Abstract
Afa/Dr diffusely adhering Escherichia coli (DAEC) bacteria that are responsible for recurrent urinary tract and gastrointestinal infections recognized as a receptor the glycosylphosphatidylinositol (GPI)-anchored protein decay-accelerating factor (DAF; CD55) at the brush border of cultured human intestinal cells. Results show that Afa/Dr DAEC C1845 bacteria were poorly associated with the mucosa of the gastrointestinal tract of infected mice. We conducted experiments with Chinese hamster ovary (CHO) cells stably transfected with mouse (GPI or transmembrane forms), pig, or human CD55 or mouse Crry cDNAs or transfected with empty vector pDR2EF1α. Recombinant E. coli AAEC185 bacteria expressing Dr or F1845 adhesins bound strongly to CHO cells expressing human CD55 but not to the CHO cells expressing mouse (transmembrane and GPI anchored), rat, or pig CD55 or mouse Crry. Positive clustering of CD55 around Dr-positive bacteria was observed in human CD55-expressing CHO cells but not around the rarely adhering Dr-positive bacteria randomly distributed at the cell surface of CHO cells expressing mouse, rat, or pig CD55.
Several microbial pathogens have been found to hijack human CD55 (decay-accelerating factor [DAF]) directly to infect the host cells or indirectly as signaling molecules (via proteins associated with the glycosylphosphatidylinositol [GPI] anchor) to establish molecular cross-talk with the mammalian target cells and trigger cellular responses. Among human pathogenic Escherichia coli bacteria (23), the Afa/Dr diffusely adhering E. coli strains (DAEC) are responsible for recurrent urinary tract and gastrointestinal infections in humans (39). This subclass of pathogenic E. coli expressed a family of gene operons with similar genetic organizations consisting of at least five genes, including the afa, dra, and daa genes. The last gene, gene E, encodes the major structural AfaE-I (29), AfaE-III (31), DraE (35), DraE-II (45), and DaaE (6) proteins that function as adhesins. All these adhesins recognized CD55 as a receptor (36, 37, 45). AfaE-VII and AfaE-VIII, two other Afa/Dr adhesins encoded by the afa-7 and afa-8 gene clusters and expressed mostly by bovine isolates (14, 30), have been recently identified. These animal afa gene clusters are plasmid and chromosome borne and are expressed by strains that produced other virulence factors such as cytonecrotizing factor toxins and F17, pyelonephritis-associated pilus, and CS31A adhesins. It was noticed that AfaE-VIII adhesin has been found in human E. coli (14) but has never been detected in diarrhea-associated human isolates (32). Importantly, in contrast with AfaE-I, AfaE-III, Dr, Dr-II, and F1845 adhesins, both AfaE-VII and AfaE-VIII adhesins failed to recognize the human CD55 molecule as a receptor, and the mechanism(s) of pathogenicity of E. coli harboring these two adhesins remain currently unknown.
CD55 is a molecule attached to the outer leaflet of the cell membrane by a GPI anchor and contains four complement control protein repeats (CCPs) followed by a serine/threonine-rich, heavily O-glycosylated C-terminal domain that serves as a nonspecific spacer projecting the molecule at the membrane surface. CD55 plays a central role in regulating the complement system by dissociating autologous C3 convertases responsible for cleaving C3, which assemble on self-cell surfaces (8, 28, 34). The classical pathway regulatory activity of CD55 resides in CCP2 and CCP3, while its alternative pathway regulatory activity resides in CCP2, CCP3, and CCP4 (12). It has been recently reported that decay-accelerating activity maps to a single face of the molecule whereas microbial pathogens recognize a variety of different sites on CD55 (61). In the case of Afa/Dr DAEC, E. coli bacteria bearing AfaE-I, AfaE-III, Dr, and F1845 adhesins primarily recognized CCP3 of human CD55 (with some contribution of CCP2) whereas E. coli bacteria bearing Dr-II adhesin recognized CCP4 (36, 44). Residues important to binding of DraE adhesin in the 148-to-171 loop at the surface of CD55 CCP3 were distinct (approximately 20 A apart) from those important to complement regulation (20). Finally, it has been shown that individual amino acid changes at positions 10, 63, 65, 75, 77, 79, and 131 on the major structural subunit DraE significantly reduced its CD55 binding capacity (57).
In culture-grown polarized epithelial intestinal cells that express CD55 at the brush border (2), infection by human Afa/Dr DAEC promoted cytoskeleton rearrangements (3, 17, 42), functional cellular injuries (40, 41), and proinflammatory responses (4, 5, 55). Most of these cellular injuries and responses follow the recognition of CD55 by Afa/Dr adhesins. Regarding these in vitro results, we conducted an experiment to examine in vivo the intestinal colonization and the epithelial cell injuries. For this purpose, we infected conventional mice with the human Afa/Dr DAEC strain C1845 (6) since conventional mice have been previously used to analyze the renal injuries by human uropathogenic Dr-positive E. coli (15, 26). Adult female germfree C3H/He/Oujco mice (Cesal, Orléans, France) 7 to 8 weeks of age previously used to investigate intestinal colonization by commensal E. coli (22) were orally infected. Results reported in Table 1 show that in infected mice an increase from proximal to distal intestine is observed for E. coli levels in both intestinal luminal content and corresponding washed tissue. Chappuis et al. (10) have previously demonstrated that mucosa-adhering bacteria are found in vivo at least at the same levels in the washed mucosa and in the intestinal content. Results show that C1845 bacteria were poorly associated with the mucosa of the gastrointestinal tract of infected mice, since the number of tissue-associated bacteria was always 10 or 100 times lower than in the intestinal contents. Therefore, our results indicate that there is no specific attachment of Afa/Dr DAEC strain C1845 to the murine intestinal epithelium. In addition, it should be noted that no sign of diarrhea and no sign of inflammation was found in the intestinal epithelium of C1845-infected mice.
TABLE 1.
Intestinal colonization in germfree mice infected with human E. coli strain C1845legend
| Intestinal segment | Intestinal content (log 10 CFU ± SD) | Washed intestinal wall (log 10 CFU ± SD) |
|---|---|---|
| Si1 | 5.9 ± 0.3 | 4.1 ± 0.4 |
| Si2 | 7.1 ± 0.4 | 4.7 ± 0.5 |
| Si3 | 7.8 ± 0.3 | 6.6 ± 0.3 |
| Caecum | 9.8 ± 0.4 | 7.8 ± 0.2 |
Germfree mice were reared in Trexler-type isolators fitted with a rapid transfer system (La Calhène, Vélizy Villacoublay Cedex, France), and mice were checked for freedom from bacterial contamination by culture of fresh feces aerobically and anaerobically. Mice were housed, fed, and sacrificed in accordance with the highest standards of humane animal care and the relevant national legislation. They were given ad libitum a commercial diet (RO3 40; UAR, Villemoisson/Orge, France) sterilized by gamma irradiation (40 kGy) and autoclaved demineralized water. Mice deprived of water from the day before infection received orally a suspension of E. coli strain C1845 (n = 6) in bottled water, i.e., approximately 2 × 106 CFU/mouse. A high population of E. coli (109 CFU/g of fresh feces) was detected 18 h after infection. Infected mice were killed by cervical dislocation 7 days after challenge. The different segments taken off were small intestine (divided in three segments corresponding approximately to the duodenum, jejunum, and ileum) and caecum. After the removal of the content, the intestinal wall was gently washed with eight successive 5-ml sterile PBS aliquots and drained before being weighed. All content samples were weighed and diluted 10-fold in PBS. Organs were weighed and homogenized with 2 ml of PBS with a Ultraturrax apparatus for 2 min and then diluted 10-fold. The number of viable bacteria in the samples was estimated by plating serial decimal dilutions on tryptic soy agar. Results are given as log 10 CFU ± standard deviation per gram of intestinal wall or per gram of content.
The results reported above showing that human E. coli bacteria expressing F1845 adhesin are not able to colonize the gastrointestinal epithelium of mice prompted us to examine whether or not this adhesin recognized mouse CD55. For this purpose, we conducted experiments with Chinese hamster ovary (CHO) cells stably transfected with mouse (GPI or transmembrane [TM] form) cDNAs (53). For a negative control, we used CHO cells transfected with empty vector pDR2EF1α. For a positive control, we used CHO cells stably transfected with human CD55 cDNA (53). CHO cells transfected with empty vector pDR2EF1α or human or mouse GPI or TM forms were grown in Dulbecco modified Eagle medium-Ham's F12 with l-glutamine (Invitrogen) supplemented with 5% fetal calf serum and 100 μg of hygromycin B (Invitrogen)/ml at 37°C in an atmosphere containing 5% CO2. The cells were infected with a recombinant E. coli strain obtained by transforming the E. coli AAEC185 strain (7), which lacks type 1 pili, with the recombinant plasmid that encodes F1845 adhesin (6). Bacteria were grown in cultures in Luria-Bertani broth at 37°C for 18 h with appropriate antibiotic. For each experiment, bacteria were washed three times with sterile phosphate-buffered saline (PBS) and recovered with Hanks' balanced salt solution (Invitrogen). Cells were inoculated with 108 CFU/ml, corresponding to a multiplicity of infection of 100 bacteria per cell. Infected monolayers were incubated for 3 h at 37°C in a humidified atmosphere containing 5% CO2. At the end of infection, monolayers were washed four times with sterile PBS to remove nonadhering bacteria and either fixed for immunofluorescence labeling or processed further to determine cell-associated CFU levels (CFU/milliliter). To quantitate total cell-associated bacteria, washed monolayers were lysed with 1% saponin for 10 min. Bacteria were suspended by vigorous pipetting, and levels of CFU/milliliter in the lysates were determined by plating of serial dilutions.
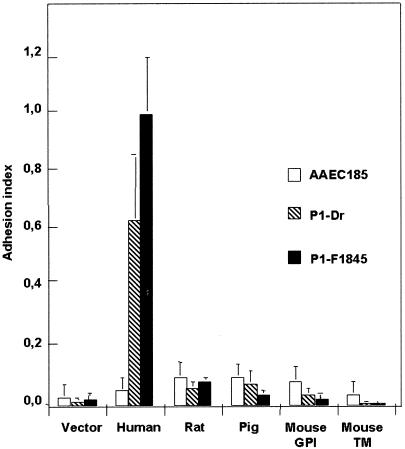
As shown in Fig. 1, recombinant E. coli bacteria expressing F1845 adhesin bound strongly onto CHO cells expressing human CD55 but not onto the CHO cells expressing mouse (TM and GPI anchored) CD55. This result prompted us to examine whether or nor other Afa/Dr adhesins such as Dr hemagglutinin (9) lack recognition of mouse CD55. For this purpose, transfected CHO cells were infected with recombinant E. coli bacteria obtained by transforming the E. coli AAEC185 strain with the recombinant plasmid that encodes Dr hemagglutinin. As shown in Fig. 1, Dr hemagglutinin did not recognize the TM- and GPI-anchored forms of mouse CD55. We examined whether or not Dr and C1845 adhesins recognized the rat and pig CD55 in CHO cells stably transfected with rat or pig CD55 cDNAs (53). In addition, considering that in mice, Crry is an evolutionarily unique, complement-regulatory protein that functions like CD55 to inhibit classical complement pathway C3 deposition on cell membranes (13, 27, 33, 48, 49), we examined whether or not Dr and F1845 adhesins bound onto the stably transfected CHO cells with mouse Crry cDNA. Results in Fig. 1 show that both Dr and C1845 failed to recognize the rat and pig CD55. Moreover, results show that Crry is not recognized as a receptor by recombinant E. coli bacteria expressing Dr or F1845 adhesins (indexes of adhesion for the AAEC strain, 0.037 ± 0.005; for P1-Dr, 0.015 ± 0.009; and for P1-F1845, 0.006 ± 0.002).
FIG. 1.
Recognition of human, rodent, and pig CD55 as receptor by Dr and F1845 adhesins. CHO cell lines expressing each CD55 were plated to confluency. Cell monolayers were inoculated with 108 bacteria/ml (E. coli strain AAEC185 [control] or recombinant strain AAEC185 expressing Dr [P1-Dr] or F1845 [P1-F1845]). After 3 h at 37°C monolayers were processed to determine CFU levels/milliliter. Indexes of cell-associated bacteria (CFU/milliliter) per cell line were calculated on the basis of the higher cell association level obtained for F1845-positive strain (P1-F1845) in CHO-human CD55 (vector-CHO, 1.58 × 104 ± 0.68 × 104 CFU/ml; human CD55-CHO, 1.85 × 106 ± 0.55 × 106 CFU/ml). Data shown represent mean results ± standard deviations of 10 experiments for each cell line. Similar results were obtained with human wild-type IH11128 and C1845 bacteria expressing Dr hemagglutinin and F1845 adhesin, respectively (data not shown).
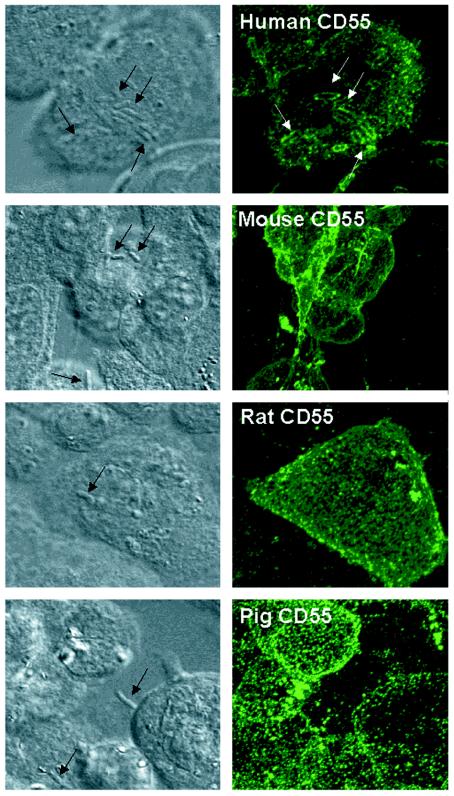
Clustering of human membrane-bound CD55 around human Afa/Dr DAEC bacteria adhering onto HeLa cells has been previously observed (16, 17). Transfected CHO cells infected with recombinant Dr-positive E. coli were processed for immunofluorescence labeling of human, rat, mouse, or pig CD55 with corresponding monoclonal antibodies (MAbs) (53) (Fig. 2). Positive clustering around Dr-positive bacteria was observed in human CD55-expressing CHO cells. In contrast, for the rarely adhering Dr-positive bacteria randomly distributed at the surface of CHO cells expressing mouse, rat, or pig CD55 CHO cells, no CD55 clustering developed around bacteria.
FIG. 2.
Observation of adherent F1845-positive bacteria and recruitment of CD55 in CHO cell lines expressing human, mouse, rat, or pig CD55. Left column, phase-contrast micrographs; right column, indirect immunofluorescence labeling of human, mouse, rat, and pig CD55 conducted with corresponding MAbs. Micrographs show optical sections (0.5 μm) representative of the apical part of the cells. Indirect immunofluorescence labeling of CHO cells transfected with human, mouse, rat, or pig CD55 cDNAs with corresponding MAbs shows a homogeneous distribution of a fine, punctuate labeling characteristic of GPI-anchored proteins. Clustering of positive immunofluorescence around F1845-positive bacteria was only observed in CHO cells transfected with human CD55 cDNA. Note that no labeling was found around F1845-positive bacteria plated on a glass coverslip and subjected to immunofluorescence labeling with anti-CD55 antibodies (data not shown).
Human CD55 functions as receptor for microbial pathogens, including Afa/Dr DAEC and several strains of enterovirus (24, 25, 47), coxsackievirus B (CVB) (50-52), and echovirus (EV) (1, 11, 21, 46, 54). CV strain A21 and enterovirus strain 70 were found to require sequences in CCP1 for binding (24, 50), while the majority of CD55-binding enteroviruses require CCP3 and CCP4 (47), although EV7 binding may require CCP2, CCP3, and CCP4 (11, 21). The specificity of binding with respect to Afa/Dr adhesins observed here resembles the specificity of binding of EV and CVB. Indeed, we have previously investigated enterovirus binding to transfected CHO cells abundantly expressing rodent or human CD55 and found EV serotypes 3, 6′, 7, 11 to 13, and 29 and CVB serotypes 1, 3, and 5 only recognized cells that expressed human CD55 (53). Molecular cloning of mouse CD55 has identified two forms of the molecule, one TM form and the other GPI anchored, and CHO cells expressing either form were protected from attack with mouse or rat complement (18). The pig analogue of CD55 only comprised three CCPs homologous with the amino-terminal three CCPs in human CD55 (therefore missing the equivalent to human CD55 CCP4), a serine/threonine-rich region, a TM domain, and a cytoplasmic tail (43). Although mouse and rat CD55 only have amino acid identities to human CD55 of approximately 60%, they function to inhibit complement as human CD55 (18, 53). Interestingly, human and rodent analogues of CD55 are not species restricted and highlight interesting differences in the capacity to regulate alternative and classical pathways (19). Consistent with the failure to bind rodent CD55, EV serotypes 3, 6′, 7, 11 to 13, and 29 and CBV serotypes 1, 3, and 5 were found to hemagglutinate human erythrocytes but not rat or mouse erythrocytes (53). Regarding these previous results and the results reported here, it is interesting that identical characteristics with respect to specificity of binding onto human CD55 have been developed by pathogenic bacteria and viruses.
Our results raise several questions concerning the animal models previously used to examine the pathogenicity of human Afa/Dr DAEC. It has been reported that the infection of mice with the human wild-type E. coli strain IH11128 bearing Dr adhesin induces tubulointerstitial nephritis, including interstitial inflammation, fibrosis, and tubular atrophy in the kidney tissue (15), and induces preterm delivery in pregnant mice (26). Goluszko et al. (15) reported that the renal interstitial E. coli binding mediated by Dr adhesin may be important for the development of chronic pyelonephritis. Indeed, an insertional inactivation of draC involved in assembly of functional adhesins of Afa/Dr family resulted in a dramatic decrease in expression of draE and the mutant strain DR14 fails to colonize the renal interstitium and to develop the histological changes. Importantly, a complementation of dra mutation restores both binding and lesions at the level of wild-type clinical isolate IH11128. Our results showing that Dr adhesin does not bind mouse CD55 do not support a mechanism involving the binding onto mouse CD55 for the colonization of renal tissue and the induction of interstitial inflammation, fibrosis, and tubular atrophy in mouse kidney tissue by human Dr-positive wild-type strain IH11128 bacteria. To explain the intriguing renal epithelial cell colonization by Dr-positive bacteria in mice (15, 26), it may be possible that another type of adhesion specific for renal epithelial cells exists in human uropathogenic Afa/Dr DAEC which remains to be characterized. One member of the family of human E. coli strains expressing Afa/Dr adhesins, the Dr-positive wild-type uropathogenic strain IH11128, has also acquired binding specificity for type IV collagen (9, 38, 56, 59, 60). Interestingly, Weissman et al. (58) indicated that the type-IV-collagen-binding phenotype is crucial for E. coli virulence in the mouse model of chronic pyelonephritis (R. Selvarangan et al., unpublished data).
Acknowledgments
We are grateful to R. Amsellem for her expert assistance in cell culture. We are also indebted to V. Nicolas for her kindly help in confocal image analysis (Imagerie Cellulaire, IFR75-ISIT, Faculté de Pharmacie Paris XI).
Editor: V. J. DiRita
REFERENCES
- 1.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut 38:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betis, F., P. Brest, V. Hofman, J. Guignot, M. F. Bernet-Camard, B. Rossi, A. Servin, and P. Hofman. 2003. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect. Immun. 71:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betis, F., P. Brest, V. Hofman, J. Guignot, I. Kansau, B. Rossi, A. Servin, and P. Hofman. 2003. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect. Immun. 71:1774-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilge, S. S., C. R. Clausen, W. Lau, and S. L. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomfield, I. C., M. S. McClain, J. A. Princ, P. J. Calie, and B. I. Eisenstein. 1991. Type 1 fimbriation and fimE mutants of Escherichia coli K-12. J. Bacteriol. 173:5298-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodbeck, W. G., L. Kuttner-Kondo, C. Mold, and M. E. Medof. 2000. Structure/function studies of human decay-accelerating factor. Immunology 101:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnoy, C., and S. L. Moseley. 1997. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol. Microbiol. 23:365-379. [DOI] [PubMed] [Google Scholar]
- 10.Chappuis, J. P., Y. Duval-Iflah, R. Ducluzeau, and P. Raibaud. 1985. Resistance of gnotobiotic large white and Chinese piglets to in vivo attachment of a K88ab enterotoxigenic Escherichia coli strain. Reprod. Nutr. Dev. 25:49-60. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson, N. A., R. Kaufman, D. M. Lublin, T. Ward, P. A. Pipkin, P. D. Minor, D. J. Evans, and J. W. Almond. 1995. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J. Virol. 69:5497-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyne, K. E., S. E. Hall, S. Thompson, M. A. Arce, T. Kinoshita, T. Fujita, D. J. Anstee, W. Rosse, and D. M. Lublin. 1992. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J. Immunol. 149:2906-2913. [PubMed] [Google Scholar]
- 13.Foley, S., B. Li, M. Dehoff, H. Molina, and V. M. Holers. 1993. Mouse Crry/p65 is a regulator of the alternative pathway of complement activation. Eur. J. Immunol. 23:1381-1384. [DOI] [PubMed] [Google Scholar]
- 14.Girardeau, J. P., L. Lalioui, A. M. Said, C. De Champs, and C. Le Bouguenec. 2003. Extended virulence genotype of pathogenic Escherichia coli isolates carrying the afa-8 operon: evidence of similarities between isolates from humans and animals with extraintestinal infections. J. Clin. Microbiol. 41:218-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goluszko, P., R. Selvarangan, V. Popov, T. Pham, J. W. Wen, and J. Singhal. 1999. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect. Immun. 67:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely-adhering family of Escherichia coli infecting the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, C. L., N. K. Rushmere, and B. P. Morgan. 1999. Molecular and functional analysis of mouse decay accelerating factor (CD55). Biochem. J. 341:821-829. [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, C. L., O. B. Spiller, and B. P. Morgan. 2000. Human and rodent decay-accelerating factors (CD55) are not species restricted in their complement-inhibiting activities. Immunology 100:462-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan, R. J., E. Pawelczyk, P. T. Urvil, M. S. Venkatarajan, P. Goluszko, J. Kur, R. Selvarangan, S. Nowicki, W. A. Braun, and B. J. Nowicki. 2002. Structure-function analysis of decay-accelerating factor: identification of residues important for binding of the Escherichia coli Dr adhesin and complement regulation. Infect. Immun. 70:4485-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He, Y., F. Lin, P. R. Chipman, C. M. Bator, T. S. Baker, M. Shoham, R. J. Kuhn, M. E. Medof, and M. G. Rossmann. 2002. Structure of decay-accelerating factor bound to echovirus 7: a virus-receptor complex. Proc. Natl. Acad. Sci. USA 99:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudault, S., J. Guignot, and A. L. Servin. 2001. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 49:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaper, J. B., J. P. Nataro, and H. L. T. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 24.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaul, A. K., S. Khan, M. G. Martens, J. T. Crosson, V. R. Lupo, and R. Kaul. 1999. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect. Immun. 67:5958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, Y. U., T. Kinoshita, H. Molina, D. Hourcade, T. Seya, L. M. Wagner, and V. M. Holers. 1995. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J. Exp. Med. 181:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuttner-Kondo, L. A., L. Mitchell, D. E. Hourcade, and M. E. Medof. 2001. Characterization of the active sites in decay-accelerating factor. J. Immunol. 167:2164-2171. [DOI] [PubMed] [Google Scholar]
- 29.Labigne-Roussel, A. F., D. Lark, G. Schoolnik, and S. Falkow. 1984. Cloning and expression of an afimbrial adhesin (Afa-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect. Immun. 46:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalioui, L., M. Jouve, P. Gounon, and C. Le Bouguenec. 1999. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect. Immun. 67:5048-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Bouguenec, C., M. I. Garcia, V. Ouin, J. M. Desperrier, P. Gounon, and A. Labigne. 1993. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect. Immun. 61:5106-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bouguenec, C., L. Lalioui, L. du Merle, M. Jouve, P. Courcoux, S. Bouzari, R. Selvarangan, B. J. Nowicki, Y. Germani, A. Andremont, P. Gounon, and M. I. Garcia. 2001. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J. Clin. Microbiol. 39:1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, B., C. Sallee, M. Dehoff, S. Foley, H. Molina, and V. M. Holers. 1993. Mouse Crry/p65. Characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J. Immunol. 151:4295-4305. [PubMed] [Google Scholar]
- 34.Lublin, D. M., and J. P. Atkinson. 1989. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7:35-58. [DOI] [PubMed] [Google Scholar]
- 35.Nowicki, B., J. P. Barrish, T. Korhonen, R. A. Hull, and S. I. Hull. 1987. Molecular cloning of the Escherichia coli O75X adhesin. Infect. Immun. 55:3168-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowicki, B., A. Labigne, S. Moseley, R. Hull, S. Hull, and J. Moulds. 1990. The Dr hemagglutinin, afimbrial adhesins AfaA-I and Afa-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect. Immun. 58:279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowicki, B., J. Moulds, R. Hull, and S. Hull. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 56:1057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 40.Peiffer, I., M. F. Bernet-Camard, M. Rousset, and A. L. Servin. 2001. Impairments in enzyme activity and biosynthesis of brush border-associated hydrolases in human intestinal Caco-2/TC7 cells infected by members of the Afa/Dr family of diffusely adhering Escherichia coli. Cell. Microbiol. 3:341-357. [DOI] [PubMed] [Google Scholar]
- 41.Peiffer, I., J. Guignot, A. Barbat, C. Carnoy, S. L. Moseley, B. J. Nowicki, A. L. Servin, and M. F. Bernet-Camard. 2000. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect. Immun. 68:5979-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peiffer, I., A. L. Servin, and M. F. Bernet-Camard. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez de la Lastra, J. M., C. L. Harris, S. J. Hinchliffe, D. S. Holt, N. K. Rushmere, and B. P. Morgan. 2000. Pigs express multiple forms of decay-accelerating factor (CD55), all of which contain only three short consensus repeats. J. Immunol. 165:2563-2573. [DOI] [PubMed] [Google Scholar]
- 44.Pham, T., A. Kaul, A. Hart, P. Goluszko, J. Moulds, S. Nowicki, D. M. Lublin, and B. J. Nowicki. 1995. dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect. Immun. 63:1663-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pham, T. Q., P. Goluszko, V. Popov, S. Nowicki, and B. J. Nowicki. 1997. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect. Immun. 65:4309-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell, R. M., T. Ward, D. J. Evans, and J. W. Almond. 1997. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J. Virol. 71:9306-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. 1999. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed] [Google Scholar]
- 48.Quigg, R. J., C. He, A. Lim, D. Berthiaume, J. J. Alexander, D. Kraus, and V. M. Holers. 1998. Transgenic mice overexpressing the complement inhibitor crry as a soluble protein are protected from antibody-induced glomerular injury. J. Exp. Med. 188:1321-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quigg, R. J., Y. Kozono, D. Berthiaume, A. Lim, D. J. Salant, A. Weinfeld, P. Griffin, E. Kremmer, and V. M. Holers. 1998. Blockade of antibody-induced glomerulonephritis with Crry-Ig, a soluble murine complement inhibitor. J. Immunol. 160:4553-4560. [PubMed] [Google Scholar]
- 50.Shafren, D. R. 1998. Viral cell entry induced by cross-linked decay-accelerating factor. J. Virol. 72:9407-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shafren, D. R., D. T. Williams, and R. D. Barry. 1997. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J. Virol. 71:9844-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiller, O. B., I. G. Goodfellow, D. J. Evans, J. W. Almond, and B. P. Morgan. 2000. Echoviruses and coxsackie B viruses that use human decay-accelerating factor (DAF) as a receptor do not bind the rodent analogues of DAF. J. Infect. Dis. 181:340-343. [DOI] [PubMed] [Google Scholar]
- 54.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tieng, V., C. Le Bouguenec, L. du Merle, P. Bertheau, P. Desreumaux, A. Janin, D. Charron, and A. Toubert. 2002. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc. Natl. Acad. Sci. USA 99:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Loy, C. P., E. V. Sokurenko, and S. L. Moseley. 2002. The major structural subunits of Dr and F1845 fimbriae are adhesins. Infect. Immun. 70:1694-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Loy, C. P., E. V. Sokurenko, R. Samudrala, and S. L. Moseley. 2002. Identification of amino acids in the Dr adhesin required for binding to decay-accelerating factor. Mol. Microbiol. 45:439-452. [DOI] [PubMed] [Google Scholar]
- 58.Weissman, S. J., S. L. Moseley, D. E. Dykhuizen, and E. V. Sokurenko. 2003. Enterobacterial adhesins and the case for studying SNPs in bacteria. Trends Microbiol. 11:115-117. [DOI] [PubMed] [Google Scholar]
- 59.Westerlund, B., and T. K. Korhonen. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9:687-694. [DOI] [PubMed] [Google Scholar]
- 60.Westerlund, B., P. Kuusela, J. Risteli, L. Risteli, T. Vartio, H. Rauvala, R. Virkola, and T. K. Korhonen. 1989. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3:329-337. [DOI] [PubMed] [Google Scholar]
- 61.Williams, P., Y. Chaudhry, I. G. Goodfellow, J. Billington, R. Powell, O. B. Spiller, D. J. Evans, and S. Lea. 2003. Mapping CD55 function. The structure of two pathogen-binding domains at 1.7 A. J. Biol. Chem. 278:10691-10696. [DOI] [PubMed] [Google Scholar]