Abstract
In order to identify regulators of the oxidative stress response in Enterococcus faecalis, an important human pathogen, several genes annotated as coding for transcriptional regulators were inactivated by insertional mutagenesis. One mutant, affected in the ef2958 locus (designated hypR [hydrogen peroxide regulator]), appeared to be highly sensitive to oxidative challenge caused by hydrogen peroxide. Moreover, testing of the hypR mutant by using an in vivo-in vitro macrophage infection model resulted in a highly significant reduction in survival compared to the survival of parent strain JH2-2. Northern blot analyses were carried out with probes specific for genes encoding known antioxidant enzymes, and they showed that the ahpCF (alkyl hydroperoxide reductase) transcript was expressed less in mutant cells. Mobility shift protein-DNA binding assays revealed that HypR regulated directly the expression of hypR itself and the ahpCF operon. Our combined results showed that HypR appeared to be directly involved in the expression of ahpCF genes under oxidative stress conditions and suggested that this regulator could contribute to the virulence of E. faecalis.
In their environments, bacteria have to sense and cope with different growth-restricting physicochemical stresses. In order to survive, microorganisms developed strategies for adaptation and resistance against multiple hostile conditions. During this adaptation, the expression of several stress-implicated genes was modified. This expression is usually under transcriptional initiation control by alternative sigma factors like σS and σB, which are general stress response regulators in Escherichia coli and Bacillus subtilis, respectively (17, 24). Furthermore, a σB homologous gene has also been identified in some nonsporulating gram-positive bacteria, like Listeria monocytogenes and Staphylococcus aureus (42, 43).
Enterococcus faecalis, a non-spore-forming gram-positive microorganism, can cause serious diseases and is one of the most common bacteria responsible for hospital-based infections (18). Nevertheless, despite the recognition of the clinical importance of enterococcal infections, their pathogenic mechanisms are not well understood (15). Surprisingly, no σB-like sigma factor gene has been found in the E. faecalis chromosome sequence (26) or in the closely related species Lactococcus lactis (3), and little information is available about the regulation of gene expression during stress conditions. Workers in our laboratory have identified some general stress proteins in E. faecalis, but the corresponding regulators of the genes are still unknown (14, 29). Furthermore, Le Breton and coworkers have recently analyzed nine two-component system regulators. When transcriptional and mutational approaches were used, eight of these regulators appeared to be implicated in stress responses in E. faecalis. However, none of the mutants were more sensitive to oxidative stress (25, 41).
Oxidative stress is one of the common stresses encountered by bacteria in many environments, especially during the infection process due to the immune response. Under such conditions, many genes encoding antioxidant enzymes are induced in order to shield the microorganisms against reactive oxygen species. The two main specific regulators implicated in the oxidative stress response, OxyR and SoxS, were first described in E. coli (8, 16, 34, 40) and Salmonella enterica serovar typhimurium (7). PerR, a functional analogue of OxyR, has been characterized as the major regulator of the inducible peroxide stress response in B. subtilis (5, 6, 19).
The oxidative stress response of E. faecalis has been analyzed in our laboratory. Physiological experiments revealed that this bacterium was able to strongly resist a hydrogen peroxide treatment and developed significant H2O2 cross-protection (10). By using two-dimensional electrophoresis, 23 H2O2-induced proteins have been detected (10). Moreover, several genes involved in the oxidative stress response, such as the ahpCF (alkyl hydroperoxide reductase), npr (NADH peroxidase), sodA (superoxide dismutase), and katA (catalase) genes, are present in the chromosome of E. faecalis, and recently, the catalase activity of this bacterium has been demonstrated (11). However, until now, no oxidative stress-specific regulator has been described in E. faecalis. In this work, we proved that a mutation in the ef2958 gene, now designated hypR, sensitized E. faecalis to an H2O2 treatment and greatly affected survival in murine peritoneal macrophages. Transcriptional analysis revealed that the hypR and ahpCF genes were repressed in the mutant, thus providing the first evidence of a new oxidative stress regulon in E. faecalis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. faecalis strain used in this study was JH2-2 (21, 44). E. coli XL1Blue (Stratagene, La Jolla, Calif.) was used as a recipient strain for internal fragment cloning, and pUCB300 was used as a cloning and integrational vector (12). Cultures of E. faecalis were grown at 37°C without shaking in 20-ml glass tubes containing 10 ml of semisynthetic medium (Bacto Folic AOAC medium; Difco, Detroit, Mich.) supplemented with 0.2% glucose. For plate counting, a sample was taken, immediately diluted in 0.9% NaCl, and poured onto M17 (38) agar (1.5% [wt/vol] agar [Difco]) supplemented with 0.5% (wt/vol) glucose. The plates were incubated at 37°C for 48 h. E. coli strains were cultivated with vigorous agitation at 37°C in Luria-Bertani medium (31) with erythromycin (300 μg/ml) when required.
General molecular methods.
Restriction endonucleases, alkaline phosphatase, and T4 DNA ligase were obtained from Roche (Mannheim, Germany) and were used according to the manufacturer's instructions. Each PCR was carried out in a 25-μl reaction mixture containing 5 μg of chromosomal DNA of E. faecalis by using Ready To Go PCR beads (Pharmacia Biotech, Little Chalfont, United Kingdom). The annealing temperature was 5°C below the melting temperature of the primers, and the PCR products were purified with a QIAquick kit (QIAGEN, Valencia, Calif.) before they were cloned into the SmaI site of the vector. E. coli and E. faecalis were transformed by electroporation by using a Gene Pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) as described by Dower et al. (9). Plasmids were purified by using QIAprep Miniprep (QIAGEN). Other standard techniques were carried out as described by Sambrook et al. (31).
Construction of the hypR (previously ef2958) insertional mutant.
To construct an insertional mutant with a disruption in the E. faecalis hypR gene, a 342-bp fragment of the hypR gene (obtained with the hypint5 and hypint3 primers [Table 1 ]) was ligated with the insertional vector pUCB300 which had been digested with SmaI. The resulting plasmid obtained after transformation of E. coli XL1Blue was used to transform competent cells of E. faecalis JH2-2. Erythromycin-resistant colonies were selected on agar plates containing 150 μg of erythromycin per ml. Integration were verified by PCR and Southern blot analysis.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′ to 3′) | Position in the corresponding gene (nucleotide no.)a |
|---|---|---|
| hypR gene | ||
| hypint5 | TAGCGAATAAAACAGTCACC | 220 to 239 |
| hypint3 | AACTTGTGCTTGTCGAGAAA | 599 to 581 |
| hyprace1 | CATGCAATTGTTTGGCTGCG | 78 to 58 |
| hyprace2 | GTCACGCCTAATTCTTCCTC | 136 to 117 |
| hyprace3 | CATAGCTTTTGTTTCGCCGC | 305 to 286 |
| hyptot5 | GCGGATCCATGGAATTACGAGTGATTCACTATTTCTTAGCAGTGGb | 1 to 37 |
| hyptot3 | GGGGTACCTTATTCAGGGCGTTGAATACTTTTTTTTATTTGTTCc | 884 to 849 |
| Phyp5 | CGTGGCAAGAAGATTCCTTAC | −272 to −252 |
| Phyp3 | GTTTGGCTGCGCCACTAATCG | 69 to 49 |
| npr gene | ||
| npr5 | TGTTCGCTATATGACTGGCG | 170 to 189 |
| npr3 | CGATTTTTTGCACACGACCG | 678 to 659 |
| ahpCF operon | ||
| ahpC5 | TGTGATGCCTATCACGATGG | 36 to 55 |
| ahpC3 | CGTTTCTGTAGCGTCAGCCC | 263 to 244 |
| Pahp5 | GTTCCTCTTGGTGATGGAGT | −306 to −287 |
| Pahp3 | CACACGAAAGAAAAATCAGC | 139 to 120 |
Position 1 corresponds to the A of the ATG start codon.
The BamHI restriction site is underlined.
The KpnI restriction site is underlined.
H2O2 challenge conditions.
Wild-type and mutants cells (grown as described above) were harvested at an optical density at 600 nm of 0.5 by centrifugation and were resuspended in 0.9% NaCl with 20 mM H2O2. The cultures were placed into a 37°C water bath, and at the desired time, samples were taken for plate counting. Numbers of CFU were determined after 48 h of incubation at 37°C. Each data point below is the average of the data for at least three experiments with duplicate plating. The level of survival at any given time point was determined by determining the ratio of the number of CFU after treatment to the number of CFU at zero time.
RNA isolation, mapping of the transcriptional start site, and Northern blot analysis.
Total RNA of E. faecalis was isolated from exponentially growing cells or stressed cells (30 min at pH 4.8, adjusted with lactic acid or with 2.4 mM H2O2, which has been shown to be the best adaptation conditions for E. faecalis [10]) by using an Rneasy Midi kit (QIAGEN). The transcriptional start point of hypR was determined by using a RACE 5′/3′ kit (Roche) with the hyprace1, hyprace2, and hyprace3 primers (Table 1) according to the manufacturer's instructions. Northern blots of exactly 5 μg of electrophoresed RNA were prepared by using Hybond-N+ membranes and standard procedures (28). The sizes of transcripts were estimated by comparing their mobilities with the band mobilities of standards in an RNA ladder (0.56 to 9.4 kb; Amersham International, Little Chalfont, United Kingdom). Membrane-bound nucleic acids were hybridized at a temperature 5°C below the melting temperature with radioactively labeled probes that were prepared by PCR amplification of an internal fragment of each targeted gene with Taq DNA polymerase and by using [α-32P]dATP. The primers used are listed in Table 1. The membranes were then exposed to a storage phosphor screen (Packard Instrument Company, Canberra, Australia) for 5 h. The intensity levels of transcripts were determined by using the Optiquant image analysis software (Packard Instrument Company). This software calculates the number of digital light units per square millimeter.
Overproduction and purification of HypR.
E. faecalis HypR was overproduced and purified as a hybrid protein with a His6 tag fused to its N terminus. First, the hypR gene was amplified by PCR (by using the hyptot5 and hyptot3 primers [Table 1]) and cloned into the pQE30 vector (QIAGEN). The protein was overproduced in E. coli M15(pREP4) carrying pQE30:hypR. E. coli was grown in 200 ml of 2× TY medium supplemented with ampicillin (100 μg/ml) and kanamycin (25 μg/ml) to an optical density at 600 nm of 0.5. HypR was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h at 37°C. Cells were harvested by centrifugation, washed in 20 ml of buffer I (50 mM Tris HCl [pH 7.5], 50 mM Na2SO4, 15% glycerol), harvested again by centrifugation, resuspended in 5 ml of buffer I, and then disrupted by one passage through the One Shot Cell Disrupter system (ConstantSystem, Northants, United Kingdom) at 2.15 × 108 Pa. The lysate was centrifuged at 13,000 × g for 30 min at 4°C, and the soluble fraction of proteins was recovered. His-tagged HypR was purified by using Ni-nitrilotriacetic acid resin (QIAGEN) according to the manufacturer's recommendations. Proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and visualized by Coomassie brilliant blue staining.
Gel mobility shift assay.
DNA fragments (about 300 bp) corresponding to the different promoter regions analyzed were amplified and labeled by PCR with [α-32P]dATP. The promoter region for npr has been identified by Ross and Claiborne (30), and for ahpCF and sodA the transcriptional start sites have been mapped (unpublished data). Each labeled DNA was incubated with purified HypR (7.8 ng to 4.0 μg) in interaction buffer [40 mM Tris HCl [pH 7.5], 200 μg of bovine serum albumin per ml, 2 mM CaCl2, 2 mM dithiothreitol, 20 μg of poly(dI-dC) per ml] at room temperature for 30 min. The DNA-HypR mixtures were electrophoresed on a 12.5% polyacrylamide gel in 0.5× Tris-borate-EDTA at 180 V. Gels were dried and analyzed by autoradiography.
Assays of survival in mouse peritoneal macrophages.
Survival of E. faecalis in mouse peritoneal macrophages was tested by using an in vivo-in vitro infection model as described previously (12). Briefly, the E. faecalis hypR mutant and JH2-2 were grown aerobically at 37°C in brain heart infusion (BHI) for 16 h, while E. coli DH5α was grown in Luria-Bertani broth and used as a negative control. Then the bacteria were pelleted and resuspended in an adequate volume of phosphate-buffered saline for injection. Male BALB/c mice (10 weeks old; Harlan Italy S.r.l., San Pietro al Natisone, Udine, Italy) were infected with 107 to 108 cells of each strain by intraperitoneal injection. After a 6 h-infection period, the peritoneal macrophages were collected by peritoneal lavage, centrifuged, and suspended in Dulbecco's modified Eagle's medium containing 10 mM HEPES, 2 mM glutamine, 10% bovine fetal serum, and 1× nonessential amino acids supplemented with vancomycin (10 μg/ml) and gentamicin (150 μg/ml). The cell suspension was dispensed into 24-well tissue culture plates and incubated at 37°C under 5% CO2 for 2 h. After exposure to antibiotics to kill extracellular bacteria (i.e., at 8 h postinfection), the infected macrophages were washed, and triplicate wells of macrophages were lysed with detergent. After dilution with BHI broth, the lysates were plated on BHI agar to quantitate the viable intracellular bacteria. The remaining wells containing infected macrophages were maintained in Dulbecco's modified Eagle's medium with the antibiotics for the duration of the experiment. The same procedure was performed at 24, 48, and 72 h postinfection. In the meantime, at 8, 24, 48, and 72 h postinfection, supernatant fluid was removed from each well, and extracellular bacteria were quantitated by counting on BHI agar plates. To assess viability, macrophages were detached from tissue culture wells with cell scrapers and stained with trypan blue, and the viable macrophages were counted with a hemacytometer. All experiments were performed three times, and the results were subjected to statistical analysis by using one-way analysis of variance with a Bonferroni correction posttest with the SPSS statistical software.
Complementation of the hypR mutant.
To complement the hypR gene in trans, a 1,156-bp PCR fragment containing hypR and its promoter (obtained with primers Phyp5 and hyptot3 [Table 1]) was cloned into the low-copy-number plasmid pNZ273 (Cmr) (27). The resulting vector (pNVhyp) was then transformed into the hypR mutant. Proper construction was verified by sequencing. In order to compare the phenotypes of the complemented strain and the wild type, plasmid pNZ273 was introduced into strain JH2-2 and the hypR mutant strain to render them chloramphenicol resistant. Assays of survival in mouse peritoneal macrophages were carried out as described above.
RESULTS
Identification of an E. faecalis mutant strain sensitive to H2O2 challenge.
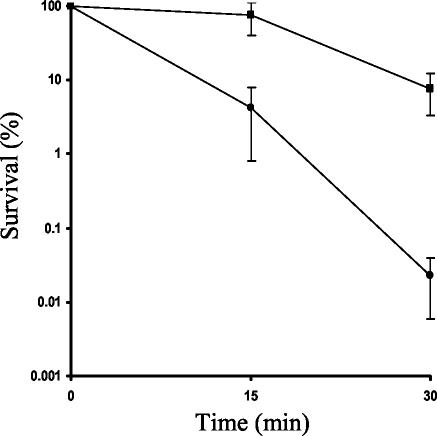
An in silico analysis by using the E. faecalis V583 genome sequence provided by The Institute for Genomic Research was carried out in order to find polypeptides homologous to bacterial regulators known to be involved in the oxidative stress response. Then our homology searches revealed that five main transcriptional regulators, members of the LysR family (encoded by ef0644, ef1656, ef1710, ef1815, and ef2958), were slightly homologous to E. coli OxyR (17 to 23.1% identity). Mutants with mutations in the corresponding genes were constructed in the JH2-2 strain by insertional mutagenesis and then tested with oxidative stresses. Only mutants affected in the ef2958 locus, which encoded the protein that exhibited the highest level of homology to OxyR, appeared to be 335-fold more sensitive after 30 min in the presence of 20 mM H2O2 than the wild-type strain (Fig. 1). It is noteworthy that the growth rate of the ef2958 mutant was identical to that of the wild-type strain (data not shown). Furthermore, the rates of survival after an H2O2 challenge following a 30-min adaptation with a 2.4 mM H2O2 pretreatment were the same for the two strains (data not shown). Moreover, no difference in survival was observed between the wild type and the ef2958 mutant after oxidative stress caused by incubation for 30 min with 200 mM t-butyl hydroperoxide, 10 mM cumene hydroperoxide, or 500 mM menadione or after other challenges, including an acid pH (pH 3.7 for 30 min), heat shock (62°C for 30 min), or incubation with bile salts (0.3% bile salts for 15 s), arguing that there is specificity of this regulator for coping with H2O2 in nonadapted cells. Therefore, the locus was designated hypR (hydrogen peroxide regulator), and in subsequent experiments we focused on this putative transcriptional regulator.
FIG. 1.
Survival of E. faecalis JH2-2 (▪) and hypR mutant (•) growing cells at 15 and 30 min after challenge with 20 mM H2O2. A value of 100% corresponds to the number of CFU before H2O2 was applied. The values are the averages of results obtained in at least three independent experiments.
Effect of the hypR mutation on the expression of genes involved in the oxidative stress response.
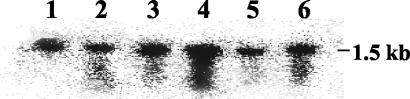
In order to characterize the HypR regulon and to correlate the phenotype of the mutant with the regulation of genes involved in the oxidative stress response, transcriptional analyses were carried out. A previous study reported that OxyR purified from E. coli was able to bind to the promoter region of the NADH peroxidase gene (npr) from E. faecalis (30). In order to analyze if HypR, which is weakly related to OxyR, regulates the npr gene in E. faecalis, Northern blotting was performed with RNA isolated from the wild-type and hypR mutant strains. Experiments were carried out with total RNA extracted from cells in the exponential growth phase (control) and from bacteria cultured with 2.4 mM H2O2 and at pH 4.8 (adjusted with lactic acid), which were used as an internal control. A signal corresponding to the expected size of the npr transcript (approximately 1.5 kb) was observed (Fig. 2). Our results and quantification of the level of transcription revealed that a mutation in the hypR locus did not significantly modify the npr expression in any situation examined, even if slight induction was observed after a H2O2 treatment (Fig. 2).
FIG. 2.
Northern blot analysis of E. faecalis RNA. Samples of total RNA were prepared from wild-type (lanes 1, 3, and 5) and hypR mutant (lanes 2, 4, and 6) cells in the exponential growth phase (lanes 1 and 2) after treatment with 2.4 mM H2O2 (lanes 3 and 4) and treatment at pH 4.8 (pH adjusted with lactic acid) (lanes 5 and 6). Hybridization was performed with a probe specific for the npr gene (by using the npr5 and npr3 primers [Table 1]). The deduced size of the transcript is indicated on the right.
We then hybridized the same RNAs with probes specific for other genes encoding antioxidant enzymes. Hybridization experiments were performed with a probe specific for the sodA gene encoding the superoxide dismutase, and they revealed a signal corresponding to a 0.6-kb monocistronic transcript. No significant difference in sodA expression between the wild-type strain and the hypR mutant was observed under H2O2 stress conditions (data not shown). Moreover, no signal was detected in our conditions by using probes complementary to the gene encoding the catalase of E. faecalis (data not shown).
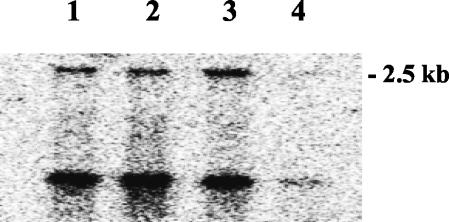
Northern blot analysis with an ahpC-specific mRNA probe allowed us to detect two transcripts (Fig. 3). The size of the larger transcript, which was nearly 2.5 kb long, corresponded to the expected size of the bicistronic ahpCF operon. The short transcript might have been due to degradation, which has also been observed in B. subtilis (2). As shown in Fig. 3, the ahpCF operon was down regulated in the hypR mutant after oxidative stress caused by H2O2 (Fig. 3, lane 4).
FIG. 3.
Transcriptional analysis of the ahpCF operon. Samples of total RNA were prepared from wild-type (lanes 1 and 3) and hypR mutant (lanes 2 and 4) cells in the exponential growth phase (lanes 1 and 2) and after treatment with 2.4 mM H2O2 (lanes 3 and 4). Hybridization was performed with a probe specific for the ahpC gene obtained with the ahpC5 and ahpC3 primers (Table 1). The deduced size of the transcript is indicated on the right.
In order to verify all our transcriptional data, quantitative real-time PCR were carried out. The levels of expression were normalized to the expression of the constitutively expressed gene encoding the 23S rRNA, which showed similar levels for all samples. We confirmed that both ahpC and ahpF were down regulated at the same level in the hypR mutant under H2O2 stress conditions, while no significant difference was observed with the sodA and npr genes (data not shown).
Protein-DNA binding test for HypR.
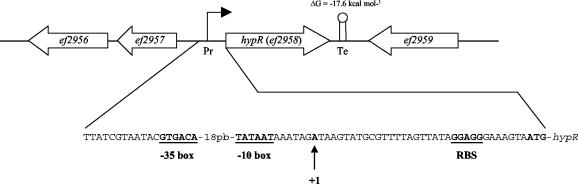
To determine whether HypR really functions as a DNA binding protein, His6-tagged HypR was produced and purified, and gel retardation experiments were performed with different 32P-labeled promoters. First, we tested the interaction of HypR with the promoter region of the hypR locus itself. Indeed, HypR is included in the LysR family represented by transcriptional regulators which are able to control positively a wide range of genes and are known to be autorepressors (32). To precisely locate the promoter, the transcriptional start point of the hypR gene was determined. Note that a Northern blot analysis with a specific labeled probe for hypR was performed, but no signal was detected; however, data obtained from amplification of cDNA ends-PCR proved that hypR is transcribed in E. faecalis. We concluded that the A at 34 bp upstream of the ATG codon of hypR corresponds to position 1 of the transcript. A perfect −10 (TATAAT) box located seven nucleotides upstream from the transcriptional start point and a sequence exhibiting similarity to a −35 box (GTGACA [difference underlined]) separated by 18 bp were identified (Fig. 4). Furthermore, the hypR locus appeared to be followed by an inverted repeat (ΔG = −17.6 kcal mol−1), which may be a transcriptional terminator (Fig. 4). Interestingly, identification of this position 1 occurred only with mRNA extracted from cells cultivated in the presence of 2.4 mM H2O2. More investigations are required to determine if hypR is overexpressed under oxidative stress conditions.
FIG. 4.
Mapping of the 5′ ends of the hypR mRNA by amplification of cDNA ends-PCR analysis. The transcriptional start site is indicated by an arrow. Potential −10 and −35 regions and the ribosome binding site (RBS) sequence are indicated by boldface type and underlining, and the start codon is indicated by boldface type.
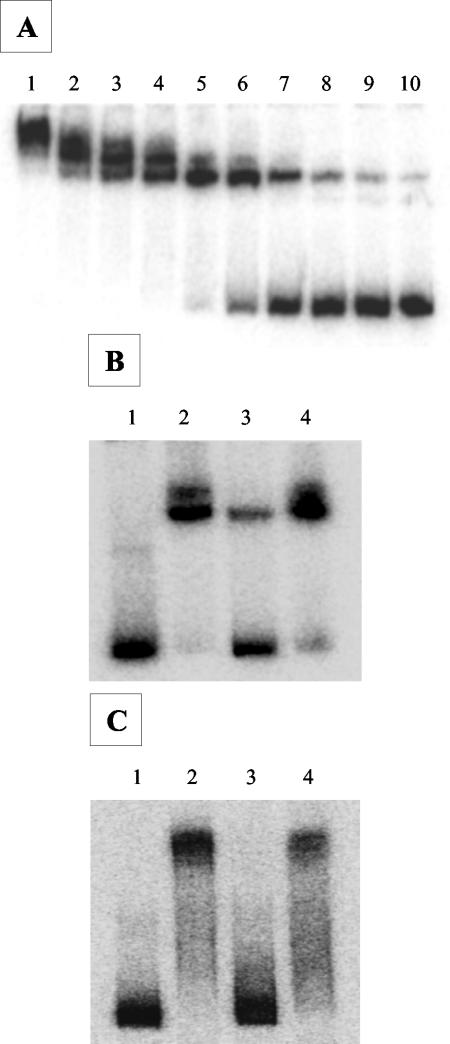
As expected, the mobility shift protein-DNA binding assay showed that purified HypR specifically binds the region upstream of the translational start site of hypR (Fig. 5A and B). An HypR concentration-dependent shift was observed (Fig. 5A). Based on these results, the following experiments were carried out with 0.25 and 0.5 μg of protein (Fig. 5A, lanes 4 and 5). Addition of an unlabeled hypR promoter as a competitor (Fig. 5B, lane 3) resulted in a loss of the shift compared with addition of a nonspecific DNA fragment (Fig. 5B, lane 4).
FIG. 5.
Electrophoretic mobility shift assay of HypR binding to the promoter region of hypR and ahpCF. (A) Effect of HypR concentration. Several half-dilutions of purified HypR were incubated with a 32P-labeled hypR promoter region obtained with the Phyp5 and Phyp3 primers (Table 1). Lanes 1 to 10 contained 4 μg, 2 μg, 1 μg, 0.5 μg, 0.25 μg, 0.125 μg, 62.5 ng, 32 ng, 16 ng, and 8 ng of HypR, respectively. (B and C) Specificity of the HypR-DNA interaction. Purified HypR was incubated with a 32P-labeled hypR (B) or ahpCF (C) promoter region obtained with the Pahp5 and Pahp3 primers (Table 1). DNA fragments were incubated without protein (lane 1), with HypR (lane 2), with an unlabeled competitor (lane 3), and with a nonspecific DNA fragment (lane 4).
Then participation of HypR as a direct regulator of ahpCF operon was examined. As shown in Fig. 5C, HypR binds in a specific manner to the promoter region upstream of the ahpC locus, indicating that HypR may participate in the control of expression of the corresponding alkyl hydroperoxide reductase genes. Finally, no shift was observed when the npr or sodA promoter regions were tested (data not shown).
Effect of the hypR mutation on survival within mouse peritoneal macrophages.
Gentry-Weeks et al. (13) demonstrated that E. faecalis was able to persist for an extended period in mouse peritoneal macrophages by using a well-established infection model which consisted of infecting BALB/c mice intraperitoneally, recovering infected macrophages 6 h later, and then monitoring over a 72-h period the survival of intracellular bacteria within peritoneal macrophages maintained in vitro.
In order to assess if the hypR gene affected the ability of E. faecalis to resist killing by macrophages, the intracellular survival of the E. faecalis hypR mutant and the intracellular survival of the wild-type strain were monitored by determining the numbers of viable bacteria inside infected mouse peritoneal macrophages over the 72-h time course (Fig. 6). In these studies, the nonpathogenic strain E. coli DH5α was used as a negative control, since this strain is known to be susceptible to killing by mouse peritoneal macrophages (13).
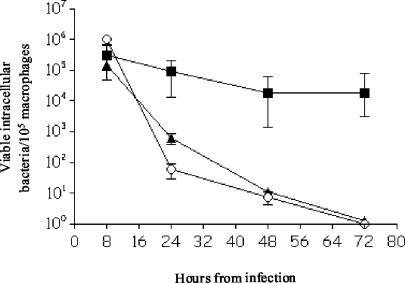
FIG. 6.
Time course of intracellular survival of the E. faecalis and E. coli DH5α strains within murine peritoneal macrophages. The data are the means ± standard deviations for the number of viable intracellular bacteria per 105 macrophages in three independent experiments with three wells. ▪, E. faecalis JH2-2; ▴, hypR mutant; ○, E. coli DH5α.
No significant difference was observed in the levels of the E. faecalis strains recovered 8 h postinfection, suggesting that the two strains possessed similar abilities to infect macrophages (Fig. 6). However, E. faecalis JH2-2 was superior to E. faecalis hypR in the ability to survive intracellularly at the 24-, 48-, and 72-h time points (P < 0.0001). Interestingly, the number of viable E. faecalis hypR organisms decreased more rapidly over the 72-h time course; there was an initial reduction of 2 log units between 8 and 24 h postinfection and a similar reduction between 24 and 48 h postinfection, followed by a decline of 1 log unit between 48 and 72 h postinfection. In contrast, E. faecalis JH2-2 exhibited a reduction of approximately 0.5 log unit over the 72-h period. As expected, E. coli DH5α was efficiently killed by the macrophages (Fig. 6).
Complementation of hypR gene.
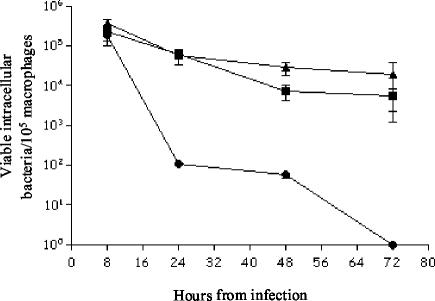
To confirm that the effect on survival within macrophages of the E. faecalis hypR mutant was due to the loss of a functional hypR gene, we complemented the mutant with plasmid pNVhyp, which contained a fragment encoding the regulator. As shown in Fig. 7, this construction restored the survival to nearly the wild-type level, indicating that the phenotype can be complemented with the hypR function provided in trans. The hypR mutant and strain JH2-2 transformed with the pNZ273 empty vector were used as controls.
FIG. 7.
Complementation of hypR in trans restores survival within murine peritoneal macrophages. ▴, E. faecalis JH2-2 (wild type) carrying the empty vector pNZ273; •, hypR mutant carrying the empty vector pNZ273; ▪, hypR mutant carrying plasmid pNVhyp.
DISCUSSION
Whereas the regulation of the oxidative stress response in E. coli or B. subtilis is well known, little information is available for non-spore-forming gram-positive bacteria like E. faecalis. OxyR was first defined in E. coli and S. enterica serovar Typhimurium as an activator that conferred increased resistance to H2O2 and led to elevated expression of H2O2-inducible proteins, such as hydroperoxidase I and alkyl hydroperoxide reductase (7). In B. subtilis, the major regulator of the inducible peroxide stress response genes was designated PerR (5, 6). The PerR regulon includes proteins such as alkyl hydroperoxide reductase or catalase (19). Thus, the main challenge of this work was to identify in E. faecalis transcriptional regulators specifically involved in such responses. An in silico analysis was performed to obtain, among the approximately 200 proteins annotated as potential regulators in E. faecalis, one or more putative candidates likely to be implicated in the oxidative stress response. We then constructed mutants affected in genes coding for five transcriptional regulators of the LysR family of E. faecalis (41). These proteins showed weak homology to OxyR from E. coli. One of the mutants (Ef2958, with the protein most homologous to OxyR) was much more sensitive to the H2O2 treatment than the wild-type strain, and the corresponding gene was designated hypR. The fact that the presence of several paralogs was not able to compensate for the hypR mutation phenotype proved the crucial role of this gene in the H2O2 oxidative stress response of E. faecalis. It should be noted that one characteristic of E. faecalis is the ability to generate superoxide and H2O2. For the OG1RF strain cultivated in an oxygenated buffer, the amount produced was 17.2 nmol of O2-/min/10 CFU. Under these conditions, the cells also produced 23 nmol of H2O2/min/CFU that was generated by spontaneous disproportionation of O2− (20). These values are very low compared to the 20 mM H2O2 used in our experiments carried out without agitation.
Our study showed that HypR is a new transcriptional regulator involved in oxidative stress. Indeed, in spite of weak homology, HypR appeared to be the polypeptide most closely related to OxyR from E. coli. Whereas OxyR contains two cysteines (at positions 199 and 208) necessary to sense oxidative stress and to convert it to the oxidative active form, no cysteine is present in the amino acid sequence of HypR (35, 45). It is also noteworthy that the HypR sequence did not exhibit significant homology with other previously characterized transcriptional regulators. Moreover, previous investigations revealed that OxyR from E. coli bound in a specific manner the promoter region of the NADH peroxidase gene (npr) from E. faecalis (30), which is not the case with HypR. Furthermore, a mutation in the HypR locus did not significantly modify the npr expression in our stress conditions. Taken together, our results revealed that HypR and OxyR are not functionally related, and these observations argue that HypR is a new oxidative stress response regulator.
The transcriptional and DNA-protein binding test approaches allowed us to define the first members of the HypR regulon. This regulon contained hypR itself and the ahpCF operon, encoding alkyl hydroperoxide reductase. The latter operon was down regulated in the HypR mutant background only under oxidative stress conditions. The ahpCF operon is under OxyR control in gram-negative bacteria and is regulated by PerR in B. subtilis. The alkyl hydroperoxide reductase was originally identified as an enzymatic activity responsible for the increased resistance of S. enterica serovar Typhimurium oxyR mutant cells to various alkyl hydroperoxides (1, 22). Later, it was shown that this enzyme is the primary scavenger of endogenous hydrogen peroxide in E. coli (33). Thus, it may be a good candidate to explain the higher sensitivity of the E. faecalis hypR mutant to H2O2 stress. However, inactivation of the ahpC gene in B. subtilis leads to development of expression from peroxide regulon promoters, and, as a result, the mutant displayed increased resistance to H2O2 (4). Thus, it will be interesting to see what role the ahpCF operon plays in the hydrogen peroxide stress response in E. faecalis.
As expected, the protein-DNA binding tests allowed us to confirm that HypR seems to regulate its own expression. These experiments also showed that HypR may directly control the expression of the alkyl hydroperoxide reductase. Interestingly, it has been reported that putative Per or Fur boxes are found upstream from the ahpCF operon of E. faecalis (39), suggesting that there is complex regulation of this operon. However, from our Northern blots, it seems that the ahpCF operon is not inducible by hydrogen peroxide stress, as is the case in the PerR-regulated homologous structure of B. subtilis (2).
HypR appears to be the first specific transcriptional regulator involved in the oxidative stress response of E. faecalis, and further study is needed to address whether the hypR gene in E. faecalis also regulates other virulence factors. This response, which relies upon the production of enzymes that inactivate reactive oxygen species generated by the oxidative burst, may explain the ability of E. faecalis to survive inside human neutrophils and macrophages. It has been hypothesized that survival and sequestration within macrophages may contribute to the pathogenesis of E. faecalis infections (23).
Recently, the resistance to killing by neutrophils and macrophages was investigated in E. faecalis strains bearing aggregation substance (AS), one of the few virulence factors postulated for E. faecalis (28, 36). These studies demonstrated that the AS promotes the intracellular survival of E. faecalis within phagocytic cells, suggesting that the AS may be a virulence factor used by some strains of E. faecalis. In addition, in interesting work Murray's group found that another virulence factor, the enterococcal polysaccharide antigen, confers some protection against human phagocytic killing (37). In contrast, studies by Gentry-Weeks et al. (13) indicated that other virulence factors, such as cytolisin or gelatinase, had no effect on intracellular survival in mouse peritoneal macrophages. Quantitation of viable, intracellular bacteria in infected macrophages revealed that the viability rates for the hypR and JH2-2 E. faecalis strains were significantly different over the 72-h infection period and that the wild-type strain was able to survive better intracellularly. Reinforced by the complementation experiments, our data suggest that HypR may play a role in macrophage survival, even though more investigations are required for a complete understanding of the definite mechanisms by which E. faecalis persists inside macrophages.
Acknowledgments
The expert technical assistance of Annick Blandin, Béatrice Gillot, Marie-Jeanne Pigny, and Valerio Cardinali is greatly appreciated. We thank A. Benachour, J.-M. Laplace, A. Rincé, and V. Pichereau for helpful discussions.
Editor: F. C. Fang
REFERENCES
- 1.Altuvia, S., M. Almiron, G. Huisman, R. Kolter, and G. Storz. 1994. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol. Microbiol. 13:265-272. [DOI] [PubMed] [Google Scholar]
- 2.Antelmann, H., S. Engelmann, R. Schmid, and M. Hecker. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolotin, A., S. Mauger, K. Malarme, S. D. Ehrlich, and A. Sorokin. 1999. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek 76:27-76. [PubMed] [Google Scholar]
- 4.Bsat, N., L. Chen, and J. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologs: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christman, M. F., R. W. Morgan, F. S. Jacobson, and B. N. Ames. 1985. Positive control of a regulon for defences against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753-762. [DOI] [PubMed] [Google Scholar]
- 8.Christman, M. F., G. Storz, and B. N. Ames. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. USA 86:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dower, W. J., J. F. Miller, and C. W. Ragsdal. 1988. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acid Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flahaut, S., J. M. Laplace, J. Frère, and Y. Auffray. 1998. The oxidative stress response in Enterococcus faecalis: relationship between H2O2 tolerance and H2O2 stress proteins. Lett. Appl. Microbiol. 26:259-264. [DOI] [PubMed] [Google Scholar]
- 11.Frankenberg, L., M. Brugna, and L. Hederstedt. 2002. Enterococcus faecalis heme-dependent catalase. J. Bacteriol. 184:6351-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frère, J., M. Novel, and G. Novel. 1993. Molecular analysis of the Lactococcus lactis subspecies lactis CNRZ270 bidirectional theta replicating lactose plasmid pUCL22. Mol. Microbiol. 10:1113-1124. [DOI] [PubMed] [Google Scholar]
- 13.Gentry-Weeks, C. R., R. Karkhoff-Schweizer, A. Pikis, M. Estay, and J. M. Keith. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 67:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giard, J. C., A. Rincé, H. Capiaux, Y. Auffray, and A. Hartke. 2000. Inactivation of the stress- and starvation-inducible gls24 operon has a pleiotropic effect on cell morphology, stress sensitivity, and gene expression in Enterococcus faecalis. J. Bacteriol. 182:4512-4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmore M. S., P. S. Coburn, S. R. Nallapareddy, and B. E. Murray. 2002. Enterococcal virulence, p. 301-354. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, D.C.
- 16.Greenberg, J. T., P. Monach, J. H. Chou, P. D. Josephy, and B. Demple. 1990. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:6181-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock, L. E., and M. S. Gilmore. 2000. Pathogenicity of enterococci, p 251-258. In V. Fischetti, R. P. Novick, J. Ferreti, D. Portnoy, and J. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 19.Herbig, A., and J. D. Helmann. 2002. Metal ion uptake and oxidative stress, p. 405-414. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives, 2nd ed. ASM Press, Washington, D.C.
- 20.Huycke, M. M., V. Abrams, and D. R. Moore. 2002. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 23:529-536. [DOI] [PubMed] [Google Scholar]
- 21.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson, F. S., R. W. Morgan, M. F. Christman, and B. N. Ames. 1989. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage: purification and properties. J. Biol. Chem. 264:1488-1496. [PubMed] [Google Scholar]
- 23.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 25.Le Breton, Y., G. Boël, A. Benachour, H. Prévost, Y. Auffray, and A. Rincé. 2003. Molecular characterization of Enterococcus faecalis two-component signal transduction pathways related to environmental stresses. Environ. Microbiol. 5:329-337. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen, I. T., L. Banerjei, G. S. A. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 27.Platteeuw, C., G. Simons, and W. M. de Vos. 1994. Use of the Escherichia coli β-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl. Environ. Microbiol. 60:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakita, R. M., N. N. Vanek, K. Jacques-Palaz, M. Mee M. M. Mariscalco, G. M. Dunny, M. Snuggs, W. B. Van Winkle, and S. I. Simon. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect. Immun. 67:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rince, A., J. C. Giard, V. Pichereau, S. Flahaut, and Y. Auffray. 2001. Identification and characterization of gsp65, an organic hydroperoxide resistance (ohr) gene encoding a general stress protein in Enterococcus faecalis. J. Bacteriol. 183:1482-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross, R. P., and A. Claiborne. 1997. Evidence for regulation of the NADH peroxidase gene (npr) from Enterococcus faecalis by OxyR. FEMS Microbiol. Lett. 151:177-183. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 33.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 35.Storz, G., L. A. Tartaglia, and B. N. Ames. 1990. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248:189-194. [DOI] [PubMed] [Google Scholar]
- 36.Sussmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng, F., K. D. Jacques-Palaz, G. M. Weinstock, and B. E. Murray. 2002. Evidence that the enterococcal polysaccharide antigen gene (epa) cluster is widespread in Enterococcus faecalis and influences resistance to phagocytic killing of E. faecalis. Infect. Immun. 70:2010-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terzaghi, B. E., and W. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 2:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 40.Tsaneva, I. R., and B. Weiss. 1990. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J. Bacteriol. 172:4197-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verneuil, N., Y. Le Breton, A. Hartke, Y. Auffray, and J.-C. Giard. Identification of a new oxidative stress transcriptional regulator in Enterococcus faecalis. Lait 84:69-76.
- 42.Wiedmann, M., T. J. Arvik, and K. J. Boor. 1998. General stress transcription factor B and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagi, Y., and D. B. Clewell. 1980. Recombination-deficient mutant of Streptococcus faecalis. J. Bacteriol. 143:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng, M., F. Aslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]