with source and receptor plants exposed to the two CO2 regimes. Receptor plants were exposed to CO2 conditions either immediately after aphid introduction (direct exposure), or two weeks prior, in order to study previous acclimation (indirect exposure).
Results
Aphid life history
To asses the effect of increased CO2 concentration on pepper plants and M. persicae, plants were grown under aCO2 (400 ppm) or eCO2 (650 ppm). Myzus persicae pre-reproductive period (d), which was measured from aphid birth to adulthood was 11% longer on pepper plants from eCO2 environment (U = 138.5, Z = −3.706, p < 0.001) (Fig. 1, Supplementary Table S1). Additionally, under eCO2, instars N3 and N4 lasted significantly longer than under ambient conditions (N3: U = 237.5, Z = −2.291, p = 0.022. N4: U = 225.0, Z = −2.366, p = 0.018) (Fig. 2a). At the same time, the effective fecundity (Md) (t = 5.537, df = 48, p < 0.001) and offspring production over 10 days (M10) (t = 8.352, df = 48, p < 0.001) dropped respectively by 27% and 37% on plants grown under eCO2 (Fig. 1, Supplementary Table S1). Daily offspring production was also significantly lower under eCO2 when compared to ambient conditions (Treatment: F = 69.749, df = 1, p < 0.001. Time: F = 13.730, df = 10, p < 0.001. Treatment × Time: F = 1.749, df = 1, p = 0.067) (Fig. 2b). This latter treatment resulted in a higher mean generation time (Td) (U = 138.5, Z = −3.706, p < 0.001), as well as a slower intrinsic rate of natural increase (rm) (t = 7.407, df = 48, p < 0.001) and mean relative growth rate (RGR) (t = 7.407, df = 48, p < 0.001) when compared to aphids reared on aCO2 peppers (Fig. 1, Supplementary Table S1).
Figure 1. Percent change under eCO2 of the life history parameters of aphid (Myzus persicae), plant growth and leaf chemical profile.
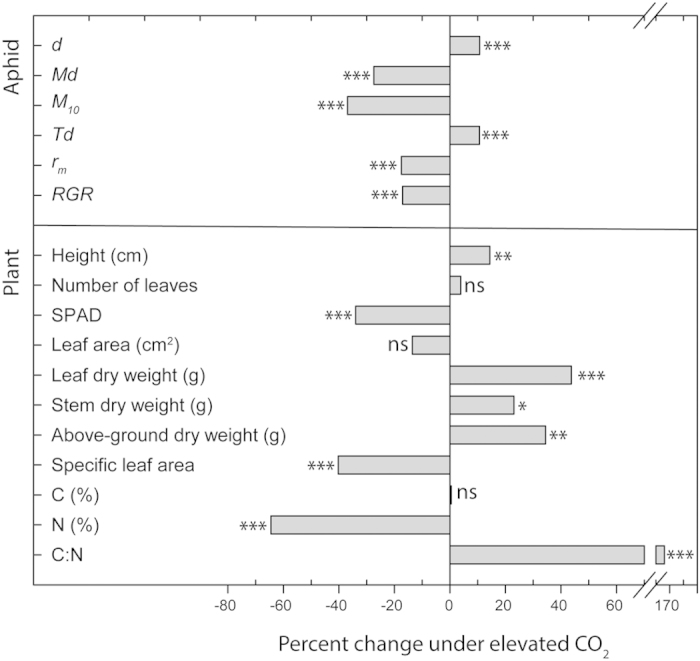
Statistical differences are calculated according to Student t-test for Gaussian variables or Mann-Whitney U-test for non-Gaussian variables (p ≤ 0.05). No significant differences are labelled as “ns”, and significant differences are indicated by stars with ***p ≤ 0.001, **p < 0.01 and *p < 0.05. d is the time (days) from birth to the onset of reproduction; Md is the reproductive output per aphid that represents the duration of d; M10 is the mean offspring number per female over the 10 day period; Td is the mean generation time; rm is the intrinsic rate of natural increase and RGR is the mean relative growth rate.
Figure 2.
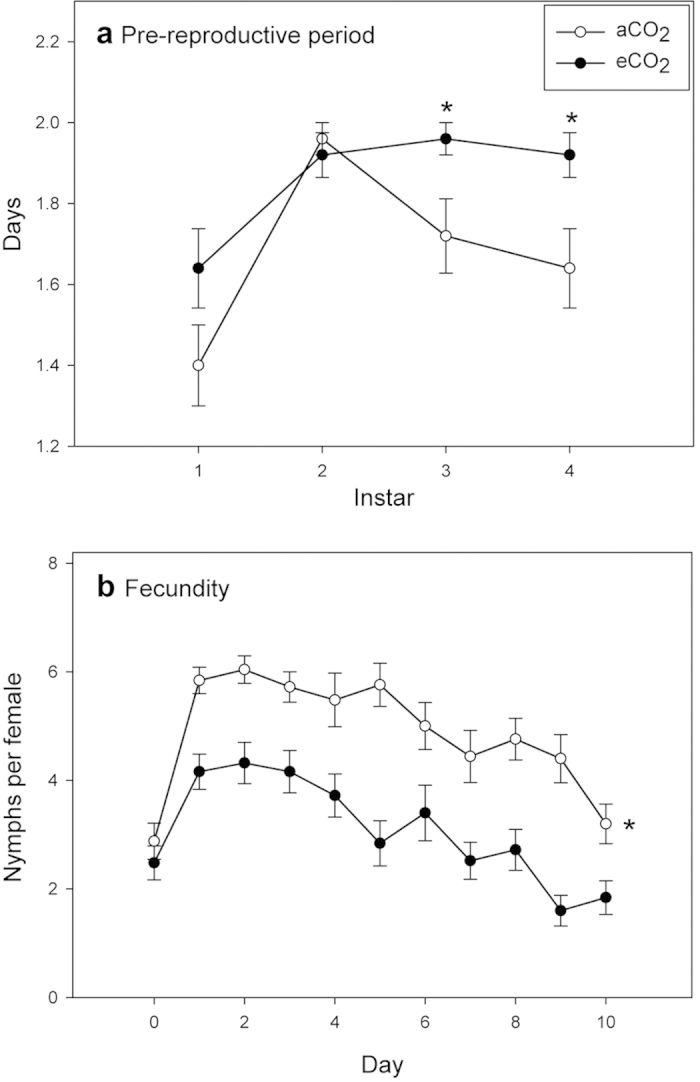
(a) Mean duration ± SEM of the four Myzus persicae nymphal instars under aCO2 and eCO2. (b) Mean number of daily nymphs per female ± SEM under aCO2 (400 ppm) and eCO2 (650 ppm) for 10 days. Asterisks indicate statistical differences according to (a) Mann-Whitney U-test and (b) GLM repeated measures analysis (p ≤ 0.05).
Feeding behaviour by Electrical Penetration Graphs
The sequential and non-sequential variable values obtained for the stylet penetration activities of M. persicae on aCO2 and eCO2-grown pepper plants showed relevant differences between treatments. Aphids made significantly fewer probes (U = 603.500, Z = −2.196, p = 0.028), intercellular stylet pathways C (U = 584.000, Z = −2.377, p = 0.017), phloem salivations E1 (U = 582.500, Z = −2.415, p = 0.016), single E1 not followed by phloem ingestion E2 (U = 567.000, Z = −2.612, p = 0.009) and probes after the first E1 (U = 546.000, Z = −2.831, p = 0.005) on eCO2 peppers (Table 1). Furthermore, the total duration of E1 per insect (U = 598.000, Z = −2.089, p = 0.037), and the time elapsed from the end of the last short intracellular puncture pd to the end of the probe (U = 540.000, Z = −2.783, p = 0.005) were significantly shorter on eCO2 peppers (Table 1). With regard to waveform duration per event, significant differences were found for non-probe activity (U = 104194.500, Z = −3.245, p = 0.001) and short intracellular punctures pd (U = 2193221.000, Z = −15.843, p < 0.001), with aphids spending less time on these activities if reared on eCO2 peppers (Table 1). When the percentage of time spent on each activity was evaluated, aphids phloem salivations E1 was reduced on eCO2 peppers (U = 591.000, Z = −2.310, p = 0.021) (Table 1). No differences were found for phloem ingestion (E2) parameters.
Table 1. Mean ± SEM (ranges in parenthesis) values of non-sequential and sequential EPG variables for the probing behaviour of Myzus persicae apterae adults on pepper plants grown under aCO2 (400 ppm) and eCO2 (650 ppm).
| Non-sequential variables | Treatment | PPW | NWEI | p | WDI | p | WDE | p | Percentage | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-probe | aCO2 | 42/42 | 23.26 ± 2.30 (4–57) | 0.027 | 2085.45 ± 262.35 (240.31–6967.96) | 0.378 | 94.22 ± 5.58 (2.33–1689.09) | 0.001 | ||
| eCO2 | 40/40 | 17.03 ± 2.51 (1–64) | 1936.62 ± 282.37 (139.10–7443.69) | 84.24 ± 7.61 (1.30–1849.54) | ||||||
| Probe | aCO2 | 42/42 | 23.17 ± 2.30 (4–57) | 0.028 | 26699.29 ± 262.35 (21816.76–28544.55) | 0.378 | 1174.15 ± 172.43 (9.77–27425.60) | 0.369 | ||
| eCO2 | 40/40 | 17.00 ± 2.51 (1–64) | 26848.12 ± 282.37 (21341.07–28645.64) | 1475.05 ± 211.92 (9.75–25800.66) | ||||||
| pd | aCO2 | 42/42 | 118.60 ± 9.39 (25–256) | 0.185 | 599.13 ± 51.79 (123.00–1560.81) | 0.170 | 5.31 ± 0.02 (2.94–12.00) | <0.001 | ||
| eCO2 | 40/40 | 107.05 ± 11.94 (21–301) | 532.77 ± 60.20 (81.82–1615.75) | 4.86 ± 0.02 (2.91–13.27) | ||||||
| C | aCO2 | 42/42 | 27.01 ± 2.53 (4–65) | 0.017 | 10041.60 ± 865.03 (2289.27–22618.31) | 0.330 | 391.90 ± 27.53 (9.77–5813.95) | 0.601 | 38.51 ± 3.54 (8.10–85.64) | 0.344 |
| eCO2 | 40/40 | 19.35 ± 2.57 (3–66) | 9109.61 ± 986.98 (1183.33–23545.48) | 428.46 ± 33.30 (9.75–6750.34) | 35.19 ± 4.09 (4.15–92.90) | |||||
| E1 | aCO2 | 42/42 | 4.79 ± 0.50 (1–14) | 0.016 | 1367.96 ± 198.98 (37.06–5410.55) | 0.037 | 251.49 ± 30.97 (21.35–1551.16) | 0.614 | 5.12 ± 0.74 (0.13–19.62) | 0.021 |
| eCO2 | 39/40 | 3.35 ± 0.47 (0–14) | 887.69 ± 155.08 (29.93–4178.15) | 328.21 ± 61.52 (5.28–3479.72) | 3.29 ± 0.62 (0.00–17.94) | |||||
| Single E1 | aCO2 | 32/42 | 2.29 ± 0.34 (0–8) | 0.009 | ||||||
| eCO2 | 21/40 | 1.23 ± 0.29 (0–8) | ||||||||
| E2 | aCO2 | 41/42 | 2.50 ± 0.30 (0–9) | 0.385 | 14030.68 ± 1310.98 (33.62–25363.72) | 0.272 | 4832.44 ± 1046.12 (9.26–25363.72) | 0.386 | 50.22 ± 4.74 (0.00–89.82) | 0.425 |
| eCO2 | 38/40 | 2.13 ± 0.25 (0–6) | 15793.52 ± 1334.13 (1409.46–27163.43) | 6217.17 ± 1112.24 (25.31–24282.08) | 54.50 ± 4.80 (0.00–95.09) | |||||
| Probe after 1st E1 | aCO2 | 32/42 | 10.17 ± 1.83 (0–44) | 0.005 | ||||||
| eCO2 | 16/40 | 5.70 ± 1.62 (0–39) | ||||||||
| Sequential variables | ||||||||||
| End of last pd to end of probe | aCO2 | 42/42 | 381.19 ± 165.28 (1.70–5788.84) | 0.005 | ||||||
| eCO2 | 40/40 | 1993.63 ± 942.89 (3.48–27379.65) |
P-values according to Mann Whitney U-test for non-Gaussian variables. Bold-type indicates significant differences (p ≤ 0.05).
PPW, proportion of individuals that produced the waveform type; NWEI, number of waveform events per insect; WDI, waveform duration (sec) per insect; WDE, waveform duration (sec) per event. Probe: probe activity. Waveforms: pd, short intracellular punctures; C, intercellular stylet pathway; E1, phloem salivation; single E1, E1 not followed by E2.
Plant growth and physiology
Peppers grown under eCO2 for four weeks were significantly taller (t = −3.277, df = 48, p = 0.002) although they had the same number of leaves than those under aCO2 (Fig. 1, Supplementary Table S1). At harvest, leaf (t = −3.732, df = 48, p = 0.001), stem (t = −2.454, df = 48, p = 0.018) and above-ground dry biomass (t = −3.367, df = 48, p = 0.002) were significantly higher under eCO2 (Fig. 1, Supplementary Table S1). Leaf area was similar in both treatments but there was a significant decrease in specific leaf area under eCO2 (t = 13.158, df = 48, p < 0.001) (Fig. 1, Supplementary Table S1). Moreover, SPAD was significantly lower under eCO2 (t = 10.044, df = 48, p < 0.001) (Fig. 1, Supplementary Table S1). Exposure to eCO2 did not alter carbon content, however it significantly decreased foliar nitrogen content (U = 0.000, Z = −5.127, p < 0.001) (Fig. 1, Supplementary Table S1). Additionally, the pepper canopy temperature measured using an IR camera was 1.2 °C higher under eCO2 (aCO2: 21.2 ± 0.8 °C, eCO2: 22.4 ± 0.6 °C) (Fig. 3a).
Figure 3.
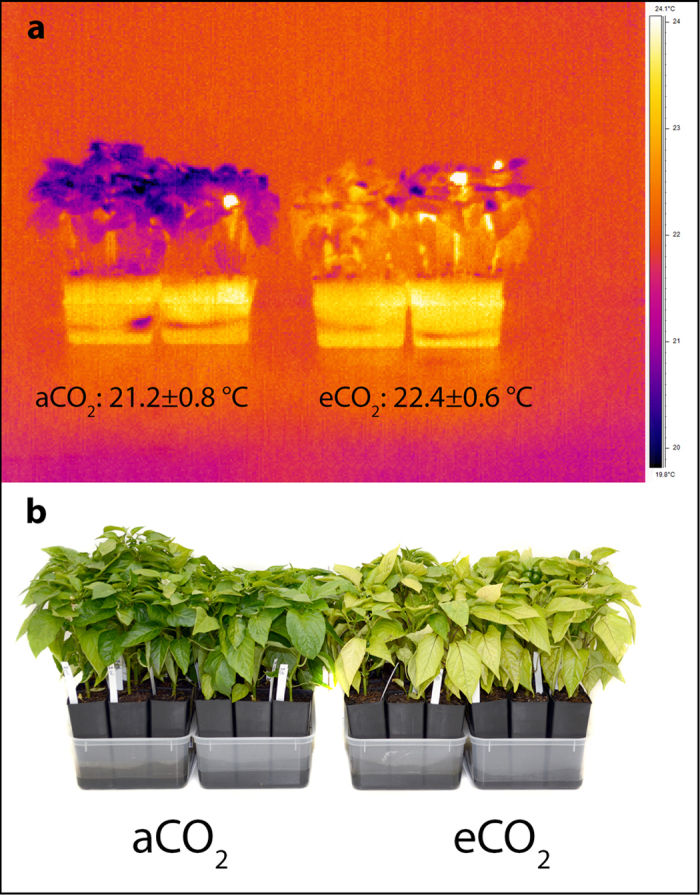
(a) Infrared thermal image of the canopy temperature of peppers, with mean ± SEM values measured under aCO2 and eCO2. (b) Picture of two-month old peppers under aCO2 and eCO2.
CMV transmission by aphids
To understand the potential effects of increased CO2 on virus transmission/acquisition efficiency, we used CMV and the same aphid/plant combination as per previous experiments. No differences were found if CO2 was applied to receptor plants straight after aphid introduction (direct exposure), when the CMV source inoculum had been grown either under aCO2 or eCO2 (Fig. 4). If the receptor plants had been previously grown under one of the CO2 regimes before CMV inoculation by aphids (indirect exposure), a two-fold decrease on CMV transmission was observed when source and receptor plants were grown under eCO2 conditions compared to aCO2 (χ2 = 4.432, p = 0.035) (Fig. 4).
Figure 4. CMV transmission (%) of direct and indirect CO2 exposure experiments, with receptor plants exposed to the two CO2 regimes after and before aphid introduction, respectively.
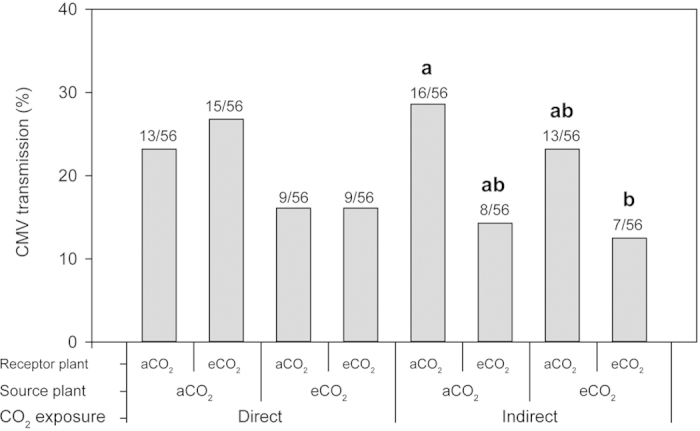
Ratios on bars refer to the number of CMV-infected receptor plants out of the total tested. Different letters in bold stand for statistical differences according to a χ2 goodness of fit test (p ≤ 0.05).
Discussion
Current carbon dioxide (CO2) concentration has exceeded 400 ppm and the concentration will continue increasing at an accelerating rate from decade to decade. This rising of CO2 levels in the Earth’s atmosphere causes general climate change and global warming46. Among the observed effects on plants, increasing CO2 leads to changes in plant growth, physiology and metabolism7,13,15. At the same time, these changes have an indirect impact on insect pest biology and behaviour, altering their population growth or feeding habits, as well as on the symptomatology and incidence of associated pathogens such as plant viruses24,26,27,35. To our knowledge, this is the first study to demonstrate the variety of effects of increasing CO2 on pepper plants and the subsequent indirect response on M. persicae fitness and feeding behavior. Moreover, these experiments have been coupled with research on CMV transmission with receptor plants exposed to ambient and elevated CO2 either before or after aphid introduction.
Overall M. persicae fitness was reduced on pepper plants grown under eCO2, being consistent with previous results for this aphid on Brassicaceae species10,15,21,33 and other aphid species such as A. pisum on broad bean or B. brassicae on Brussels sprout6,13,18. Not only was the time from birth to adulthood longer, but they also produced less offspring, which resulted in lowered growth rates. Again, this highlights the specificity of responses to hosts, as eCO2 has been reported to increase the abundance of the green peach aphid on herbaceous species in the past6,39. The response to eCO2 also differs among insect guilds. It is generally agreed that the decline in the foliar nitrogen content of host plants under eCO2 prolongs the development of chewing insects, and that they compensate for decreased nutritional quality by consuming more foliage5,22,47. By contrast, eCO2 has species-specific effects on phloem-sucking insects22.
The amount of foliar nitrogen in pepper was significantly lower under eCO2 when compare to ambient conditions, which may well explain the poor aphid performance we observed. Aphids which fed on plants grown under eCO2 had a reduced efficiency, with an 11% longer pre-reproductive period and 37% fewer nymphs. This is probably mediated by reduced nitrogen content in the pepper source as aphids tend to be limited by the amount of nitrogen rather than carbohydrates47. Additionally, it has been shown that this aphid species is particularly sensitive to low nitrogen contents48,49. However, it has to be also pointed out that we measured whole foliar tissue nitrogen rather than phloem nitrogen but it is likely that our results could be extended to sap composition11,42,50. Chlorophyll content of leaves was also significantly lower under eCO2, this result being consistent with significantly lower nitrogen7,15,17,18. Also, it has been reported that C:N ratio of leaves can be more affected by increasing CO2 than that of the plant as a whole51.
Carbon levels were similar in both treatments, as opposed to other plant species under eCO2, in which carbon content increased14,15,16. As expected, the increased height and above-ground dry weight of peppers are in agreement with previous studies, reporting enhanced growth under eCO2 in several plant species3,11,12,13,21,33,35,36. Finally, using infrared imagery an increase of 1.2 °C in plant canopy temperature was measured for peppers grown under eCO2. Cotton and wheat also show an increase ranging from 0.6 to 1.1 °C, and closure of leaf stomata appears to be the main driver behind this response52. Increased plant canopy temperature can further modify the microclimate for the aphid and higher temperature has been shown to increase virus titer in wheat53.
In this study, EPG recordings showed decreased aphid salivation into sieve elements and shorter non-probe phase under rising CO2, similar to the effects found for A. pisum31,42. However, no differences were found for the duration and number of events of phloem ingestion, suggesting that eCO2 was not a factor impeding sap feeding by M. persicae, as opposed to A. pisum31. This result may also indicate that the diminished fitness observed could be mostly due to poorer tissue quality. What analysed parameters seem to stress is that there were no differences in feeding behaviour explaining a worse performance in eCO2 plants. That is, aphids did not reduce or increase feeding, but simply ingested sap that was less nutritious. Therefore, with equal ingested sap, aphids grew slower under eCO2 because they acquired a much more unfavourable N content diet.
Furthermore, the number of single E1 waveforms (phloem salivation) not followed by E2 events (phloem ingestion) and the percentage of time spent on E1 were lower under eCO2. Altogether, these results might imply that the ingestion success rate once aphids reached the phloem was higher under eCO2. An explanation could be found in the natural defense responses triggered under herbivory pressure, resulting in a rejection of sustained feeding under ambient conditions. Indeed, eCO2 is likely to reduce the resistance ability of hosts against aphids by the down-regulation of genes involved in plant defence31,39,54. Without these barriers, aphids might be able to ingest phloem more easily under eCO2. Several mechanisms have been proposed, and include but are not limited to: 1) decreased activity of enzymes mediating the accumulation of reactive oxygen species, 2) reduced polymerization of phenolics absorbed by aphids that causes browning of cells in contact with the saliva, a disadvantage for aphid feeding, 3) down-regulation of ethylene signalling pathways31, and 4) decreased jasmonic and salicylic acid defences39,54.
Plants used for EPG recordings were grown inside CO2 chambers for four weeks, whereas those used for the life history experiment were exposed to CO2 during the aphid cycle as well, reaching a total of seven weeks, so it is likely that the nutritional profile of these plants declined with time. Additional work testing different periods of CO2 exposure should be addressed to evaluate if aphid response changes with time.
Work on the interactions between pepper plants and aphids was complemented with the study of CMV transmission by M. persicae when source and receptor plants were either previously exposed to ambient and elevated CO2 before aphid introduction (indirect effect) or straight after insect inoculation (direct effect). Elevated CO2 lessened virus transmission risk if receptor plants had been previously grown under eCO2, but not if CO2 was applied directly after pest introduction, which suggests that this difference may be associated with early exposure to elevated CO2. CMV is transmitted in a non-circulative manner during brief probes in the host epidermis55. As we observed a similar number of stylet punctures (pd) under both treatments, but shorter duration under eCO2, the decrease in CMV transmission might be due to plant resistance mechanisms once the virus infected the host, rather than an altered aphid feeding behaviour during the stages involved in virus inoculation.
Under ambient CO2 conditions, it has been shown that plant pathogens are responsible for triggering changes in nutritional quality or mediating attractiveness to increase the chances of vectors spreading viruses56. In our experiments, the decreased CMV transmission found under eCO2 may be associated with lower aphid attraction to virus-infected plants, resulting in fewer viruliferous aphids, which would ultimately reduce the spread of CMV under future climate conditions.
Consecutive rising of CO2 concentration and global temperature are a reality of future scenarios. If disease severity changes under forthcoming environmental conditions, then additional research becomes imperative to understand the impact of viral infections on crop production and to minimise losses due to vector infestation. Additional studies could add to knowledge on insect-plant interactions under elevated CO2. Further studies with other insect vectors and beneficials are required to identify if the causal factors for the observed changes are conserved among different insect guilds. With regard to subsequent virus transmission by vectors, studying the causes of a delay in symptom expression after CMV infection and the decrease in CMV transmission under indirect eCO2 conditions may benefit the overall knowledge on the interactions among pathogens, host plants and vectors and help predict potential outbreaks.
Methods
Plants and CO2 growth chambers
Pepper seeds (Capsicum annum L.) cv. ‘California Wonder’ (D. T. Brown & Co Ltd., South Windsor, Australia) were sown in plastic pots filled with potting mix containing slow release fertilizer, additional trace elements, iron and lime. At two true-leaf stage, pots were placed inside growth chambers (internal dimensions 1.2 × 0.7 × 1.5 m, 1260 L) with five 400W high pressure sodium lights (Lucagrow GE Lighting, Richmond, Australia) and four 77W halogen lights (Osram Pty. Ltd., Sydney, Australia) at two different CO2 regimes, aCO2 (400 ppm) and eCO2 (650 ppm) (CLIMATRON-1260, Thermoline Scientific, Smithfield, Australia). Growing conditions were 24 °C, 70% RH, 16-hour photoperiod and 1000 μmol m−2 s−1 light intensity at canopy level. Water in trays was maintained at a similar level between treatments and plants were rotated within the chambers to ensure there was no positional influence. CO2 regime was switched between chambers once a week to ensure there was no potential chamber effect.
Aphid population
A clonal source population of M. persicae was established from a virus free female naturally infesting marshmallow (Malva parviflora L.) at Grains Innovation Park (Horsham, Australia). Aphids were continuously reared on pepper plants in a chamber at 22 °C, 70% RH, 16-hour photoperiod and 800 μmol m−2 s−1 light intensity. Individuals were synchronised prior to assays to ensure they were the same age.
Aphid life history
Peppers were previously grown under aCO2 (400 ppm) and eCO2 (650 ppm) for four weeks since two-leaf stage. A single wingless M. persicae adult was placed in a clip-cage on the adaxial side of the youngest fully developed leaf of each pepper and allowed to produce nymphs for 24 hours. Surplus nymphs were then removed leaving one nymph per plant, which was monitored until adulthood. Offspring were counted by removing nymphs daily for 10 days after the onset of reproduction. Duration of the four nymphal instars, pre-reproductive period (d), effective fecundity (offspring for a period equal to the pre-reproductive period (Md)), offspring for 10 days, mean generation time (Td = d/0.738), intrinsic rate of natural increase (rm = 0.738*(logeMd)/d) and mean relative growth rate (RGR = rm/0.86) were calculated (n = 25).
Feeding behaviour by Electrical Penetration Graphs
Myzus persicae probing and feeding behaviour was monitored by EPG, a tool for determining the activities of the aphid’s stylets, including probing, salivation into sieve elements and passive uptake of phloem in real time40,57. Recordings were conducted inside a Faraday cage to avoid noise and interference for eight hours on peppers previously grown under aCO2 (400 ppm) and eCO2 (650 ppm) for four weeks from the two-leaf stage. Each recording was conducted using a new plant and aphid. Recordings were made simultaneously over an eight-week period (n = 42 for aCO2 and n = 40 for eCO2). Young adult aphids were immobilized using a vacuum device57. A thin gold wire (12.5 μm diameter, 3 cm length) was attached to the dorsum of the insect with a small droplet of water-based silver glue (EPG-Systems, Wageningen, The Netherlands) using an entomological pin. The gold wire was glued to a copper wire (0.2 m diameter) attached to a brass pin, which was inserted into the input connector of the first-stage amplifier. The output electrode was a copper post (10 cm × 2 mm), which was inserted into the pot. Recordings were performed using a Giga-8 DC amplifier with a 1 Giga Ω input resistance (EPG-Systems, Wageningen, The Netherlands). EPG output was set to 50x gain and data was acquired at 100 Hz using a DATAQ Di700 A/D data acquisition USB device card (Dataq Instruments, Ohio, USA). Data was analysed with Stylet + a software (EPG-Systems, Wageningen, The Netherlands). All behavioural variables were then processed using the MS Excel Workbook for automatic EPG data calculation58.
Selected variables were compared between aCO2 and eCO259: PPW, proportion of individuals that produced a specific waveform type; NWEI, number of waveform events per insect, that is the sum of the number of events of a particular waveform divided by the total number of insects under each treatment; WDI, waveform duration per insect, that is the sum of durations of each event of a particular wave-form made by each individual insect that produced that waveform divided by the number of insects that performed that particular waveform under each treatment; and WDE, waveform duration per event, that is the sum of the duration of the events for a particular waveform divided by the total number of events of that particular waveform under each treatment.
Pepper growth and physiology
Peppers grown under aCO2 and eCO2 were monitored for height, number of leaves and leaf chlorophyll content before insect introduction. Chlorophyll was measured in SPAD (Soil Plant Analytical Development) units, commonly used as an indirect indicator of nitrogen foliar content (Chlorophyll meter SPAD-502Plus, Konica Minolta, Japan). This device measures leaf transmittance at two wavelengths, 660 and 940 nm. SPAD readings were taken on every leaf and averaged per plant.
Canopy temperature was recorded by infrared thermal imaging (ThermaCAM P40, Flir Systems Pty. Ltd., Notting Hill, Australia) at the end of the fitness experiment. Thermal imaging was taken within five minutes after plants were removed from the chambers and placed in a climate controlled room (matching chamber temperatures) and against a contrast temperature background. Images were processed, and minimum, maximum and average temperature values were recorded for each treatment. Using box area tool, an equal sized rectangle was overlaid over each treatment, which covered around 50% of the plant canopy (ThermaCAM Researcher Professional 2.8 software, Flir Systems Pty. Ltd., Notting Hill, Australia).
Plants were harvested and leaf area recorded (Portable area meter LI-300C, LI-COR Biosciences, Nebraska, USA). Leaves and stems were dried at 65 °C for 48 hours (Dehydrating oven TD-150FTS, Thermoline Scientific, Smithfield, Australia). Specific leaf area (SLA = leaf area/leaf dry weight) was calculated. Leaves were finely ground (<0.5 mm) (Tissuelyser MM300, Quiagen Retsch, Haan, Germany). Total carbon (C) and nitrogen (N) concentration of leaf tissue was determined by the Dumas combustion method using a CHN analyser (CHN 2000, LECO, St. Joseph, USA) at the University of Melbourne, Creswick. C:N ratio was calculated by dividing the concentration of C by the concentration of N for each leaf sample.
Aphid ability to transmit CMV and systemic infection
Pepper seeds cv. ‘California Wonder’ (Ramiro Arnedo S.A., La Rioja, Spain) were sown in plastic pots filled with a 1:1 mixture of substrate (Kekkilä Iberia, Quart de Poblet, Spain) and vermiculite (No. 3, Asfaltex S.A., Barcelona, Spain). Plants were watered three times a week using 20:20:20 (N:P:K) Nutrichem 60 fertiliser at a dose of 3 g L−1 (Miller Chemical & Fertilizer Corp., Pennsylvania, USA).
CMV source plants
Seedlings were infected with CMV strain M6, subgroup IA at two-true leaf stage using a non-persistent virus type protocol. Fifteen M. persicae with one-hour starvation were allowed to feed for five minutes (acquisition access period) on a CMV positive leaf and were transferred to the seedlings for 24 hours (inoculation access period). These virus sources were then maintained either under aCO2 (427 ppm) or eCO2 (612 ppm) conditions for four weeks until symptom development inside climate chambers with 24:20 °C (D:N), 70% RH and 14-hour photoperiod. After four weeks, these plants were used as CMV inoculum for the transmission experiments.
CMV transmission experiments
Two types of experiments were designed based on the moment of CO2 exposure of receptor plants: 1) an experiment where receptor plants were exposed to the CO2 regimes, either aCO2 or eCO2, straight after virus inoculation by aphids, with the objective of studying the direct effect of CO2 on the development of the viral disease, and b) an experiment where receptor plants had been previously grown for two weeks under each of the two CO2 regimes before aphid introduction, to study the effect of the indirect acclimation of receptor plants on CMV transmission. In both experiments, plants providing CMV inoculum were the virus sources obtained in section 2.6.1, grown either under aCO2 or eCO2.
CMV inoculation of receptor plants for both experiments was done with the same procedure mentioned above, placing five M. persicae which had previously fed on the CMV source plants for five minutes onto each receptor plant (n = 56). After 24 hours, receptor plants were sprayed with imidacloprid (Confidor® 20 LS, Bayer CropScience, Valencia, Spain) and maintained either under aCO2 or eCO2 conditions inside climate chambers with 24:20 °C (D:N), 70% RH and 14-hour photoperiod for four weeks until visual inspection of symptoms.
Statistical analysis
Data was transformed with either √(x + 0.5), x2, Ln(x + 1) or 2*arcsin√x if needed to reduce heteroscedasticity. Differences in parameters of aphid life history, EPG recordings and plant physiology between CO2 treatments were assessed with Student t-test (p ≤ 0.05) using IBM Statistics SPSS 21.0 software (SPSS Inc.). When data did not follow the ANOVA assumptions, a non-parametric Mann-Whitney U-test (p ≤ 0.05) was performed. Daily offspring for 10 days over the fitness assay was assessed with GLM repeated measures analysis (p ≤ 0.05) (SPSS Inc.). CMV transmission was compared by a χ2 goodness of fit test (p ≤ 0.05) to check if the observed frequency distribution was related to the expected frequency distribution using Statview 4.01 software (Abacus Concepts Inc.).
Additional Information
How to cite this article: Dáder, B. et al. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 6, 19120; doi: 10.1038/srep19120 (2016).
Supplementary Material
Acknowledgments
This work was funded by Victorian State Government Australia Bioscience Research, AGL2013-47603-C2-2-R project in Spain, and fellowships BES-2011-045885 and EEBB-I-14-08266 to B.D. We thank Glenn Fitzgerald for the IR thermal camera and Alan Yen for assisting with the project proposal.
Footnotes
Author Contributions B.D., A.F., A.M. and P.T. conceived the experiments; B.D. and P.T. conducted the experiments; B.D., A.M. and P.T. analysed the results; all authors wrote the main manuscript text; P.T. prepared figures; all authors reviewed the manuscript.
References
- IPCC. Climate Change 2014: Synthesis Report (eds Pachauri, R. K. & Meyer, L. A.) (IPCC, 2014).
- Keeling C. D. & Whorf T. P. In Trends online: A compendium of data on global change (ed Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory) (USA Department of Energy, 2004). [Google Scholar]
- Ziska L. H. Rising atmospheric carbon dioxide and plant biology: The overlooked paradigm. DNA Cell Biol. 27, 165–172 (2008). [DOI] [PubMed] [Google Scholar]
- Woorward F. I., Thomson G. B. & McKee I. F. How plants respond to climate change: migration rates, individualism and the consequences for plant communities. Ann. Bot. 67, 23–38 (1991). [Google Scholar]
- Coviella C. E. & Trumble J. T. Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Conserv. Biol. 13, 700–712 (1999). [Google Scholar]
- Hughes L. & Bazzaz F. A. Effects of elevated CO2 on five plant-aphid interactions. Entomol. Exp. Appl. 99, 87–96 (2001). [Google Scholar]
- Ainsworth E. A. & Long S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE) ? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372 (2005). [DOI] [PubMed] [Google Scholar]
- Ainsworth E. A. & Rogers A. The response of photosynthesis and stomatal conductance to rising (CO2): mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 (2007). [DOI] [PubMed] [Google Scholar]
- Gao F. et al. Interactive effects of elevated CO2 and cotton cultivar on tri-trophic interaction of Gossypium hirsutum, Aphis gossyppii, and Propylaea japonica. Environ. Entomol. 37, 29–37 (2008). [DOI] [PubMed] [Google Scholar]
- Himanen S. J. et al. Interactions of elevated carbon dioxide and temperature with aphid feeding on transgenic oilseed rape: Are Bacillus thuringiensis (Bt) plants more susceptible to nontarget herbivores in future climate? Glob. Chang. Biol. 14, 1–18 (2008). [Google Scholar]
- Sun Y. C., Chen F. J. & Ge F. Elevated CO2 changes interspecific competition among three species of wheat aphids: Sitobion avenae, Rhopalosiphum padi, and Schizaphis graminum. Environ. Entomol. 38, 26–34 (2009). [DOI] [PubMed] [Google Scholar]
- Johnson S. N. & Riegler M. Root damage by insects reverses the effects of elevated atmospheric CO2 on eucalypt seedlings. Plos One, 8, e79479 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber J., Najar-Rodriguez A. J., Piskorski R. & Dorn S. Plant acclimation to elevated CO2 affects important plant functional traits, and concomitantly reduces plant colonization rates by an herbivorous insect. Planta 237, 29–42 (2013). [DOI] [PubMed] [Google Scholar]
- Ainsworth E. A. Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Chang. Biol. 14, 1642–1650 (2008). [Google Scholar]
- Oehme V., Högy P., Zebitz C. P. W. & Fangmeier A. Effects of elevated atmospheric CO2 concentrations on phloem sap composition of spring crops and aphid performance. J. Plant Interact. 8, 74–84 (2013). [Google Scholar]
- Ryan G. D., Emiljanowicz L., Härri S. A. & Newman J. A. Aphid and host-plant genotype × genotype interactions under elevated CO2. Ecol. Entomol. 39, 309–315 (2014). [Google Scholar]
- Taub D. R. & Wang X. Z. Why are nitrogen concentrations in plant tissues lower under elevated CO2 ? A critical examination of the hypotheses. J. Integrat. Plant Biol. 50, 1365–1374 (2008). [DOI] [PubMed] [Google Scholar]
- Ryan G. D., Rasmussen S., Xue H., Parsons A. J. & Newman J. A. Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum. Plant Cell Environ. 37, 204–212 (2014). [DOI] [PubMed] [Google Scholar]
- Stiling P. et al. Direct and legacy effects of long-term elevated CO2 on fine root growth and plant-insect interactions. New Phytol. 200, 788–795 (2013). [DOI] [PubMed] [Google Scholar]
- Bezemer T. M., Hefin Jones T. & Knight K. J. Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae. Oecologia 116, 128–135 (1998). [DOI] [PubMed] [Google Scholar]
- Stacey D. A. & Fellowes M. D. E. Influence of elevated CO2 on interspecific interactions at higher trophic levels. Glob. Chang. Biol. 8, 668–678 (2002). [Google Scholar]
- Sun Y. C., Yin J., Chen F. J., Wu G. & Ge F. How does atmospheric elevated CO2 affect crop pests and their natural enemies ? Case histories from China. Insect Sci. 18, 393–400 (2011). [Google Scholar]
- Hentley W. T., Vanbergen A. J., Hails R. S., Hefin Jones T. H. & Johnson S. N. Elevated atmospheric CO2 impairs aphid escape responses to predators and conspecific alarm signals. J. Chem. Ecol. 40, 1110–1114 (2014). [DOI] [PubMed] [Google Scholar]
- Malmstrom C. M. & Field C. B. Virus-induced differences in the response of oat plants to elevated carbon dioxide. Plant Cell Environ. 20, 178–88 (1997). [Google Scholar]
- Luck J. et al. Climate change and diseases of food crops. Plant Pathol. 60, 113–121 (2011). [Google Scholar]
- Jones E. A. C. & Barbetti M. J. Influence of climate change on plant disease infections and epidemics caused by viruses and bacteria. CAB Rev. 7, 1–33 (2012). [Google Scholar]
- Trebicki P. et al. Virus disease in wheat predicted to increase with a changing climate. Glob. Chang. Biol. 21, 3511–3519 (2015). [DOI] [PubMed] [Google Scholar]
- Newman J. A., Gibson D. J., Parsons A. J. & Thorney J. H. M. How predictable are aphid population responses to elevated CO2 ? J. Anim. Ecol. 72, 556–566 (2003). [DOI] [PubMed] [Google Scholar]
- Chen F. J., Ge F. & Parajulee M. N. Impact of elevated CO2 on tri-trophic interaction of Gossypium hirsutum, Aphis gossypii, and Leis axyridis. Environ. Entomol. 34, 37–46 (2005). [DOI] [PubMed] [Google Scholar]
- Chen F. J., Wu G. & Ge F. Impacts of elevated CO2 on the population abundance and reproductive activity of aphid Sitobion avenae Fabricius feeding on spring wheat. J. Appl. Entomol. 128, 723–730 (2004). [Google Scholar]
- Guo H. et al. Elevated CO2 decreases the response of the ethylene signaling pathway in Medicago truncatula and increases the abundance of the pea aphid. New Phytol. 201, 279–291 (2014). [DOI] [PubMed] [Google Scholar]
- Xie A. et al. Changes in life history parameters of Rhopalosiphum maidis (Homoptera: Aphididae) under four different elevated temperature and CO2 combinations. J. Econ. Entomol. 107, 1411–1418 (2014). [DOI] [PubMed] [Google Scholar]
- Oehme V., Högy P., Franzaring J., Zebitz C. P. W. & Fangmeier A. Response of spring crops and associated aphids to elevated atmospheric CO2 concentrations. J. Appl. Bot. Food Qual. 84, 151–157 (2011). [Google Scholar]
- Flynn D. F. B., Sudderth E. A. & Bazzaz F. A. Effects of aphid herbivory on biomass and leaf-level physiology of Solanum dulcamara under elevated temperature and CO2. Environ. Exp. Bot. 56, 10–18 (2006). [Google Scholar]
- Johnson S. N., Ryalls J. M. W. & Karley A. J. Global climate change and crop resistance to aphids: contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann. Appl. Biol. 165, 62–72 (2014). [Google Scholar]
- Awmack C. S. & Harrington R. Elevated CO2 affects the interactions between aphid pests and host plant flowering. Agricul. Forest Entomol. 2, 57–61 (2000). [Google Scholar]
- Ryalls J. M. W., Riegler M., Moore B. D., Lopaticki G. & Johnson S. N. Effects of elevated temperature and CO2 on aboveground-belowground systems: a case study with plants, their mutualistic bacteria and root/shoot herbivores. Front. Plant Sci. 4, 445 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman R. L. & Eastop V. F. In Aphids as crop pests (eds van Emden H. F. & Harrington R.) Pp. 1–29 (CABI, 2007). [Google Scholar]
- Sun Y., Guo H., Zhu-Salzman K. & Ge F. Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 210, 128–140 (2013). [DOI] [PubMed] [Google Scholar]
- Tjallingii W. F. In Aphids: Their biology. Natural enemies and control, Vol. 2B (eds Minks A. K. & Harrewijn P. J.) Pp. 95–108 (Elsevier, 1988). [Google Scholar]
- Sun Y. et al. Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Chang. Biol. 21, 2739–2748 (2015). [DOI] [PubMed] [Google Scholar]
- Guo H. et al. Pea aphid promotes amino acid metabolism in Medicago truncatula and bacteriocytes to favour aphid population growth under elevated CO2. Glob. Chang. Biol. 19, 3210–3223 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 66, 1951–1963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matros A. et al. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 29, 126–37 (2006). [DOI] [PubMed] [Google Scholar]
- Fu X., Ye L., Kang L. & Ge F. Elevated CO2 shifts the focus of tobacco plant defences from cucumber mosaic virus to the green peach aphid. Plant Cell Environ. 33, 2056–2064 (2010). [DOI] [PubMed] [Google Scholar]
- Carter T. R. et al. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Parry M. L. et al.) Ch. 2, 133–171 (Cambridge University Press, 2007). [Google Scholar]
- Fajer E. D., Bowers M. D. & Bazzaz F. A. The effects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions. Science 243, 1198–1200 (1989). [DOI] [PubMed] [Google Scholar]
- Dadd R. H. & Krieger D. L. Dietary amino acid requirements of aphid Myzus persicae. J. Insect Physiol. 14, 741–764 (1968). [Google Scholar]
- Srivastava P. N. & Auclair J. L. Role of single amino acids in phagostimulation, growth and survival of Acyrthosiphon pisum. J. Insect Physiol. 21, 1865–1871 (1975). [Google Scholar]
- Ryan G. D., Sylvester E. V. A., Shelp B. J. & Newman J. A. Towards an understanding of how phloem amino acid composition shapes elevated CO2-induced changes in aphid population dynamics. Ecol. Entomol. 40, 247–257 (2015). [Google Scholar]
- Lawler I. R., Foley W. J., Woodrow I. E. & Cork S. J. The effects of elevated CO2 atmospheres on the nutritional quality of Eucalyptus foliage and its interaction with soil nutrient and light availability. Oecologia 109, 59–68 (1997). [DOI] [PubMed] [Google Scholar]
- Kimball B. A., Kobayashi K. & Bindi M. Responses of agricultural crops to Free-Air CO2 Enrichment. Adv. Agron. 77, 293–368 (2002). [PubMed] [Google Scholar]
- Nancarrow N. et al. The effect of elevated temperature on Barley yellow dwarf virus-PAV in wheat. Vir. Res. 186, 97–103 (2014). [DOI] [PubMed] [Google Scholar]
- Zavala J. A., Nabity P. D. & DeLucia E. H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 58, 79–97 (2013). [DOI] [PubMed] [Google Scholar]
- Ng J. C. K. & Falk B. W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Ann. Rev. Phytopathol. 44, 183–212 (2006). [DOI] [PubMed] [Google Scholar]
- Ingwell L. L., Eigenbrode S. D. & Bosque-Pérez N. A. Plant viruses alter insect behaviour to enhance their spread. Sci. Rep. 2, 1–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebicki P., Tjallingii W. F., Harding R. M., Rodoni B. & Powell K. S. EPG monitoring of the probing behaviour of the common brown leafhopper Orosius orientalis on artificial diet and selected host plants. Arthropod Plant Interact. 6, 405–415 (2012). [Google Scholar]
- Sarria E., Cid M., Garzo E. & Fereres A. Excel workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 67, 35–42 (2009). [Google Scholar]
- Backus E. A., Cline A. R., Ellerseick M. R. & Serrano M. S. Lygus hesperus (Hemiptera: Miridae) feeding on cotton: new methods and parameters for analysis of non-sequential electrical penetration graph data. Ann. Entomol. Soc. Am. 100, 296–310 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


