Abstract
There is increasing evidence for the emergence of long noncoding RNAs (IncRNAs) as important components, especially in the regulation of gene expression. In the event of X chromosome inactivation, robust epigenetic marks are established in a long noncoding Xist RNA-dependent manner, giving rise to a distinct epigenetic landscape on the inactive X chromosome (Xi). The X inactivation center (Xic is essential for induction of X chromosome inactivation and harbors two topologically associated domains (TADs) to regulate monoallelic Xist expression: one at the noncoding Xist gene and its upstream region, and the other at the antisense Tsix and its upstream region. The monoallelic expression of Xist is tightly regulated by these two functionally distinct TADs as well as their constituting IncRNAs and proteins. In this review, we summarize recent updates in our knowledge of IncRNAs found at the Xic and discuss their overall mechanisms of action. We also discuss our current understanding of the molecular mechanism behind Xist RNA-mediated induction of the repressive epigenetic landscape at the Xi.
Introduction
The XX/XY sex-determination system is the most common designation in mammals whereby SRY on the Y-chromosome mainly acts to determine sex and activates a cascade of genetic pathways to develop testis in mice and humans. In this system, females have two X chromosomes in somatic cells, while males have one X and one Y chromosome. Since more than 1,300 genes reside on the X chromosome, there is a potential risk for females to possess a twofold abundance of X-linked genes, which is harmful for cellular viability and embryonic development. To overcome this problem, the mammalian females have developed a unique dosage compensation system called X chromosome inactivation (XCI) to balance the X-linked gene dosage between females and males [1]. As a result of XCI, which is induced at an early stage of embryonic development, one of the two X chromosomes becomes inactive (Xi), meaning the majority of genes on the Xi are transcriptionally silenced. About 15% of X-linked genes on the Xi in humans and 3–13% of X-linked genes in mice escape XCI; dubbed escape genes, these may have female-specific roles during development [2–4]. Epigenetic marks for active transcription, such as trimethylation of histone H3 lysine-4 (H3K4me3), are known to be enriched at escape loci on the Xi, indicating the establishment of transcriptionally active compartments within an otherwise transcriptionally inactive environment [5].
Although XCI occurs in early developmental stages, the exact process varies across species [6]. XCI is triggered by overexpression of Xist IncRNA from the Xi, followed by Xist RNA coating and chromatin modifications across the Xi. In mice, XCI can be divided into two distinct forms: imprinted and random XCI [7]. Xist, a master regulator of XCI, is transcribed from the paternal X-chromosome (Xp) at low levels as early as the two-cell embryonic stage. Timing of X-linked gene silencing on the Xp is diverse in each X-linked gene and the gene silencing is induced from the 4-cell stage to the post-blastocyst stage, which is referred to as imprinted XCI [8–11]. During the peri-implantation stage, cells in the trophectoderm and primitive endoderm, which give rise to extraembryonic tissue such as the placenta, sustain imprinted XCI; meanwhile, the epiblast lineage cells at the inner cell mass (ICM) erase imprinted XCI and reactivate X-linked gene expression [8,12]. Tsix, an antagonist and antisense long noncoding gene to Xist, and the germline factor PRDM14 cooperatively play a critical role in X-chromosome reactivation at the ICM in the blastocyst [13]. X-chromosome reactivation is then followed by random XCI, wherein either the Xp or Xm is randomly inactivated in the epiblast lineage around gastrulation [14]. Once random XCI is completed, the Xi is inherited to the next generation of cells. Mouse embryonic stem (ES) cells derived from the ICM of the blastocyst provides us an ideal system to study random XCI. Using an ex vivo embryoid body differentiation method, we can recapitulate random XCI during early embryo development [15]. The random nature of XCI in somatic cells results in mosaicism of cells, which contributes to its physiological diversity and is usually beneficial for the survival of females. However, defective XCI causes developmental anomalies and embryonic lethality during embryogenesis and diseases such as cancer [16,17]. Proper XCI regulation is hence indispensable to mammalian females.
In this review, we aim to cover recent findings regarding the operation of XCI, with a special focus on describing the molecular mechanism underlying: (1) induction of monoallelic Xist expression through the cooperation of multiple IncRNAs at the X-inactivation center and (2) Xist RNA-induced recruitment of repressive factors along the Xi. An elucidation of the molecular mechanism driving XCI would provide valuable insight towards understanding IncRNA-mediated gene regulation, which is critical for various biological processes.
2. Molecular mechanism to induce monoallelic Xist upregulation - Dynamic interplay of multiple long noncoding genes within Xic
To date, numerous IncRNAs are known to be expressed all over our genome and an increasing number of reports have indicated that IncRNAs regulate a wide variety of biological processes [18–21]. Xist RNA is one of the extensively studied representations of a regulatory IncRNA, which triggers dynamic alteration of the epigenetic landscape on the future Xi. The Xist gene is located within the X-inactivation center (Xic), a genetic locus which has been mapped within XqD and Xq13 on the X chromosome in mice and humans, respectively, through a series of cytological experiments using X-autosome translocation [22–24]. Prior studies have determined Xist is exclusively expressed from the Xi and is required for induction of XCI [25–28]. Furthermore, a series of transgene experiments delineated the Xic region [29–31]. When a mouse 450-kb multicopy DNA fragment containing Xist was introduced into autosomes in male ES cells, the autosomes containing the fragment underwent inactivation similarly to the Xi in female cells. This suggests that the 450-kb fragment can fulfill the functions of the Xic [29]. Since a single copy of a YAC transgene containing Xist in mice could not lead to random inactivation [32], copy number of transgenes could be a critical factor for transgenic Xic function. Interestingly, the same single copy transgene inherited paternally induced autosome inactivation associated with H3K27me3 accumulation and late replication, which were observed in the inactivated Xp in the imprinted XCI [33], suggesting different factors might be required for inducing Xist expression in imprinted and random XCI. To date, the minimal Xic region required for induction of inactivation has been reduced to ~100 kb including Xist [34]. Several IncRNAs within the Xic have been identified (Tsix, Xite, DxPas34, Tsx, Jpx/Enox, Ftx and RepA) which act cooperatively together for the induction of monoallelic Xist expression from the future Xi in order to initiate XCI [35,36]. Furthermore, various approaches surveying a neighboring region of the Xic have identified several additional factors, such as IncRNA Linx, as potential transcriptional regulators of Tsix, in addition to the E3 ubiquitin ligase Rnf12 as an activator of Xist [37,38]. These findings indicate that various RNA and protein factors are involved in coordinating the regulation of Xist mono-allelic expression from the Xi.
2.1. Xist IncRNA
Xist plays crucial roles in both imprinted and random XCI [16,28]. Xist resides at the central region of the Xic locus on the Xi (Fig. 1) and is expressed from promoter P1 and P2 in a differentiation-specific manner [25–27,39,40]. Nascent Xist RNA is processed to −17 kb-length RNA by splicing, and different polyadenylation sites and alternative splicing patterns contribute to the generation of multiple isoforms of Xist RNA [41–43]. Interestingly, some isoforms of Xist RNA is produced in a differentiation- or sex-specific manner [43,44].
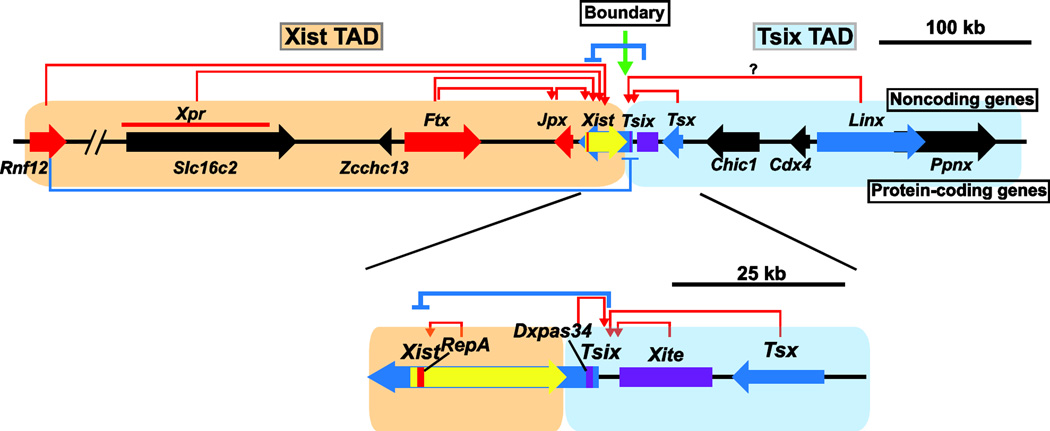
Fig. 1.
Functionally distinct topologically associating domains (TADs) at the Xic. The schematic of Xist and Tsix TADs, long noncoding genes, and a protein-coding gene at the Xic locus is shown; each of these plays a role in XCI. Yellow bold arrow indicates Xist. Red and Blue bold arrows indicate genes that activate and repress Xist, respectively. Black arrows indicate non-XCI-related genes to date. Purple boxes indicate enhancer-like region for Tsix. Names of long noncoding and protein-coding genes are shown above and below these bold arrows, respectively. Red and blue arrows indicate gene’s function as activator and repressor for Xist expression, respectively. The green arrow indicates the Xist/Tsix TAD boundary.
Xist IncRNA initiates XCI in cis by covering the entire Xi and recruiting multiple chromatin modifying enzymes for repressive epigenetic modification, including polycomb repressive complex 2 (PRC2) for histone H3 trimethylation at lysine 27 (H3K27me3), during embryogenesis [45–47]. Exclusion of RNA polymerase II occurs during the first wave of transcriptional alteration as induced by Xist RNA, which is followed by the loss of active histone markers like histone H3K4 methylation and histone H3 acetylation [48]. Then, repressive epigenetic modifications such as H3K27me3 and H2Aub which are catalyzed by PRC2 and PRC1, respectively, are deposited onto the Xi [48–50]. Consistent with the Xist RNA-dependent recruitment of PRC2 to the Xi, deposition of H3K27me3 and PRC2 is well-correlated with Xist RNA recruitment onto the Xi during X-inactivation: a limited number of binding loci were seen to frequently overlap with CpG islands in gene-rich regions at the initiation of XCI, along with a robust number of binding loci at the intergenic regions and chromosome-wide spreading as differentiation progressed [5,51]. Thereafter, further repressive epigenetic modifications such as histone macro H2A and DNA methylation are incorporated into the entire Xi in an Xist RNA-dependent manner and are maintained through subsequent generations for stable X-linked gene silencing [52,53].
2.2. Functionally distinct topologically associating domains (TADs) which regulate monoallelic Xist expression
Xic can be divided into two topologically associating domains (TADs) or active chromatin hubs (ACHs) which could facilitate the interplay of long noncoding genes within the same TAD to activate or repress Xist [37,54]. Interestingly, each TAD is functionally distinct in Xist expression. The Tsix TAD is located downstream of Xist and contains long noncoding genes and IncRNA-associated elements (Tsix, Dxpas34, Xite, Tsx and Linx) and primarily plays a role in upregulating Tsix expression, which in turn downregulates Xist expression. The Xist TAD contains Xist itself and its upstream region including long noncoding genes, Jpx and Ftx, and the protein-coding Rnf12 gene encoding an ubiquitin ligase, all of which eventually promote Xist upregulation at the onset of random XCI [55–57]. These two TADs are separated by a boundary region, which resides around the Xist/Tsix region [37,58]. Interestingly, functionally relevant genes and elements for Xist regulation reside within the same TAD in close proximity to each other and exhibit similar expression profiles. Thus, genes that facilitate coordinated expression and cooperative action in Xist regulation are clustered within the Xist and Tsix TADs. Disruption of the boundary between Xist and Tsix TADs by Xist-Tsix deletion induces ectopic contacts between sequences in the Xist and Tsix TADs, altered organization of the Tsix TAD, and long-range transcriptional misregulation of genes within the Xist TAD [37]. Furthermore, the deletion of boundary elements between the Xist and Tsix ACHs also leads to aberrant XCI in female mutant ES cells [58], suggesting an essential role of TAD/ACH in XCI to both separate functionally distinct chromosomal domains as well as to cooperatively induce Xist expression.
2.2.1. Tsix TAD antagonizes Xist expression though Tsix upregulation
As a central player in the Tsix TAD, the expression of Tsix is cooperatively promoted by the other Tsix TAD elements. Tsix is important as an antagonist of Xist expression in cis on the future active X chromosome (Xa) and is required in both imprinted and random XCI [59–61]. Tsix is transcribed to the antisense orientation of Xist from minor and major promoters [60,62]. Based on a study using ex vivo differentiation of mouse ES cells, Xist and Tsix exhibit an interesting expression pattern in random XCI which reflects Tsix function in Xist expression [62]. Prior to the onset of random XCI, both Tsix and to a lesser extent Xist are biallelically expressed from both X chromosomes. At the onset of random XCI, Tsix expression becomes monoallelic and designates the future Xa; consequently, Xist expression is upregulated on the future Xi and repressed on the future Xa. When the XCI is established, the expression of Tsix is repressed on both alleles. While random XCI is tightly linked with cellular differentiation, Tsix expression is regulated by pluripotent factors [63–65]. Tsix is known to exert its antagonistic function against Xist by promoting DNA methylation and histone modification at the promoter of Xist [66–69]. Since a Tsix deficient X chromosome does not undergo XCI in male embryonic lineages and ES cells, there is a Tsix-independent mechanism for the prevention of Xist upregulation [59,70]. Double mutation of Eed, a component of PRC2, and Tsix in male ES cells leads to Xist hyperactivation upon differentiation: this does not occur in cells carrying either single mutation, suggesting that PRC2 is involved in the repression of Xist [71]. Although Tsix was believed to be involved in choosing the Xi upon initiation of random XCI [59,66–68], a recent report proposes that Tsix is only involved in the maintenance of random XCI to prevent Xist induction from the Xa, not both the “counting” and “choice” steps at the onset of X-inactivation [72]. The observation of bi-allelic Xist RNA clouds in a subset of Tsix mutant female ES cells and epiblast cells at the onset of random XCI prompted the authors to propose that non-random XCI in Tsix heterozygous mutant female cells is due to secondary cell selection. That is, cells exhibiting bi-allelic Xist RNA clouds choose the wild-type X-chromosome to become the Xi, while the Tsix-mutant X becomes the Xa. However, this Tsix-mutant Xa eventually reverts to an inactive state due to a failure in the repression of Xist induction. To support this model, the authors found that Tsix heterozygous mutant epiblast stem cells (EpiSCs), which represent an early phase of random XCI, exhibited a wide-range of skewed X-chromosome choices in each undifferentiated EpiSC clones, which became almost exclusively skewed in the favoring of Tsix mutant X-chromosomes as the Xi in a majority of EpiSC clones upon differentiation. Their findings stand in contrast to previous works which found Xist expression to be partially upregulated and completely skewed in favor of the Tsix mutant X-chromosome in Tsix heterozygous mutant undifferentiated ES cells [59,73–76]. Due to a lack of Xist RNA cloud formation in Tsix mutant undifferentiated ES cells, it is possible that further Xist induction and Xist RNA cloud formation would be dependent on cell differentiation. On the other hand, it appears as if the initial Xist induction and Xi/Xa choice can be completed within Tsix heterozygous mutant female ES cells without additional support. Further cautious examination would be necessary to clarify whether Tsix is required for the choice between Xi and Xa or maintenance of the Xa.
In imprinted XCI, the disruption of maternal Tsix results in ectopic Xist expression from the maternal X chromosome (Xm) in the extraembryonic tissues and early embryonic lethality in females [60,61], attributed mainly to the inactivation of both Xp and Xm. These data suggest a crucial role for Tsix in the maintenance of Xist repression in cis on the Xm in extraembryonic tissues. Since Tsix expression cannot be detected until the blastocyst stage, and Xist is monoallelically expressed from the Xp even in the female embryo carrying a maternal Tsix mutation, it can be inferred that Tsix expression is not required for initial monoallelic Xist expression at the early stage of imprinted XCI. Thereafter, Tsix is critical for the prevention of Xist expression from the Xm as trophectodermal progenitor cells differentiate [77].
Two enhancer-like elements preserve Tsix expression and repress Xist on the future Xa at the onset of random XCI [73,78,79]. One of the enhancer elements for Tsix, Xite (X-inactivation intergenic transcription elements), is located between the major and minor Tsix promoters and lies 20–32 kb downstream of Xist. Xite mutation results in a significant downregulation of Tsix coupled with Xist upregulation on the Xite mutant X chromosome upon differentiation, leading to a skewed XCI which favors the mutant X chromosome as the Xi. A 1.2-kb core enhancer element embedded within the Xite is associated with differentiation-specific bidirectional low-level transcription and DNasel hypersensitive sites, both of which are characteristic features of an enhancer. During ES cells differentiation, the expression pattern of Xite is similar to that of Tsix but at a lower level: Xite expression is highest in undifferentiated cells and downregulated upon differentiation. The other enhancer element for Tsix is DXPas34, which resides <1-kb downstream of the Tsix major promoter and comprises a 34 mer tandem repeat and flanking sequence [73,79]. DXPas34 as well as Xite promotes Tsix only in undifferentiated ES cells prior to XCI and in differentiating cells during the early stage of XCI; their enhancer activity gradually diminishes upon cellular differentiation. However, once the XCI is established, DXPas34 is needed for proper repression of Tsix [73]. While each Xite and DXPas34 enhancer unit of Tsix can act in a separate fashion, their combination leads to a greater stimulation of the Tsix promoter [79].
Other noncoding genes in the Tsix TAD are also implicated in Tsix repression [37,80]. Tsx (Testes specific X-linked gene) located 40 kb downstream to the 3’ end of Xist was originally proposed as a coding gene, but further investigation indicated Tsx is a non-coding gene [80,81]. Tsx is comprised of 7 exons, and is transcribed from the opposite orientation of Xist. Tsx expression is found in many tissues with the highest expression level seen in testis. The expression of Tsx decreases upon differentiation similar to that of Tsix and Xite. Since the Tsx knockout mutant leads to repression of Tsix and abnormal accumulation of Xist RNA onto the Xa in both male and female ES cells during differentiation, Tsx is proposed to function in Tsix activation [80]. Linx, which resides within the Tsix TAD, has recently been identified as a long noncoding gene that may be a potential regulator for Tsix expression. Although its function in Tsix expression remains unknown, there is a positive correlation between Linx and Tsix expression reminiscent of the correlation between Tsix and Xite expression, indicating a possible similarity in function [37]. Further investigation will reveal the role of Linx in XCI, especially for Tsix and Xist regulation.
2.2.2. Xist TAD promotes Xist upregulation
Several long noncoding genes and one protein-coding gene have been identified as a positive regulator for Xist within the Xist TAD. RepA RNA, a 1.6kb-long IncRNA, is transcribed from the same DNA strand as Xist [82]. RepA’s transcription start site is located within repeat A, which is located ~300 bp downstream of Xist’s P1 promoter and contains 7.5 tandem repeats of a 28 nucleotide-long sequence which may fold into specific secondary structures [83,84]. RepA RNA is present in both male and female undifferentiated ES cells, but is only maintained in female cells after XCI [82]. RepA RNA knock-down in ES cells showed poor embryonic body differentiation with less Xist expression and H3K27me3 levels on the Xi compared with control cells. Furthermore, RepA RNA interacts with Ezh2, a component of the PRC2 complex, in vivo and in vitro. Thus, RepA RNA is proposed to function in upregulation of Xist expression by altering chromatin modification at the Xist promoter.
Jpx (also called Enox [expressed neighbor of Xist]) is located 10-kb upstream of Xist and is transcribed in the opposite direction of Xist [85,86]. Jpx is known as an escape gene in both random and imprinted XCI and Jpx expression increases in both male and female ES cells during ex vivo differentiation [55,86,87]. The Jpx heterozygous knockout results in inefficient Xist upregulation and leads to cell death in mutant female ES cells upon differentiation; thus, Jpx works as an activator of Xist [55]. Interestingly, transgenic expression of Jpx rescues defects in Jpx heterozygous mutant cells while anti-Jpx siRNA destroys its ability to promote Xist expression; thus, Jpx RNA can promote Xist upregulation in trans. Since the Jpx heterozygous mutation shows mild Xist repression on the mutant X chromosome, Jpx might function in cis in vivo. Moreover, the XCI and differentiation defects observed in Jpx heterozygous mutant ES cells can be restored by disrupting Tsix function on the same X chromosome, indicating multiple long noncoding genes regulate Xist expression in females. Further studies found that the Jpx transgene released CTCF from the Xist promoter, which led to an upregulation of Xist expression, while transgenic CTCF expression reduced Xist expression [56]. Based on these findings, it has been proposed that Jpx RNA activates Xist by reducing CTCF from the Xist promoter at the onset of XCI.
In mice, the Ftx (five prime to Xist) gene is localized about 150-kb upstream of Xist, with the same transcription orientation as Xist [85]. The 5’ region of Ftx and the microRNA cluster which resides in intron 12 of Ftx are conserved among species [88]. Similar to Jpx, Ftx is upregulated during ex vivo ES cell differentiation and is also considered as an escape gene [87,88]. In male ES cells, Ftx disruption induced gene repression in a wide-range of neighboring regions from Cnbp2 to Tsix [88]. In this Ftx mutant male ES cells, the expression of Xist was also reduced whereas CpG methylation at the Xist promoter was increased. Thus, Ftx is proposed as a positive regulator of Xist. Since Ftx mutation also led to Jpx repression, Ftx function for Xist upregulation might be mediated by Jpx. However, Ftx might be dispensable for imprinted XCI [89]: Ftx mutant homozygous and heterozygous female embryos were both normal and Ftx knockout on the paternal X did not affect the silencing of paternal X-linked genes nor expression profiles of imprinted paternal X-linked genes, Xist and Rnf12. Since this work examined silencing of only a subset of X-linked genes, it is unclear whether the paternal Ftx mutation affects the chromosome-wide integrity of imprinted XCI in Ftx heterozygous mutant female mice. Like trans-action of Jpx RNA in Xist activation, Ftx on the maternal X might be able to induce Xist upregulation in trans. Alternatively, it is possible that Ftx has different functions in imprinted and random XCI. Ftx gene targeting and ex vivo differentiation experiments using female ES cells could delineate Ftx function in random XCI.
Xpr (X-pairing region) was originally identified as a region required for X-X pairing at the initiation of XCI [90]. Although X-X pairing mediated by Xpr is not essential for induction of XCI, Xpr cooperates with Jpx, Ftx and Rnf12 to induce Xist upregulation upon differentiation [91]. Although Xpr overlaps with the protein-coding Slc16a2 gene, also known as monocarboxylate transporter 8 (Mct8), it is unclear whether the SLC16A2 protein is involved in Xist expression. However, since Slc16a2 is expressed in many tissues and plays a critical role in the development of the central nervous system as a transporter of the thyroid hormone [92,93], it is unlikely to directly control Xist expression and XCI. Future studies might lead to the discovery of novel critical elements for Xist expression such as an enhancer and an additional regulatory IncRNA within this region.
Protein-coding Rnf12 is also included within Xist TAD and functions to promote Xist upregulation in both imprinted and random XCI [37,38,94]. Rnf12 encodes an ubiquitin ligase and is the only functional protein-coding gene in Xist regulation within Xist and Tsix TADs. While the Rnf12 transgene induces XCI in male ES cells and produces abnormal XCI at both X chromosomes in female ES cells, Rnf 12 heterozygous knockout in female ES cells leads to significantly delayed XCI upon differentiation. These results suggest that Rnf12 has a critical role for dose-dependent activation of Xist induction [38]. Rnf12 expression is upregulated upon differentiation to induce Xist upregulation at the onset of random XCI and then gradually reduced during the course of differentiation, indicating a differentiation-specific function of Rnf12 at the onset of random XCI. ChIP analysis showed the pluripotent factors Nanog, Sox2 and Oct4 bind together upstream of Rnf12 and may play a role in the repression of Rnf12 in undifferentiated ES cells, suggesting a further restriction of Rnf12 function upon differentiation [95]. Although Rnf12 was originally proposed to activate Xist directly in random XCI [57], more recent studies have indicated that Rnf12 regulates Tsix expression by ubiquitination-mediated proteasomal degradation of REX1, a putative transcription factor for Tsix, at the initiation of random XCI [65,91]. Although Rnf12 is essential for Xist upregulation in imprinted XCI, knocking out Rnf12 leads to only a mild impact on Tsix expression; thus, it is likely that Tsix repression by Rnf12 is not involved in activation of Xist expression in the embryonic day 3.5–4.5 blastocyst [94]. Two prominent REX1 binding regions reside within Xist/Tsix. one exists between Xist promoter P1 and P2 while the other exists between Tsix promoter and DXPas34. Although it remains unknown how RNF12 directly functions on Xist expression, REX1 might act as a repressor at the Xist promoter in opposition to its putative function as a transcriptional activator for Tsix because REX1 binds upstream of the differentiation-specific Xist promoter P2. However, recent in vivo studies have provided some evidence to show that Rnf12 is dispensable in random XCI [96]. Conditional knockout of Rnf12 in female embryos post imprinted XCI but before random XCI did not result in any defects in the embryos nor reduced fertility. It is possible that a compensation mechanism induces XCI for normal embryonic development since Rnf12 heterozygous knockout female ES cells undergo delayed XCI but can be induced to random XCI upon differentiation [38].
2.2.3. Boundary element separates functionally distinct TAD
The boundary between Xist and Tsix TADs resides in the Xist/Tsix region. Deletion of Xist-Xite, including this putative boundary region led to increased contact between Xist and Tsix TADs, which is not observed in wildtype, and an altered organization of Tsix TAD, suggesting the boundary region is essential for partitioning and compartmentalization of two distinct TADs [37]. Interestingly, within this putative boundary region, the RS14 element has been previously identified at the 3’-end of Xist [58]. RS14 exhibits high cross-species conservation among eutherian mammals, implying the critical function of this element. RS14 is associated with the DNasel hypersensitive site and contains multiple binding sites of CTCF, a critical factor for genome architecture and gene expression. One of the CTCF binding sites within RS14 has been shown to bind with CTCF in vivo and in vitro and functions as a chromatin insulator and boundary factor in reporter assay. The deletion of RS14 failed to induce Xist upregulation and instead retained a high expression of Tsix from the mutant X in a female-specific manner; hence, RS14 is presumed to be a boundary element to separate Xist and Tsix ACHs for proper Xist and Tsix expression. Further studies are required to reveal whether RS14 is a primary functional boundary element which is necessary for the division of two functionally distinct Xist and Tsix TADs. A recent report using CLIP-seq (UV crosslinking and immunoprecipitation followed by deep sequencing) indicated that CTCF binds thousands of RNAs including Xist, Tsix and Xite RNAs in mouse ES cells [97]. Knockdown of Tsix and Xite RNAs resulted in reduced occupancy of CTCF at known CTCF binding sites within the Tsix and Xite region, indicating the potential role of RNA in targeting CTCF. It has been shown that CTCF has multiple roles in XCI: transcriptional regulation of Xist [56], X-chromosome pairing through binding to the Tsix and Xite loci at the onset of random XCI [98–100], and service as a putative boundary factor between the Xist and Tsix TADs/ACHs [58]. Because CTCF binds to multiple sites across the Xic, it may also be a contributor to the organization of Xist and Tsix TADs for the coordination of precise Xist transcriptional regulation.
3. Mechanism of Xist RNA-mediated gene silencing on the Xi
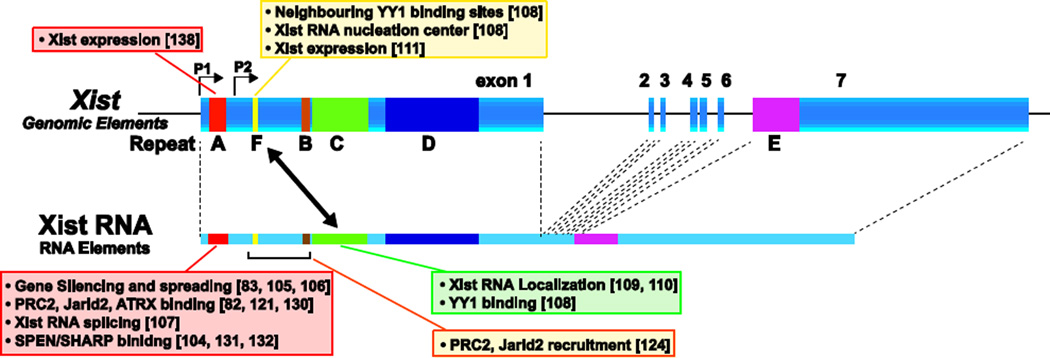
As mentioned above, monoallelic upregulation of Xist expression triggers random XCI. It is not entirely clear how Xist RNA spreads and covers across the future Xi and recruits chromatin modifying enzymes to the Xi. The epigenetic landscape at the Xi undergoes a number of repressive epigenetic modifications such as H3K27me3 and H2AK119ub, brought about by the chromatin modifying enzymes PRC2 and PRC1, respectively. Within the Xist gene, six xist-specific repetitive elements (repeats A-F) that are conserved among eutherian mammals, have been identified (Fig. 2) [39,101–103]. These unique repeat elements make Xist RNA a hub for a variety of protein factors, which induce multiple layers of repressive modification on the Xi. Repeat A was identified as a crucial element for gene silencing using an inducible Xist transgene system [83], and has been the most intensively studied. In addition to induction of gene silencing, the repeat A region of Xist RNA is considered to be a multi-functional element which serves many important roles including: acting as a hub for several chromatin modifying enzymes [82,104], spreading Xist RNA across the Xi [105,106], repositioning active gene regions into the Xist silenced compartment [48], and splicing Xist RNA [107]. All repeat elements reside within exon 1, which is known to be the largest exon, except for repeat E which resides in the second largest exon, exon 6 in humans and exon 7 in mice. These conserved repeat domains interact with various proteins and confer a wide variety of function to Xist RNA, as described below.
Fig. 2.
Xist repeat elements confer a variety of function to Xist. Summary of function of Xist repeat elements within Xist RNA and the Xist gene. While a majority of essential repeat elements on the Xist gene and Xist RNA are located across exonl, many have not yet been explored across the Xist gene and Xist RNA. Colored bold line indicates Xist exon structure and Xist repeat elements (A-F). Xist RNA is shown below the Xist gene map. Function of each repeat in genomic elements within Xist and Xist RNA are shown above and below the map, respectively, with reference numbers in brackets. Two-headed arrow indicates interaction between a genomic element within Xist and Xist RNA through YY1.
3.1. Role of Xist repeat elements in Xist RNA targeting onto the Xi
Although the detailed molecular mechanism underlying c/s-limited action of Xist RNA remains unknown, a recent report proposes that the transcription factor YY1 (Yin Yang 1) anchors Xist RNA within the Xist gene locus to function as a nucleation center for chromosome-wide Xist RNA spreading and X-linked gene silencing in cis [108]. In vivo and in vitro data indicates that YY1 binds to Xist RNA through Xist RNA repeat C as well as DNA through YY1 binding sites that are proximal to Xist repeat F. Further evidence supports repeat C in Xist RNA playing an important role for Xist RNA localization on the Xi. When the function of Xist RNA repeat C is interfered with by peptide nucleic acids (PNAs) or locked nucleic acids (LNAs), which are complementary to the repeat C domain of Xist RNA, then Xist RNA is displaced from the Xi and a loss of PRC2 complex localization occurs [109,110]. YY1 and its binding sites proximal to repeat F are also shown to be essential for Xist upregulation upon differentiation, although previous studies have indicated that YY1 does not affect Xist expression but its localization [108,111]. In addition to YY1, hnRNP U (also called Saf-A/SP120) is known to be a protein factor required for chromosome-wide Xist RNA localization [112]. Since hnRNP U has DNA- and RNA-binding domains at its N- and C-terminal, respectively, and both domains are essential for Xist RNA localization on the Xi, it has been proposed that hnRNP U anchors Xist RNA at the scaffold/matrix attachment region (S/MAR) on the Xi. Since hnRNP U localization on the Xi depends on Xist RNA, hnRNP U does not seem to simply serve as a bridge between MAR/SAR on the Xi. Cooperative action such as the formation of a ribonucleoprotein complex between hnRNP U and Xist RNA might be required for Xist RNA recruitment to the Xi. The interplay between YY1 and hnRNP U might facilitate the efficient localization and spreading of Xist RNA and X-linked gene silencing.
Recent attempts to delineate the spreading mechanism of Xist RNA and X-linked gene silencing across the Xi have revealed an unique orderly fashion of Xist RNA and PRC2 spreading upon differentiation [5,51,106]. Using ChlP-seq (chromatin immunoprecipitation with deep sequencing) of Ezh2, it has been revealed that PRC2 binds to a limited number of sites (about 1,500) frequently associated with H3K4me3/H3K27me3 bivalent domains and CpG islands, which are followed by additional binding sites (about 4,000) at intergenic regions and spreading across the Xi as differentiation progresses [5]. Furthermore, recent technical advancements have contributed to uncovering the detailed molecular mechanism of Xist RNA spreading. Using biotinylated antisense oligonucleotides to Xist RNA, CHART-seq (capture hybridization analysis of RNA targets with deep sequencing) and RAP (RNA antisense purification), a genome-wide chromatin region associated with Xist RNA has been isolated and analyzed [51,106]. Binding of PRC2 onto the Xi is correlated with Xist RNA recruitment and H3K27me3 deposition. Interestingly, before the discovery of Xist, the well-ordered spreading of XCI had been proposed as the ‘way-station model,’ wherein X-linked gene silencing happens at a number of way-stations across the X-chromosome and then spreads outward along adjacent regions [113]. Similar to this model, the quick and efficient propagation of Xist RNA across the Xi is driven by utilizing spatially proximal regions to the Xic and spreading towards distal regions which serve as way-stations to further gene silencing at the onset of XCI [51,106,114]. Consistent with the previous observation of inefficient Xist RNA spreading in the repeat A mutant Xist [105], RAP analysis using an inducible Xist transgene lacking repeat A showed a reduction in Xist RNA occupancy levels across the Xi, implicating the role of repeat A in the spreading of Xist RNA across the Xi [106]. Interestingly, the repeat A-mutant Xist RNA accumulation was observed on the edges of the active gene-dense. A previous study also showed that transcriptionally active gene regions on the Xi are looped out of the silencing compartment created by Xist RNA lacking repeat A [48]. These results indicate the role of repeat A in spreading Xist RNA to the active gene region for induction of gene silencing. Because repeat A is known as a hub of various chromatin modifying enzymes [82,104], chromatin modifications which are induced in an Xist RNA-dependent manner might be recognized by other Xist RNA-binding factors, thus cooperatively facilitating Xist RNA spreading across the Xi.
3.2. Xist RNA-mediated PRC2 recruitment to the Xi
Previous studies have shown that PRC2 is recruited to the Xi and deposits H3K27me3, one of the hallmarks of facultative heterochromatin, in an Xist RNA-dependent manner [46,47,105]. However, the molecular mechanism behind PRC2 recruitment to the Xi (target chromatin sites) by Xist RNA remains disputed. PRC2 binds to a variety of RNA, which raises the question of how it discriminates between bound and unbound RNA species without a distinct consensus binding sequence [115–119]. Recently, in vivo UV cross-linking and immunoprecipitation (CLIP) approaches revealed that PRC2 binds to the 5’ region of nascent RNA from a number of genes [117]. Interestingly, the majority of Ezh2-bound nascent RNAs (ezRNAs) originate from transcriptionally active genes with moderate PRC2 binding but without H3K27me3. In the model proposed, PRC2 interacts with a majority of transcription start sites and surveys the transcription state by interacting with ezRNAs. Interaction with ezRNA inhibits the methyltransferase activity of PRC2. Once transcription is repressed, extinction of ezRNA promotes H3K27me3 deposition by PRC2, followed by stable maintenance of gene silencing. The function of ezRNA seems to be restricted at the promoter where ezRNA is transcribed. Since Xist RNA plays a role in repressing chromosome-wide X-linked genes, the molecular mechanism behind Xist RNA’s recruitment of PRC2 to its target loci might be distinct from the mechanism driving ezRNAs. The PRC2 core complex consists of four subunits: SUZ12, EED, RBBP4/7, and catalytic subunit EZH2. Among the four core components of PRC2, EZH2 and SUZ12 exhibit RNA binding activity against Xist RNA in vivo and in vitro [82,84,115,116,120]. Repeat A in the 5’ of Xist RNA is crucial for induction of X-linked gene silencing, and repeat A RNA (RepA RNA) containing two long stem-loop structures has been identified as a PRC2 binding motif [82,83]. Since induced mutant Xist expression lacking repeat A can still recruit PRC2 to the Xi, albeit only as a small Xist RNA cloud and weak H3K27me3 signal [105], repeat A is not the sole Xist RNA domain to recruit PRC2 to the Xi. Recent studies using in vitro RNA electrophoretic mobility shift assays (EMSAs) indicated that PRC2 exhibits promiscuous binding but has a higher binding affinity to RepA RNA [118,121,122]. The catalytic subunit of PRC2, EZH2, binds to RNA in a somewhat non-specific manner, but its combination with the other PRC2 components EED and SUZ12 enhances the specificity of EZH2 binding to target RNAs, including RepA RNA [121]. Interestingly, RNA binding to PRC2 inhibits the methyltransferase activity of PRC2, suggesting that RNA is involved in not only PRC2 recruitment to its targets but also regulation of histone methyltransferase activity [121,123].
Jarid2, a co-factor of the PRC2, is involved in recruiting PRC2 onto the Xi [121,124]. Jarid2 is known to possess both RNA and nucleosome binding activity, regulate PRC2 recruitment to chromatin, and activate the enzymatic activity of PRC2 [123,125–129]. Since Jarid2 depletion by shRNA knockdown resulted in impaired recruitment of PRC2 and H3K27me3 modification to the Xi, Jarid2 plays an important role in PRC2 recruitment to the Xi in an Xist RNA-dependent manner [124]. The region corresponding to repeat F through B in Xist RNA is considered as minimum essential region for JARID2 and PRC2 accumulation to the Xi, although repeat A is also proposed as a JARID2 binding site [121,124]. As mentioned above, while Xist RNA is essential for PRC2 targeting to the Xi, PRC2-RNA interaction represses histone methyltransferase activity of PRC2 [121,123]. JARID2 promotes PRC2 histone methyltransferase activity by attenuating PRC2-RNA interaction and promoting PRC2 binding to chromatin [121,123,128], suggesting that JARID2 is a crucial player for PRC2 recruitment to the Xi and for the regulation of PRC2 enzymatic activation.
ATRX, known as a member of the SWI/SNF chromatin remodeler family, also binds to the repeat A motif and promotes localization of the PRC2 to the Xi [130]. Since ATRX knockdown in female mouse embryonic fibroblast (MEF) and Xist-inducible female ES cells resulted in severely impaired Ezh2 localization and H3K27me3 accumulation on the Xi, the role of ATRX in Xist RNA-dependent PRC2 recruitment to the Xi is proposed. In vitro experiments proved that ATRX has a high affinity for both RepA RNA and double strand DNA. Since inducible-Xist expression lacking repeat A abolished the localization of ATRX to the Xist locus, RepA RNA is essential for ATRX targeting to the Xi. Furthermore, in the presence of ATP, ATRX enhances the binding of PRC2 to RepA RNA; meanwhile, ATRX itself binds less well with RNA and DNA in the presence of ATP. On the basis of these findings, it is proposed that ATRX binds to RepA RNA and remodels its configuration, which in turn promotes PRC2 binding to RepA RNA. ATRX ChlP-seq data showed that the distribution of ATRX overlaps with that of EZH2, H3K27me3 and Xist RNA, suggesting the link between ATRX and Xist RNA-dependent PRC2 recruitment.
Collectively, PRC2 recruitment and spreading in XCI is a complex process regulated by multiple co-factors. Induction of Xist triggers downstream processes, including the concerted post-translational histone modifications and dynamic alteration of chromatin structure. It is interesting and should be delineated how these epigenetic modifications communicate and are coordinated to establish the highly coordinated heterochromatic landscape of the Xi.
3.3. SPEN/SHARP as a Novel Xist Repeat A binding Protein
Most recently three studies utilizing ChlRP-MS (Chromatin isolation of RNA purification-mass spectrometry), RAP-MS (RNA antisense purification-mass spectrometry) and iDRiP (identification of direct RNA interacting proteins), which are potential ways of harnessing the protein interactome associated with any RNA of interest, were able to comprehensively identify various potential Xist RNA binding proteins [104,131,132]. Two of them, hnRNP U and SPEN (mouse homolog of Drosophila Split ends)/SHARP (SMAT and HDAC associated repressor protein), were common in all three of the independent works. While hnRNP U was already known to bind directly with Xist RNA and have an essential role in Xist RNA localization on the Xi [112], SPEN/SHARP was novel and its critical function in XCI was unknown. SPEN/SHARP is a transcriptional repressor that recruits histone deacetylase (HDAC) complexes and contains RNA-recognition motifs [133,134]. Interestingly, SPEN/SHARP could not bind to Xist RNA lacking repeat A, suggesting specific binding of SPEN/SHARP to repeat A, a region which is essential for Xist RNA-mediated gene silencing [83,104]. Furthermore, knockdown of SPEN/SHARP did not affect Xist RNA localization but instead resulted in a failure to exclude RNA polymerase II from the Xi territory, poor recruitment of the PRC2 complex to the Xi, and compromised X-linked gene silencing. SPEN/SHARP is known to interact with various repressor complexes such as the SMRT nuclear compressor complex that interacts with HDAC3 histone deacetylase and the MBD3-NuRD (Nucleosome Remodeling and Deacetylation) complex [133,135,136]. Similar to SPEN/SHARP, HDAC3 knockdown also led to defective X-linked gene silencing with normal Xist RNA localization, suggesting that Xist RNA can induce X-linked gene silencing through HDAC activity recruited to the Xi by interaction between SPEN/SHARP and repeat A of Xist RNA. Because deacetylation of H3K27ac can permit H3K27me3 modification by PRC2 and gene silencing [137], SPEN/SHARP repressor complex-mediated deacetylation of H3K27ac might be essential to trigger H3K27me3 deposition onto the Xi at the onset of XCI.
Interestingly, iDRiP only identified PRC1 and PRC2 components while ChlRP-MS identified components of PRC1 but not PRC2 [82,121,122]. ChlRP-MS, RAP-MS and iDRiP identified 30, 10 and ~250 proteins, respectively, as potential Xist RNA binding proteins. Only three proteins were commonly isolated among these three approaches, suggesting that various experimental conditions used in different methods could largely affect the isolation of RNA-protein complexes. It is curious how Xist RNA binding proteins identified in the recent works interact with and cooperatively play with proteins such as PRC2, which is recruited to the Xi in a Xist RNA-dependent manner to establish facultative heterochromatin on the Xi.
Conclusions and perspectives
Over the past decade, our knowledge about IncRNA biology has increased intensely, owing to advances and developments in analytical tools and techniques. Further advancements in both the techniques to investigate and in the instruments to analyze IncRNAs would propel forward our understanding of their function in various biological processes including XCI. Indeed, most recently a study utilizing ChlRP-MS, RAP-MS and iDRiP were able to comprehensively identify novel Xist-RNA binding proteins [104,131,132]. XCI is executed through a complex molecular mechanism in which multiple IncRNAs, protein factors and chromosomal elements are involved. Discovery of novel IncRNAs and their associated proteins, as well as characterizing IncRNAs at the Xic, could provide additional insight towards the molecular mechanism of IncRNA function in XCI. Further investigations in this direction could enhance our understanding and delineate the detailed molecular mechanism underlying the establishment of such highly ordered and robust repressive Xi.
Highlights.
X-chromosome inactivation is an excellent model of long noncoding RNA (IncRNA)-mediated gene regulation. This review summarizes recent updates in our knowledge of IncRNAs and protein factors found at the X-inactivation center (Xic) and discusses their overall mechanisms of action in inducing monoallelic Xist expression. We also discuss our current understanding of the molecular mechanism behind Xist RNA-mediated induction of the repressive epigenetic landscape at the inactive X-chromosome.
Acknowledgements
We would like to thank all Ogawa lab members for helpful comments and Serenity Curtis for editing the manuscript. This work was supported by grants from the NIH (R01-GM102184) and the March of Dimes Research Foundation (#6-FY12-337) to Y.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L) Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, et al. Site-specific silencing of regulatory elements as a mechanism of x inactivation. Cell. 2012;151:951–963. doi: 10.1016/j.cell.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinter SF, Sadreyev R, Yildirim E, Jeon Y, Ohsumi TK, Borowsky M, et al. Spreading of X chromosome inactivation via a hierarchy of defined Polycomb stations. Genome Res. 2012;22:1864–1876. doi: 10.1101/gr.133751.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamoto I, Heard E, Patrat C, Thepot D, Peynot N, Fauque P, et al. Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature. 2011 doi: 10.1038/nature09872. [DOI] [PubMed] [Google Scholar]
- 7.Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science. 2004;303:644–649. doi: 10.1126/science.1092727. [DOI] [PubMed] [Google Scholar]
- 9.Huynh KD, Lee JT. Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature. 2003;426:857–862. doi: 10.1038/nature02222. [DOI] [PubMed] [Google Scholar]
- 10.Takagi N, Sasaki M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature. 1975;256:640–642. doi: 10.1038/256640a0. [DOI] [PubMed] [Google Scholar]
- 11.Patrat C, Okamoto I, Diabangouaya P, Vialon V, Le Baccon P, Chow JC, et al. Dynamic changes in paternal X-chromosome activity during imprinted X-chromosome inactivation in mice. Proc Natl Acad Sci USA. 2009;106:5198–5203. doi: 10.1073/pnas.0810683106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mak W, Nesterova TB, de Napoles M, Appanah R, Yamanaka S, Otte AP, et al. Reactivation of the paternal X chromosome in early mouse embryos. Science. 2004;303:666–669. doi: 10.1126/science.1092674. [DOI] [PubMed] [Google Scholar]
- 13.Payer B, Hayashi K, Rosenberg M, Yamaji M, Yabuta Y, Koyanagi-Aoi M, et al. Tsix RNA and the Germline Factor, PRDM14, Link X Reactivation and Stem Cell Reprogramming. Mol Cell. 2013 doi: 10.1016/j.molcel.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monk M, Harper MI. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979;281:311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- 15.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci USA. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marahrens Y, Panning B, Dausman J, Strauss WM, Jaenisch R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 1997;11:156–166. doi: 10.1101/gad.11.2.156. [DOI] [PubMed] [Google Scholar]
- 17.Yildirim E, Kirby JE, Brown DE, Mercier FE, Sadreyev R, Scadden DT, et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell. 2013;152:727–742. doi: 10.1016/j.cell.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinn J, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br. J. Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 21.Heward JA, Lindsay MA. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014;35:408–419. doi: 10.1016/j.it.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastan S. Non-random X-chromosome inactivation in mouse X-autosome translocation embryos-location of the inactivation centre. J Embryol Exp Morphol. 1983;78:1–22. [PubMed] [Google Scholar]
- 23.Rastan S, Robertson E. X-chromosome deletions in embryo-derived (EK) cell lines associated with lack of X-chromosome inactivation. J Embryol Exp Morphol. 1985;90:379–388. [PubMed] [Google Scholar]
- 24.Brown CJ, Lafreniere RG, Powers VE, Sebastio G, Ballabio A, Pettigrew AL, et al. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature. 1991;349:82–84. doi: 10.1038/349082a0. [DOI] [PubMed] [Google Scholar]
- 25.Borsani G, Tonlorenzi R, Simmler MC, Dandolo L, Arnaud D, Capra V, et al. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991;351:325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- 26.Brockdorff N, Ashworth A, Kay GF, Cooper PJ, Smith S, McCabe VM, et al. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991;351:329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- 27.Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 28.Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 29.Lee JT, Strauss WM, Dausman J, Jaenisch R. A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell. 1996;86:83–94. doi: 10.1016/s0092-8674(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 30.Heard E, Kress C, Mongelard F, Courtier B, Rougeulle C, Ashworth A, et al. Transgenic mice carrying an Xist-containing YAC. Hum Mol Genet. 1996;5:441–450. doi: 10.1093/hmg/5.4.441. [DOI] [PubMed] [Google Scholar]
- 31.Lee JT, Jaenisch R. Long-range cis effects of ectopic X-inactivation centres on a mouse autosome. Nature. 1997;386:275–279. doi: 10.1038/386275a0. [DOI] [PubMed] [Google Scholar]
- 32.Heard E, Mongelard F, Arnaud D, Avner P. Xist yeast artificial chromosome transgenes function as X-inactivation centers only in multicopy arrays and not as single copies. Mol Cell Biol. 1999;19:3156–3166. doi: 10.1128/mcb.19.4.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto I, Heard E, Arnaud D, Le Baccon P, Otte AP, Disteche CM, et al. Evidence for de novo imprinted X-chromosome inactivation independent of meiotic inactivation in mice. Nature. 2005;438:369–373. doi: 10.1038/nature04155. [DOI] [PubMed] [Google Scholar]
- 34.Lee JT, Lu N, Han Y. Genetic analysis of the mouse X inactivation center defines an 80-kb multifunction domain. Proc Natl Acad Sci U S A. 1999;96:3836–3841. doi: 10.1073/pnas.96.7.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augui S, Nora EP, Heard E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet. 2011;12:429–442. doi: 10.1038/nrg2987. [DOI] [PubMed] [Google Scholar]
- 36.Maclary E, Hinten M, Harris C, Kalantry S. Long nonoding RNAs in the X-inactivation center. Chromosome Res. 2013;21:601–614. doi: 10.1007/s10577-013-9396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nora EP, Heard E, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonkers I, Barakat TS, Achame EM, Monkhorst K, Kenter A, Rentmeester E, et al. RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell. 2009;139:999–1011. doi: 10.1016/j.cell.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Brockdorff N, Ash worth A, Kay GF, McCabe VM, Norris DP, Cooper PJ, et al. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71:515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- 40.Johnston CM, Nesterova TB, Formstone E, Newall A, Duthie S, Sheardown SA, et al. Developmentally regulated Xist promoter switch mediates initiation of X inactivation. Cell. 1998;94:809–817. doi: 10.1016/s0092-8674(00)81739-0. [DOI] [PubMed] [Google Scholar]
- 41.Hong YK, Ontiveros SD, Chen C, Strauss WM. A new structure for the murine Xist gene and its relationship to chromosome choice/counting during X-chromosome inactivation. Proc Natl Acad Sci USA. 1999;96:6829–6834. doi: 10.1073/pnas.96.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memili E, Hong YK, Kim DH, Ontiveros SD, Strauss WM. Murine Xist RNA isoforms are different at their 3’ ends: a role for differential polyadenylation. Gene. 2001;266:131–137. doi: 10.1016/s0378-1119(01)00353-5. [DOI] [PubMed] [Google Scholar]
- 43.Ma M, Strauss WM. Analysis of the Xist RNA isoforms suggests two distinctly different forms of regulation. Mamm Genome. 2005;16:391–404. doi: 10.1007/s00335-004-2464-3. [DOI] [PubMed] [Google Scholar]
- 44.Kim JS, Choi HW, Arauzo-Bravo MJ, Schbler HR, Do JT. Reactivation of the inactive X chromosome and post-transcriptional reprogramming of Xist in iPSCs. J Cell Sci. 2015;128:81–87. doi: 10.1242/jcs.154294. [DOI] [PubMed] [Google Scholar]
- 45.Clemson CM, Willard HF, McNeil JA, Lawrence JB. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol. 1996;132:259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- 47.Silva J, Mak W, Zvetkova I, Appanah R, Nesterova TB, Webster Z, et al. Establishment of Histone H3 Methylation on the Inactive X Chromosome Requires Transient Recruitment of Eed-Enx1 Polycomb Group Complexes. Dev Cell. 2003;4:481–495. doi: 10.1016/s1534-5807(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 48.Chaumeil J, Le Baccon P, Wutz A, Heard E. A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev. 2006;20:2223–2237. doi: 10.1101/gad.380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell. 2004;7:663–676. doi: 10.1016/j.devcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Fang J, Chen T, Chadwick BP, Li E, Zhang Y. Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in initiation of X inactivation. J Biol Chem. 2004;279:52812–52815. doi: 10.1074/jbc.C400493200. [DOI] [PubMed] [Google Scholar]
- 51.Simon MD, Pinter SF, Fang R, Sarma K, Rutenberg-Schoenberg M, Bowman SK, et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature. 2013;504:465–469. doi: 10.1038/nature12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow JC, Heard E. X inactivation and the complexities of silencing a sex chromosome. Curr Opin Cell Biol. 2009;21:359–366. doi: 10.1016/j.ceb.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Wutz A. Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat Rev Genet. 2011;12:542–553. doi: 10.1038/nrg3035. [DOI] [PubMed] [Google Scholar]
- 54.Tsai C-L, Rowntree RK, Cohen DE, Lee JT. Higher order chromatin structure at the X-inactivation center via looping DNA. Dev Biol. 2008;319:416–425. doi: 10.1016/j.ydbio.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian D, Sun S, Lee JT. The long noncoding RNA Jpx is a molecular switch for X chromosome inactivation. Cell. 2010;143:390–403. doi: 10.1016/j.cell.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun S, Del Rosario BC, Szanto A, Ogawa Y, Jeon Y, Lee JT. Jpx RNA Activates Xist by Evicting CTCF. Cell. 2013;153:1537–1551. doi: 10.1016/j.cell.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barakat TS, Gunhanlar N, Pardo CG, Achame EM, Ghazvini M, Boers R, et al. RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet. 2011;7:e1002001. doi: 10.1371/journal.pgen.1002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spencer RJ, Del Rosario BC, Pinter SF, Lessing D, Sadreyev R, Lee JT. A Boundary Element Between Tsix and Xist Binds the Chromatin Insulator Ctcf and Contributes to Initiation of X Chromosome Inactivation. Genetics. 2011;189:441–454. doi: 10.1534/genetics.111.132662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JT, Lu N. Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell. 1999;99:47–57. doi: 10.1016/s0092-8674(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 60.Sado T, Wang Z, Sasaki H, Li E. Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development. 2001;128:1275–1286. doi: 10.1242/dev.128.8.1275. [DOI] [PubMed] [Google Scholar]
- 61.Lee JT. Disruption of imprinted X inactivation by parent-of-origin effects at Tsix. Cell. 2000;103:17–27. doi: 10.1016/s0092-8674(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 62.Lee JT, Davidow LS, Warshawsky D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nat Genet. 1999;21:400–404. doi: 10.1038/7734. [DOI] [PubMed] [Google Scholar]
- 63.Navarro P, Chambers I, Karwacki-Neisius V, Chureau C, Morey C, Rougeulle C, et al. Molecular Coupling of Xist Regulation and Pluripotency. Science. 2008;321:1693–1695. doi: 10.1126/science.1160952. [DOI] [PubMed] [Google Scholar]
- 64.Donohoe ME, Silva SS, Pinter SF, Xu N, Lee JT. The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature. 2009;460:128–132. doi: 10.1038/nature08098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gontan C, Achame EM, Demmers J, Barakat TS, Rentmeester E, van Ijcken W, et al. RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature. 2012;485:386–390. doi: 10.1038/nature11070. [DOI] [PubMed] [Google Scholar]
- 66.Navarro P. Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X-chromosome inactivation. Genes Dev. 2005;19:1474–1484. doi: 10.1101/gad.341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sado T, Hoki Y, Sasaki H. Tsix silences Xist through modification of chromatin structure. Dev Cell. 2005;9:159–165. doi: 10.1016/j.devcel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Sun BK, Deaton A, Lee JT. A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol Cell. 2006;21:617–628. doi: 10.1016/j.molcel.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 69.Ohhata T, Hoki Y, Sasaki H, Sado T. Crucial role of antisense transcription across the Xist promoter in Tsix-mediated Xist chromatin modification. Development. 2008;135:227–235. doi: 10.1242/dev.008490. [DOI] [PubMed] [Google Scholar]
- 70.Ohhata T, Hoki Y, Sasaki H, Sado T. Tsix-deficient X chromosome does not undergo inactivation in the embryonic lineage in males: implications for Tsix-independent silencing of Xist. Cytogenet Genome Res. 2006;113:345–349. doi: 10.1159/000090851. [DOI] [PubMed] [Google Scholar]
- 71.Shibata S, Yokota T, Wutz A. Synergy of Eed and Tsix in the repression of Xist gene and X-chromosome inactivation. Embo J. 2008;27:1816–1826. doi: 10.1038/emboj.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gayen S, Maclary E, Buttigieg E, Hinten M, Kalantry S. A Primary Role for the Tsix IncRNA in Maintaining Random X-Chromosome Inactivation. Cell Rep. 2015;11:1251–1265. doi: 10.1016/j.celrep.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen DE, Davidow LS, Erwin JA, Xu N, Warshawsky D, Lee JT. The DXPas34 repeat regulates random and imprinted X inactivation. Dev Cell. 2007;12:57–71. doi: 10.1016/j.devcel.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Ogawa Y, Sun BK, Lee JT. Intersection of the RNA interference and X-inactivation pathways. Science. 2008;320:1336–1341. doi: 10.1126/science.1157676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morey C, Navarro P, Debrand E, Avner P, Rougeulle C, Clerc P. The region 3I[prime]I to Xist mediates X chromosome counting and H3 Lys-4 dimethylation within the Xist gene. Embo J. 2004;23:594–604. doi: 10.1038/sj.emboj.7600071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sado T, Li E, Sasaki H. Effect of TSIX disruption on XIST expression in male ES cells. Cytogenet Genome Res. 2002;99:115–118. doi: 10.1159/000071582. [DOI] [PubMed] [Google Scholar]
- 77.Maclary E, Buttigieg E, Hinten M, Gayen S, Harris C, Sarkar MK, et al. Differentiation-dependent requirement of Tsix long non-coding RNA in imprinted X-chromosome inactivation. Nat Commun. 2014;5:4209. doi: 10.1038/ncomms5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogawa Y, Lee JT. Xite X-inactivation intergenic transcription elements that regulate the probability of choice. Mol Cell. 2003;11:731–743. doi: 10.1016/s1097-2765(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 79.Stavropoulos N, Rowntree RK, Lee JT. Identification of developmentaly specific enhancers for Tsix in the regulation of X chromosome inactivation. Mol Cell Biol. 2005;25:2757–2769. doi: 10.1128/MCB.25.7.2757-2769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anguera MC, Ma W, Clift D, Namekawa SH, Kelleher RJ, Lee JT. Tsx produces a long noncoding RNA has general functions in the germline, stem cells, and brain. PLoS Genet. 2011;7:e1002248. doi: 10.1371/journal.pgen.1002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simmler MC, Cunningham DB, Clerc P, Vermat T, Caudron B, Cruaud C, et al. A 94 kb genomic sequence 3’ to the murine Xist gene reveals an AT rich region containing a new testis specific gene Tsx. Hum Mol Genet. 1996;5:1713–1726. doi: 10.1093/hmg/5.11.1713. [DOI] [PubMed] [Google Scholar]
- 82.Zhao J, Sun BK, Erwin JA, Song J-J, Lee JT. Polycomb Proteins Targeted by a Short Repeat RNA to the Mouse X Chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wutz A, Rasmussen TP, Jaenisch R. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet. 2002;30:167–174. doi: 10.1038/ng820. [DOI] [PubMed] [Google Scholar]
- 84.Maenner S, Blaud M, Fouillen L, Savoye A, Marchand V, Dubois A, et al. 2-D structure of the A region of Xist RNA and its implication for PRC2 association. Plos Biol. 2010;8:e1000276. doi: 10.1371/journal.pbio.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chureau C, Prissette M, Bourdet A, Barbe V, Cattolico L, Jones L, et al. Comparative sequence analysis of the X-inactivation center region in mouse, human and bovine. Genome Res. 2002;12:894–908. doi: 10.1101/gr.152902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnston CM, Newall AET, Brockdorff N, Nesterova TB. Enox a novel gene that maps 10 kb upstream of Xist and partially escapes X inactivation. Genomics. 2002;80:236–244. doi: 10.1006/geno.2002.6819. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi S, Totoki Y, Soma M, Matsumoto K, Fujihara Y, Toyoda A, et al. Identification of an imprinted gene cluster in the x-inactivation center. PLoS ONE. 2013;8:e71222. doi: 10.1371/journal.pone.0071222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chureau C, Chantalat S, Romito A, Galvani A, Duret L, Avner P, et al. Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet. 2011;20:705–718. doi: 10.1093/hmg/ddq516. [DOI] [PubMed] [Google Scholar]
- 89.Soma M, Fujihara Y, Okabe M, Ishino F, Kobayashi S. Ftx is dispensable for imprinted X-chromosome inactivation in preimplantation mouse embryos. Sci Rep. 2014;4:5181. doi: 10.1038/srep05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Augui S, Filion GJ, Huart S, Nora EP, Guggiari M, Maresca M, et al. Sensing X chromosome pairs before X inactivation via a novel X-pairing region of the Xic. Science. 2007;318:1632–1636. doi: 10.1126/science.1149420. [DOI] [PubMed] [Google Scholar]
- 91.Barakat TS, Loos F, van Staveren S, Myronova E, Ghazvini M, Grootegoed JA, et al. The Trans-Activator RNF12 and Cis-Acting Elements Effectuate X Chromosome Inactivation Independent of X-Pairing. Mol Cell. 2014 doi: 10.1016/j.molcel.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Friesema ECH, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 93.Dumitrescu AM, Liao X-H, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shin J, Bossenz M, Chung Y, Ma H, Byron M, Taniguchi-lshigaki N, et al. Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature. 2010;467:977–981. doi: 10.1038/nature09457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Navarro P, Moffat M, Mullin NP, Chambers I. The X-inactivation trans-activator Rnf 12 is negatively regulated by pluripotency factors in embryonic stem cells. Hum Genet. 2011 doi: 10.1007/s00439-011-0998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin J, Wallingford MC, Gallant J, Marcho C, Jiao B, Byron M, et al. RLIM is dispensable for X-chromosome inactivation in the mouse embryonic epiblast. Nature. 2014 doi: 10.1038/nature13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kung JT, Kesner B, An JY, Ahn JY, Cifuentes-Rojas C, Colognori D, et al. Locus-specific targeting to the X chromosome revealed by the RNA interactome of CTCF. Mol Cell. 2015;57:361–375. doi: 10.1016/j.molcel.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu N, Donohoe ME, Silva SS, Lee JT. Evidence that homologous X-chromosome pairing requires transcription and Ctcf protein. Nat Genet. 2007;39:1390–1396. doi: 10.1038/ng.2007.5. [DOI] [PubMed] [Google Scholar]
- 99.Xu N, Tsai C-L, Lee JT. Transient homologous chromosome pairing marks the onset of X inactivation. Science. 2006;311:1149–1152. doi: 10.1126/science.1122984. [DOI] [PubMed] [Google Scholar]
- 100.Bacher CP, Heard E, Guggiari M, Brors B, Augui S, Clerc P, et al. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat Cell Biol. 2006;8:293–299. doi: 10.1038/ncb1365. [DOI] [PubMed] [Google Scholar]
- 101.Nesterova TB, Slobodyanyuk SY, Elisaphenko EA, Shevchenko AI, Johnston CM, Pavlova ME, et al. Characterization of the genomic Xist locus in rodents reveals conservation of overall gene structure and tandem repeats but rapid evolution of unique sequence. Genome Res. 2001;11:833–849. doi: 10.1101/gr.174901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yen ZC, Meyer IM, Karalic S, Brown CJ. A cross-species comparison of X-chromosome inactivation in Eutheria. Genomics. 2007;90:453–463. doi: 10.1016/j.ygeno.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 103.Elisaphenko EA, Kolesnikov NN, Shevchenko AI, Rogozin IB, Nesterova TB, Brockdorff N, et al. A dual origin of the Xist gene from a protein-coding gene and a set of transposable elements. PLoS ONE. 2008;3:e2521. doi: 10.1371/journal.pone.0002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu C, Zhang QC, da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, et al. Systematic discovery of xist RNA binding proteins. Cell. 2015;161:404–416. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kohlmaier A, Savarese F, Lachner M, Martens J, Jenuwein T, Wutz A. A Chromosomal Memory Triggered by Xist Regulates Histone Methylation in X Inactivation. Plos Biol. 2004;2:e171. doi: 10.1371/journal.pbio.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engreitz JM, Pandya-Jones A, Mcdonel P, Shishkin A, Sirokman K, Surka C, et al. The Xist IncRNA Exploits Three-Dimensional Genome Architecture to Spread Across the X Chromosome. Science. 2013;341:1237973–1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Royce-Tolland ME, Panning B, Andersen AA, Koyfman HR, Talbot DJ, Wutz A, et al. The A-repeat links ASF/SF2-dependent Xist RNA processing with random choice during X inactivation. Nat Struct Mol Biol. 2010;17:948–954. doi: 10.1038/nsmb.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeon Y, Lee JT. YY1 Tethers Xist RNA to the Inactive X Nucleation Center. Cell. 2011;146:119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beletskii A, Hong YK, Pehrson J, Egholm M, Strauss WM. PNA interference mapping demonstrates functional domains in the noncoding RNA Xist. Proc Natl Acad Sci USA. 2001;98:9215–9220. doi: 10.1073/pnas.161173098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sarma K, Levasseur P, Aristarkhov A, Lee JT. Locked nucleic acids (LNAs) reveal sequence requirements and kinetics of Xist RNA localization to the X chromosome. Proc Natl Acad Sci U S A. 2010;107:22196–22201. doi: 10.1073/pnas.1009785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makhlouf M, Ouimette J-F, Oldfield A, Navarro P, Neuillet D, Rougeulle C. A prominent and conserved role for YY1 in Xist transcriptional activation. Nat Commun. 2014;5 doi: 10.1038/ncomms5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hasegawa Y, Brockdorff N, Kawano S, Tsutui K, Tsutui K, Nakagawa S. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev Cell. 2010;19:469–476. doi: 10.1016/j.devcel.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 113.Gartier SM, Riggs AD, Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- 114.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanhere A, Viiri K, Araujo CC, Rasaiyaah J, Bouwman RD, Whyte WA, et al. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell. 2010;38:675–688. doi: 10.1016/j.molcel.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaneko S, Son J, Shen SS, Reinberg D, Bonasio R. PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells. Nat Struct Mol Biol. 2013;20:1258–1264. doi: 10.1038/nsmb.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davidovich C, Zheng L, Goodrich KJ, Cech TR. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol. 2013;20:1250–1257. doi: 10.1038/nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaneko S, Li G, Son J, Xu C-F, Margueron R, Neubert TA, et al. Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 2010;24:2615–2620. doi: 10.1101/gad.1983810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cifuentes-Rojas C, Hernandez AJ, Sarma K, Lee JT. Regulatory interactions between RNA and polycomb repressive complex 2. Mol Cell. 2014;55:171–185. doi: 10.1016/j.molcel.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davidovich C, Wang X, Cifuentes-Rojas C, Goodrich KJ, Gooding AR, Lee JT, et al. Toward a Consensus on the Binding Specificity and Promiscuity of PRC2 for RNA. Mol Cell. 2015;57:552–558. doi: 10.1016/j.molcel.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaneko S, Son J, Bonasio R, Shen SS, Reinberg D. Nascent RNA interaction keeps PRC2 activity poised and in check. Genes Dev. 2014;28:1983–1988. doi: 10.1101/gad.247940.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.da Rocha ST, Boeva V, Escamilla-Del-Arenal M, Ancelin K, Granier C, Matias NR, et al. Jarid2 Is Implicated in the Initial Xist-Induced Targeting of PRC2 to the Inactive X Chromosome. Mol Cell. 2014;53:301–316. doi: 10.1016/j.molcel.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 125.Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, et al. Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, et al. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li G, Margueron R, Ku M, Chambon P, Bernstein BE, Reinberg D. Jarid2 PRC2 partners in regulating gene expression. Genes Dev. 2010;24:368–380. doi: 10.1101/gad.1886410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaneko S, Bonasio R, Saldana-Meyer R, Yoshida T, Son J, Nishino K, et al. Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell. 2014;53:290–300. doi: 10.1016/j.molcel.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Son J, Shen SS, Margueron R, Reinberg D. Nucleosome-binding activities within JARID2 and EZH1 regulate the function of PRC2 on chromatin. Genes Dev. 2013;27:2663–2677. doi: 10.1101/gad.225888.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sarma K, Cifuentes-Rojas C, Ergun A, del Rosario A, Jeon Y, White F, et al. ATRX Directs Binding of PRC2 to Xist RNA and Polycomb Targets. Cell. 2014;159:869–883. doi: 10.1016/j.cell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McHugh CA, Chen C-K, Chow A, Surka CF, Tran C, Mcdonel P, et al. The Xist IncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015 doi: 10.1038/nature14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Minajigi A, Froberg JE, Wei C, Sunwoo H, Kesner B, Colognori D, et al. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science. 2015 doi: 10.1126/science.aab2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y, Downes M, Xie W, Kao H-Y, Ordentlich P, Tsai C-C, et al. Sharp an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev. 2001;15:1140–1151. doi: 10.1101/gad.871201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arieti F, Gabus C, Tambalo M, Huet T, Round A, Thore S. The crystal structure of the Split End protein SHARP adds a new layer of complexity to proteins containing RNA recognition motifs. Nucleic Acids Res. 2014;42:6742–6752. doi: 10.1093/nar/gku277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Ng H-H, Erdjument-Bromage H, Tempst P, Bird AP, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ariyoshi M, Schwabe JWR. A conserved structural motif reveals the essential transcriptional repression function of Spen proteins and their role in developmental signaling. Genes Dev. 2003;17:1909–1920. doi: 10.1101/gad.266203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reynolds N, Latos P, Hynes-Allen A, Loos R, Leaford D, O’Shaughnessy A, et al. NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment. Cell Stem Cell. 2012;10:583–594. doi: 10.1016/j.stem.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoki Y, Kimura N, Kanbayashi M, Amakawa Y, Ohhata T, Sasaki H, et al. A proximal conserved repeat in the Xist gene is essential as a genomic element for X-inactivation in mouse. Development. 2009;136:139–146. doi: 10.1242/dev.026427. [DOI] [PubMed] [Google Scholar]