Abstract
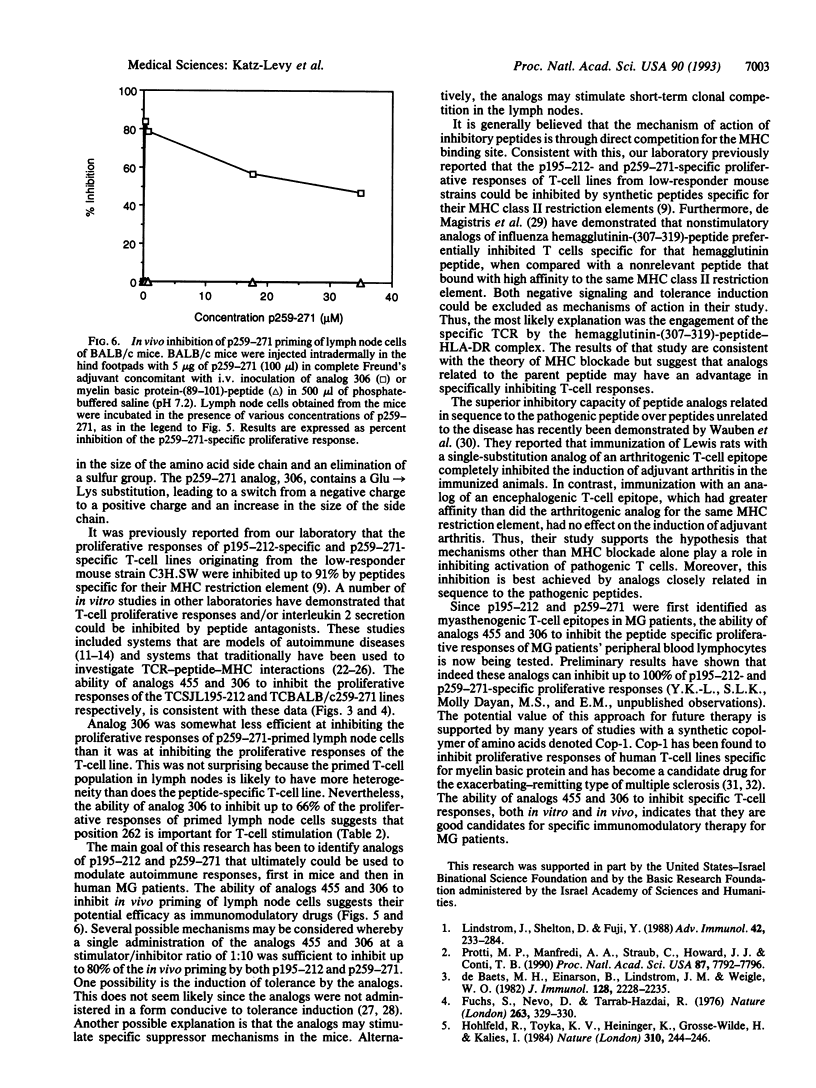
The synthetic peptides p195-212 and p259-271, representing amino acids 195-212 and 259-271 of the alpha subunit of the human acetylcholine receptor, preferentially stimulate T cells of patients with myasthenia gravis and are immunodominant T-cell epitopes in SJL and BALB/c mice, respectively. We designed and synthesized analogs of these peptides that contain single amino acid substitutions. An analog of peptide p195-212, no. 455 (Met-207-->Ala), was capable of inhibiting up to 100% of the proliferative responses of a p195-212-specific T-cell line originating from the high-responder strain SJL. Similarly, an analog of p259-271, no. 306 (Glu-262-->Lys), was capable of inhibiting up to 93% of the proliferative responses of the p259-271-specific T-cell line originating from high-responder BALB/c mice. Analog 306 also inhibited up to 43% of the proliferative responses of p259-271-primed lymph node cells in an in vitro proliferation assay. To test the in vivo inhibitory activity of the analogs, mice were primed with the myasthenogenic peptides in complete Freund's adjuvant concomitant with administration of the analogs in aqueous solution. Administration of analogs 455 and 306 led to decreased proliferative responses of up to 70% by peptide p199-212-primed lymph node cells and up to 85% by peptide p259-271-primed lymph node cells. Similar results were obtained whether the analogs were administered i.v. or i.p. Thus, these analogs are good candidates for specific immunomodulatory therapy for patients with myasthenia gravis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Nagy Z. A. Peptide competition for antigen presentation. Immunol Today. 1990 Jan;11(1):21–24. doi: 10.1016/0167-5699(90)90006-u. [DOI] [PubMed] [Google Scholar]
- Adorini L., Nagy Z. Inhibition of T cell activation by MHC blockade. Int Rev Immunol. 1990;6(1):23–35. doi: 10.3109/08830189009056615. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Matsueda G. R., Evans R. J., Dunbar J. B., Jr, Marshall G. R., Unanue E. R. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. 1987 Jun 25-Jul 1Nature. 327(6124):713–715. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- Axelrod O., Mozes E. Analysis of the biological functions and fine specificity of (T,G)-A--L specific T cell clones. Immunobiology. 1986 Aug;172(1-2):99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- Bonavida B., Mozes E., Shearer G. M., Sela M. Immunological unresponsiveness induced in adult mice to synthetic polypeptides built on multichain polyproline and multichain polyalanine. Immunochemistry. 1974 Jul;11(7):347–353. doi: 10.1016/0019-2791(74)90187-6. [DOI] [PubMed] [Google Scholar]
- Bornstein M. B., Miller A., Slagle S., Weitzman M., Crystal H., Drexler E., Keilson M., Merriam A., Wassertheil-Smoller S., Spada V. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987 Aug 13;317(7):408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- Brocke S., Brautbar C., Steinman L., Abramsky O., Rothbard J., Neumann D., Fuchs S., Mozes E. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J Clin Invest. 1988 Dec;82(6):1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke S., Dayan M., Rothbard J., Fuchs S., Mozes E. The autoimmune response of different mouse strains to T-cell epitopes of the human acetylcholine receptor alpha subunit. Immunology. 1990 Apr;69(4):495–500. [PMC free article] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- De Baets M. H., Einarson B., Lindstrom J. M., Weigle W. O. Lymphocyte activation in experimental autoimmune myasthenia gravis. J Immunol. 1982 May;128(5):2228–2235. [PubMed] [Google Scholar]
- De Magistris M. T., Alexander J., Coggeshall M., Altman A., Gaeta F. C., Grey H. M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992 Feb 21;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Nevo D., Tarrab-Hazdai R., Yaar I. Strain differences in the autoimmune response of mice to acetylcholine receptors. Nature. 1976 Sep 23;263(5575):329–330. doi: 10.1038/263329a0. [DOI] [PubMed] [Google Scholar]
- Gautam A. M., Glynn P. Competition between foreign and self proteins in antigen presentation. Ovalbumin can inhibit activation of myelin basic protein-specific T cells. J Immunol. 1990 Feb 15;144(4):1177–1180. [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Krieger J. I., Karr R. W., Grey H. M., Yu W. Y., O'Sullivan D., Batovsky L., Zheng Z. L., Colón S. M., Gaeta F. C., Sidney J. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991 Apr 1;146(7):2331–2340. [PubMed] [Google Scholar]
- Krolick K. A., Urso O. E. Influence of T cell specificity on the antibody response to the acetylcholine receptor. J Neuroimmunol. 1986 Nov;13(1):75–87. doi: 10.1016/0165-5728(86)90051-2. [DOI] [PubMed] [Google Scholar]
- Lamont A. G., Powell M. F., Colón S. M., Miles C., Grey H. M., Sette A. The use of peptide analogs with improved stability and MHC binding capacity to inhibit antigen presentation in vitro and in vivo. J Immunol. 1990 Apr 1;144(7):2493–2498. [PubMed] [Google Scholar]
- Lamont A. G., Sette A., Fujinami R., Colón S. M., Miles C., Grey H. M. Inhibition of experimental autoimmune encephalomyelitis induction in SJL/J mice by using a peptide with high affinity for IAs molecules. J Immunol. 1990 Sep 15;145(6):1687–1693. [PubMed] [Google Scholar]
- Lindstrom J., Shelton D., Fujii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- Mozes E., Dayan M., Zisman E., Brocke S., Licht A., Pecht I. Direct binding of a myasthenia gravis related epitope to MHC class II molecules on living murine antigen-presenting cells. EMBO J. 1989 Dec 20;8(13):4049–4052. doi: 10.1002/j.1460-2075.1989.tb08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Howard J. F., Jr, Conti-Tronconi B. M. Immunodominant regions for T helper-cell sensitization on the human nicotinic receptor alpha subunit in myasthenia gravis. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7792–7796. doi: 10.1073/pnas.87.19.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Zamvil S. S., Mitchell D. J., Hodgkinson S., Rothbard J. B., Steinman L. Prevention of experimental encephalomyelitis with peptides that block interaction of T cells with major histocompatibility complex proteins. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9470–9474. doi: 10.1073/pnas.86.23.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Parhami B., Mozes E., Sela M. Change in specificity of antibodies to a random synthetic branched polypeptide in mice tolerant to its ordered analogs. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5286–5288. doi: 10.1073/pnas.76.10.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Smith J. A., Miles C., Grey H. M. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. 1987 Jul 30-Aug 5Nature. 328(6129):395–399. doi: 10.1038/328395a0. [DOI] [PubMed] [Google Scholar]
- Sette A., Sidney J., Albertson M., Miles C., Colón S. M., Pedrazzini T., Lamont A. G., Grey H. M. A novel approach to the generation of high affinity class II-binding peptides. J Immunol. 1990 Sep 15;145(6):1809–1813. [PubMed] [Google Scholar]
- Smilek D. E., Wraith D. C., Hodgkinson S., Dwivedy S., Steinman L., McDevitt H. O. A single amino acid change in a myelin basic protein peptide confers the capacity to prevent rather than induce experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9633–9637. doi: 10.1073/pnas.88.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Aharoni R., Arnon R., Sela M. Specific inhibition of the T-cell response to myelin basic protein by the synthetic copolymer Cop 1. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9724–9728. doi: 10.1073/pnas.85.24.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Milo R., Arnon R., Sela M. Synthetic copolymer 1 inhibits human T-cell lines specific for myelin basic protein. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):137–141. doi: 10.1073/pnas.89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauben M. H., Boog C. J., van der Zee R., Joosten I., Schlief A., van Eden W. Disease inhibition by major histocompatibility complex binding peptide analogues of disease-associated epitopes: more than blocking alone. J Exp Med. 1992 Sep 1;176(3):667–677. doi: 10.1084/jem.176.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]


