THE TELOMERE “CLOCK”
Telomeres are the TTAGGG nucleotide repeats at the ends of mammalian chromosomes, and they progressively shorten with each replication of somatic cells. The metaphor of telomeres as a clock dominates the tale of telomeres and atherosclerosis. The telomere “clock” does not chronicle time but rather cell replication. Furthermore, in cultured human somatic cells, the clock not only records replication, it also triggers an irreversible growth arrest. Thus, in vitro, telomere length is both a biomarker of replicative history and a determinant of replicative potential. Without a doubt, the intrinsic counting mechanism of the telomere clock also registers replication of human somatic cells in vivo. The lingering puzzle that is of great interest to the medical community is whether the telomere clock, expressed in age-related telomere shortening, is an epiphenomenon of atherosclerosis or an active player in it. That question particularly applies to hematopoietic stem cells (HSCs), given that epidemiological studies have linked leukocyte telomere length (LTL) with atherosclerosis.
The model we propose to explain the links between atherosclerosis and telomere dynamics (birth telomere length and its age-dependent shortening) in leukocytes takes cues from (a) the fact that atherosclerosis is a disease of the vascular endothelium, (b) the common embryonic origin of the hematopoietic system and the vascular endothelium, and (c) genes that explain interindividual variation in LTL in the general population. The model posits that LTL dynamics mirror telomere dynamics in HSCs, where telomere length is an index of HSC reserves. Diminished HSC reserves at birth, their accelerated attrition rate afterward, or both are expressed in the form of shortened LTL during adulthood—a phenomenon that confers increased risk for atherosclerosis. This risk largely relates to the declining ability of HSC reserves to sustain a sufficient circulating number of adequately functioning endothelial progenitor cells (EPCs), which are derived from the bone marrow and circulate in the blood, to mend the vascular endothelium. In addition, an augmented rate of LTL shortening indicates an increased burden of oxidative stress and inflammation, which not only marks the atherosclerotic process but also taxes the HSC reserves. In this way, telomere length in HSCs is both a biomarker of atherosclerosis and a determinant of its development. Our model comes down to this proposition: If shortened LTL predicts increased atherosclerotic risk—and the evidence suggests that it does—it is because the injurious component of atherosclerosis exceeds the repair capacity of HSC reserves, which largely depend on HSC telomere length.
This article on the biological meaning of the nexus between LTL and atherosclerosis primarily draws on studies in humans. Excellent general reviews have addressed telomere biology in the context of monogenic diseases, atherosclerosis, and aging (1–4). Because this review reflects our own perspective on the LTL–atherosclerosis connection, we were selective in our citations and extend our apology to investigators in the telomere and atherosclerosis fields whose meritorious works are not cited.
TELOMERE BIOLOGY IN CULTURED HUMAN CELLS
Telomeres progressively shorten in proliferating cells because of two main factors: the “end replication problem” and oxidative stress. The end replication problem stems from the inability of DNA polymerase to replicate the lagging DNA strand to its terminus (5), which causes a small and fixed telomere shortening with each replication. In addition, because of the sensitivity of the G triplets of the TTAGGG telomere repeats to the superoxide radical, telomeres are exquisitely sensitive to oxidative stress. Accordingly, increased oxidative stress causes a greater loss of telomere repeats per cell replication (6). Replication of cultured somatic cells ultimately comes to a halt in two stages—M1 and M2. M1, known as replicative senescence, is triggered when the shortest telomere among the telomeres of all chromosomes attains a critical threshold (7). At that stage, the telomere T-loop (8)—a structure that comprises the telomere repeats and telomere binding proteins—becomes unraveled, leading to the exposure of the telomeric terminus. The cellular machinery recognizes the exposed terminus as a double strand break that activates DNA damage checkpoints, including p16INK4a/pRB and p53, and it stops replication. On rare occasions, a subset of cells continues to replicate until M2, which is marked by cycles of chromosomal fusion and breakage, leading to apoptosis (9).
M1 and M2 can be thwarted by telomerase, a reverse transcriptase that adds back telomere repeats onto the chromosomes (10). The enzyme comprises two major components: a catalytic (protein) subunit (TERT) and an RNA template (TERC). In addition, telomere binding proteins maintain telomeres by stabilizing the T-loop and regulating accessibility of the telomeric terminus to telomerase.
IN VIVO HUMAN TELOMERE DYNAMICS
Telomere Shortening After Birth
Robust telomerase activity is expressed in fetal tissues during the first trimester (11), with resultant synchronization of telomere length in all somatic cells. During extrauterine life, telomerase activity is repressed in somatic cells, including HSCs (12), but it is strongly expressed in the germ line (11) and in cancers (13). Telomere length in somatic tissues is longest at birth. From then on, telomeres progressively shorten with age in replicating somatic cells. In postmitotic cells such as skeletal muscle cells, which divide infrequently (unless injured) during extrauterine life, there appears to be only little age-dependent telomere shortening (14). If replicative senescence figures in aging-related diseases, it most likely occurs in highly proliferative somatic tissues. And the most proliferative tissue in humans and other mammals is the hematopoietic system.
HSCs experience the highest rate of telomere attrition during childhood and adolescence, as they divide to expand the HSC and hematopoietic progenitor cell (HPC) pools in tandem with the growing soma (15). During adulthood, HSC replication primarily accommodates the tremendous turnover rate of blood cells. Persons suffering from catastrophic (rare) mutations in telomere maintenance genes (1) are susceptible to aplastic anemia because the HSCs of these patients might be the first cells to run out of telomeres early in life. However, in the population at large, a less drastic interindividual variation in HSC reserves, which are contingent on telomere dynamics, is probably expressed in two ways: variation among individuals in the manifestations of aging-related immune senescence (16), and age-dependent decline in the numbers of adequately functioning EPCs.
HSC Telomere Dynamics and Atherosclerosis
By virtue of its anatomy, the vascular endothelium interacts with elements on its luminal and counter-luminal sides, playing key roles not only in the biology of the vasculature but also in that of the blood. Importantly, the vascular endothelium is where atherosclerosis evolves.
Based on a few autopsy cases, it was proposed that telomere shortening in the endothelium and consequently endothelial cell senescence contribute to the atherosclerotic process in the coronary arteries (17). Given the relatively low replicative index of endothelial cells (and smooth muscle cells) in the vascular wall, it is unlikely that these cells would experience senescence from telomere shortening during the human lifespan. If telomere dynamics are involved in atherosclerosis, their main impact might be not on native endothelial cells in the vascular wall but on EPCs, which originate from the HSC pool. Telomere dynamics in this pool are mirrored in LTL dynamics.
LTL reflects the mean length of telomeres in all leukocyte lineages, which show some variation in telomere dynamics within the individual. For instance, lymphocytes display a faster rate of age-dependent telomere shortening than granulocytes (18), and memory CD4+ T lymphocytes have shorter telomeres than naïve ones (19). However, the variation in telomere length among subsets of leukocytes within an individual is small compared to the interindividual variation in LTL (20).
Atherosclerosis is driven in part by chronic, low-grade inflammation (21) and oxidative stress (22). The conventional view about the LTL–atherosclerosis connection is as follows: over many years, an increased tempo of HSC replication to accommodate the inflammatory response and a greater loss of telomere repeats per HSC replication due to oxidative stress would bring about a shorter LTL in individuals with atherosclerosis than in their peers. Appealing as it may be, the view that shortened LTL in atherosclerosis primarily stems from accelerated telomere shortening in HSCs overlooks the 4–6-kb range in LTL among newborns (23, 24). Birth LTL is hence a major determinant of LTL at any age, as persons who are born with short (or long) LTL are likely to display short (or long) LTL later in life (14). Thus, a quickened pace of LTL shortening is unlikely to be the sole explanation for the shortened LTL in atherosclerosis.
ATHEROSCLEROSIS AND EPCs: POTENTIAL ROLE OF HSC TELOMERE DYNAMICS
LTL may be tied to atherosclerosis not only through oxidative stress and inflammation, but also via the vital repair countermeasures that attenuate endothelial damage. EPCs are one of these essential countermeasures. Originating from the HSC pool, EPCs are engaged in maintaining the integrity of the vascular endothelium, but their number and proliferative potential, expressed in colony-forming units (CFUs), are low in atherosclerosis. The numbers of circulating EPCs, their CFUs, or both are also diminished in older persons (25), in individuals with insulin resistance (26) or sedentary lifestyle (27), in smokers (28), and in men more than in women (29). These conditions are the same as those associated with a shortened LTL. Not only older persons and individuals with clinical manifestations of atherosclerosis display shortened LTL (2). Those at risk for atherosclerosis after adjustment for age also show shortened LTL, including men (30–33), cigarette smokers (30, 33), people with insulin resistance (34, 35), and those with a sedentary lifestyle (36).
In atherosclerosis, the partial exhaustion of the HSC reserves—indicated by diminished numbers of EPCs, a decline in their CFUs, or both—apparently relates to mechanisms that govern telomere shortening (37–39). Moreover, insights into telomere dynamics in HSC reserves and their relation to atherosclerosis have been gained from monitoring recipients of hematopoietic stem cell transplantation (HSCT). In general, the survival of HSCT recipients is inversely related to the donor's age (40). Moreover, these recipients experience substantial LTL shortening (41, 42), which evidently occurs because of extensive proliferation of donor-derived HSCs to fill the bone marrow niches of the recipients and produce more committed HPCs. Importantly, recipients of HSCT from donors older than 18 years display a shorter LTL than recipients of HSCT from younger donors (42). Recipients of HSCT also show a decline in myeloid CFUs (43). In light of these observations and our model, it is not surprising that long-term survivors of HSCT show not only substantial LTL shortening (44) but also a heightened risk of atherosclerosis (45). HSC telomere dynamics might also explain the disappointing outcomes of patients with myocardial infarction treated with autologous bone marrow–derived cells (46). These outcomes do not, however, challenge the link between telomeres and EPCs in atherosclerosis. If atherosclerosis is marked by diminished HSC reserves, bone marrow–derived cells of patients with severe atherosclerosis are likely to display diminished replication potential, which would limit their effectiveness.
It is noteworthy that recent studies have questioned the criteria that have been used to characterize EPCs and their exact role in vascular repair. Some investigators have suggested that EPCs are largely bone-marrow-derived myeloid cells that secrete paracrine factors and stimulate vascular repair and angiogenesis through the activation of nearby cells (47–50). But regardless of the true nature of EPCs, the evidence points to their engagement in vascular repair, the effectiveness of which depends on HSC telomere length, as expressed in LTL.
And so we return to the question of whether telomere length is merely a biomarker of atherosclerosis, an active player in its pathogenesis, or both. Might it be that shortened LTL, regardless of its etiology, confers diminished HSC reserves, expressed in lower numbers or reduced function of EPCs? Such a paradigm would integrate and reconcile findings of wide interindividual variation in birth LTL with the relatively narrow differences in mean LTL between persons with atherosclerosis and those without it. Birth LTL would thus reflect the initial size of the HSC reserves, and changes in LTL after birth due to growth (15) and the accruing burden of oxidative stress and inflammation would track the contraction of these reserves. Moreover, the absolute telomere length in HSCs might be a determinant of the ability of the HSC pool to generate adequate numbers of EPCs that are dispatched by the bone marrow to the site of endothelial injury to engage in mending the vascular endothelium. As such, telomere dynamics in HSCs, mirrored in their proxies, the leukocytes, may conform to the behavior of telomeres in cultured somatic cells by being both an index of replicative history and a determinant of future replicative potential.
It is not just that the conventional model linking LTL with atherosclerosis via oxidative stress and inflammation is incomplete. The model ignores the common embryonic precursor of vascular endothelial cells and blood cells, referred to as the hemogenic endothelium (51). This multipotent precursor gives rise not only to the endothelium but also to HSCs (52). Because EPCs share their embryonic and perhaps postembryonic history with HSCs, their telomere lengths and hence their endothelial repair capacity might be defined by their common origin. From the perspective of telomere biology, atherosclerosis and the aging of the vasculature might then be better understood when we consider the hematopoietic system and the vascular endothelium as a single entity—the “hemothelium.”
LTL-REGULATING GENES AND THEIR POTENTIAL ROLE IN ATHEROSCLEROSIS
Because LTL is heritable, variant genes may not only cause rare monogenic diseases (1) but also contribute to interindividual variation in LTL in the general population. Theoretically, LTL-regulating genes might belong to two major categories: genes that are directly involved in telomere maintenance and those whose function impacts HSC replication kinetics, which would ultimately affect LTL. Recent genome-wide association studies (GWAS) of LTL support this supposition by discovering loci of LTL-regulating genes (53, 54). These loci include the telomerase RNA conponent (TERC), oligonucleotide/oligosaccharide-binding fold containing 1 (OBFC1), and the chemokine (CX-C motif) receptor 4 gene (CXCR4).
TERC, as indicated above, is the reverse transcriptase component of telomerase, and OBFC1 is the human homolog of yeast Stn1, which negatively regulates telomerase action on telomeres (55). Moreover, OBFC1 interacts and colocalizes with telomeric proteins in human cells, indicating that OBFC1 regulates telomere length and/or function in humans. Whereas TERC and OBFC1 are telomere-maintenance genes, CXCR4 regulates neutrophil release from the bone marrow (56), and consequently it modulates inflammation; it follows that CXCR4 variants might impact the pace of HSC replication. Equally important, CXCR4 participates in the control of inflammatory response in atherosclerosis (57, 58). CXCR4 and its cognate ligand, CXCL12, are central elements in the feedback loop between HSCs and the endothelium, which regulates the chemotactic signals that guide the homing of EPCs to the area of vascular injury. Furthermore, the CXCL12 locus was found by GWAS to be associated with myocardial infarction (59).
As telomerase activity is robust during intrauterine life but repressed during extrauterine life, it is reasonable to hypothesize that OBFC1 and TERC variants primarily affect birth LTL and, by inference, the size of HSC reserves at birth. In contrast, CXCR4 might contribute to interindividual variation in LTL by its impact on HSC replication and consequently influence the contraction rate of HSC reserves. In this way, LTL-regulating genes might be determinants in the atherosclerotic process.
PROPOSED MODELS LINKING HSC RESERVES, EXPRESSED IN LTL DYNAMICS, WITH ATHEROSCLEROSIS
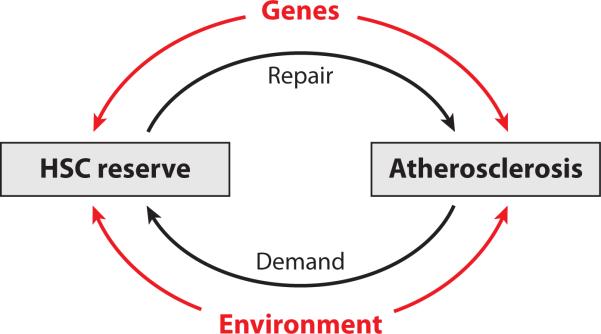
Figure 1 portrays our overall model explaining the relationship between atherosclerosis and HSC reserves as expressed in LTL. The outer loops of the diagram represent genetic and environmental elements that might independently affect the HSC reserves and atherosclerosis. The genetic elements include (a) gene networks that affect HSC reserves (i.e., genes that directly regulate telomere biology and genes that modulate HSC replication), and (b) gene networks that impact aging-related vascular injury (e.g., genes that participate in lipid homeostasis, insulin resistance, inflammation, etc.). The environmental loop includes elements that presumably affect both atherosclerosis and the HSC reserves (e.g., smoking, caloric intake, and sedentary lifestyle). For instance, smoking might injure the vascular endothelium by increasing oxidative stress but it can also impact HSC reserves, since smoking-induced inflammation and oxidative stress would increase HSC replication and augment telomere loss per HSC replication. The inner loop comprises feedback mechanisms that regulate gene-mediated interactions between the vascular endothelium and HSC reserves. The CXCR4-CXCL12 network exemplifies such interactions.
Figure 1.
Overall model linking the hematopoietic stem cell (HSC) reserves with atherosclerosis. Atherosclerosis is partially the outcome of demand for repair, exerted by injury to the vascular endothelium on the HSC reserves. The repair arm of the HSC reserves is mediated by endothelial progenitor cells, and the size of these reserves is expressed in leukocyte telomere length. Both genetic and environmental factors continuously impact the injury/repair processes.
CONCLUSIONS
If telomere dynamics figure in atherosclerosis, their main impact would be exerted on HSC reserves because of the tremendous turnover rate of blood cells. Although the size of the HSC reserves is largely determined at birth, atherosclerosis itself can tax these reserves, since oxidative stress and inflammation, the hallmarks of the disease, evidently accelerate telomere shortening in HSCs and consequently augment the contraction of HSC reserves. Thus, the atherosclerosis–LTL link might be the outcome of a feedback loop in which diminished HSC reserves beget atherosclerosis and atherosclerosis begets diminished HSC reserves. It is unlikely, however, that all HSCs would completely senesce due to telomere shortening during the human lifespan. Rather, as humans get older, their dwindling HSC reserves might encounter increasing difficulties in maintaining endothelial repair functions by EPCs. At the basic level, this concept fits the broader view of atherosclerosis as a state of chronic imbalance in which the injurious effect of oxidative stress/inflammation outstrips endothelial repair that depends on HSC reserves, which, in turn, depend on telomere length. In this sense, the metaphor of telomeres as a clock that both passively counts replication and actively sustains vitality of somatic cells may turn out to be true for human HSCs. Moreover, because birth LTL largely determines LTL at any age and LTL-maintenance genes such as TERC and OBFC1 probably affect birth LTL, it seems that for many individuals the die for atherosclerosis is cast at birth.
How important would it be to record the individual's LTL dynamics from birth to adulthood to old age? That depends on whether optimal methods of LTL measurement will be developed for clinical use, how much the knowledge of LTL will add to the present prognostication of atherosclerosis risk factors, and whether preventive and/or therapeutic steps can be guided by LTL results.
HSC: hematopoietic stem cell
LTL: leukocyte telomere length
EPCs: endothelial progenitor cells
HPCs: hematopoietic progenitor cells
CFUs:
colony-forming units
ACKNOWLEDGMENTS
A.A.'s telomere research is supported by grants AG16592, AG020132, and AG030678 from the National Institute on Aging. D.L. is an intramural scientist with the Center for Population Studies, National Heart, Lung, and Blood Institute. His funding is from the NIH Intramural Research Program.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Calado RT, Young NS. Telomere diseases. N. Engl. J. Med. 2009;361:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samani NJ, van der Harst P. Biological ageing and cardiovascular disease. Heart. 2008;94:537–39. doi: 10.1136/hrt.2007.136010. [DOI] [PubMed] [Google Scholar]
- 3.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–28. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–22. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 5.Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp. Gerontol. 1996;31:443–48. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Houben JM, Moonen HJ, van Schooten FJ, et al. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008;44:235–46. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Hemann MT, Strong MA, Hao LY, et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 8.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–40. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 9.Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–74. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Wright WE, Piatyszek MA, Rainey WE, et al. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–79. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Chiu CP, Dragowska W, Kim NW, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–48. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 13.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–15. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 14.Gardner JP, Kimura M, Chai W, et al. Telomere dynamics in macaques and humans. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:367–74. doi: 10.1093/gerona/62.4.367. [DOI] [PubMed] [Google Scholar]
- 15.Sidorov I, Kimura M, Yashin A, et al. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp. Hematol. 2009;37:514–24. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: Is it ever too old to become young again? Nat. Rev. Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 17.Ogami M, Ikura Y, Ohsawa M, et al. Telomere shortening in human coronary artery diseases. Arterioscler. Thromb. Vasc. Biol. 2004;24:546–50. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 18.Rufer N, Brümmendorf TH, Kolvraa S, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999;190:157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat. Rev. Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 20.Kimura M, Gazitt Y, Cao X. Synchrony of telomere length among hematopoietic cells. Exp. Hematol. 2010;38:854–59. doi: 10.1016/j.exphem.2010.06.010. e al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 2008;8:802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez J, Ballinger SW, Darley-Usmar VM, et al. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ. Res. 2006;99:924–32. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 23.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr. Res. 2002;52:377–81. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Akkad A, Hastings R, Konje JC, et al. Telomere length in small-for-gestational-age babies. BJOG. 2006;113:318–23. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Q, Kiechi S, Patel S, et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis—results from a large population-based study. PLoS One. 2007;10:e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy C, Kanganayagam GS, Jiang B, et al. Vascular dysfunction in healthy Indian Asians is associated with insulin resistance and reduced endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:936–42. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 27.Van Craenenbroeck EM, Vrints CJ, Haine SE, et al. A maximal exercise bout increases the number of circulating CD34+/KDR+ endothelial progenitor cells in healthy subjects. Relation with lipid profile. J. Appl. Physiol. 2008;104:1006–13. doi: 10.1152/japplphysiol.01210.2007. [DOI] [PubMed] [Google Scholar]
- 28.Michaud SE, Dussault S, Haddad P, et al. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–32. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Fadini GP, de Kreutzenberg S, Albiero M, et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler. Thromb. Vasc. Biol. 2008;28:997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation. 2008;117:1138–44. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeanclos E, Schork NJ, Kyvik KO, et al. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 32.Bekaert S, De Meyer T, Rietzschel ER, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–47. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 33.Nawrot TS, Staessen JA, Gardner JP, et al. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–10. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 34.Gardner JP, Li S, Srinivasan SR, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–77. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 35.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 36.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008;168:154–58. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 37.Satoh M, Ishikawa Y, Takahashi Y, et al. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–53. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 38.Oeseburg H, Westenbrink BD, de Boer RA, et al. Can critically short telomeres cause functional exhaustion of progenitor cells in postinfarction heart failure? J. Am. Coll. Cardiol. 2007;50:1909–13. doi: 10.1016/j.jacc.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 39.Kissel CK, Lehmann R, Assmus B, et al. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J. Am. Coll. Cardiol. 2007;49:2341–49. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 40.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–51. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 41.Wynn RF, Cross MA, Hatton C, et al. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet. 1998;351:178–81. doi: 10.1016/S0140-6736(97)08256-1. [DOI] [PubMed] [Google Scholar]
- 42.Lee JJ, Kook H, Chung IJ, et al. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:411–15. doi: 10.1038/sj.bmt.1701923. [DOI] [PubMed] [Google Scholar]
- 43.Lewis NL, Mullaney M, Mangan KF, et al. Measurable immune dysfunction and telomere attrition in long-term allogeneic transplant recipients. Bone Marrow Transplant. 2004;33:71–78. doi: 10.1038/sj.bmt.1704300. [DOI] [PubMed] [Google Scholar]
- 44.Baerlocher GM, Rovó A, Müller A, et al. Cellular senescence of white blood cells in very long-term survivors after allogeneic hematopoietic stem cell transplantation: the role of chronic graft-versus-host disease and female donor sex. Blood. 2009;114:219–22. doi: 10.1182/blood-2009-03-209833. [DOI] [PubMed] [Google Scholar]
- 45.Tsakiris DA, Tichelli A. Thrombotic complications after haematopoietic stem cell transplantation: early and late effects. Best Pract. Res. Clin. Haematol. 2009;22:137–45. doi: 10.1016/j.beha.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Möllmann H, Nef H, Elsässer A, et al. Stem cells in myocardial infarction: from bench to bedside. Heart. 2009;95:508–14. doi: 10.1136/hrt.2007.125054. [DOI] [PubMed] [Google Scholar]
- 47.Steinmetz M, Nickenig G, Werner N. Endothelial-regenerating cells: an expanding universe. Hypertension. 2010;55:593–99. doi: 10.1161/HYPERTENSIONAHA.109.134213. [DOI] [PubMed] [Google Scholar]
- 48.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–69. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 49.Rohde E, Bartmann C, Schallmoser K, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–52. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 50.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimoto M, Yoder MC. Developmental biology: birth of the blood cell. Nature. 2009;457:801–3. doi: 10.1038/457801a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–35. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy D, Neuhausen BL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc. Natl. Acad. Sci. USA. 2010;107:9293–98. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Codd V, Mangino M, van der Harst P, et al. Common variants near TERC are associated with mean telomere length. Nat. Genet. 2010;42:197–99. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S, Makovets S, Matsuguchi T, et al. Cdk1-dependent phosphorylation of Cdc13 coordinates telomere elongation during cell-cycle progression. Cell. 2009;136:50–61. doi: 10.1016/j.cell.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eash KJ, Means JM, White DW, et al. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–19. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 58.Sainz J, Sata M. Open sesame! CXCR4 blockade recruits neutrophils into the plaque. Circ. Res. 2008;102:154–56. doi: 10.1161/CIRCRESAHA.107.170241. [DOI] [PubMed] [Google Scholar]
- 59.Myocardial Infarction Genetics Consortium Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009;41:334–41. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]