Abstract
Steroid hormones are an important class of regulatory molecules that are synthesized in steroidogenic cells of the adrenal, ovary, testis, placenta, brain and skin, and influence a spectrum of developmental and physiological processes. The steroidogenic acute regulatory protein (STAR) predominantly mediates the rate-limiting step in steroid biosynthesis, i.e., the transport of the substrate of all steroid hormones, cholesterol, from the outer to the inner mitochondrial membrane. At the inner membrane, cytochrome P450 cholesterol side chain cleavage enzyme cleaves the cholesterol side-chain to form the first steroid, pregnenolone, which is converted by a series of enzymes to various steroid hormones in specific tissues. Both basic and clinical evidence have demonstrated the crucial involvement of the STAR protein in the regulation of steroid biosynthesis. Multiple levels of regulation impinge on STAR action. Recent findings demonstrate that hormone-sensitive lipase, through its action on the hydrolysis of cholesteryl esters, plays an important role in regulating StAR expression and steroidogenesis which involve the liver X receptor pathway. Activation of the latter influences macrophage cholesterol efflux that is a key process in the prevention of atherosclerotic cardiovascular disease. Appropriate regulation of steroid hormones is vital for proper functioning of many important biological activities, which are also paramount for geriatric populations to live longer and healthier. This review summarizes the current level of understanding on tissue-specific and hormone-induced regulation of STAR expression and steroidogenesis, and provides insights into a number of cholesterol and/or steroid coupled physiological and pathophysiological consequences.
Keywords: STAR protein, steroid biosynthesis, STAR deficiency, lipoid CAH, endometriosis, HSL, LXR, ABCA1, atherosclerosis, aging
Introduction
The maintenance of normal reproductive development and function, and bodily homeostasis, is dependent on steroid hormones. Although the steroid hormones are diverse, they are synthesized from a common precursor substrate, cholesterol, which can be derived from a number of sources, i.e., de novo synthesis of cellular cholesterol, lipoprotein-derived cholesteryl esters (CEs), and hydrolysis of CEs stored in lipid droplets [1–3]. Even so, the conversion of CEs into free cholesterol serves as a crucial step in controlling cholesterol availability for steroidogenesis. Regulation of steroid biosynthesis is primarily mediated by trophic hormones, although multiple intracellular events and signaling pathways have been demonstrated to play permissive roles [3–10]. Hormonal control of steroid biosynthesis occurs within minutes (acute) to hours (chronic) and is mediated by cAMP signaling. The biosynthesis of steroid hormones is initiated upon mobilization of cholesterol from cellular stores to the mitochondrial inner membrane and to the site of cytochrome P450 cholesterol side chain cleavage enzyme (P450scc or CYP11A1) (reviewed in Refs. [6, 10–12]). The precise mechanism by which cholesterol is transported to the mitochondria for steroidogenesis remains unknown; however, considerable evidence suggests the involvement of a dynamic mitochondrial protein complex in this process. These proteins include acyl-CoA synthetase 4, steroidogenic acute regulatory protein (STAR), peripheral benzodiazepine receptor/translocator protein, and AAA domain containing protein 3 [12–20]. Among them, the STAR protein has essentially all of the characteristics to become an acute regulator of steroid biosynthesis in steroidogenic tissues [6, 8, 10, 11, 13, 21–24]. STAR consists of several forms of a newly synthesized 30 kDa protein which has a 37 kDa precursor form containing an N-terminal mitochondrial targeting sequence. In describing STAR’s role in cholesterol transport, the preponderance of evidence indicates that the 37 kDa STAR acts on the outer mitochondrial membrane [13, 25]. Alternatively, it has been demonstrated that the 30 kDa phosphorylated form acting on the inner mitochondrial membrane allowing for the transfer of the majority of cholesterol [14].
The compelling evidence for the critical role of STAR in the regulation of steroidogenesis has been demonstrated in patients suffering from lipoid congenital adrenal hyperplasia (lipoid CAH), an autosomal recessive disorder in which both adrenal and gonadal steroid biosyntheses are severely impaired due to mutations in the STAR gene [23, 26–29]. Targeted disruption of the STAR gene in mouse results in an essentially identical phenotype to that found in lipoid CAH in humans [30–32]. In fact, the STAR protein plays an important role in the regulation of steroid hormones required for life itself, in the case of adrenal steroids, and for maintaining reproductive capacity, in the case of gonadal steroids [3, 4, 6, 8, 21, 25]. Nonetheless, in virtually every system studied, agents that influence STAR expression also influence steroid biosynthesis through endocrine, autocrine and paracrine regulation. Regardless of the regulatory events, studies have demonstrated a tight correlation between the synthesis of STAR protein and the synthesis of steroids in a variety of classical (e.g. adrenal and gonadal) and non-classical (e.g. glial and skin) steroidogenic tissues [3, 6, 33, 34].
Whereas STAR plays an indispensable role in controlling steroid biosynthesis, a complete understanding of the regulation of its expression and function in steroidogenesis is not available. Recent findings have demonstrated that hormone-sensitive lipase (HSL), a neutral cholesteryl ester hydrolase (NCEH), plays a vital role in regulating STAR expression in adrenal and gonadal cells [3, 9, 35]. Of note, HSL catalyzes the hydrolysis of CEs in steroidogenic tissues and macrophages. In addition, the hydrolysis of CEs has been shown to be influenced by several enzymes, including acyl coenzyme A:cholesterol acyltransferase-1, neutral CE hydrolase 1 (also known as KIAA1363 or arylacetamide deacetylase-like 1), carboxylesterase 3, and CE hydrolase (identical to either human liver carboxylesterase 1 or macrophage serine esterase 1) [36–41]. Studies have demonstrated that regulation of HSL mediated STAR expression and steroid biosynthesis involves the liver X receptor (LXR) pathway [3, 9, 34]. Oxysterols act as ligands for LXRs (LXRα and LXRβ; also known as NR1H3 and NR1H2, respectively), which are members of the nuclear receptor superfamily of ligand activated transcription factors [42, 43]. LXRs form obligate heterodimers with retinoid X receptors (RXRs), which also dimerize with retinoid acid receptors (RARs), and regulate the transcription of a number of genes involved in cholesterol utilization, metabolism, and balance, including sterol regulatory element-binding proteins (SREBPs), ATP-binding cassette transporter 1 (ABCA1), and STAR [9, 42–44]. In this review, we will summarize the significant findings that have been made with regards to expression of the STAR protein and, thus, steroid biosynthesis, and their relevance to a number of endocrinological health issues and/or relevant abnormalities.
STAR protein and regulation of steroidogenesis
Steroid biosynthesis in response to trophic hormones is a de novo protein synthesis requiring process that is regulated by the delivery of cholesterol from the outer to the inner mitochondrial membrane. A large body of evidence indicates that the intramitochondrial transport of cholesterol is primarily mediated by the STAR protein, a rapidly synthesized mitochondrial phosphoprotein whose expression, activation, and extinction is mediated by protein kinase A (PKA), PKC, as well as a host of other signaling pathways that produce both acute and chronic effects on steroidogenesis [3, 4, 6, 11, 33]. The first step in the steroid biosynthetic pathway is the conversion of cholesterol to pregnenolone by the action of CYP11A1 in the inner mitochondria (Figure 1). Pregnenolone exits the mitochondria and then it is converted to various steroid hormones in specific tissues. STAR is mostly associated with steroid producing tissues, suggesting its critical role in a number of cholesterol and/or steroid led events. Since STAR plays an indispensable role in steroid biosynthesis, the mechanism surrounding the regulation of this gene is extremely important. It has been demonstrated that expression of the STAR gene is regulated in a tissue-, stimulus-, and species-specific manner that involve both positive and negative regulatory events [45–48]. There is a wealth of information indicating that regulation of steroid biosynthesis is mediated by mechanisms that enhance transcription, translation, or activity of STAR [6, 11, 45, 47, 49–51]. In accordance with this, transcriptional and/or translational inhibition of STAR expression results in a marked decrease, but not abolished, in steroid synthesis [8, 9, 47, 52]. This suggests that other proteins, in addition to STAR, play important roles for the intramitochondrial transport of cholesterol in controlling steroidogenesis. Molecular modeling, structure-based thermodynamics and biophysical studies suggest that StAR, upon binding cholesterol, interacts with an import complex at the surface of the mitochondria for the transfer of cholesterol [53–55]. Molecular events associated with transcriptional and/or translational regulation of STAR have been previously reviewed by us [6, 11, 45, 47] and others [24, 48, 56–58], and will not be elaborated upon in great detail here.
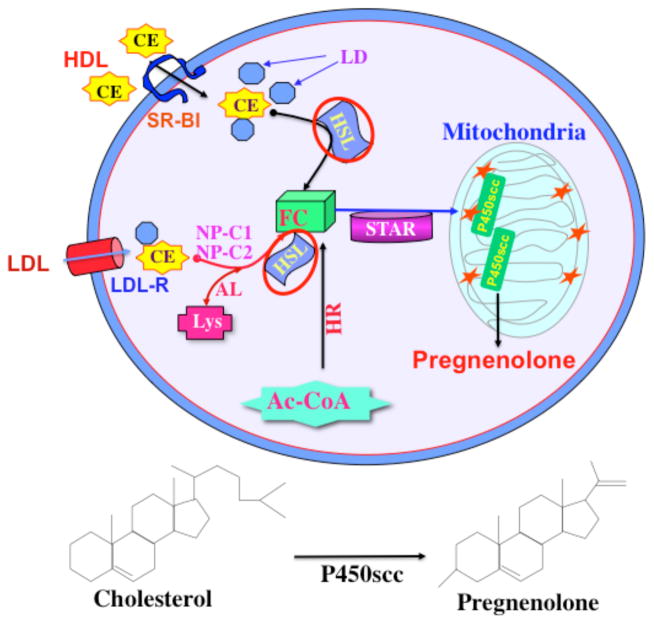
Fig. 1.
A model illustrating CE metabolism and its relevance to steroidogenesis. Cholesterol utilized for steroidogenesis is derived from a number of sources. The hydrolysis of CEs stored in lipid droplets is an important source of cholesterol for optimum steroid biosynthesis. HSL is a multifunctional enzyme that is responsible for NCEH activity. Circulating lipoprotins (HDL or LDL) bind to SR-B1 and release CEs into the cells. In rodents, free cholesterol (FC) utilized for steroid synthesis is mostly obtained via HDL mediated CE internalization and followed by cleavage by HSL. Receptor-mediated uptake of lipoprotein-derived CEs is processed via the LDL receptor in the human systems. De novo synthesis of cholesterol from acetyl-coenzyme A (AC-CoA) provides also FC for steroid synthesis. The STAR protein regulates steroid biosynthesis by controlling the transport of cholesterol from the outer to the inner mitochondrial membrane. Conversion of cholesterol to pregnenolone is the first enzymatic step in steroid hormone biosynthesis (bottom panel). Pregnenolone is then converted to various steroid hormones by a series of enzymes in specific tissues. LD, lipid droplets; AL, acid lipase; NP-C1 and C2, Niemann Pick C1 and C2; Lys, lisosome; HR, HMG-Coenzme A reductase. Revised and represented with permission from Molecular Human Reproduction (3).
Analysis of STAR protein sequences across different species exhibits considerable homology and demonstrates the presence of PKA phosphorylation sites [51, 59, 60]. Two PKA phosphorylation sites have been identified at Ser56/57 and Ser194/195, in mouse and human respectively, and mutations in these sites (Ser→Ala) demonstrated the importance of the latter in biological activity of STAR [59]. As such, phosphorylation of STAR is a plausible mechanism for the optimal cholesterol transferring ability of the STAR protein in steroid biosynthesis [9, 35, 51, 61, 62].
It is unequivocal that the cAMP/PKA signaling cascade is the major pathway regulating STAR expression and steroid biosynthesis; however, many studies have demonstrated the involvement of cAMP/PKA-independent events in these processes [3, 5, 11, 61, 63–66]. For example, whereas LH/hCG is the main regulator of various Leydig cell functions, including steroidogenesis, there is a large body of evidence indicating that the steroidogenic responsiveness of Leydig cells can be modulated by circulating peptides and locally produced factors. Indeed, several extracellular factors and/or signaling have been shown to enhance STAR expression and steroid production without altering intracellular cAMP/PKA levels. These include growth factors, macrophage derived factors, arachidonic acid and its metabolites, mitogen-activated protein kinase/extracellular signal-regulated kinase cascades, chloride ions, and calcium messenger systems [9, 51, 61, 67–70]. It should be noted, however, that the induction of cAMP/PKA-independent steroid biosynthesis (in the absence of STAR phosphorylation) is quite modest when compared to cAMP/PKA/STAR phosphorylation-dependent signaling. Therefore, phosphorylation of StAR is a potential mechanism between cAMP/PKA-independent and cAMP/PKA-dependent steroid biosynthesis, and point to the involvement of crosstalk in these signaling pathways. Even though cAMP/PKA-independent effects of various factors on steroidogenesis are quite small, many of them are capable of potentiating the steroidogenic response of gonadal and adrenal cells to gonadotropins and/or cAMP analogs. In fact, cAMP/PKA-independent pathways could be very important in modulating local regulation of steroid synthesis in various steroidogenic tissues. Consequently, the importance of cAMP/PKA-independent factors has been demonstrated in controlling a number of testicular and/or ovarian functions, including developmental and reproductive events [3, 6, 11, 61, 63, 71, 72].
Non-functional STAR protein, Lipoid CAH, and physiological consequences
Mutations in the STAR gene results in a protein that is non-functional and inactive, resulting in lipoid CAH, the rarest and most severe form of CAH [8, 26–28]. This potentially life-threatening disorder is characterized by an inborn error of steroid hormone biosynthesis resulting in near complete inability of the newborn to synthesize steroids. Clinical manifestations of lipoid CAH include a marked adrenocortical insufficiency, hypergonadotropic hypogonadism, and severe salt wasting, and affected newborns die shortly after birth as a result of glucocorticoid and mineralocorticoid deficiencies. Individuals afflicted with lipoid CAH are phenotypically female irrespective of chromosomal sex, have large adrenals containing high CE and cholesterol levels, and also have cholesterol deposition in steroidogenic cells [8, 26, 27, 73–77]. Affected individuals (who fail to metabolize cholesterol in mitochondria of the adrenal glands and gonads) die shortly after birth due to glucocorticoid and mineralocorticoid insufficiencies; however, appropriate hormone replacement therapy results in the survival of lipoid CAH patients to adulthood. Analysis of the STAR gene isolated from testicular tissue of lipoid CAH patients demonstrated the presence of nonsense and deletion mutations, which demonstrated initial proof of this disease and the critical role of STAR in steroid biosynthesis [23]. Whereas overexpression of wild type STAR in monkey kidney COS-1 cells (rendered steroidogenic by transfection with the P450scc system) increased steroid production, cells expressing mutant STAR were completely inactive in promoting steroidogenesis [23, 26, 78]. Therefore, expression of the STAR protein was an obligatory requirement for intracellular trafficking of cholesterol and that lipoid CAH represents a natural knockout of STAR with consequences consistent with the role of STAR on steroid biosynthesis.
Molecular genetic analyses have identified approximately three-dozen mutations in the STAR gene producing lipoid CAH and include nonsense and missense mutations, splicing errors, and frameshifts causing deletions/insertions [27, 28, 32, 76, 77, 79]. Notably, two patients diagnosed with lipoid CAH lacked mutations in the STAR gene. However, these patients have heterozygous mutations in the CYP11A1 gene, allowing afflicted individuals to survive longer periods without hormone replacement therapy. Thus, haploinsufficiency of CYP11A1 can lead to a late onset form of lipoid CAH or these patients may harbor mutations in other gene(s) whose function is dependent on STAR action. Noteworthy, however, CAH can be induced by the deficiency of one of four steroidogenic enzymes involved in cortisol biosynthesis, i.e., 21-hydroxylase, 11-hydroxylase, 3β-hydroxysteroid dehydrogenase, and 17α hydroxylase/17,20-lyase [29, 80–82]. All of the STAR mutations identified in lipoid CAH are found in the C-terminus of the STAR protein, alter its structure/function, and result in a biologically inactive non-functional STAR that lacks the ability to deliver cholesterol to CYP11A1 in supporting steroidogenesis. While a correlation between STAR mutations and lipoid CAH is documented, molecular analyses of female subjects led to the formulation of the two hit model [8, 26, 28, 76]. The first hit is caused by the inability of STAR to transfer cholesterol to the inner mitochondrial membrane for acute steroid synthesis due to mutations in the STAR gene. However, cells can continue to make small amounts of steroid by a STAR independent mechanism, which allows for the survival of some lipoid CAH patients for 1 to 2 months without treatment [26, 83]. The second hit results in the prolonged and massive accumulation of lipids in affected cells that eventually interferes with normal cellular processes and results in death of cells. Thus, the feminization occurring at puberty with lipoid CAH is due to the small amount of STAR independent steroids synthesized by ovarian follicles recruited during each cycle. Since STAR is not present in the granulosa cells of preovulatory follicles, the first hit cannot occur. In the second hit, which utilizes a STAR-independent process, recruited follicles synthesize small amounts of estrogen but ultimately accumulate excessive amounts of lipids resulting in destruction of the follicle prior to luteinization and progesterone production [26, 76, 84–86]. Factors responsible for the production of small amount of steroids include members of the START (STAR-related lipid-transfer) domain family (that have been reported to be involved in steroidogenesis) and oxysterols [43, 87–92].
Further insights into these mechanisms have been documented with targeted disruption of the STAR gene in a mouse model [30, 31, 93]. Similar to lipoid CAH in humans, STAR null mice have female external genitalia, fail to grow normally and die shortly after birth as a result of adrenocortical insufficiency. The adrenal glands were smaller in STAR knockout mice than those of wild type littermates and demonstrated profound morphological anomalies in the cortex [8, 30, 31]. Moreover, mice lacking the STAR gene had multiple abnormalities in adrenal and gonadal functions caused by the massive lipid accumulation in these tissues. Serum corticosterone and aldosterone levels were low with elevated ACTH and corticotropin-releasing hormone levels, representing impaired adrenal steroid production and loss of feedback regulation at the hypothalamic-pituitary level. Despite the dramatic effects of the absence of STAR on adrenal and gonadal steroid formation, prepubertal serum testosterone levels in STAR null mice did not differ from wild type littermates [30]. The physio-pathological characteristics of STAR knockout mice were similar to those seen in lipoid CAH in humans, supporting the two hit model, demonstrating the consequences of a non-functional STAR, and reinforcing the crucial role of this protein in the regulation of steroid biosynthesis (reviewed in Refs. [6, 8, 26–28]).
STAR expression and its correlation to estrogen dependent disorders
As mentioned above, the STAR protein predominantly regulates steroid biosynthesis. It is well known that estrogen, derived from a number of sources, plays a central role in the pathogenesis of gynecological disorders and/or cancers, i.e., endometriosis and, endometrial, breast, and ovarian cancers [94–97]. The key enzyme for biosynthesis of estrogens is aromatase cytochrome P450 (P450arom, the product of CYP19 gene) that catalyzes the conversion of androstenedione and testosterone to estrone and estradiol [94, 98]. Consequently, aromatase inhibitors (that eliminate and/or block estrogen production) have been successfully used for prevention and treatment of these diseases.
One of the most common, inherited, chronic, and estrogen-dependent gynecological disorder of complex multifactorial etiology is endometriosis. This disease is defined by the presence of endometrial glands and stroma within pelvic peritoneum and other extrauterine tissues and is associated with pelvic pain and infertility [99, 100]. It is well established that estrogen from the ovaries is critical for maintenance of endometriosis, which affects approximately 5–10% in reproductive-aged women and 40–50% with infertility [101–104]. Endometriotic tissue expresses STAR, CYP11A1, aromatase, and other steroidogenic enzymes, thereby producing estrogen from cholesterol de novo [99, 105–107]. However, normal endometrium is not steroidogenic. Studies have shown that both mouse and rat peritoneal macrophages produce 25-hydroxycholesterol (25-HC; an endogenous metabolite of cholesterol) that can serve as a substrate for inducing STAR expression and steroid production in different steroidogenic tissues [108–110]. Hence, macrophages in the peritoneal cavity might be involved in providing 25-HC to endometriotic cells, thus increasing the estrogen load to the lesions. Studies have also reported that altered expression and DNA binding activity of a number of transcription factors involved in controlling steroidogenic genes are responsible for the abnormalities connected with endometriosis [94, 98, 104].
We observed recently that both oxysterols (22R-, 25- and 27-HC) and peritoneal fluids (collected from woman without or with endometriosis of stages from I through IV; Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center; IRB# L13-033) were capable of increasing STAR expression and pregnenolone synthesis in human endometrial stromal (ATCC, Manassas, VA) cells (Manna PR et al., unpublished observations). In addition, STAR mRNA expression was found to be strikingly higher in stage IV endometriosis when compared with control endometrium. It is likely that 25-HC derived estrogen plays an important role in endometriosis, has the ability to induce non-steroidogenic cells to become steroidogenic, and is elevated in women with endometriosis. As such, determination of 25-HC in the peritoneal fluid and/or blood of patients with endometriosis could be a novel noninvasive procedure for diagnosing this disorder. Collectively, there are two sources of estrogen stimulating the endometriotic tissue, one from the ovary and the other from the lesions themselves. Regardless of estrogen sources, drugs that inhibit the production or action of estrogen have been shown to reduce the severity of endometriosis [104, 111–113].
Role of HSL-LXR signaling on STAR mediated physiological events
HSL, a multifunctional enzyme, by catalyzing the hydrolysis of CEs, plays an important role in a number of physiological processes [3, 9, 38, 114, 115]. Notably, HSL is the primary NCEH in steroidogenic tissues, and disruption of HSL in mice results in a marked attenuation of NCEH activity in the adrenals and testes accompanied with profound morphological alterations in these tissues [3, 9, 38, 39, 116, 117]. Male, but not female, mice homozygous for the mutant HSL allele were sterile [38]; thus, the inactivation of HSL mainly affected spermatogenesis and not oogenesis. HSL null male mice exhibit several testicular anomalies, including decreased weight, vacuolated seminiferous tubules, reduced spermatids, and sterility [38, 117–119]; however, circulating steroid hormone levels were normal in these mice. The mechanism responsible for persistent steroidogenesis in HSL null mice, resulting in little to no HSL/NCEH activity, remains unclear, and may involve one or more compensatory event(s), including acyl-coenzyme A:cholesterol-acyltransferase activity and/or de novo cholesterol synthesis.
Hormonal control of HSL activity is chiefly mediated by phosphorylation of several serine (Ser) residues i.e. Ser563, Ser565, Ser600, Ser659, and Ser660 (sequences corresponding to rat HSL), by cAMP-dependent PKA as well as other kinases [9, 120–122]. Previously, we have demonstrated that activation of cAMP/PKA signaling enhances phosphorylation of HSL at Ser660 and Ser563, concomitant with its increased hydrolytic activity, and these events are tightly connected with STAR expression and steroid biosynthesis in gonadal and adrenal cells [3, 9]. In keeping with this, deficiency of HSL decreases STAR and steroid levels, demonstrating that HSL plays a vital role in regulating the steroidogenic response. However, the interaction of HSL with a number of proteins, including STAR and perilipin (a lipid droplet associated protein), in trafficking of intracellular cholesterol from lipid droplets into the mitochondria thereby supporting steroidogenesis has been demonstrated [2, 123]. Whether members of the START domain family, specifically STARD3 (also called as MLN64, metastatic lymph node 64) and STARD4-STARD6, interact with HSL and influence cholesterol trafficking, metabolism and balance requires additional investigation.
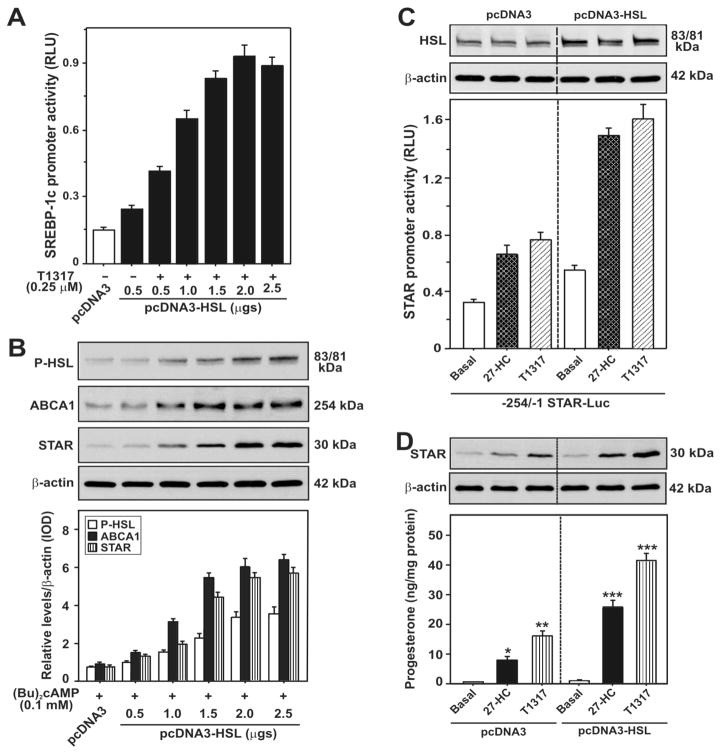
Lipoprotein-derived selective uptake of CEs, via the scavenger-receptor class B type 1 ((SR-B1, a high-density lipoprotein (HDL) receptor)), provides most of the cholesterol for steroidogenesis in rodents, with lesser contributions from low-density lipoprotein (LDL) and de novo synthesis [1, 61, 124, 125]. Receptor-mediated endocytic uptake of lipoprotein-derived CEs is processed via the LDL receptor in the human systems [126, 127]. The roles of the Niemann Pick C1 (NP-C1) and NP-C2 proteins in cholesterol trafficking via LDL receptor-mediated endocytosis and cleavage of CEs by lysosomal acid lipase have also been reported [128–130]. Nonetheless, a striking correlation between hormonal induction of SR-B1 expression and steroid synthesis has been reported in different steroidogenic cells. High levels of HSL and SR-B1 are also linked with constitutive expression of STAR and steroid production in R2C rat Leydig cells [124]. It is worth mentioning that knock-down of HSL decreases SR-B1, cholesterol, and STAR levels in gonadal and adrenal cells, representing a direct connection between HSL action and STAR expression [3, 9, 124]. Alternatively, overexpression of HSL increases not only the efficacy of LXR ligands on STAR transcription and steroid biosynthesis but also the LXR target genes, SREBP-1c and ABCA1 (Figure 2), demonstrating the involvement of LXR signaling in controlling HSL mediated steroidogenesis.
Fig. 2.
Overexpression of HSL on 27-HC and/or T1317 and (Bu)2cAMP stimulated SREBP-1c and STAR promoter activity, and HSL, P-HSL, ABCA1, and STAR and steroid levels. MA-10 cells were transfected with pcDNA3-HSL at either increasing (0.5–2.5 μg; A and B) or fixed (2.0 μg; C and D) amounts of cDNAs, within the context of either the −2.7 kb/+1 bp SREBP-1c (A) or −254/−1 bp STAR (C) promoter-driven luciferase reporter plasmid, in the presence of pRL-SV40. Following 36h of transfection, cells were treated without or with 27-HC (0.25 μM) and T1317 (0.25 μM) for an additional 6h. Luciferase activity in the cell lysates was determined and expressed as SREBP-1c (A) and STAR (C) promoter activity, RLU (luciferase/renilla). Cells were also processed for immunoblotting (B). Representative immunoblots illustrate HSL, P-HSL, ABCA1, and STAR in different groups using 20–30 μg of total cellular protein (B–D). Immunoblots shown are representative of four independent experiments. β-actin expression was assessed as a loading control (C and D). *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. basal. (Revised and represented following copyright permission, “this research was originally published in The Journal of Biological Chemistry, Manna PR, Cohen-Tannoudji J, Counis R, Garner CW, Huhtaniemi I, Kraemer FB, Stocco DM, Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J. Biol. Chem., 2013, 288(12):8505–18. © the American Society for Biochemistry and Molecular Biology”).
LXRs bind to oxysterol ligands, activate transcription of many genes, including STAR, and play essential roles in regulating intracellular cholesterol trafficking and balance [9, 43, 131]. Oxysterols are involved in STAR expression and steroidogenesis. Mice lacking LXRs (both α and β) display reduced fertility, underscoring the importance of these receptors in reproduction [132, 133]. In keeping with this, we have shown that silencing of LXRα/β diminishes cAMP/PKA responsive HSL activity, accompanied with decreased expression and phosphorylation of STAR and steroid biosynthesis in mouse Leydig cells [9]. An increase in HSL levels modulates the steroidogenic response mediated by LXR ligands, suggesting HSL dependent steroidogenesis entails enhanced oxysterol production. HSL is also capable of increasing ABCA1 protein expression. Recent findings provide evidence that LXRs interact/cooperate with RXRs/(RARs) and result in synergistic activation of cAMP/PKA mediated STAR expression and steroid synthesis [9, 35]. Additionally, it has been demonstrated that retinoids (vitamin A and its derivatives), especially all-trans retinoic acid (atRA) and 9-cis RA (which primarily act through RXRs and RARs), by interacting with an LXR-RXR/RAR heterodimeric motif in the STAR promoter, plays an important role in transcriptional regulation of the STAR gene [34, 35]. Retinoids are hormone-like molecules that exert a multifaceted array of effects on development, differentiation, reproduction, and epidermal homeostasis [35, 134–140]. Retinoid signaling enhances HSL activity, and HSL mediated up-regulation of StAR expression involves the LXR regulatory pathway [3, 9, 35]. Based on these observations, it is plausible that regulation of HSL-LXR-RXR/RAR by retinoid signaling may have important implications on prevention of many complications and diseases.
Role of STAR in macrophage cholesterol efflux and its relevance to atherosclerosis
The deposition of excess lipids/CEs in the arterial walls is a hallmark of atherosclerosis in which macrophages play a vital role to form the foam cells. Atherosclerosis is the most prevalent event within cardiovascular diseases and is the leading cause of morbidity and mortality worldwide. Accumulating evidence indicates that stimulation of CE hydrolysis following overexpression of HSL correlates with ABCA1 expression in macrophages, a crucial event in cellular lipid transport and atherosclerosis [141–145]. ABCA1, a key protein in cholesterol efflux, transports cellular cholesterol from macrophages to apolipoprotein A1 (Apo-A1). The importance of ABCA1 has been documented by its absence in patients afflicted with Tangier disease, which is linked to HDL deficiency and premature atherosclerosis [146]. Removal of excess CEs from macrophage-derived foam cells is critical for the progression of atherosclerotic lesions [145, 147–149]. Studies have shown that overexpression of STAR increases macrophage cholesterol efflux and decreases intracellular lipids and the secretion of inflammatory factors [148, 149].
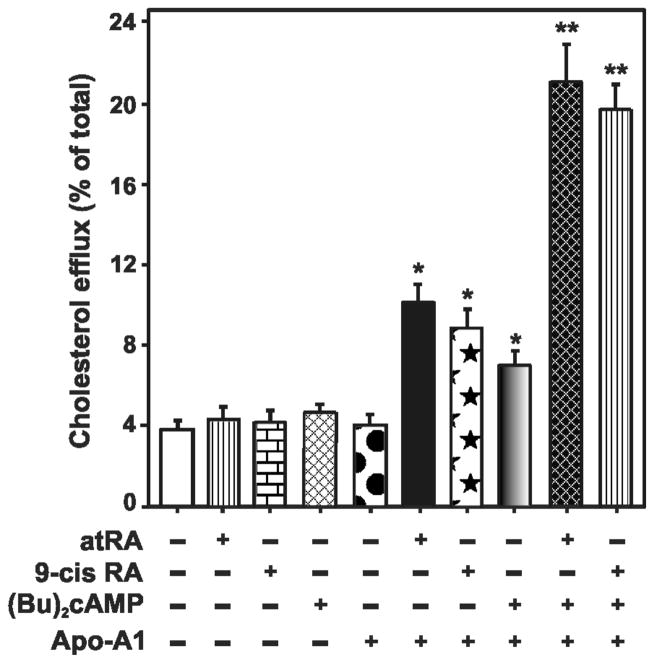
LXR-RXR/RAR and their heterodimerization partners, especially lipid homeostasis-related transcription factors, SREBPs (particularly SREBP-1c) and peroxisome proliferator-activated receptors (PPARs), are targets for intervention in atherosclerosis [144, 150–152]. Our recent findings demonstrate that retinoids strikingly increase cAMP/PKA responsive STAR and steroid levels in a variety of steroidogenic cell models [9, 34, 35]. These results suggest that retinoid signaling is capable of enhancing cholesterol clearance from cells, an approach that may be effective in limiting plaque stability and progression of atherosclerotic cardiovascular disease. Consistent with this, in a recent study [153] we have reported that retinoids enhance cholesterol efflux to Apo-A1 in mouse RAW 264.7 macrophages, and this effect was further augmented in the presence of cAMP/PKA signaling (Figure 3). Besides, macrophages overexpressing HSL increased the hydrolysis of CEs resulting in a depletion of CE content and elevated StAR mRNA expression. Concurrently, HSL overexpression was capable of enhancing the efficacy of RAR and LXR ligands on StAR and ABCA1 protein levels [153]. These findings imply that an increase in HSL levels promotes oxysterol production, which, in turn, activates LXR and results in up-regulation of retinoid mediated macrophage cholesterol efflux. In support of this, previous studies have demonstrated the role of LXRs and PPARs in the control of cholesterol trafficking in macrophages [142, 154]. Taken together, it is plausible that LXR activation enhances plasma membrane cholesterol trafficking and efflux, and modulates cholesterol esterification, thus contributing to the effects of retinoids in controlling cholesterol balance for limiting/stabilizing atherosclerotic cardiovascular disease. An understanding of the process of macrophage foam cell formation and its connection to HSL-LXR regulated events will help develop novel therapeutic interventions for atherosclerosis.
Fig. 3.
Effects of atRA and 9-cis RA on (Bu)2cAMP stimulated macrophage cholesterol efflux. Mouse RAW 264.7 macrophages were labeled with 3H-cholesterol for 24h. Macrophages were then treated without or with atRA (10 μM), 9-cis RA (10 μM), (Bu)2cAMP (0.1 mM), or their combination, for 12h, in the absence or presence of Apo-A1 (20 mg/ml), as indicated. Following treatments, media and cells from different groups were collected separately and counted in a liquid scintillation counter, and cholesterol efflux was calculated as the percentage of radioactivity recovered in the media over total (cells plus media) radioactivity. Data represent the mean ± SE of 4 independent experiments. *, p < 0.05; **, p <0.01; vs. control. Revised and represented with permission from Biochemical Biophysical Research Communication (153).
STAR expression and its correlation to aging
Complex endocrine changes, affecting the morphology and function of a multitude of organs, occur as life progresses from adulthood into senescence. This aging process results in a decline of various hormones and, as a consequence, affects a number of physiological functions [155–160]. The occurrence of hormone deficiencies (or endocrinosenescence) is constituted to be the major cause of human senescence and it is associated with numerous complications and disabilities [156, 160–162]. Endocrinosenescence includes growth hormone/insulin-like growth factor-1 axis (somatopause), hypothalamic-pituitary gonadal axis (hypogonadism), testosterone (andropause), estradiol (menopause), and dehydroepiandrosterone (adrenopause) [159, 160, 163–166]. The manifestations of these deficiencies include, but are not limited to, inefficient hypothalamic-pituitary-thyroidal-adrenal-gonadal (HPTAG) axes, neurodegenerative disorders, diminished eyesight, impaired memory and cognitive function, decreased muscle mass and bone density, decreased steroid biosynthesis, sexual dysfunction and depression, increased risk in cardiovascular disease, and skin disorders [65, 139, 155, 159, 162, 167, 168]. These conditions profoundly affect geriatric populations worldwide within the context of a substantial rise in life expectancy. Preservation of hormonal balance is the key to proper functioning of various biological activities during aging. We have demonstrated that retinoids, especially RAs, up-regulate STAR expression and steroid biosynthesis in adrenal, gonadal, glial, and epidermal cells [34, 35], indicating that retinoid signaling is capable of influencing a number of cholesterol/steroid coupled physiological activities that are frequently impaired in geriatric populations.
Aging is an inevitable heterogeneous phenomenon involving the whole organism and results in a decline of the central nervous system and the endocrine system [156, 169–172]. The maintenance of a well-balanced endocrine circadian rhythmicity is critical to health. Age-associated hormonal imbalance, involving a reduction in the steroidogenic output, is linked to numerous health complications along with a host of pathologies [138, 173–177]. There is increasing evidence that aging is connected to the progressive accumulation of dysfunctional mitochondria and oxidative damage, which modulate the immune system and contribute to increased morbidity and mortality [177–181]. With aging, excessive production of free radicals and reactive oxygen species (ROS) occurs in the mitochondria, which affects the function of the HTPAG axis auto-regulating system. The increase in free radicals is inversely correlated with antioxidant capacity in the central nervous system and its associated glands [179, 182]. It is conceivable that an imbalance between production of free radicals/ROS and protective antioxidant systems, affecting cellular oxidative damage, might induce age-related complications and diseases [179, 180, 182]. Previous studies have reported that ROS disrupts mitochondria and decreases in STAR expression and steroidogenesis in a variety of steroid producing cells, and is tightly connected with age-related decline in steroid biosynthesis [177, 178, 183, 184]. As such, oxidative damage induced by ROS is deleterious to the functional efficiency of various cellular processes and is implicated in the pathogenesis of numerous conditions including age-related complications and disabilities.
Physiological aging results in most of the phenotypic changes observed in skin. The latter forms an essential barrier between the external environment and the biological milieu, and it is tightly networked to central regulatory systems [34, 139, 176, 185–187]. Several lines of evidence demonstrate that regulation of glucocorticosteroidogenesis in the skin is similar to those operating in classical steroidogenic tissues [188–191]. Human skin cells express STAR, synthesize cholesterol, and possess the functional biochemical apparatus for the synthesis of glucocorticoids, androgens, and estrogens, which play vital roles in epidermal homeostasis [139, 188, 190, 192–194]. It should be noted that in human skin CYP11A1 can also use 7-dehydrocholesterol (precursor to cholesterol and vitamin D) as an alternative substrate leading to production of 7Δ-steroids [176, 195]. Expression levels of STAR and aromatase have been correlated with androgen and estrogens in male and female skin tissues, respectively, demonstrating the relevance of STAR and sex steroids in homeostasis of the human skin [194]. In contrast, malfunction in skin cholesterol synthesis, involving a global reduction in steroids, is associated with down-regulation of epidermal differentiation, leading to many skin complications/disorders [34, 139, 187, 193, 196]. We and others have demonstrated that expression of STAR mRNA is decreased or aberrant in several inflammatory skin diseases, including eczema, intertrigo, atopic dermatitis, signifying that steroid biosynthesis is disrupted in these diseased conditions [34, 139, 197–199].
As mentioned above, retinoids influence an array of functions, ranging from vision to reproduction to homeostasis [35, 139, 199–202]. The therapeutic and preventive effects of retinoids in numerous skin conditions and diseases, including premature skin aging, skin cancer prevention, squamous cell carcinoma, and skin rejuvenation and hyperpigmentation have long been established [137, 191, 203–205]. Retinoid metabolism and signaling also decreases in a variety of complications and diseases [138, 140, 168, 206]. The systemic administration of RAs has been shown to reverse most reproductive and developmental blocks in vitamin A deficient (VAD) rats and mice, demonstrating that retinoid signaling rescues reproductive defects as well as steroidogenesis in VAD animals [201, 207, 208]. An unanswered question is if retinoids are able to reverse the decline in steroid biosynthesis in target tissues and thereby restore steroid coupled impaired biological activities that particularly evolve during aging. It has been shown that retinoids, especially RAs, elevate expression of steroidogenic enzymes, STAR, and steroid biosynthesis in a variety of target tissues [35, 209–213]. Recently, we observed that retinoids were capable of enhancing and/or restoring STAR expression and pregnenolone synthesis in isolated epidermal keratinocytes of elderly (64–83 years) individuals (Manna PR et al., unpublished observations). These de-identified human skin tissues were obtained upon various surgeries from the Department of Dermatology clinic, Texas Tech University Health Sciences Center (IRB# L14-085). This implies that retinoid signaling is capable of reversing the decline in steroid biosynthesis and relevant skin complications and disorders in aging populations. Hence, it is conceivable that therapeutic strategies involving the use of retinoids will have benefits in the restoration of many impaired physiological activities that are important for healthy aging. Future studies on tissue-specific regulation of retinoid mediated restoration of cholesterol/steroid dependent events provide better understanding on many important physiological and/or patho-physiological processes.
Acknowledgments
The authors would like to thank many co-workers and collaborators, and the studies of several research groups whose contributions helped in preparing this review. This review was supported, in part, by NIH grants CA155223 to KP and, AR052190, 1R01AR056666-01A2, and R21AR066505-01A1 to ATS.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Azhar S, Reaven E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol Cell Endocrinol. 2002;195:1–26. doi: 10.1016/s0303-7207(02)00222-8. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer FB, Shen WJ, Harada K, Patel S, Osuga J, Ishibashi S, Azhar S. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol. 2004;18:549–557. doi: 10.1210/me.2003-0179. [DOI] [PubMed] [Google Scholar]
- 3.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15:321–333. doi: 10.1093/molehr/gap025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:93–108. doi: 10.2174/1568008053174714. [DOI] [PubMed] [Google Scholar]
- 5.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- 6.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo AF, Orlando U, Helfenberger KE, Poderoso C, Podesta EJ. The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol Cell Endocrinol. 2015;408:73–79. doi: 10.1016/j.mce.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Stocco D. Star protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 10.Miller WL. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- 11.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manna PR, Cohen-Tannoudji J, Counis R, Garner CW, Huhtaniemi I, Kraemer FB, Stocco DM. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J Biol Chem. 2013;288:8505–8518. doi: 10.1074/jbc.M112.417873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arakane F, Kallen CB, Watari H, Foster JA, Sepuri NB, Pain D, Stayrook SE, Lewis M, Gerton GL, Strauss JF., 3rd The mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem. 1998;273:16339–16345. doi: 10.1074/jbc.273.26.16339. [DOI] [PubMed] [Google Scholar]
- 14.Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem. 2001;276:46583–46596. doi: 10.1074/jbc.M107815200. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Liu J, Culty M, Papadopoulos V. Acyl-coenzyme A binding domain containing 3 (ACBD3; PAP7; GCP60): an emerging signaling molecule. Prog Lipid Res. 2010;49:218–234. doi: 10.1016/j.plipres.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poderoso C, Duarte A, Cooke M, Orlando U, Gottifredi V, Solano AR, Lemos JR, Podesta EJ. The spatial and temporal regulation of the hormonal signal. Role of mitochondria in the formation of a protein complex required for the activation of cholesterol transport and steroids synthesis. Mol Cell Endocrinol. 2013;371:26–33. doi: 10.1016/j.mce.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Orlando U, Cooke M, Cornejo Maciel F, Papadopoulos V, Podesta EJ, Maloberti P. Characterization of the mouse promoter region of the acyl-CoA synthetase 4 gene: role of Sp1 and CREB. Mol Cell Endocrinol. 2013;369:15–26. doi: 10.1016/j.mce.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci. 2015;112:7261–7266. doi: 10.1073/pnas.1502670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 22.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 23.Lin D, Sugawara T, Strauss JF, III, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 24.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- 26.Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. International Congenital Lipoid Adrenal Hyperplasia Consortium. N Engl J Med. 1996;335:1870–1878. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- 27.Stocco DM. Clinical disorders associated with abnormal cholesterol transport: mutations in the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 2002;191:19–25. doi: 10.1016/s0303-7207(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 28.King SR, Bhangoo A, Stocco DM. Functional and physiological consequences of StAR deficiency: role in lipoid congenital adrenal hyperplasia. Endocr Dev. 2011;20:47–53. doi: 10.1159/000321214. [DOI] [PubMed] [Google Scholar]
- 29.Auchus RJ, Miller WL. Congenital adrenal hyperplasia--more dogma bites the dust. J Clin Endocrino Metab. 2012;97:772–775. doi: 10.1210/jc.2012-1080. [DOI] [PubMed] [Google Scholar]
- 30.Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci. 1997;94:11540–11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14:1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- 32.Bose HS, Sato S, Aisenberg J, Shalev SA, Matsuo N, Miller WL. Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:3636–3639. doi: 10.1210/jcem.85.10.6896. [DOI] [PubMed] [Google Scholar]
- 33.Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015 doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manna PR, Slominski AT, King SR, Stetson CL, Stocco DM. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: Involvement of cAMP/PKA signaling. Endocrinology. 2014;155:576–591. doi: 10.1210/en.2013-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Li H, Papadopoulos V. PAP7, a PBR/PKA-RIalpha-associated protein: a new element in the relay of the hormonal induction of steroidogenesis. J Steroid Biochem Mol Biol. 2003;85:275–283. doi: 10.1016/s0960-0760(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Zhao B, Bie J, Song J. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascul Pharmacol. 2010;52:1–10. doi: 10.1016/j.vph.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 40.Zhao B, Bie J, Wang J, Marqueen SA, Ghosh S. Identification of a novel intracellular cholesteryl ester hydrolase (carboxylesterase 3) in human macrophages: compensatory increase in its expression after carboxylesterase 1 silencing. Am J Physiol Cell Physiol. 2012;303:C427–435. doi: 10.1152/ajpcell.00103.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai K, Igarashi M, Yamamuro D, Ohshiro T, Nagashima S, Takahashi M, Enkhtuvshin B, Sekiya M, Okazaki H, Osuga J, Ishibashi S. Critical role of neutral cholesteryl ester hydrolase 1 in cholesteryl ester hydrolysis in murine macrophages. J Lipid Res. 2014;55:2033–2040. doi: 10.1194/jlr.M047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 43.Cummins CL, Volle DH, Zhang Y, McDonald JG, Sion B, Lefrancois-Martinez AM, Caira F, Veyssiere G, Mangelsdorf DJ, Lobaccaro JM. Liver X receptors regulate adrenal cholesterol balance. J Clin Invest. 2006;116:1902–1912. doi: 10.1172/JCI28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volle DH, Lobaccaro JM. Role of the nuclear receptors for oxysterols LXRs in steroidogenic tissues: beyond the “foie gras”, the steroids and sex?, Mol. Cell Endocrinol. 2007;265–266:183–189. doi: 10.1016/j.mce.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol. 2003;30:381–397. doi: 10.1677/jme.0.0300381. [DOI] [PubMed] [Google Scholar]
- 46.Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J Mol Endocrinol. 2007;39:261–277. doi: 10.1677/JME-07-0065. [DOI] [PubMed] [Google Scholar]
- 47.Manna PR, Dyson MT, Stocco DM. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol Cell Endocrinol. 2009;302:1–11. doi: 10.1016/j.mce.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood) 2009;234:880–907. doi: 10.3181/0903-MR-97. [DOI] [PubMed] [Google Scholar]
- 49.Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- 50.King SR, Ronen-Fuhrmann T, Timberg R, Clark BJ, Orly J, Stocco DM. Steroid production after in vitro transcription, translation, and mitochondrial processing of protein products of complementary deoxyribonucleic acid for steroidogenic acute regulatory protein. Endocrinology. 1995;136:5165–5176. doi: 10.1210/endo.136.11.7588255. [DOI] [PubMed] [Google Scholar]
- 51.Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol Endocrinol. 2006;20:362–378. doi: 10.1210/me.2004-0526. [DOI] [PubMed] [Google Scholar]
- 52.Clark BJ, Combs R, Hales KH, Hales DB, Stocco DM. Inhibition of transcription affects synthesis of steroidogenic acute regulatory protein and steroidogenesis in MA-10 mouse Leydig tumor cells. Endocrinology. 1997;138:4893–4901. doi: 10.1210/endo.138.11.5535. [DOI] [PubMed] [Google Scholar]
- 53.Mathieu AP, Fleury A, Ducharme L, Lavigne P, LeHoux JG. Insights into steroidogenic acute regulatory protein (StAR)-dependent cholesterol transfer in mitochondria: evidence from molecular modeling and structure-based thermodynamics supporting the existence of partially unfolded states of StAR. J Mol Endocrinol. 2002;29:327–345. doi: 10.1677/jme.0.0290327. [DOI] [PubMed] [Google Scholar]
- 54.Roostaee A, Barbar E, Lehoux JG, Lavigne P. Cholesterol binding is a prerequisite for the activity of the steroidogenic acute regulatory protein (StAR) Biochem J. 2008;412:553–562. doi: 10.1042/BJ20071264. [DOI] [PubMed] [Google Scholar]
- 55.Barbar E, Lehoux JG, Lavigne P. Toward the NMR structure of StAR. Mol Cell Endocrinol. 2009;300:89–93. doi: 10.1016/j.mce.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Clark BJ, Ranganathan V, Combs R. Steroidogenic acute regulatory protein expression is dependent upon post-translational effects of cAMP-dependent protein kinase A. Mol Cell Endocrinol. 2001;173:183–192. doi: 10.1016/s0303-7207(00)00410-x. [DOI] [PubMed] [Google Scholar]
- 57.Christenson LK, Strauss JF., 3rd Steroidogenic acute regulatory protein: an update on its regulation and mechanism of action. Arch Med Res. 2001;32:576–586. doi: 10.1016/s0188-4409(01)00338-1. [DOI] [PubMed] [Google Scholar]
- 58.Yivgi-Ohana N, Sher N, Melamed-Book N, Eimerl S, Koler M, Manna PR, Stocco DM, Orly J. Transcription of steroidogenic acute regulatory protein in the rodent ovary and placenta: alternative modes of cyclic adenosine 3′, 5′-monophosphate dependent and independent regulation. Endocrinology. 2009;150:977–989. doi: 10.1210/en.2008-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 60.Fleury A, Mathieu AP, Ducharme L, Hales DB, LeHoux JG. Phosphorylation and function of the hamster adrenal steroidogenic acute regulatory protein (StAR) J Steroid Biochem Mol Biol. 2004;91:259–271. doi: 10.1016/j.jsbmb.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Manna PR, Chandrala SP, Jo Y, Stocco DM. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol. 2006;37:81–95. doi: 10.1677/jme.1.02065. [DOI] [PubMed] [Google Scholar]
- 62.Manna PR, Dyson MT, Jo Y, Stocco DM. Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A- and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action. Endocrinology. 2009;150:187–199. doi: 10.1210/en.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooke BA. Signal transduction involving cyclic AMP-dependent and cyclic AMP- independent mechanisms in the control of steroidogenesis. Mol Cell Endocrinol. 1999;151:25–35. doi: 10.1016/s0303-7207(98)00255-x. [DOI] [PubMed] [Google Scholar]
- 64.Richards JS. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 65.Manna PR, Joshi L, Reinhold VN, Aubert ML, Suganuma N, Pettersson K, Huhtaniemi IT. Synthesis, purification and structural and functional characterization of recombinant form of a common genetic variant of human luteinizing hormone. Hum Mol Genet. 2002;11:301–315. doi: 10.1093/hmg/11.3.301. [DOI] [PubMed] [Google Scholar]
- 66.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57:3–18. doi: 10.1016/s0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 67.Manna PR, Pakarinen P, El-Hefnawy T, Huhtaniemi IT. Functional assessment of the calcium messenger system in cultured mouse Leydig tumor cells: regulation of human chorionic gonadotropin-induced expression of the steroidogenic acute regulatory protein. Endocrinology. 1999;140:1739–1751. doi: 10.1210/endo.140.4.6650. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Walsh LP, Reinhart AJ, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem. 2000;275:20204–20209. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- 69.Manna PR, Huhtaniemi IT, Wang XJ, Eubank DW, Stocco DM. Mechanisms of epidermal growth factor signaling: regulation of steroid biosynthesis and the steroidogenic acute regulatory protein in mouse leydig tumor cells. Biol Reprod. 2002;67:1393–1404. doi: 10.1095/biolreprod.102.007179. [DOI] [PubMed] [Google Scholar]
- 70.Cornejo Maciel F, Maloberti P, Neuman I, Cano F, Castilla R, Castillo F, Paz C, Podesta EJ. An arachidonic acid-preferring acyl-CoA synthetase is a hormone-dependent and obligatory protein in the signal transduction pathway of steroidogenic hormones. J Mol Endocrinol. 2005;34:655–666. doi: 10.1677/jme.1.01691. [DOI] [PubMed] [Google Scholar]
- 71.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 72.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakae J, Tajima T, Sugawara T, Arakane F, Hanaki K, Hotsubo T, Igarashi N, Igarashi Y, Ishii T, Koda N, Kondo T, Kohno H, Nakagawa Y, Tachibana K, Takeshima Y, Tsubouchi K, Strauss JF, 3rd, Fujieda K. Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum Mol Genet. 1997;6:571–576. doi: 10.1093/hmg/6.4.571. [DOI] [PubMed] [Google Scholar]
- 74.Okuyama E, Nishi N, Onishi S, Itoh S, Ishii Y, Miyanaka H, Fujita K, Ichikawa Y. A novel splicing junction mutation in the gene for the steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 1997;82:2337–2342. doi: 10.1210/jcem.82.7.4045. [DOI] [PubMed] [Google Scholar]
- 75.Miller WL. Congenital lipoid adrenal hyperplasia: the human gene knockout for the steroidogenic acute regulatory protein. J Mol Endocrinol. 1997;19:227–240. doi: 10.1677/jme.0.0190227. [DOI] [PubMed] [Google Scholar]
- 76.Miller WL, Strauss JF., 3rd Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 77.Fujieda K, Okuhara K, Abe S, Tajima T, Mukai T, Nakae J. Molecular pathogenesis of lipoid adrenal hyperplasia and adrenal hypoplasia congenita. J Steroid Biochem Mol Biol. 2003;85:483–489. doi: 10.1016/s0960-0760(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 78.Tee MK, Lin D, Sugawara T, Holt JA, Guiguen Y, Buckingham B, Strauss JF, 3rd, Miller WL. T-->A transversion 11 bp from a splice acceptor site in the human gene for steroidogenic acute regulatory protein causes congenial lipoid adrenal hyperplasia. Hum Mol Genet. 1995;4:2299–2305. doi: 10.1093/hmg/4.12.2299. [DOI] [PubMed] [Google Scholar]
- 79.Christenson LK, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) and the intramitochondrial translocation of cholesterol. Biochim Biophys Acta. 2000;1529:175–187. doi: 10.1016/s1388-1981(00)00147-5. [DOI] [PubMed] [Google Scholar]
- 80.New MI. Inborn errors of adrenal steroidogenesis. Mol Cell Endocrinol. 2003;211:75–83. doi: 10.1016/j.mce.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 81.Krone N, Grotzinger J, Holterhus PM, Sippell WG, Schwarz HP, Riepe FG. Congenital adrenal hyperplasia due to 11-hydroxylase deficiency--insights from two novel CYP11B1 mutations (p.M92X, p.R453Q) Horm Res. 2009;72:281–286. doi: 10.1159/000245930. [DOI] [PubMed] [Google Scholar]
- 82.Brett EM, Auchus RJ. Genetic forms of adrenal insufficiency. Endocr Pract. 2015;21:395–399. doi: 10.4158/EP14503.RA. [DOI] [PubMed] [Google Scholar]
- 83.Hauffa BP, Miller WL, Grumbach MM, Conte FA, Kaplan SL. Congenital adrenal hyperplasia due to deficient cholesterol side-chain cleavage activity (20, 22-desmolase) in a patient treated for 18 years. Clin Endocrinol. 1985;23:481–493. doi: 10.1111/j.1365-2265.1985.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 84.Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab. 1997;82:1511–1515. doi: 10.1210/jcem.82.5.3962. [DOI] [PubMed] [Google Scholar]
- 85.Fujieda K, Tajima T, Nakae J, Sageshima S, Tachibana K, Suwa S, Sugawara T, Strauss JF., 3rd Spontaneous puberty in, 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 1997;99:1265–1271. doi: 10.1172/JCI119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shima M, Tanae A, Miki K, Katsumata N, Matsumoto S, Nakajima S, Harada T, Shinagawa T, Tanaka T, Okada S. Mechanism for the development of ovarian cysts in patients with congenital lipoid adrenal hyperplasia. Eur J Endocrinol. 2000;142:274–279. doi: 10.1530/eje.0.1420274. [DOI] [PubMed] [Google Scholar]
- 87.Strauss JF, 3rd, Kishida T, Christenson LK, Fujimoto T, Hiroi H. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol Cell Endocrinol. 2000;202:59–65. doi: 10.1016/s0303-7207(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 88.Kishida T, Kostetskii I, Zhang Z, Martinez F, Liu P, Walkley SU, Dwyer NK, Blanchette-Mackie EJ, Radice GL, Strauss JF., 3rd Targeted mutation of the MLN64 START domain causes only modest alterations in cellular sterol metabolism. J Biol Chem. 2004;279:19276–19285. doi: 10.1074/jbc.M400717200. [DOI] [PubMed] [Google Scholar]
- 89.King SR, Matassa AA, White EK, Walsh LP, Jo Y, Rao RM, Stocco DM, Reyland ME. Oxysterols regulate expression of the steroidogenic acute regulatory protein. J Mol Endocrinol. 2004;32:507–517. doi: 10.1677/jme.0.0320507. [DOI] [PubMed] [Google Scholar]
- 90.Manna PR, Jo Y, Stocco DM. Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol. 2007;193:53–63. doi: 10.1677/JOE-06-0201. [DOI] [PubMed] [Google Scholar]
- 91.Bose HS, Whittal RM, Ran Y, Bose M, Baker BY, Miller WL. StAR-like activity and molten globule behavior of StARD6, a male germ-line protein. Biochemistry. 2008;47:2277–2288. doi: 10.1021/bi701966a. [DOI] [PubMed] [Google Scholar]
- 92.Esparza-Perusquia M, Olvera-Sanchez S, Flores-Herrera O, Flores-Herrera H, Guevara-Flores A, Pardo JP, Espinosa-Garcia MT, Martinez F. Mitochondrial proteases act on STARD3 to activate progesterone synthesis in human syncytiotrophoblast. Biochim Biophys Acta. 2015;1850:107–117. doi: 10.1016/j.bbagen.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Caron KM, Soo SC, Parker KL. Targeted disruption of StAR provides novel insights into congenital adrenal hyperplasia. Endocr Res. 1998;24:827–834. doi: 10.3109/07435809809032693. [DOI] [PubMed] [Google Scholar]
- 94.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 95.Bulun SE, Simpson ER. Aromatase expression in women’s cancers. Adv Exp Med Biol. 2008;630:112–132. doi: 10.1007/978-0-387-78818-0_8. [DOI] [PubMed] [Google Scholar]
- 96.Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, Chen D. Regulation of aromatase expression in breast cancer tissue. Ann N Y Acad Sci. 2009;1155:121–131. doi: 10.1111/j.1749-6632.2009.03705.x. [DOI] [PubMed] [Google Scholar]
- 97.Holloway KR, Barbieri A, Malyarchuk S, Saxena M, Nedeljkovic-Kurepa A, Cameron Mehl M, Wang A, Gu X, Pruitt K. SIRT1 positively regulates breast cancer associated human aromatase (CYP19A1) expression. Mol Endocrinol. 2013;27:480–490. doi: 10.1210/me.2012-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase--a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 99.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S. Estrogen production and metabolism in endometriosis. Ann N Y Acad Sci. 2002;955:396–406. doi: 10.1111/j.1749-6632.2002.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 100.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 101.Barbieri RL, Missmer S. Endometriosis and infertility: a cause-effect relationship? Ann N Y Acad Sci. 2002;955:396–406. doi: 10.1111/j.1749-6632.2002.tb02762.x. [DOI] [PubMed] [Google Scholar]
- 102.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:396–406. doi: 10.1111/j.1749-6632.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 103.Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155:217–224. doi: 10.1093/aje/155.3.217. [DOI] [PubMed] [Google Scholar]
- 104.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsai SJ, Wu MH, Lin CC, Sun HS, Chen HM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86:5765–5773. doi: 10.1210/jcem.86.12.8082. [DOI] [PubMed] [Google Scholar]
- 106.Yang S, Fang Z, Suzuki T, Sasano H, Zhou J, Gurates B, Tamura M, Ferrer K, Bulun S. Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPbeta in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab. 2002;87:2336–2345. doi: 10.1210/jcem.87.5.8486. [DOI] [PubMed] [Google Scholar]
- 107.Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49–56. doi: 10.1093/humupd/dmi034. [DOI] [PubMed] [Google Scholar]
- 108.Nes WD, Lukyanenko YO, Jia ZH, Quideau S, Howald WN, Pratum TK, West RR, Hutson JC. Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis. Endocrinology. 2000;141:953–958. doi: 10.1210/endo.141.3.7350. [DOI] [PubMed] [Google Scholar]
- 109.Lukyanenko YO, Chen JJ, Hutson JC. Production of 25-hydroxycholesterol by testicular macrophages and its effects on Leydig cells. Biol Reprod. 2001;64:790–796. doi: 10.1095/biolreprod64.3.790. [DOI] [PubMed] [Google Scholar]
- 110.Crow JA, Herring KL, Xie S, Borazjani A, Potter PM, Ross MK. Inhibition of carboxylesterase activity of THP1 monocytes/macrophages and recombinant human carboxylesterase 1 by oxysterols and fatty acids. Biochim Biophys Acta. 2010;1801:31–41. doi: 10.1016/j.bbalip.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril. 2002;78:699–704. doi: 10.1016/s0015-0282(02)03373-3. [DOI] [PubMed] [Google Scholar]
- 112.Rice VM. Conventional medical therapies for endometriosis. Ann N Y Acad Sci. 2002;955:396–406. doi: 10.1111/j.1749-6632.2002.tb02795.x. [DOI] [PubMed] [Google Scholar]
- 113.Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81:290–296. doi: 10.1016/j.fertnstert.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 114.Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feingold KR, Shigenaga JK, Kazemi MR, McDonald CM, Patzek SM, Cross AS, Moser A, Grunfeld C. Mechanisms of triglyceride accumulation in activated macrophages. J Leukoc Biol. 2012;92:829–839. doi: 10.1189/jlb.1111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holm C, Kirchgessner TG, Svenson KL, Fredrikson G, Nilsson S, Miller CG, Shively JE, Heinzmann C, Sparkes RS, Mohandas T, et al. Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science. 1988;241:1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- 117.Li H, Brochu M, Wang SP, Rochdi L, Cote M, Mitchell G, Gallo-Payet N. Hormone-sensitive lipase deficiency in mice causes lipid storage in the adrenal cortex and impaired corticosterone response to corticotropin stimulation. Endocrinology. 2002;143:3333–3340. doi: 10.1210/en.2002-220341. [DOI] [PubMed] [Google Scholar]
- 118.Chung S, Wang SP, Pan L, Mitchell G, Trasler J, Hermo L. Infertility and testicular defects in hormone-sensitive lipase-deficient mice. Endocrinology. 2001;142:4272–4281. doi: 10.1210/endo.142.10.8424. [DOI] [PubMed] [Google Scholar]
- 119.Wang SP, Chung S, Soni K, Bourdages H, Hermo L, Trasler J, Mitchell GA. Expression of human hormone-sensitive lipase (HSL) in postmeiotic germ cells confers normal fertility to HSL-deficient mice. Endocrinology. 2004;145:5688–5693. doi: 10.1210/en.2004-0919. [DOI] [PubMed] [Google Scholar]
- 120.Osterlund T. Structure-function relationships of hormone-sensitive lipase. Eur J Biochem. 2001;268:1899–1907. doi: 10.1046/j.1432-1327.2001.02097.x. [DOI] [PubMed] [Google Scholar]
- 121.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 122.Krintel C, Morgelin M, Logan DT, Holm C. Phosphorylation of hormone-sensitive lipase by protein kinase A in vitro promotes an increase in its hydrophobic surface area. FEBS J. 2009;276:4752–476. doi: 10.1111/j.1742-4658.2009.07172.x. [DOI] [PubMed] [Google Scholar]
- 123.Shen WJ, Patel S, Natu V, Hong R, Wang J, Azhar S, Kraemer FB. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J Biol Chem. 2003;278:43870–43876. doi: 10.1074/jbc.M303934200. [DOI] [PubMed] [Google Scholar]
- 124.Rao RM, Jo Y, Leers-Sucheta S, Bose HS, Miller WL, Azhar S, Stocco DM. Differential regulation of steroid hormone biosynthesis in R2C and MA-10 Leydig tumor cells: role of SR-B1-mediated selective cholesteryl ester transport. Biol Reprod. 2003;68:114–121. doi: 10.1095/biolreprod.102.007518. [DOI] [PubMed] [Google Scholar]
- 125.Uda S, Spolitu S, Angius F, Collu M, Accossu S, Banni S, Murru E, Sanna F, Batetta B. Role of HDL in cholesteryl ester metabolism of lipopolysaccharide-activated P388D1 macrophages. J Lipid Res. 2013;54:3158–3169. doi: 10.1194/jlr.M042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gwynne JT, Strauss JF., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 127.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 128.Blanchette-Mackie EJ. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta. 2000;1486:171–183. doi: 10.1016/s1388-1981(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 129.Watari H, Blanchette-Mackie EJ, Dwyer NK, Sun G, Glick JM, Patel S, Neufeld EB, Pentchev PG, Strauss JF., 3rd NPC1-containing compartment of human granulosa-lutein cells: a role in the intracellular trafficking of cholesterol supporting steroidogenesis. Exp Cell Res. 2000;255:56–66. doi: 10.1006/excr.1999.4774. [DOI] [PubMed] [Google Scholar]
- 130.Gevry NY, Murphy BD. The role and regulation of the Niemann-Pick C1 gene in adrenal steroidogenesis. Endocr Res. 2002;28:403–412. doi: 10.1081/erc-120016815. [DOI] [PubMed] [Google Scholar]
- 131.Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C, Bouhlel MA, Bultel S, Fruchart JC, Ikonen E, Clavey V, Staels B, Chinetti-Gbaguidi G. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ Res. 2005;97:682–689. doi: 10.1161/01.RES.0000184678.43488.9f. [DOI] [PubMed] [Google Scholar]
- 132.Robertson KM, Schuster GU, Steffensen KR, Hovatta O, Meaney S, Hultenby K, Johansson LC, Svechnikov K, Soder O, Gustafsson JA. The liver X receptor-{beta} is essential for maintaining cholesterol homeostasis in the testis. Endocrinology. 2005;146:2519–2530. doi: 10.1210/en.2004-1413. [DOI] [PubMed] [Google Scholar]
- 133.Volle DH, Mouzat K, Duggavathi R, Siddeek B, Dechelotte P, Sion B, Veyssiere G, Benahmed M, Lobaccaro JM. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol Endocrinol. 2007;21:1014–1027. doi: 10.1210/me.2006-0277. [DOI] [PubMed] [Google Scholar]
- 134.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 135.Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325:94–105. doi: 10.1016/j.ydbio.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 137.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54:489–495. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2010;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bonhomme D, Minni AM, Alfos S, Roux P, Richard E, Higueret P, Moisan MP, Pallet V, Touyarot K. Vitamin A status regulates glucocorticoid availability in Wistar rats: consequences on cognitive functions and hippocampal neurogenesis? Front Behav Neurosci. 2014;8:1–13. doi: 10.3389/fnbeh.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Srivastava N. ATP binding cassette transporter A1--key roles in cellular lipid transport and atherosclerosis. Mol Cell Biochem. 2002;237:155–164. doi: 10.1023/a:1016506221047. [DOI] [PubMed] [Google Scholar]
- 142.Chinetti-Gbaguidi G, Rigamonti E, Helin L, Mutka AL, Lepore M, Fruchart JC, Clavey V, Ikonen E, Lestavel S, Staels B. Peroxisome proliferator-activated receptor alpha controls cellular cholesterol trafficking in macrophages. J Lipid Res. 2005;46:2717–2725. doi: 10.1194/jlr.M500326-JLR200. [DOI] [PubMed] [Google Scholar]
- 143.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 144.Bultel S, Helin L, Clavey V, Chinetti-Gbaguidi G, Rigamonti E, Colin M, Fruchart JC, Staels B, Lestavel S. Liver X receptor activation induces the uptake of cholesteryl esters from high density lipoproteins in primary human macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2288–2295. doi: 10.1161/ATVBAHA.108.175042. [DOI] [PubMed] [Google Scholar]
- 145.Yuan Y, Li P, Ye J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell. 2012;3:173–181. doi: 10.1007/s13238-012-2025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 147.Tazoe F, Yagyu H, Okazaki H, Igarashi M, Eto K, Nagashima S, Inaba T, Shimano H, Osuga J, Ishibashi S. Induction of ABCA1 by overexpression of hormone-sensitive lipase in macrophages. Biochem Biophys Res Commun. 2008;376:111–115. doi: 10.1016/j.bbrc.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 148.Ning Y, Bai Q, Lu H, Li X, Pandak WM, Zhao F, Chen S, Ren S, Yin L. Overexpression of mitochondrial cholesterol delivery protein, StAR, decreases intracellular lipids and inflammatory factors secretion in macrophages. Atherosclerosis. 2009;204:0114–120. doi: 10.1016/j.atherosclerosis.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taylor JM, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc Res. 2010;86:526–534. doi: 10.1093/cvr/cvq015. [DOI] [PubMed] [Google Scholar]
- 150.Nohara A, Kobayashi J, Mabuchi H. Retinoid X receptor heterodimer variants and cardiovascular risk factors. J Atheroscler Thromb. 2009;16:303–318. doi: 10.5551/jat.no786. [DOI] [PubMed] [Google Scholar]
- 151.Hozoji-Inada M, Munehira Y, Nagao K, Kioka N, Ueda K. Liver X receptor beta (LXRbeta) interacts directly with ATP-binding cassette A1 (ABCA1) to promote high density lipoprotein formation during acute cholesterol accumulation. J Biol Chem. 2011;286:20117–20124. doi: 10.1074/jbc.M111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ayaori M, Yakushiji E, Ogura M, Nakaya K, Hisada T, Uto-Kondo H, Takiguchi S, Terao Y, Sasaki M, Komatsu T, Iizuka M, Yogo M, Uehara Y, Kagechika H, Nakanishi T, Ikewaki K. Retinoic acid receptor agonists regulate expression of ATP-binding cassette transporter G1 in macrophages. Biochim Biophys Acta. 2012;1821:561–572. doi: 10.1016/j.bbalip.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 153.Manna PR, Sennoune SR, Martinez-Zaguilan R, Slominski AT, Pruitt K. Regulation of retinoid mediated cholesterol efflux involves liver X receptor activation in mouse macrophages. Biochem Biophys Res Commun. 2015;464:312–317. doi: 10.1016/j.bbrc.2015.06.150. [DOI] [PubMed] [Google Scholar]
- 154.Chinetti G, Fruchart JC, Staels B. Transcriptional regulation of macrophage cholesterol trafficking by PPARalpha and LXR. Biochem Soc Trans. 2006;34:1128–1131. doi: 10.1042/BST0341128. [DOI] [PubMed] [Google Scholar]
- 155.Krone N, Hanley NA, Arlt W. Age-specific changes in sex steroid biosynthesis and sex development. Best Pract Res Clin Endocrinol Metab. 2007;21:393–401. doi: 10.1016/j.beem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Chahal HS, Drake WM. The endocrine system and aging. J Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 157.Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- 158.Huhtaniemi I, Perheentupa A. Diagnosis and therapy of male hormonal changes related to aging. Duodecim. 2009;125:1099–1106. [PubMed] [Google Scholar]
- 159.Traub ML, Santoro N. Reproductive aging and its consequences for general health. Ann N Y Acad Sci. 2010;1204:179–187. doi: 10.1111/j.1749-6632.2010.05521.x. [DOI] [PubMed] [Google Scholar]
- 160.Manor B, Lipsitz LA. Physiologic complexity and aging: implications for physical function and rehabilitation. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:287–293. doi: 10.1016/j.pnpbp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hertoghe T. The “multiple hormone deficiency” theory of aging: is human senescence caused mainly by multiple hormone deficiencies? Ann N Y Acad Sci. 2005;1057:448–465. doi: 10.1196/annals.1322.035. [DOI] [PubMed] [Google Scholar]
- 162.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J Clin Endocrinol Metab. 2009;94:3259–3264. doi: 10.1210/jc.2009-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huhtaniemi I, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat Rev Urol. 2011;8:335–344. doi: 10.1038/nrurol.2011.47. [DOI] [PubMed] [Google Scholar]
- 165.Veldhuis JD. Changes in pituitary function with ageing and implications for patient care. Nat Rev Endocrinol. 2013;9:205–215. doi: 10.1038/nrendo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Janovick JA, Stewart MD, Jacob D, Martin LD, Deng JM, Stewart CA, Wang Y, Cornea A, Chavali L, Lopez S, Mitalipov S, Kang E, Lee HS, Manna PR, Stocco DM, Behringer RR, Conn PM. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci. 2013;110:21030–21035. doi: 10.1073/pnas.1315194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Feart C, Pallet V, Boucheron C, Higueret D, Alfos S, Letenneur L, Dartigues JF, Higueret P. Aging affects the retinoic acid and the triiodothyronine nuclear receptor mRNA expression in human peripheral blood mononuclear cells. Eur J Endocrinol. 2005;152:449–458. doi: 10.1530/eje.1.01858. [DOI] [PubMed] [Google Scholar]
- 168.Ono K, Yamada M. Vitamin A and Alzheimer’s disease. Geriatr Gerontol Int. 2012;12:180–188. doi: 10.1111/j.1447-0594.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- 169.Blagosklonny MV, Campisi J, Sinclair DA, Bartke A, Blasco MA, Bonner WM, Bohr VA, Brosh RM, Jr, Brunet A, Depinho RA, Donehower LA, Finch CE, Finkel T, Gorospe M, Gudkov AV, Hall MN, Hekimi S, Helfand SL, Karlseder J, Kenyon C, Kroemer G, Longo V, Nussenzweig A, Osiewacz HD, Peeper DS, Rando TA, Rudolph KL, Sassone-Corsi P, Serrano M, Sharpless NE, Skulachev VP, Tilly JL, Tower J, Verdin E, Vijg J. Impact papers on aging in 2009. Aging. 2010;2:111–121. doi: 10.18632/aging.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Batrinos ML. The aging of the endocrine hypothalamus and its dependent endocrine glands. Hormones. 2012;11:241–253. doi: 10.14310/horm.2002.1354. [DOI] [PubMed] [Google Scholar]
- 171.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ortuno-Sahagun D, Pallas M, Rojas-Mayorquin AE. Oxidative stress in aging: advances in proteomic approaches. Oxi Med Cell Longev. 2014;2014:1–18. doi: 10.1155/2014/573208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Age-specific changes in the regulation of LH-dependent testosterone secretion: assessing responsiveness to varying endogenous gonadotropin output in normal men. Am J Physiol Regul Integr Comp Physiol. 2005;289:R721–728. doi: 10.1152/ajpregu.00138.2005. [DOI] [PubMed] [Google Scholar]
- 174.Wang X, Stocco DM. The decline in testosterone biosynthesis during male aging: a consequence of multiple alterations. Mol Cell Endocrinol. 2005;238:1–7. doi: 10.1016/j.mce.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 175.Makrantonaki E, Schonknecht P, Hossini AM, Kaiser E, Katsouli MM, Adjaye J, Schroder J, Zouboulis CC. Skin and brain age together: The role of hormones in the ageing process. Exp Gerontol. 2010;45:801–813. doi: 10.1016/j.exger.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 176.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat leydig cells. Biol Reprod. 2013;88:1–7. doi: 10.1095/biolreprod.112.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou L, Beattie MC, Lin CY, Liu J, Traore K, Papadopoulos V, Zirkin BR, Chen H. Oxidative stress and phthalate-induced down-regulation of steroidogenesis in MA-10 Leydig cells. Reprod Toxicol. 2013;42:95–101. doi: 10.1016/j.reprotox.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9:228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- 180.Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip Top Gerontol. 2014;39:86–107. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- 181.Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbabio.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rodrigues Siqueira I, Fochesatto C, da Silva Torres IL, Dalmaz C, Alexandre Netto C. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 183.Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- 184.Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299:23–31. doi: 10.1016/j.mce.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 185.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 186.Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–295. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 189.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 190.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key Role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, Zjawiony J, Tuckey RC. Cytochromes P450 and Skin Cancer: Role of Local Endocrine Pathways. Anticancer Agents Med Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 194.Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, Kakuo S, Fujimura T, Hachiya A, Honma S, Aiba S, Sasano H. Sex steroid synthesis in human skin in situ: The roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol Cell Endocrinol. 2012;362:19–28. doi: 10.1016/j.mce.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 195.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, CTR A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Elias PM, Crumrine D, Paller A, Rodriguez-Martin M, Williams ML. Pathogenesis of the cutaneous phenotype in inherited disorders of cholesterol metabolism: Therapeutic implications for topical treatment of these disorders. Dermatoendocrinol. 2011;3:100–106. doi: 10.4161/derm.3.2.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Suomela S, Elomaa O, Skoog T, Ala-aho R, Jeskanen L, Parssinen J, Latonen L, Grenman R, Kere J, Kahari VM, Saarialho-Kere U. CCHCR1 is up-regulated in skin cancer and associated with EGFR expression. PLoS One. 2009;4:e6030. doi: 10.1371/journal.pone.0006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 199.Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol. 2014;23:369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 201.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gericke J, Ittensohn J, Mihaly J, Alvarez S, Alvarez R, Torocsik D, de Lera AR, Ruhl R. Regulation of retinoid-mediated signaling involved in skin homeostasis by RAR and RXR agonists/antagonists in mouse skin. PLoS One. 2013;8:e62643. doi: 10.1371/journal.pone.0062643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin, Interv Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ortonne JP. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006;19:280–288. doi: 10.1111/j.1529-8019.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 205.So PL, Fujimoto MA, Epstein EH., Jr Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol Cancer Ther. 2008;7:1275–1284. doi: 10.1158/1535-7163.MCT-07-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mihaly J, Gamlieli A, Worm M, Ruhl R. Decreased retinoid concentration and retinoid signalling pathways in human atopic dermatitis. Exp Dermatol. 2011;20:326–330. doi: 10.1111/j.1600-0625.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 207.Chung SS, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res. 2004;105:189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 209.Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF, 3rd, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4858–4865. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- 210.Tucci P, Cione E, Genchi G. Retinoic acid-induced testosterone production and retinoylation reaction are concomitant and exhibit a positive correlation in Leydig (TM-3) cells. J Bioenerg Biomembr. 2008;40:111–115. doi: 10.1007/s10863-008-9132-3. [DOI] [PubMed] [Google Scholar]
- 211.Itoh K, Hiromori Y, Kato N, Yoshida I, Itoh N, Ike M, Nagase H, Tanaka K, Nakanishi T. Placental steroidogenesis in rats is independent of signaling pathways induced by retinoic acids. Gen Comp Endocrinol. 2009;163:285–291. doi: 10.1016/j.ygcen.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 212.Munetsuna E, Hojo Y, Hattori M, Ishii H, Kawato S, Ishida A, Kominami SA, Yamazaki T. Retinoic acid stimulates 17beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology. 2009;150:4260–4269. doi: 10.1210/en.2008-1644. [DOI] [PubMed] [Google Scholar]
- 213.Kushida A, Tamura H. Retinoic acids induce neurosteroid biosynthesis in human glial GI-1 Cells via the induction of steroidogenic genes. J Biochem. 2009;146:917–923. doi: 10.1093/jb/mvp142. [DOI] [PubMed] [Google Scholar]